Abstract
Immune suppression remains a consistent obstacle to successful anti-tumor immune responses. As tumors develop, they create a microenvironment that not only supports tumor growth and metastasis but also reduces potential adaptive immunity to tumor antigens. Among the many components of this tumor microenvironment is a population of dendritic cells which exert profound immune suppressive effects on T cells. In this review, we discuss our recent findings related to these tumor-associated dendritic cells and how targeting them may serve to generate more durable anti-tumor immune responses.
Keywords: Tumor microenvironment, T cell, Tolerance, Suppression, CITIM 2011
Immune tolerance, suppression, and tumors
One of the unique features of the immune system is its exquisite ability to discriminate between self and non-self antigens. Immune tolerance sets a balance between immunity to foreign antigens and immunity to self-antigens [1, 2]. Immune tolerance starts in the thymus, where most self-reactive T cells are eliminated, and because thymic selection is imperfect, it continues in the periphery. As a result, the repertoire of T cells with the capacity to recognize tumor antigens is somewhat restricted [3]. Most potential tumor antigens are “self,” non-mutated epitopes, and therefore, overcoming this balance may require induction of an immune response analogous to autoimmunity [4].
As tumors develop, they “work hard” to evade recognition by the immune system, essentially creating a tolerant microenvironment. This commonly involves down-regulation of MHC expression which renders them insensitive to T cells but may paradoxically increase susceptibility to NK cells. In addition, tumors can secrete factors that suppress T cell responsiveness. This includes the expression of immune suppressive or anti-inflammatory cytokines such as TGF-β and IL-10 as well as enzymes that catabolize amino acids that are critical for T cell effector functions (e.g., arginase and indoleamine-2,3-dioxygenase, IDO) [5–7]. Another trick that tumors use to escape the adaptive immune response is recruiting or converting inflammatory cells that suppress T cell responses. This includes regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSC), and tolerogenic dendritic cells (DC) [8]. These cells use a variety of mechanisms to inhibit T cells in both lymphoid tissues and in the tumor microenvironment (TME).
T cell tolerance to tumor antigens
It has been well documented that despite efficient priming, T cells often lose their responsiveness to tumor antigens [8, 9]. This may include a variety of mechanisms including cross-presentation of tumor antigens, suppression by regulatory T cells, or suppression by the factors described above. In any event, the TME represents a formidable barrier to maintaining T cell effector functions.
Previously, we reported that tumor-specific CD8+ T cells that enter into a developing tumor rapidly become tolerized (Fig. 1a). Using the TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model [10], we observed that CD8+ T cells specific for the TRAMP tumor antigen, SV40 T antigen (Tag), rapidly lost their ability to secrete IFN-γ and display reduced cytolytic ability, both are key features of T cell tolerance [9]. This loss of function was dependent on tumor infiltration as restricting T cells from leaving the lymph node using FTY720 (Fingolimod®, a sphingosine-1-phosphate receptor agonist), allowed retention of these effector functions, yet the small number of cells that escaped FTY720 and trafficked to the prostate were tolerized [11].
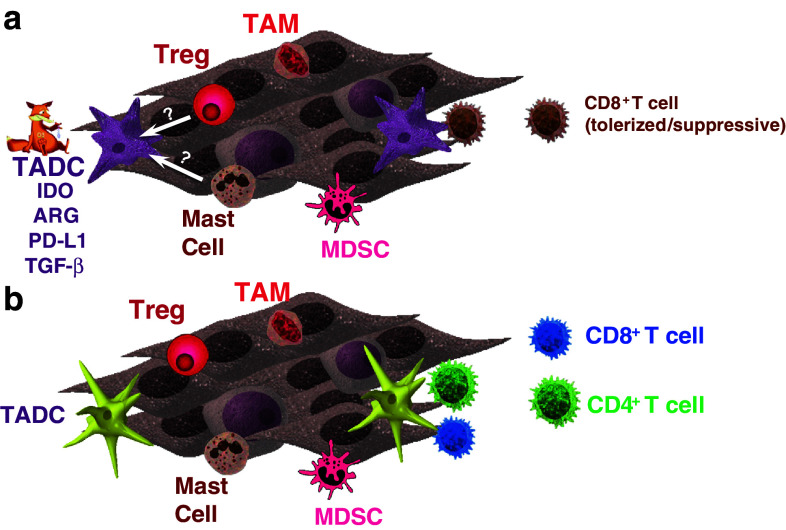
Fig. 1.
