Abstract
To develop a systems biology model of fibrosis progression within the human lung we performed RNA sequencing and microRNA analysis on 95 samples obtained from 10 idiopathic pulmonary fibrosis (IPF) and 6 control lungs. Extent of fibrosis in each sample was assessed by microCT-measured alveolar surface density (ASD) and confirmed by histology. Regulatory gene expression networks were identified using linear mixed-effect models and dynamic regulatory events miner (DREM). Differential gene expression analysis identified a core set of genes increased or decreased before fibrosis was histologically evident that continued to change with advanced fibrosis. DREM generated a systems biology model (www.sb.cs.cmu.edu/IPFReg) that identified progressively divergent gene expression tracks with microRNAs and transcription factors that specifically regulate mild or advanced fibrosis. We confirmed model predictions by demonstrating that expression of POU2AF1, previously unassociated with lung fibrosis but proposed by the model as regulator, is increased in B lymphocytes in IPF lungs and that POU2AF1-knockout mice were protected from bleomycin-induced lung fibrosis. Our results reveal distinct regulation of gene expression changes in IPF tissue that remained structurally normal compared with moderate or advanced fibrosis and suggest distinct regulatory mechanisms for each stage.
Keywords: Pulmonology
Keywords: Fibrosis
Regulatory gene transcription model of lung fibrosis from tissue samples with varying disease of disease severity in the IPF lung.
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common interstitial lung disease and the most lethal, with mortality of 50% of patients 3–5 years after diagnosis (1, 2). It is a chronic, progressive fibrosing disease that occurs more commonly in older male subjects. Diagnosis is defined based on a combination of radiological or histological patterns of usual interstitial pneumonia (UIP) in the absence of known causes for these changes (3). IPF was initially considered a chronic inflammatory disease due to association of inflammatory infiltrates and the fibrosis present within these lungs (4, 5) and treated with immunosuppression. More recently, IPF has become thought of as being a disease of repetitive injury of the alveolar epithelial cells, with aberrant wound healing resulting in fibrosis (2). This view has been further supported by convincing evidence that immunosuppressive therapies have a detrimental effect on the patient and that recently developed antifibrotic drugs are effective in slowing the progression of the disease (6, 7).
While the diagnosis of IPF relies on the integration of histologic, radiologic, and clinical criteria, the underlying pathologic changes are rooted in lung morphology, described as UIP (8). UIP is characterized by marked fibrosis with or without honeycombing in a predominantly subpleural and paraseptal location, with central areas being relatively spared. The fibrosis must be heterogeneous, with normal lung adjacent to established fibrosis. At the boundary between these regions are myofibroblast foci, defined by accumulation of immature hyaluronic acid–rich matrix underneath epithelial cells undergoing injury and apoptosis. UIP is therefore characterized by temporal heterogeneity — the presence of acute or active disease (fibroblastic foci with or without epithelization) along with progressive disease (mature fibrotic scar) and spatial heterogeneity — the presence of fibrotic lung adjacent to histologically normal lung. In most cases there is no necrosis or substantial inflammatory cell infiltration, although some mild inflammatory response can be seen, especially in areas of collagen deposition. These patterns are not replicated in animal models of pulmonary fibrosis, which is the reason that despite the significant progress in our understanding of the molecular events underlying pulmonary fibrosis in animals (9) we have no information about what regulates the progression of IPF in the human lung. Understanding the molecular changes that characterize the progression of IPF from histologically normal regions into end-stage disease within the human lung would allow development of therapeutic strategies in all stages of disease.
To develop a model of the gene regulatory changes associated with progressive fibrosis in the human lung, and given the fact that it is impossible to directly observe the progression of fibrosis by sampling the same lung multiple times, we hypothesized that analysis of differentially affected regions within the IPF lung could provide an approximation of the trajectory of pathological changes suitable for a systems biology approach. We sampled multiple regions in human IPF or control lungs. We determined the extent of fibrosis using quantitative microCT imaging and tissue histology and classified the samples as controls, IPF with no fibrosis, IPF with moderate fibrosis, and IPF with advanced fibrosis. We then performed RNA sequencing (RNA-seq) and microRNA profiling to determine gene expression at these selected stages, applied a linear mixed-effects model to identify differentially expressed genes, and discovered a core set of gene expression changes that preceded the occurrence of fibrosis in the IPF lung. We used computational modeling to reconstruct a dynamic gene regulatory model of IPF, which uncovered distinct expression changes between different levels of fibrosis severity and identified well-known as well as potentially novel regulators of disease progression. We confirmed model predictions that POU2AF1, a lymphocyte transcription factor previously unassociated with pulmonary fibrosis, is a regulator of fibrosis by immunohistochemistry and an animal model of lung fibrosis. This study is accompanied by a supplemental web resource (10) that allows interactive and detailed mining of the model.
Results
Patient demographics.
All subjects used in this study were males and matched for age. The use of only male subjects was due to limited numbers of female transplant recipients for IPF with only 1 lung being available for research over a period of 5 years. Patient demographics and lung function are described in Table 1.
Table 1. Demographic data for human subjects.
MicroCT quantitation and histological analysis of differentially affected samples in the IPF lungs.
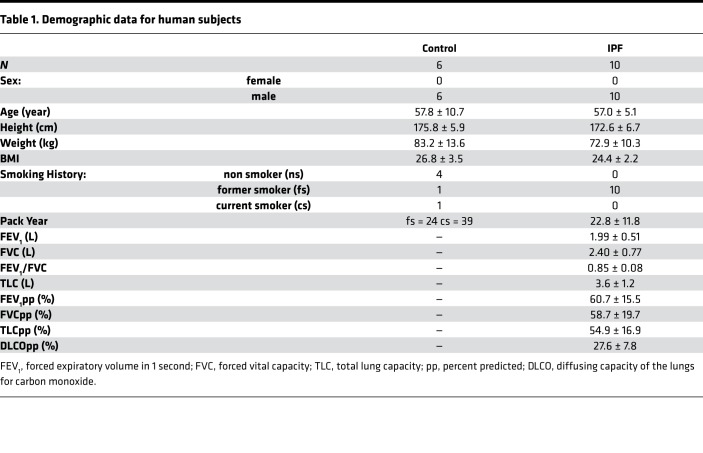
In total, 59 samples were available from IPF lungs and 36 samples from controls for microCT imaging (Figure 1, A–C). Samples were histologically assessed by a pathologist and 11 samples with emphysema, large airway, or large vessels were excluded from the analysis. Alveolar surface density (ASD) computed from microCT images as previously described (11) was significantly negatively correlated (r = –0.81, P = 1.74 × 10–12) with the Ashcroft histology fibrosis score (12), validating the use of ASD as an unbiased quantitative measure of fibrosis (Figure 1D). For our analysis, we clustered samples based on the principal components surface density and collagen 1 staining into control (n = 35), samples obtained from IPF patients with ASD similar to control — IPF1 (n = 19), samples with lower surface density that reflects moderate fibrosis — IPF2 (n = 16), and samples with the lowest surface density that reflects advanced fibrosis — IPF3 (n = 14) (Figure 1E) and referred to them as distinct stages of fibrosis progression. While ASD was decreased in most IPF samples, IPF1 showed a large overlap with controls, reflecting conserved normal lung structure (Figure 1F). This was consistent with the pathological report of tissue samples, which found that 14 of 19 IPF1 samples had normal lung histology and the remaining 5 of 19 samples with minimal levels of fibrosis. As expected, total collagen staining was not increased in IPF1, but was significantly increased in later stages of IPF, starting with IPF2 (Figure 1G). Staining for collagen 1 revealed a trend towards increased staining with stage that reached significance in IPF3 (Figure 1H). The staining for collagen 3 did not reveal any changes, potentially reflecting reduced sensitivity (Figure 1I).
Figure 1. Sampling of the lung.
(A) Schematic for sampling of the lung. (B) Image of the inflated whole IPF lung with a slice taken before and after sampling. (C) Representative microCT images of a control sample and for each stage of IPF. (D) A significant negative correlation (r = –0.81, P = 1.74 × 10–12) was found between alveolar surface density (ASD) compared with Ashcroft scores. Gray area represents the 95% confidence interval. (E) Principal component and expectation-maximization clustering separated samples into control (green) and early-stage (IPF1; blue), progressive (IPF2; magenta), and end-stage (IPF3; black) IPF. Each subject is identified by number (IPF: 1–10; control: 11–16). (F) ASD was significantly reduced in IPF stages compared with control but a large overlap remained between IPF1 and control. (G) Volume fraction (Vv) of total collagen was significantly increased in IPF2 and IPF3 stages compared with control. (H) Collagen 1 Vv was significantly increased in IPF3 compared with controls. (I) Collagen 3 Vv showed no difference compared to control but a significant decrease in IPF1 compared with IPF3. For panels F–I, data are presented as box plots showing median and interquartile ranges for each group compared using linear mixed-effects models. *P < 0.05; **P < 0.001.
Differential gene expression identified a core set of IPF genes that are changed before fibrosis is histologically evident.
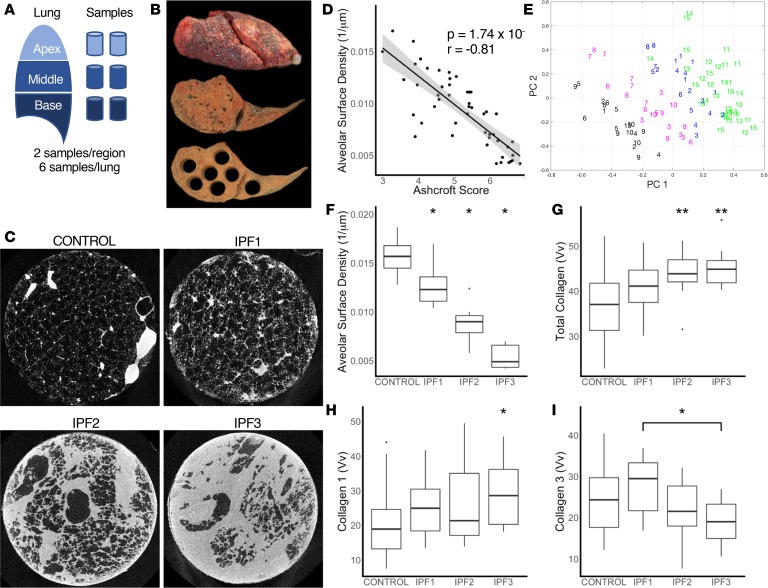
Using a linear mixed-effects model we identified 3672 genes that were differentially expressed in at least one IPF group with 2141 genes increased and 1531 genes decreased. In IPF1, 591 genes were increased and 990 were decreased, potentially reflecting the gene expression changes that precede overt histological changes. In IPF2, 1299 genes were increased and 890 were decreased and in IPF3, 1753 genes were increased and 932 were decreased. Of these, 908 genes were common to all groups of IPF, suggesting they represented the core IPF genes (Figure 2A), which are changed even before fibrosis is histologically evident and continue to change as disease progresses.
Figure 2. Expression of genes and regulators.
(A) Euler diagrams showing the number of upregulated or downregulated genes or microRNAs at each stage of IPF. (B) Heatmap of the 100 genes with the greatest increased or decreased expression in all stages of IPF. (C) Box plots for expression of key genes and regulators showing median and interquartile ranges for each group compared using linear mixed-effects models. *P < 0.05, **P < 0.001, ***P < 0.0001 versus control.
The 100 genes with the greatest increase or decrease in expression at any stage (Figure 2B and Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.131597DS1) were significantly overrepresented among these core genes (67 of 100, P < 2.2 × 10–16). The 67 most highly differentially expressed core genes included several collagens and extracellular matrix (ECM) proteins showing overexpression (COL1A1, COL1A2, COL2A1, COL3A1, COL5A1, COL10A1, COL15A1, and COMP), with COL1A1 being the most highly expressed gene in every stage of IPF (Figure 2C). Despite COL1A1 being highly expressed, we did not find a significant increase in the degree of staining of collagen 1 on tissue sections until IPF3, potentially reflecting RNA changes that precede changes in protein, or more likely limited sensitivity of the staining (Figure 1H). Some chemokines (CCL13, CCL18, CXCL6, CXCl13, and CXCL14) were also among the most highly expressed genes in our data set. CXCL14, a chemokine expressed by fibroblasts and associated with monocyte recruitment and inhibition of angiogenesis (13), and CCL18, a chemokine secreted by alveolar macrophages, have both been proposed as IPF biomarkers (14, 15). Among the highly overexpressed genes were the fibroblast genes CTHRC1, FAP, ISLR, and THY1, and the bronchial epithelial cell markers KRT5, KRT14, KRT15, and KRT17. The metalloproteinase MMP7, previously observed in the alveolar epithelium and whose blood levels have been consistently associated with IPF disease progression and survival (16, 17), was overexpressed in all groups of IPF, as was MMP11 (Figure 1C). Genes related to B cell function were also highly expressed and included CD79A, MZB1, previously identified in IPF from a proteomic screen (18), as well as IGJ, the immunoglobulin J-chain linking IgM and IgA antibodies, and IGLL5, the immunoglobulin lambda light chain.
Among the most highly decreased genes in IPF were AGER, an alveolar type I marker previously shown to be decreased in IPF (19), innate immunity–related genes including the S100 family genes S100A9, S100A9, and S100A12, and the neutrophil defensins DEFA1, DEFA3, and DEFA4. The solute carrier SLCO4A1 was also significantly underexpressed in all stages of IPF. This gene is involved in the transport of thyroid hormones recently implicated as potential therapies in IPF (20) (Figure 2B).
Globally, results were consistent with previous transcriptomic studies. Half (34 of 69) of the genes recently highlighted in a review of transcriptomic studies of IPF (21) were differentially expressed in at least one group of IPF in our data set (Supplemental Table 2) and 16 were among our core set of IPF genes. This included 4 genes (CTHRC1, GREM1, FHL2, and MMP7) previously associated with disease severity as measured by percentage predicted diffusing capacity of carbon monoxide (%DLCO) (22). GREM1, FHL2, and MMP7 showed a progressive increase related to disease severity in IPF, whereas CTHRC1 was increased in IPF1, but did not increase further in IPF2 or IPF3 (Supplemental Figure 1). In comparing our core list of genes to differentially expressed genes from the Lung Tissue Research Consortium data set of IPF lung tissues (GSE47460), as recently analyzed by us (23), we found 535 genes differentially expressed in both data sets, with the vast majority of genes (527 of 535 genes, P < 2.2 × 10–16) showing a similar direction of expression (Supplemental Figure 2).