Tumor-associated dendritic cells regulate T cell responsiveness in the tumor microenvironment. a The prostatic tumor microenvironment (TME) contains a variety of inflammatory cells that contribute to immune suppression. Tumor-associated dendritic cells (TADCs) tolerize CD8+ T cells that enter the TME in a FOXO3-dependent mechanism. Induction of this tolerogenic behavior by TADCs may be mediated by Treg cells, mast cells, or tumor-derived factors. b Provision of tumor-specific CD4+ T cells alters the TME and prevents tolerization of CD8+ T cells. This is in part mediated by the activation of TADC and down-regulation of FOXO3 expression by TADC
Similarly striking was our observation that the tumor-specific CD8+ T cells also acquired the ability to suppress other T cells [11]. Again, infiltration into the tumor was required for the induction of this activity (Fig. 1a). These CD8 suppressor cells could reduce the proliferative response of both CD4+ and C8+ T cells in a TGF-β-dependent mechanism. Of interest was our observation that only a small subfraction of the tumor-infiltrating CD8+ T cells expressed FOXP3, the transcription factor previously associated with suppressive activity in natural regulatory T (Treg) cells. Thus, it is not clear whether FOXP3 directs suppression in TRAMP tumor-infiltrating CD8+ T cells, and this is currently under investigation in our laboratory.
In a separate study, we reported that provision of tumor-specific CD4+ T cell help can alter tolerization of CD8+ T cells [11]. A single co-transfer of the CD4+ T cells along with the CD8+ T cells delayed tolerization, while repeated delivery of CD4+ T cells (every 5 days) was capable of preventing CD8+ T cell tolerization. The CD8+ T cells isolated from “co-transferred” mice also had reduced suppressive function. The retained effector function was associated with reduced tumor burden. Our initial studies suggested that the CD4+ T cells entered into the developing tumor microenvironment and altered the resident antigen-presenting cells (APCs), making them more immune-stimulatory [12]. These findings led us to identify the population of cells responsible for tolerization of the T cells within the TME (Fig. 1b).
Tumor-associated dendritic cells tolerize T cells by a FOXO3-dependent mechanism
Upon examination of the leukocytes that infiltrate TRAMP tumors, we noted a population of DC with a phenotype consistent with plasmacytoid DC [13]. These tumor-associated DC (TADC) were B220+, CD317+, CD11cdim, and expressed low levels of the costimulatory ligands CD80 and CD86 and CD40. In addition, they expressed genes classically associated with immune suppression in tumors, including PD-L1, arginase (ARG), and IDO (Fig. 1a). These TADC represented approximately 30% of the total CD45+ cells and could be depleted using an antibody directed against CD317. Interestingly, a phenotypically similar population of pDC was found in the prostate of wild-type (WT) mice, although as described below, their function was profoundly different.
Based on the previous reports suggesting an immune suppressive role for pDC, we tested whether elimination of the TADC would lead to enhanced responsiveness of tumor-infiltrating T cells. When the tumor-specific CD8+ T cells were transplanted into TRAMP mice depleted of TADC, they retained effector function and had reduced suppressive activity, and the tumor burden was reduced. These findings suggested that the TADC were at least in part responsible for tolerization of the tumor-infiltrating T cells.
To test directly whether the TADC could tolerize T cells, we developed an in vitro assay that would assess the immune suppressive function of the TADC. Highly enriched TADC were co-cultured with T cells and their cognate antigen. Several days later, the T cells were re-isolated and tested for their secondary response to antigen as presented by competent APCs. Using this assay, we observed that only the TRAMP TADC, and not WT prostatic pDC, could tolerize T cells. Not surprisingly, the TADC induced suppressive activity in the CD8+ T cells, as well. Taken together with the depletion studies, these findings directly implicated TADC as a potent immune suppressive cell population within the TRAMP TME (Fig. 1a).
In parallel studies, we identified a similar population of pDC in human prostate tissues [13]. The human pDC were CD11c−, CD123+, and also expressed low levels of costimulatory ligands CD80 and CD86 and elevated levels of IDO. We reported that the pDC isolated from prostate tumor tissues were also able to tolerize peripheral blood T cells and induce suppressive activity. Thus, TADC in prostate tumors represent a potential target for enhancing immune responsiveness to tumor antigens.