While many gene changes relevant to IPF disease were present from the earliest stages of IPF, other genes that have been associated with fibrosis or IPF pathogenesis were increased only in the severe stages. Among these genes are MUC5B, known to be increased and genetically associated with IPF (24–26). MUC5B was not changed in IPF1 but was increased in IPF2 and IPF3 (MUC5B: IPF1 P = 0.235, fold-change [fc] = 2.1; IPF2 P = 0.013, fc = 3.1; IPF3 P = 0.015, fc = 3.7; Figure 2C). FKBP10 has been shown to be increased in IPF and involved in regulating collagen production (27). In our data, FKBP10 was significantly increased only in IPF3 compared with controls (FKBP10: IPF1 P = 0.1, fc = 1.9; IPF2 P = 0.1, fc = 1.7; IPF3 P = 0.027, fc = 2.0). WNT signaling has also been associated with IPF disease pathogenesis, in particular involving epithelial cell developmental pathways (28). Similar to what has been shown previously (29), we found decreased WNT3A (WNT3A: IPF1 P = 0.089, fc = 0.61; IPF2 P = 0.035, fc = 0.53; IPF3 P = 0.016, fc = 0.51) and increased WNT10A (WNT10B: IPF1 P = 0.122, fc = 1.4; IPF2 P = 0.023, fc = 1.6; IPF3 P = 0.023, fc = 1.7) in IPF compared with controls, but this was only significant in the severe stages of disease. Overall, these data indicate that many of the transcriptional changes in IPF are already present in regions with relatively normal lung structure and minimal change in cellular infiltration (IPF1), while other gene expression changes only occur in more severely affected regions with an obvious UIP pattern (IPF2 or IPF3), potentially reflecting cellular changes associated with lung remodeling such as honeycomb formation.
MicroRNAs differentially expressed in IPF are regulators of many of the differentially expressed genes but are stage specific.
Of the 800 measured microRNAs, we identified 86 as differentially expressed between IPF and controls in at least 1 stage of disease (Supplemental Table 3). Examining the microRNA targets using the validated target data set (miRTarBase) in the multi-miR database (30), we found that 50% of differentially expressed genes (1860 of 3672 genes, P = 9.97 × 10–8) were predicted to be regulated by these 86 microRNAs. In focusing our observations on significant (P < 0.05) microRNAs and target genes with at least a 2-fold change in IPF compared with controls, we found 4 microRNAs were significant with greater than 2-fold increased expression (miR-127-3p, miR-21-5p, miR-382-5p, and miR-495) that were known to downregulate 20 genes from the core gene set (Supplemental Table 4), with miR-21 being a regulator for all 20 of these genes. miR-21 has previously been shown to have an important role in fibrogenesis (31). Pathway enrichment for these 20 genes reveals that they are related to HIF-1 (Kyoto Encyclopedia of Genes and Genomes [KEGG]: 04066, HIF-1 signaling pathway, P = 5.65 × 10–3) and thyroid hormone signaling (KEGG: 04919, thyroid hormone signaling pathway, P = 8.79 × 10–3).
Notably, microRNA changes seemed to be specific to distinct IPF groups (see Supplemental Table 4 for details). In IPF1, 6 microRNAs were decreased and associated with 16 increased target genes that included several collagens (COL1A1, COL1A2, COL3A1, COL5A2, COL10A1, and COL15A1), all known to be regulated by miR-29c-3p, a well-established antifibrotic microRNA (32, 33), as well as MMP7 and TGFBI (Supplemental Table 4). Five microRNAs that were increased in IPF1 (miR-21-5p, miR-127-3p, miR-382-5p, miR-451a, and miR-495) targeted 43 decreased genes. These genes were enriched for the hypoxia pathway (KEGG: 04066, HIF-1 signaling pathway, P = 2.83 × 10–7) and included CLOCK, MAPK1, and VEGFA. In IPF2, 11 microRNAs, including let-7d-5p known to be decreased in IPF (34), targeted 18 increased genes that included collagens (COL3A1, COL5A2, and COL16A1), regulators of ECM such as MMP1 and HAS2, and the myofibroblast transcription factor TWIST1. Ten microRNAs were increased that targeted 114 downregulated genes with miR-155-5p, previously implicated in IPF (35), accounting for 48 of these genes and miR-21 accounting for 39. For IPF3, 8 microRNAs, including 3 from the let-7 microRNA family, were decreased. These microRNAs targeted 31 increased genes including ECM proteins (COL1A2, COL3A1, COL16A1, and FN1), ECM regulators (LOXL2, HAS2, MMP2, and MMP14), and transcription factors (SOX9, TWIST1). Twelve microRNAs were increased in this stage that targeted 130 decreased genes with miR-34a-5p (targeting 33 genes), joining previously mentioned miR-155-5p (43 genes) and miR-21-5p (targeting 35 genes) genes. As also observed more formally in the dynamic regulatory events miner (DREM) analysis, decreased microRNAs exhibited a shifting regulatory effect on gene expression with disease severity, in which the miR-29 family dominated in mild disease and the let-7 in severe disease, whereas increased microRNAs exhibited an additive effect, with miR-21 being the main regulator in IPF1, with miR-155 joining in IPF2 and miR-34a in IPF3.
DREM analysis identified distinctly regulated gene expression tracks related to extent of fibrosis in the lung.
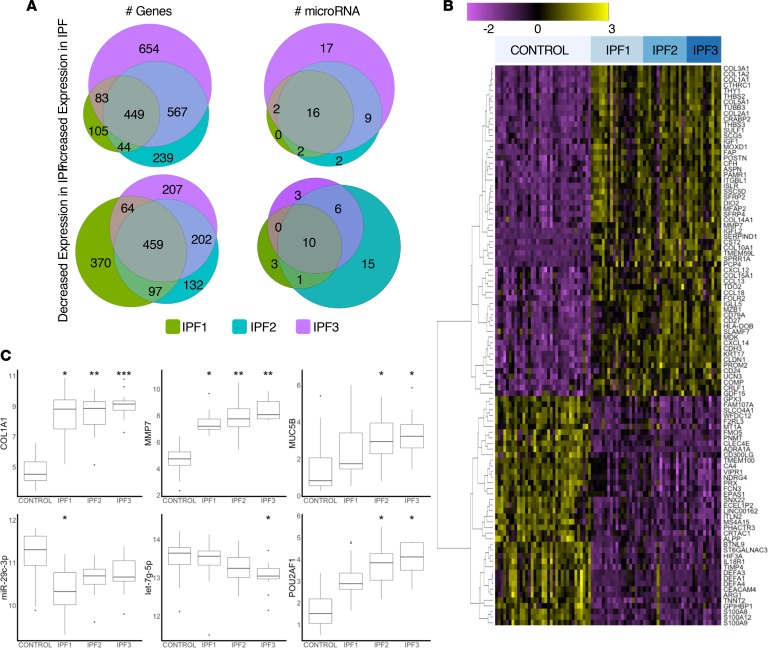
To develop a systems biology model of the distinct regulatory events active in the differentially affected IPF microenvironments, we applied the DREM algorithm to the differentially expressed genes and microRNAs. The model identified 13 gene expression tracks regulated at 4 nodes (nodes 1–4, Figure 3A) that were established in the minimally or nonaffected regions of IPF (i.e., from control to IPF1) that differed in their patterns of gene expression (increased, nodes 1–3 and decreased, node 4), as well as regulation and biological function (see below). At IPF2, there was divergence with each track being regulated by a distinct set of regulators (nodes 5–17, Figure 3A), indicating a shift in gene expression regulation between more normal regions and advanced stages of the disease. At this stage, 8 tracks were characterized by increased gene expression (tracks A–H, Figure 3A) and 5 decreased tracks (I–M, Figure 3A). Only 1 decreased advanced track (M, Figure 3A) diverged from a node that was previously characterized by increased gene expression). The tracks also differed in their slope of gene expression. Increased tracks A and B and all of the decreased tracks (I–M) exhibited their largest changes between control and IPF1, potentially reflecting changes that occurred in IPF tissue before overt changes in tissue histology were present (Figure 3B: A, B, and I–M). Tracks C, D, E, and H exhibited the majority of gene expression changes between IPF1 and IPF2, potentially reflecting changes occurring during the development of more advanced disease (Figure 3B: C–E and H). Tracks F and G were characterized by genes that changed mostly at the transition of IPF2 and IPF3, potentially reflecting the changes that occur in the transition to end-stage disease (Figure 3B: F and G).
Figure 3. DREM tracks at each stage of IPF.
(A) Flow diagram showing the 4 primary tracks established in IPF1 which split into 13 secondary tracks in the IPF2 stage. Numbers denote regulatory node for that junction where regulators and gene expression diverge. Letters denote track name. (B) Number of differentially expressed genes for each track at each stage of IPF. (C) Sankey diagram of biological function terms identified for each DREM track. (D) Heatmap of enriched genes between DREM tracks and a published IPF single-cell gene marker list scaled to the number of genes per cell type (40).
The gene expression tracks differed in their functional enrichments (Figure 3C and dynamic Sankey image in supplemental website; ref. 10), suggesting a distinct trajectory for changes in molecular function associated with increased fibrosis severity. Both increased tracks A and B were highly enriched for ECM genes (Figure 3C, BIOCARTA M5889: ensemble of genes encoding ECM and ECM-associated proteins; track A: P = 1.79 × 10–12; track B: P = 2.46 × 10–16). Genes in track A included the fibrillar collagen types 1 and 3 (COL1A1, COL1A2, and COL3A1) and track B included genes for FACIT collagens type 14, 15, and 16 (COL14A1, COL15A1, and COL16A1). The third track (M) to emerge from node 1 (Figure 3, A and B) was decreased in expression and enriched with antimicrobial peptides (Reactome: 1457777, P = 1.21 × 10–3) that included the neutrophil defensins (DEFA1, DEFA3, and DEFA4). This track also included AGER, expressed in type I alveolar epithelium and previously shown to be decreased in IPF (19). The main regulator of node 1 was the antifibrotic microRNA miR-29c in the mild stage (IPF1, node 1), and the transcriptions factors NF1, NKX6-1, RUNX2, and POU2AF1. As mentioned before, we observed the biphasic regulation of tracks A and B, with miR-29 mainly regulating node 1 and let-7 family taking over the advanced stages (nodes 5 and 6, Figure 3A and supplemental website; ref. 10). The key transcription factor for node 6 (increased genes from IPF1 to IPF2, track B) was POU2AF1, a lymphocyte transcription factor previously not associated with lung fibrosis, which we went on to validate (see below). HMGA2, the transcription factor and target gene of let-7 and previously been reported to be involved in fibrosis, was also a regulator of this node (34). The transcription tracks C, D, and E, with gene expression mainly increased at the moderately affected regions between minimally and most affected regions (IPF2, Figure 3B), were enriched for genes related to activation of the lymphocyte immune response. Specifically, track C was associated with regulation of lymphocyte activation (Gene Ontology [GO]: 0051249, P = 8.98 × 10–10) and included the WNT gene WNT10A, track D with immunological synapse (GO: 0001772, P = 0.027), and track E with hematopoietic or lymphoid organ development (GO: 0048534, P = 3.18 × 10–4) (Figure 3C). The transcription factor LEF1, the canonical WNT signaling transcription factor (36), was the main regulator for node 2. Tracks F, G, and H, where gene expression was mainly increased in most affected IPF regions (IPF3), were enriched for DNA-related functions: track F for cell division (GO: 0051301, P = 0.049) and chromosome organization (GO: 0051276, P = 0.049); track G for chromosome breakage (GO:0031052, p = 0.047) and DNA elimination (GO:0031049, P = 0.047); and track H for ion channel proteins (GO:0006812, P = 3.46 × 10–5) (Figure 3C). These tracks were regulated by numerous microRNAs, including the miR-30 family (miR-30A/B/D/E) (node 3). Tracks I–M were composed of genes decreased in minimally affected IPF tissue (IPF1) and reflected the extent of functions lost in the IPF lung even before overt histological changes were observed. Track I was enriched for olfactory signaling (Reactome: 1269583, P = 4.02 × 10–17) and track J for mitochondrial transport (GO: 0006839, P = 0.030), protein targeting to mitochondria (GO: 0006626, P = 0.038), and mitochondrial organization (GO: 0007005, P = 0.049), potentially reflecting the loss of normal mitochondrial function recently reported in IPF (37). Track K was enriched for alcohol metabolism (GO: 1902652, P = 4.01 × 10–4) and cholesterol biosynthesis (Reactome: 1270037, P = 1.02 × 10–4), potentially reflecting the metabolic shift that occurs in the IPF lung (Figure 3C). Track L was highly enriched for genes related to the circulatory system (GO: 0003013, circulatory system process, P = 7.21 × 10–7), reflecting inhibition of angiogenesis in progressive pulmonary fibrosis (Figure 3C). The primary regulator of node 4 that regulated tracks I–L was the microRNA miR-205, which had increased expression in IPF compared with control and has been demonstrated to have an extensive role in inhibiting angiogenesis (38, 39). Interestingly, regulators for node 15, which regulated path K, included the thyroid hormone receptors A and B (THRA and THRB), suggesting their role in altered metabolic regulation in IPF. The decreased track M was enriched for antimicrobial peptides (Reactome: 1457777, P = 1.21 × 10–3) that included the neutrophil defensins (DEFA1, DEFA3, and DEFA4), potentially reflecting early changes in innate immunity during fibrosis.
Analysis of cellular enrichment of gene expression tracks.
To identify the contribution of shifts in cellular populations to the gene expression tracks, we used the annotated cell types from a publicly available single-cell RNA-seq data set of lung tissues (40). In general, decreased tracks were enriched for AT1, AT2, and endothelial vascular cell markers (tracks J–M, Figure 3D), early increased tracks were enriched for fibroblast and ciliated epithelial cell markers (track B, Figure 3D), and progressive tracks were enriched for B lymphocyte, basal cell, and ciliated cell markers (tracks C–E, Figure 3D). Macrophage and monocyte genes were enriched in the early decreased tracks (tracks J–L, Figure 3D) and in the advanced increased tracks (tracks D and E, Figure 3D), potentially reflecting the enhanced recruitment of these cells to areas with advanced remodeling. Tracks A, G, and H were not enriched for cell markers, potentially representing modular changes in fibrosis that are not dependent on shifts in cell populations.
Pou2af1–/– mice exhibit attenuated lung fibrosis in bleomycin model.
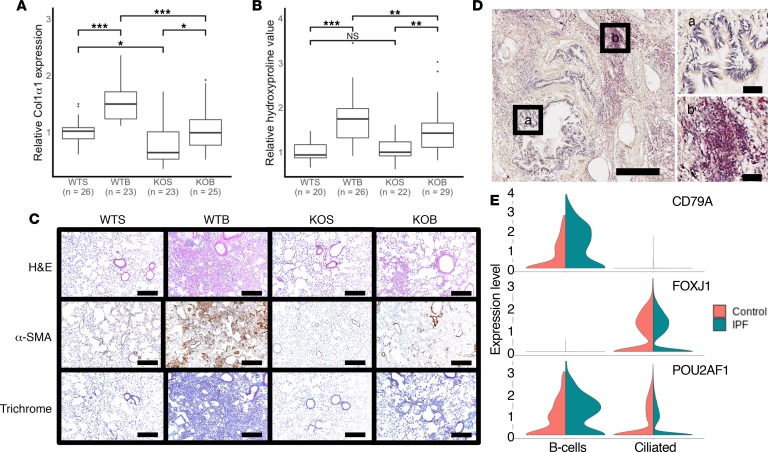
As our DREM model identified POU2AF1 as being a regulator of node 6 in the fibrotic track B, we sought to confirm a mechanistic link between POU2AF1 and fibrosis using a transgenic mouse model. For this, we administered bleomycin to Pou2af1–/– mice and wild-type (WT) (Pou2af1+/+) littermates and measured collagen and hydroxyproline levels. Pou2af1–/– mice exhibited significantly lower expression of Col1α1 mRNA levels in comparison with WT counterpart with saline or after administration of bleomycin (Figure 4A). Saline treatment of WT and Pou2af1–/– mice showed no difference in hydroxyproline levels. On day 14 after intratracheal bleomycin administration, WT littermates developed pulmonary fibrosis, while Pou2af1–/– mice exhibited lower hydroxyproline levels, indicative of reduced deposition of collagen in the lung (Figure 4B). In line with these findings, Pou2af1–/– mice showed reduced histological evidence of fibrosis by H&E, α-smooth muscle actin (α-SMA), and Masson’s trichrome staining as compared with WT littermates (Figure 4C).