To identify the mechanism by which these TADC tolerize T cells, we performed microarray-based gene expression analyses. While many genes were up-regulated in the TADCs, including those associated with immune suppression in tumors (IDO, ARG, TGF-β, PD-L1, etc.), one gene that appeared as over-represented among the human and mouse TADC relative to control pDC populations was the transcription factorFoxo3. Both mRNA and protein levels of FOXO3 were elevated eightfold to tenfold in TADC [13]. FOXO3 was initially described as tumor suppressor that regulates expression of genes associated with cell cycle progression and survival; subsequent studies demonstrated its involvement in stress responses and longevity [14]. More recent reports have suggested FOXO3 to have an anti-inflammatory function, as it negatively regulated NF-κB activity and was implicated in regulating DC stimulatory activity [15].
Based on these functions, we tested whether silencing FOXO3 in TADC would reverse their tolerogenic behavior [13]. Using Foxo3-specific siRNAs, we observed that in both human and murine TADC, FOXO3 was critical for suppressive activity, and targeting FOXO3 led to more immune stimulatory function of the TADC. This was reflected in a down-regulation of ARG, IDO, and TGF-β and up-regulation of CD80 and IL-6 [13]. While a similar population of FOXO3+ TADC was noted in B16 melanoma tumors, the TADC isolated from B16 tumors growing in Foxo3 −/− mice were not tolerogenic, confirming a critical role for FOXO3 in the development of suppressive activity [13]. We believe these findings all support the identification of FOXO3 as another putative immunoregulatory gene product that can be targeted to enhance immunity to tumor antigens.
What’s left to learn and how do we use it?
Our data, along with many other studies, demonstrate that the TME is a difficult place to maintain tumor immunity. A variety of cell populations have been identified to contribute to the immune suppressive environment. Some cells are recruited to the TME from the periphery. This includes Treg cells, tumor-associated macrophages (TAMs), and MDSC [8, 16, 17]. Our identification of a suppressive population of TADC and a similar population of pDC in non-cancerous tissue suggests that as tumors develop, resident pDC are modified to become more suppressive. This may be the result of a variety of different cell populations that reside in the developing tumor. We believe it is critical to identify the signals that are involved in the development of these immune suppressive TADCs.
One possibility is that transformed prostatic epithelium expresses factors that can alter prostate-resident pDCs. This may include cytokines, chemokines, enzymes that catabolize critical nutrients for maintaining pDC immune stimulatory function, or other factors that alter pDC function [18]. By using tumor cell culture supernatants and co-culture systems employing DCs and tumor cells, we are attempting to identify both secreted factors and intercellular interactions that convert DCs into immune suppressive, TADC-like cells. Identifying these mechanisms and approaches that inhibit them will enable us to alter the TME and generate more productive anti-tumor immune responses.
Another possibility is that the developing tumor recruits cells that convert pDCs into an immune suppressive phenotype. Previously, Hedrick and colleagues reported that CTLA-4, an inhibitory receptor expressed by activated T cells and Treg cells, can reverse signal through CD80/86 in DC and up-regulate FOXO3 expression [19]. This may be yet another suppressive mechanism by which Treg cells control tumor immunity. In fact, TRAMP and human prostate tumors are reported to be heavily infiltrated by Treg cells [20–22], so confirming this mechanism will further support a role for Treg cells in retarding T cell responses to tumor antigens and the importance of targeting this cell population.
In addition, other leukocyte populations may regulate FOXO3 expression and TADC function. This includes mast cells, which have been reported to be increased in many cancers and were recently suggested to regulate TRAMP tumor development [23–25]. Mast cells have unique and pleiotropic effects that both support and inhibit inflammatory and immune responses, including regulation of tolerance to alloantigens [26–28]. Understanding how mast cells might contribute to the development of TADC suppressive functions will again reveal novel approaches to elicit more durable tumor immunity.
Similarly, it is important to understand how FOXO3 regulates this suppressive effect. As a transcription factor, it may control the expression of genes that induce or mediate the tolerogenicity of TADC. A BLAST search of the 5′ untranslated region of IDO and TGF-β revealed that both genes contain FOXO consensus binding motifs. Therefore, on-going studies will examine whether FOXO3 alters the transcriptional landscape of dendritic cells, and if so, which genes are critical to conferring immune suppressive behavior to the TADC. Alternatively, FOXO3 may alter existing signaling cascades. Previously, it was reported that FOXO3 can regulate NF-κB function by an as yet unidentified mechanism [15]. Thus, FOXO3 may also serve to regulate proinflammatory cascades, leaving the TADC crippled in immune stimulatory functions and by default, tolerogenic. By identifying these additional regulatory pathways, we may reveal novel ways to re-program TADCs and restore their immunogenicity.