Figure 4. Pou2af1–/– mice show less fibrotic severity in bleomycin-induced lung fibrosis.
(A) qRT-PCR analysis of relative change in collagen type I, α1 (Col1α1) mRNA levels in wild-type (Pou2af1+/+) and Pou2af1–/– mice on day 14 after treatment with saline or bleomycin. Data are presented as box plots showing median and interquartile ranges for each group, with groups compared using linear mixed-effects models. Wild-type saline (WTS) n = 26; wild-type bleomycin (WTB) n = 23; Pou2af1-knockout saline (KOS) n = 23; and Pou2af1-knockout bleomycin (KOB) n = 25. (B) Relative collagen deposition assessed by hydroxyproline content per right lung in wild-type (Pou2af1+/+) and Pou2af1–/– mice as indicated by treatment groups. Data are shown as box plots with median and interquartile ranges and compared using linear mixed-effect models. WTS n = 20; WTB n = 26; KOS n = 22; and KOB n = 29. (C) H&E staining (upper panels), α-SMA (middle panels), and Masson’s trichrome (lower panels) staining of representative lung sections (n = 2 per group). Scale bars: 200 μm. (D) POU2AF1 immunostaining (red) in human IPF lung tissue showed no staining in bronchial epithelium (a) but lymphocytic aggregates (b) were positive. Scale bars: 400 μm (left) or 50 μm (right 2 images). (E) Violin plots from single-cell RNA-sequencing data of control (orange) and IPF (green) lung tissues showing specific cell types expressing POU2AF1. Upper panel shows CD79 expression identifying the B cell population, middle panel shows FOXJ1 expression identifying the ciliated epithelial cell population. POU2AF1 (bottom panel) was highly expressed in CD79 expressing B cells with some expression in ciliated epithelium (FOXJ1). In IPF, POU2AF1 showed increased expression in B cells and a slight reduction of expression in the ciliated cells. *P < 0.05, **P < 0.001, ***P < 0.0001, ns = no significance.
Human POU2AF1 was localized in lymphocytic aggregates in the IPF lung.
As we confirmed that POU2AF1 was able to directly regulate fibrosis, we sought to identify the cell types which may be expressing this gene in the human lung. In examining human lung samples from patients with IPF using immunostaining, we found extensive POU2AF1/BOB1 staining in lymphocytic aggregates present in fibrotic regions but no staining in bronchial epithelium (Figure 4D), suggesting lymphocytes are the primary source of POU2AF1. Reanalysis of a single-cell RNA-seq data set from Adams et al. (41) showed that POU2AF1 is highly expressed in B cells (identified by expression of CD79), with some expression in ciliated epithelial cells (identified by FOXJ1). In IPF, POU2AF1 is increased only in B cells but not the ciliated airway bronchial cells (Figure 4E).
Discussion
In this study, we quantitatively characterized differentially affected regions in human lungs to model progression of IPF in the human lung. We performed RNA-seq and microRNA profiling of microCT-characterized regions in IPF lungs and compared gene expression between normal-histology lungs from donors and IPF lungs where the lung tissue was quantified and categorized into minimally affected, moderately affected, and end-stage disease severity. Our differential expression analysis identified a core set of genes that changed even before fibrosis is histologically evident and continue to change as disease progresses. Our systems biology analysis allowed us to identify distinct tracks of gene expression that increase or decrease with progressive fibrosis in the lung, as well as their regulation by microRNAs and transcription factors. Among these were regulators previously identified such as the miR-29, let-7, and miR-21 microRNAs. These regulators drive profibrotic programs but seemed to have distinct and previously unrecognized roles at different stages of the histological progression of IPF. Finally, we confirmed our model prediction that POU2AF1, a lymphocyte transcriptional coactivator previously not associated with lung disease, was a regulator of profibrotic transcriptional programs using immunohistochemistry and the bleomycin-induced fibrosis model.
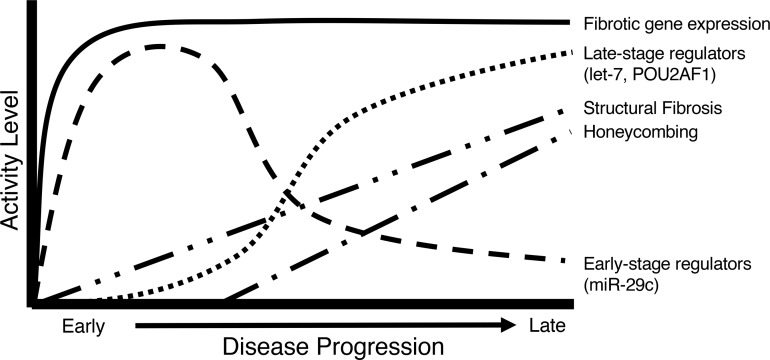
Studies in IPF using transcriptomic technologies have led to the identification of numerous genes and microRNAs involved in pulmonary fibrosis such as MMP7, CCL18, COMP, SPP1, RXFP1, miR-29, miR-21, and let-7, among others (16, 24, 32, 33). However, few studies have considered these results in the context of the tissue microenvironment and whether they relate to early- or late-stage events in disease progression. This is important, as IPF is a disease that progresses from the periphery to the center, with different lung regions having distinct pathological features likely corresponding to different stages of disease, as occurs in a heterogeneous and progressive disease (42). In contrast with previous studies, we focused on analyzing gene expression in distinct IPF microenvironments, by cutting out differentially affected regions in the same lung, and then characterizing these samples by microCT and performing RNA-seq and microRNA profiling on exactly the same core that was imaged. Thus, we were able to correlate gene expression changes with the extent of fibrosis in the tissues, which led to some unexpected and potentially novel insights. The first was that normal-histology tissue in IPF is very different from similar-histology tissue obtained from a human without IPF. The changes are impressive, with over 1000 genes differentially expressed, including known IPF genes. A recent study examining mild and severely fibrotic regions in 3 IPF explant lungs also showed changes were present in macroscopically normal but microscopically fibrotic tissues (43). We extended these findings by demonstrating that even microscopically normal tissues, as quantitatively measured by microCT and pathological assessment, showed these molecular changes and accounted for the multiple samples examined per subject, providing a more accurate description of these changes. These gene expression changes continue to progress, as can be seen in tracks A and B, corresponding with increased fibrosis, but these changes are already present in normal-histology lung. For instance, the gene expression of many collagens is increased in the minimally affected IPF lung, before changes at the protein levels are seen. Similarly, miR-29 microRNA, potentially the most universal antifibrotic microRNA (32, 44–46), is also decreased at this stage, demonstrating that histologically normal tissue from patients with IPF is molecularly abnormal. The second finding was that different genes, commonly thought of as characteristic of IPF, showed distinct patterns, some increasing early and with minimal further changes (those in tracks A and B), some consistently increasing with progression of fibrosis (tracks C and D), and some changing only in the most severe disease (tracks F and G). The third finding was that the regulation of fibrosis is, at least to some extent, modular and thus the end results, increased ECM proteins, are driven by different microRNAs and transcription factors in minimally affected tissue compared with progressively affected or end-stage tissue (Figure 5). For example, the regulation of fibrillar collagens is particularly relevant for IPF, as it is the primary component of fibrotic ECM. The microRNA miR-29c, considered a master regulator of fibrosis (47, 48), has a direct effect on inhibiting fibrillar collagen expression and has been studied as a possible therapeutic for pulmonary fibrosis (33). Despite high levels of collagen expression throughout IPF disease progression, we found that miR-29c is mostly a regulator at early stages of disease, with the let-7 family of microRNAs taking over at later stages. Various members of the let-7 family of microRNAs are decreased in the IPF lung at progressive stages and regulate HMGA2, collagen 1, and potentially other profibrotic molecules (32, 34); however, little was known about their regulation in the IPF lung. Taken together, these results uncover for the first time to our knowledge the diversity of gene expression, highlight the complexity and potential stability of the fibrotic networks, and the need to develop therapeutics that target the early events and potentially prevent the development of disease, as well as therapeutics that target late events to potentially reverse the areas of progressive disease.