Conclusions
Our studies have identified a novel population of dendritic cells that exerts profound immune suppressive activity [29]. These TADC exploit a transcription factor, FOXO3, to tolerize T cells and induce suppressive activity. Targeting this inhibitory pathway will provide a novel avenue for therapeutic enhancement of immunity to tumor antigens. One could envision employing FOXO3 blockade with current vaccine strategies, such as the recently FDA-approved vaccine, Provenge® [30]. Alternatively, approaches that diminish the expression of FOXO3 by TADC in situ, in combination with T cell-based therapies, would also provide enhanced responses to tumor antigens. In addition, on-going studies aimed at elucidating the mechanism by which the TME induces FOXO3 expression, as well as mechanisms by which FOXO3 exerts this suppressive activity, will provide a greater array of therapeutic options. Thus, combinatorial approaches that target multiple immune suppressive pathways will, in all likelihood, provide durable immunity and hold the most promise for successful immune-based therapies for cancer.
Acknowledgments
The work described in this review was supported in part by the intramural research programs of the NCI, NIH. The authors appreciate the critical review of Dr. Joost Oppenheim.
Conflict of interest
The authors declare they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Second International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2011), held in Budapest, Hungary, 2nd–5th May 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cools N, Ponsaerts P, Van TV, Berneman ZN. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol. 2007;82:1365–1374. doi: 10.1189/jlb.0307166. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Belladonna ML, Volpi C, Bianchi R, et al. Cutting edge: autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- 6.Cools N, Van TV, Smits EL, Lenjou M, Nijs G, Van Bockstaele DR, Berneman ZN, Ponsaerts P. Immunosuppression induced by immature dendritic cells is mediated by TGF-beta/IL-10 double-positive CD4+ regulatory T cells. J Cell Mol Med. 2008;12:690–700. doi: 10.1111/j.1582-4934.2007.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2, 3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 8.Ganss R, Hanahan D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998;58:4673–4681. [PubMed] [Google Scholar]
- 9.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178:1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED (2001) The TRAMP mouse as a model for prostate cancer. Curr.Protoc.Immunol (Chapter 20) [DOI] [PubMed]
- 11.Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA. Cutting edge: tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol. 2009;183:4848–4852. doi: 10.4049/jimmunol.0900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shafer-Weaver KA, Watkins SK, Anderson MJ, Draper LJ, Malyguine A, Alvord WG, Greenberg NM, Hurwitz AA. Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells. Cancer Res. 2009;69:6256–6264. doi: 10.1158/0008-5472.CAN-08-4516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Fallarino F, Bianchi R, Orabona C, Vacca C, Belladonna ML, Fioretti MC, Serreze DV, Grohmann U, Puccetti P. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med. 2004;200:1051–1062. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest. 2006;35:459–483. doi: 10.1080/08820130600803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 18.Aalamian M, Pirtskhalaishvili G, Nunez A, Esche C, Shurin GV, Huland E, Huland H, Shurin MR. Human prostate cancer regulates generation and maturation of monocyte-derived dendritic cells. Prostate. 2001;46:68–75. doi: 10.1002/1097-0045(200101)46:1<68::AID-PROS1010>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, Castrillon DH, DePinho RA, Hedrick SM. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 22.Kurinna S, Stratton SA, Tsai WW, Akdemir KC, Gu W, Singh P, Goode T, Darlington GJ, Barton MC. Direct activation of forkhead box O3 by tumor suppressors p53 and p73 is disrupted during liver regeneration in mice. Hepatology. 2010;52:1023–1032. doi: 10.1002/hep.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khazaie K, Blatner NR, Khan MW, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30:45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 24.Pittoni P, Tripodo C, Piconese S, Mauri G, Parenza M, Rigoni A, Sangaletti S, Colombo MP. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res. 2011;71:5987–5997. doi: 10.1158/0008-5472.CAN-11-1637. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Zhang B, Li D, Lv M, Huang C, Shen GX, Huang B. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS One. 2010;5:e8922–2. doi: 10.1371/journal.pone.0008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vries VC, Noelle RJ. Mast cell mediators in tolerance. Curr Opin Immunol. 2010;22:643–648. doi: 10.1016/j.coi.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- 29.Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KER, Sklavos MM, Ambs S, Yagita H, Hurwitz AA (2011) Foxo3a programs tumor associated dendritic cells to become tolerogenic in human and murine prostate cancer. J Clin Investig 121 [DOI] [PMC free article] [PubMed] [Retracted]
- 30.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]