Figure 5. A model of fibrotic activity in IPF.
A common difficulty in studies that apply high-throughput profiling approaches to systems biology models of disease is the requirement to provide some validations to the model predictions. In our study we applied several layers of validation. The first was that we assessed whether findings of our model were consistent with what was previously known. This was done at the level of the genes and showed our results were highly consistent with previous transcriptomic analyses of IPF lungs (22, 49, 50), including a recent paper that applied gene expression module networks to identify coexpression pathways in IPF (23). A second layer of validation was whether our model would identify pathways and regulators consistent with what is known in the literature — 2 examples are the WNT pathway and the presence of mitochondrial dysfunction. The WNT pathway is well known to be involved in pulmonary fibrosis (29, 51). We found increased WNT10A, in track C, associated with the major transcription factor for WNT signaling, LEF1, at node 2. The observed decrease in WNT3A was also consistent with previous studies (29). In recent years, mitochondrial dysfunction has been of significant focus in IPF (20, 37). Impressively, we have found that 2 decreased tracks, J and K, were enriched for mitochondrial organization, structure, and function, as well metabolic pathways required for cellular function. The third layer was experimental and as it was impossible to check every single prediction, we chose to focus on POU2AF1, to our knowledge not previously associated with fibrosis. POU2AF1, a transcriptional coactivator that associates with POU2F1 or with POU2F2 is mainly expressed in B cells in relation to the formation of germinal centers, and at much lower levels in T cells (52, 53). Because POU2AF1 was identified from our model as a regulator at node 6 of the fibrotic track B, we confirmed that it was increased in the IPF lung using immunohistochemistry and single-cell RNA-seq data. Using a POU2AF1-knockout mouse demonstrated that it has a role in bleomycin-induced pulmonary fibrosis. We had previously found POU2AF1 to be coexpressed with B cell–related genes in IPF (23), and by reanalyzing IPF single-cell data (41) we also showed that the predominant expression and increase of POU2AF1 in IPF is in B cells. While these data support a role for POU2AF1 in B lymphocytes, which are increasingly implicated in fibrosis (18, 54, 55), they are of course limited, especially considering that POU2AF1 was also recently found in airway epithelial cells (56). Thus, while our results establish a functional link between POU2AF1 and pulmonary fibrosis, and confirm our model predictions, more detailed studies using cell-specific loss- and gain-of-function experiments will be required to assess the specific role of POU2AF1 in fibrosis.
Probably the key limitation of our study is our inability to directly determine whether changes we observed are the result of a change in gene expression in tissue-resident cells or a shift in the cellular composition of the tissue. To address this concern, we used a recently published single-cell IPF RNA-seq data set (40) and computational approaches to determine the source of the signal in each of our tracks. This does not resolve the issue of potential rare cell populations but does allow us to assess the source of the signal and incorporate it in our model. Some events, such as the increase in airway basal cells or B cell–related genes in certain tracks are, of course, the result of cellular infiltrations, but such an analysis had not been done thus far, and the identification of early and late molecular changes may have importance regardless of the cell type they happen in. Additionally, some of the findings are probably not the result of major changes in cell populations. For example, one of our most impressive findings, the significant molecular changes in IPF tissue with conserved histology, is probably not significantly affected by cellular infiltration, as these were observed in samples that were histologically similar to controls without IPF. Last, it is important to note that our findings probably represent the exact gene expression in the lung, as explanted lungs were inflated, snap frozen over liquid N2, and then carefully phenotyped without induction of post-harvesting gene expression changes, whereas many of the common approaches to single-cell profiling of tissue require dissociation and sometimes sorting, processes that are not yet standardized, and make the detailed imaging-based quantitation of tissue fibrosis impossible (57). While clearly, analysis of carefully characterized, differentially affected tissues at single-cell resolution would be ideal, we believe that our insights, as well as our data set with the accompanying user-friendly interactive website will have significant impact on our understanding of the progression of fibrosis within the human lung.
In this study, we provide a dynamic regulatory model of human IPF based on mRNA and microRNA profiles of carefully characterized differentially affected microenvironments in the IPF lung. Our analysis allowed us to determine that histologically normal IPF tissue is dramatically different from lung tissue from individuals without IPF, identify molecular changes that are specific to tissue with early, progressive, and end-stage histological changes, and identify microRNA and transcriptional regulators of these stages. We have compared our results to previously available data sets, incorporated single-cell data, and validated the importance of a previously unrecognized transcription factor. Our results suggest that different regulatory mechanisms are active in minimally affected, progressively affected, and end-stage tissues in IPF. This finding should drive the identification of novel biomarkers and therapeutic interventions that address the specific stages of disease in IPF.
Methods
Subject selection and study approval.
Patients with IPF undergoing lung transplantation at University Hospital Leuven, Belgium, were selected. Donor lungs that were not suitable for transplantation were collected to be used as “healthy” controls.
Lung processing and sample imaging.
In total, we analyzed 95 samples from 10 IPF lungs and 6 donor lungs for this study. Following transplant surgery, explanted lungs were inflated to 30 cmH2O and held at 10 cmH2O pressure inflation while frozen over liquid nitrogen vapor according to previously established protocols (58). The frozen lung was imaged using a high-resolution CT scanner (Siemens Somatom) and cut into 2-cm-thick slices along the transverse plane for sampling. Cores were systematically sampled from each slice using a 1.4-cm-diameter coring drill. For this study, 2 cores were randomly selected from each of upper, mid, and lower lung regions for 6 samples per lung (Figure 1, A and B). Frozen lung samples were scanned by microCT (Skyscan 1172, Bruker) using a cooling stage according to a previously established protocol (59). MicroCT scans were set at 40 kV, 226 mA, and 0.5° rotation step at –30°C, with temperature maintained throughout the scan. Scans were reconstructed using NRecon software (Bruker) and images analyzed using CTAn software (Bruker) with a manual threshold to segment tissue from air to measure tissue percentage (tissue%) and surface area/volume (surface density, SD) from each core (Figure 1C). Tissue% and SD changes are both correlated with increased tissue from fibrosis and reduction in surface area due to loss of normal parenchymal tissues, allowing these measures to quantify the extent of disease within each sample. As expected, more severely fibrotic regions were in the basal regions of the lung (Supplemental Figure 3).
A portion of each core was embedded for sectioning with additional samples cut for gene expression. Sections were stained for H&E, picrosirius red (total collagen), and Van Gieson (elastin). Antibodies were used to detect collagen type I (ab6308, Abcam), collagen type III (2150-0100, Bio-Rad), and POU2AF1/BOB1 (ab238036, Abcam). Detailed methods for histology and mRNA and microRNA labeling and processing steps are available in the supplemental material.
Single-cell RNA-seq analysis.
Using a previously published data set (41), we examined the cell types expressing POU2AF1 using the violin plot function in Seurat (version 3.0.2) and R (version 3.5.1) in 243,472 cells from 29 healthy control lungs and 32 IPF lungs.
Differential expression analysis.
Samples were divided based on principal component analysis of surface density and collagen 1 and collagen 3 volume fractions followed by expectation-maximization clustering. Three groups were defined representing early-stage disease with normal lung structure (IPF1), moderate-stage disease where progressive changes are occurring (IPF2), and late-stage disease with severe fibrosis and end-stage pathology (IPF3). Genes were filtered and log2 transformed and then differential expression calculated using linear mixed-effects models for each IPF group with a false discovery rate (FDR) adjusted P < 0.05 considered significant for gene expression and P < 0.1 for microRNA expression used in DREM analysis and P < 0.05 used for identifying miR target genes with greater than 2-fold increased/decreased expression. Detailed descriptions are available in the supplemental material.
Fibrosis progression analysis using the DREM.
The DREM (60, 61) was applied to our data set to develop a model of regulatory tracks associated with disease progression in IPF. DREM uses a machine-learning method based on graphical models to identify places in the gene expression profile where a set of genes that were previously coexpressed start to diverge. These locations denote the branching points and are associated with regulators (transcription factors and microRNAs) predicted to impact these events. Cell types associated with each track were determined using single-cell expression data from lung tissues (40). Detailed descriptions of these methods are in the supplemental material.
Mouse model of lung fibrosis.
C57BL/6 WT and Pou2af1–/– mice (KO) on the C57BL/6 background (62) were administered bleomycin or saline and then sacrificed on day 14. A total of 104 mice from 3 batches were used in this study (WT-saline [WTS], n = 26; WT-bleomycin [WTB], n = 26; KO-saline [KOS], n = 23; KO-bleomycin [KOB], n = 29). Total RNA was extracted from 30–50 mg of frozen lung tissue by tissue disruption and homogenization. Gene expression was determined by Life Technologies TaqMan real-time PCR (Thermo Fisher Scientific). β-Glucuronidase was employed as an internal standard control and collagen 1α1 primer was obtained from Thermo Fisher Scientific. Each reaction was performed in triplicate and control reactions without RNA performed as negative control. Relative gene expression was normalized to the unstimulated control group and a linear mixed-effects model was used to compare relative expression of Col1α1 between groups using batch as the random effect. Lung hydroxyproline content was analyzed with the hydroxyproline colorimetric assay kit (BioVision Inc). The number of micrograms of hydroxyproline per right lung was calculated with each assay normalized to the unstimulated control group. A linear mixed-effects model was used to compare relative hydroxyproline levels between groups using batch as the random effect. Paraffin-embedded tissue sections were stained with Masson’s trichrome (collagen/connective tissue) or H&E using 2 slices per animal and 2 animals per group. Immunohistochemistry for α-SMA (ab5694, Abcam) was performed with nonimmune serum used for negative controls. Detailed methods are further described in the supplemental material.
Data sharing.
All RNA-seq data are available in NCBI’s Gene Expression Omnibus (GEO GSE124685). The complete DREM model is available as an interactive model online (10). This website allows for detailed track analysis and data mining of differentially expressed genes and associated regulators.
Statistics.
Differential expression was calculated using linear mixed-effects models for each IPF group with an FDR-adjusted P < 0.05 considered significant for gene expression and P < 0.1 for microRNA expression. DREM modeling is based on input-output hidden Markov modeling to identify regulatory tracks (see supplemental material for additional details). For comparing groups in the mouse model, a linear mixed-effects model was used with treatment group as the fixed effect and each batch considered as the random effect; significance was considered as P < 0.05.
Study approvals.
Human lungs were collected following local hospital ethical committee approval (ML6385) and informed patient consent. According to Belgian legislation, declined donor lungs can be used for research purposes. All animals included in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Yale University (2016-20084).
Author contributions
PVB, ZBJ, JCH, BMV, and NK contributed to study conception and design. JEM, FA, QL, SJ, MV, GD, AT, JHM, NT, FC, and XY contributed to the acquisition of data and conducted the experiments. DT provided the transgenic mouse model. JEM, SJ, XY, RJH, JHM, DVM, JZ, JD, PVB, ZBJ, and NK contributed to the analysis and writing. Samples were collected and processed by JEM, SEV, JV, KM, LDS, RV, AN, and WAW. All authors reviewed and contributed comments that were incorporated into the final version of this manuscript. Study supervision was provided by NK.
Supplementary Material
Acknowledgments
The work was funded by NIH grants R01HL127349, U01HL145567, U01HL122626, and U54HG008540 to NK, U01HL137159 and R01LM012087 to PVB, and KU Leuven grant C24/15/30 to BV4. JEM is a senior research fellow of the ERS (RESPIRE2-2015-9192). WW and RV are senior clinical investigators and SEV a senior research fellow of the Research Foundation Flanders (FWO).
Version 1. 10/10/2019
In-Press Preview
Version 2. 11/14/2019
Electronic publication
Footnotes
Conflict of interest: NK reports personal fees from Biogen Idec, Boehringer Ingelheim, Third Rock, Miragen, Pliant, Samumed, NuMedii, Indaloo, Theravance, LifeMax, Optikira, and Three Lake Partners; in addition, NK has patents (9913819, 20180101642, 10036069) on New Therapies in Pulmonary Fibrosis Peripheral Blood Gene Expression in IPF.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(22):e131597.https://doi.org/10.1172/jci.insight.131597.
Contributor Information
John E. McDonough, Email: john.e.mcdonough@yale.edu.
Farida Ahangari, Email: farida.ahangari@yale.edu.
Qin Li, Email: qin.li@yale.edu.
Siddhartha Jain, Email: sj1@csail.mit.edu.
Stijn E. Verleden, Email: stijn.verleden@kuleuven.be.
Jose Herazo-Maya, Email: jose.herazo-maya@nchmd.org.
Milica Vukmirovic, Email: milica.vukmirovic@yale.edu.
Giuseppe DeIuliis, Email: joseph.deiuliis@yale.edu.
Argyrios Tzouvelekis, Email: argyrios.tzouvelekis@fleming.gr.
Naoya Tanabe, Email: ntana@kuhp.kyoto-u.ac.jp.
Fanny Chu, Email: fanny.chu@hli.ubc.ca.
Xiting Yan, Email: xiting.yan@yale.edu.
Johny Verschakelen, Email: johny.verschakelen@uzleuven.be.
Robert J. Homer, Email: robert.homer@yale.edu.
Dimitris V. Manatakis, Email: dimitris@pitt.edu.
Junke Zhang, Email: JUZ19@pitt.edu.
Jun Ding, Email: phoenixjunding@gmail.com.
Karen Maes, Email: karen.maes@kuleuven.be.
Laurens De Sadeleer, Email: laurens.desadeleer@kuleuven.be.
Robin Vos, Email: robin.vos@uzleuven.be.
Arne Neyrinck, Email: arne.neyrinck@kuleuven.be.
Panayiotis V. Benos, Email: benos@pitt.edu.
Ziv Bar-Joseph, Email: zivbj@cs.cmu.edu.
Dean Tantin, Email: dean.tantin@path.utah.edu.
James C. Hogg, Email: jim.hogg@hli.ubc.edu.
Bart M. Vanaudenaerde, Email: bart.vanaudenaerde@kuleuven.be.
Wim A. Wuyts, Email: wim.wuyts@uzleuven.be.
Naftali Kaminski, Email: naftali.kaminski@yale.edu.
References
- 1.Kim DS, Collard HR, King TE. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 3.Lynch DA, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 4.Scadding JG, Hinson KF. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax. 1967;22(4):291–304. doi: 10.1136/thx.22.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crystal RG, Bitterman PB, Rennard SI, Hance AJ, Keogh BA. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract (first of two parts) N Engl J Med. 1984;310(3):154–166. doi: 10.1056/NEJM198401193100304. [DOI] [PubMed] [Google Scholar]
- 6.King TE, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 7.Richeldi L, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 9.Carrington R, Jordan S, Pitchford SC, Page CP. Use of animal models in IPF research. Pulm Pharmacol Ther. 2018;51:73–78. doi: 10.1016/j.pupt.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 10. [No authors listed]. IPF regulatory iDREM model. Carnegie Mellon University. http://sb.cs.cmu.edu/IPFReg/ Accessed October 18, 2019.
- 11.McDonough JE, et al. A role for telomere length and chromosomal damage in idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):132. doi: 10.1186/s12931-018-0838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41(4):467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augsten M, et al. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc Natl Acad Sci USA. 2009;106(9):3414–3419. doi: 10.1073/pnas.0813144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasse A, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173(7):781–792. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 15.Jia G, et al. CXCL14 is a candidate biomarker for Hedgehog signalling in idiopathic pulmonary fibrosis. Thorax. 2017;72(9):780–787. doi: 10.1136/thoraxjnl-2015-207682. [DOI] [PubMed] [Google Scholar]
- 16.Zuo F, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99(9):6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards TJ, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185(1):67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiller HB, et al. Deep proteome profiling reveals common prevalence of MZB1-positive plasma B cells in human lung and skin fibrosis. Am J Respir Crit Care Med. 2017;196(10):1298–1310. doi: 10.1164/rccm.201611-2263OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Englert JM, et al. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol. 2008;172(3):583–591. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu G, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. 2018;24(1):39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vukmirovic M, Kaminski N. Impact of transcriptomics on our understanding of pulmonary fibrosis. Front Med (Lausanne) 2018;5:87. doi: 10.3389/fmed.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer Y, et al. A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;52(2):217–231. doi: 10.1165/rcmb.2013-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonough JE, Kaminski N, Thienpont B, Hogg JC, Vanaudenaerde BM, Wuyts WA. Gene correlation network analysis to identify regulatory factors in idiopathic pulmonary fibrosis. Thorax. 2019;74(2):132–140. doi: 10.1136/thoraxjnl-2018-211929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seibold MA, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunninghake GM, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368(23):2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fingerlin TE, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staab-Weijnitz CA, et al. FK506-binding protein 10, a Potential novel drug target for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192(4):455–467. doi: 10.1164/rccm.201412-2233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baarsma HA, Königshoff M. ’WNT-er is coming’: WNT signalling in chronic lung diseases. Thorax. 2017;72(8):746–759. doi: 10.1136/thoraxjnl-2016-209753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Königshoff M, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3(5):e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ru Y, et al. The multiMiR R package and database: integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014;42(17):e133. doi: 10.1093/nar/gku631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cushing L, et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45(2):287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery RL, et al. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol Med. 2014;6(10):1347–1356. doi: 10.15252/emmm.201303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandit KV, et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182(2):220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pottier N, et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial-mesenchymal interactions. PLoS One. 2009;4(8):e6718. doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;11(2):233–240. doi: 10.1016/S0955-0674(99)80031-3. [DOI] [PubMed] [Google Scholar]
- 37.Mora AL, Bueno M, Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J Clin Invest. 2017;127(2):405–414. doi: 10.1172/JCI87440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salajegheh A, Vosgha H, Md Rahman A, Amin M, Smith RA, Lam AK. Modulatory role of miR-205 in angiogenesis and progression of thyroid cancer. J Mol Endocrinol. 2015;55(3):183–196. doi: 10.1530/JME-15-0182. [DOI] [PubMed] [Google Scholar]
- 39.Vosgha H, Ariana A, Smith RA, Lam AK. miR-205 targets angiogenesis and EMT concurrently in anaplastic thyroid carcinoma. Endocr Relat Cancer. 2018;25(3):323–337. doi: 10.1530/ERC-17-0497. [DOI] [PubMed] [Google Scholar]
- 40.Reyfman PA, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199(12):1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adams TS, et al. Single cell RNA-seq reveals ectopic and aberrant lung resident cell populations in idiopathic pulmonary fibrosis. bioRxiv. https://www.biorxiv.org/content/10.1101/759902v2 Published September 9, 2019. Accessed October 18, 2019.
- 42.Mai C, et al. Thin-section CT features of idiopathic pulmonary fibrosis correlated with micro-CT and histologic analysis. Radiology. 2017;283(1):252–263. doi: 10.1148/radiol.2016152362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luzina IG, et al. Transcriptomic evidence of immune activation in macroscopically normal-appearing and scarred lung tissues in idiopathic pulmonary fibrosis. Cell Immunol. 2018;325:1–13. doi: 10.1016/j.cellimm.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rooij E, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105(35):13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin W, et al. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22(8):1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roderburg C, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53(1):209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 47.Cushing L, Kuang P, Lü J. The role of miR-29 in pulmonary fibrosis. Biochem Cell Biol. 2015;93(2):109–118. doi: 10.1139/bcb-2014-0095. [DOI] [PubMed] [Google Scholar]
- 48.Deng Z, et al. MicroRNA-29: a crucial player in fibrotic disease. Mol Diagn Ther. 2017;21(3):285–294. doi: 10.1007/s40291-016-0253-9. [DOI] [PubMed] [Google Scholar]
- 49.Kim S, et al. Integrative phenotyping framework (iPF): integrative clustering of multiple omics data identifies novel lung disease subphenotypes. BMC Genomics. 2015;16:924. doi: 10.1186/s12864-015-2170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steele MP, et al. Relationship between gene expression and lung function in idiopathic interstitial pneumonias. BMC Genomics. 2015;16:869. doi: 10.1186/s12864-015-2102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burgy O, Königshoff M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 2018;68–69:67–80. doi: 10.1016/j.matbio.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 52.Schubart DB, Rolink A, Kosco-Vilbois MH, Botteri F, Matthias P. B-cell-specific coactivator OBF-1/OCA-B/Bob1 required for immune response and germinal centre formation. Nature. 1996;383(6600):538–542. doi: 10.1038/383538a0. [DOI] [PubMed] [Google Scholar]
- 53.Zwilling S, Dieckmann A, Pfisterer P, Angel P, Wirth T. Inducible expression and phosphorylation of coactivator BOB.1/OBF.1 in T cells. Science. 1997;277(5323):221–225. doi: 10.1126/science.277.5323.221. [DOI] [PubMed] [Google Scholar]
- 54.François A, et al. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res Ther. 2013;15(5):R168. doi: 10.1186/ar4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue J, et al. Plasma B lymphocyte stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients. J Immunol. 2013;191(5):2089–2095. doi: 10.4049/jimmunol.1203476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou H, et al. POU2AF1 functions in the human airway epithelium to regulate expression of host defense genes. J Immunol. 2016;196(7):3159–3167. doi: 10.4049/jimmunol.1502400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiller HB, et al. The human lung cell atlas: a high-resolution reference map of the human lung in health and disease. Am J Respir Cell Mol Biol. 2019;61(1):31–41. doi: 10.1165/rcmb.2018-0416TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonough JE, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasilescu DM, et al. Nondestructive cryomicro-CT imaging enables structural and molecular analysis of human lung tissue. J Appl Physiol. 2017;122(1):161–169. doi: 10.1152/japplphysiol.00838.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding J, Hagood JS, Ambalavanan N, Kaminski N, Bar-Joseph Z. iDREM: Interactive visualization of dynamic regulatory networks. PLoS Comput Biol. 2018;14(3):e1006019. doi: 10.1371/journal.pcbi.1006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding J, et al. Integrating multi-omics longitudinal data to reconstruct networks underlying lung development. Am J Physiol Lung Cell Mol Physiol. doi: 10.1152/ajplung.00554.2018. doi: 10.1152/ajplung.00554.2018. [published online ahead of print August 21, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin XF, Reichlin A, Luo Y, Roeder RG, Nussenzweig MC. OCA-B integrates B cell antigen receptor-, CD40L- and IL 4-mediated signals for the germinal center pathway of B cell development. EMBO J. 1998;17(17):5066–5075. doi: 10.1093/emboj/17.17.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.