Abstract
BACKGROUND
Innate immune activation impacts lung transplant outcomes. Dectin-1 is an innate receptor important for pathogen recognition. We hypothesized that genotypes reducing dectin-1 activity would be associated with infection, graft dysfunction, and death in lung transplant recipients.
METHODS
We assessed the rs16910526 CLEC7A gene polymorphism Y238X, which results in dectin-1 truncation, in 321 lung allograft recipients at a single institution and in 1,129 lung allograft recipients in the multicenter Lung Transplant Outcomes Group (LTOG) cohort. Differences in dectin-1 mRNA, cytokines, protein levels, immunophenotypes, and clinical factors were assessed.
RESULTS
Y238X carriers had decreased dectin-1 mRNA expression (P = 0.0001), decreased soluble dectin-1 protein concentrations in bronchoalveolar lavage (P = 0.008) and plasma (P = 0.04), and decreased monocyte surface dectin-1 (P = 0.01) compared with wild-type subjects. Y238X carriers had an increased risk of fungal pathogens (HR 1.17, CI 1.0–1.4), an increased risk of graft dysfunction or death (HR 1.6, CI 1.0–2.6), as well increased mortality in the UCSF cohort (HR 1.8, CI 1.1–3.8) and in the LTOG cohort (HR 1.3, CI 1.1–1.6), compared with wild-type CLEC7A subjects.
CONCLUSION
Increased rates of graft dysfunction and death associated with this dectin-1 polymorphism may be amplified by immunosuppression that drives higher fungal burden from compromised pathogen recognition.
FUNDING
The UCSF Nina Ireland Program for Lung Health Innovative Grant program, the Clinical Sciences Research & Development Service of the VA Office of Research and Development, and the Joel D. Cooper Career Development Award from the International Society for Heart and Lung Transplantation.
Keywords: Immunology, Transplantation
Keywords: Fungal infections, Genetic diseases, Innate immunity
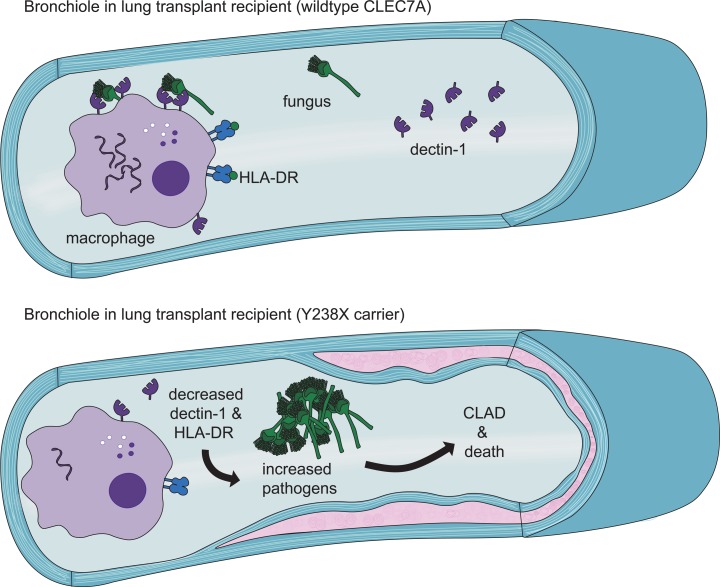
Genetic variation in the fungal recognition receptor, dectin-1, is associated with increased risk of graft dysfunction and death in lung transplant recipients, representing an important evoked immune phenotype.
Introduction
Even though lung transplantation is a life-prolonging option for many patients with advanced pulmonary disease, lung allograft recipients experience lower survival rates relative to other solid organ transplant patients (1). Chronic lung allograft dysfunction (CLAD) remains the primary driver of poor long-term survival (2). More than 50% of lung allograft recipients alive at 5 years have CLAD, manifesting as obstructive and/or restrictive defects in pulmonary function (3).
CLAD is associated with constrictive bronchiolitis and parenchymal fibrosis pathology, both of which result from the integration of multiple stressors on the allograft (4–9). Importantly, CLAD risk is increased following infection or colonization with Aspergillus and other airway pathogens (10, 11). Local tissue inflammation enhances recipient alloimmune responses, leading to airway fibrosis (3, 12). Thus, increased innate immune system activation may link airway injury from infection to CLAD.
Dectin-1 is a C-type lectin innate immune receptor that recognizes β-glucans on common transplant-associated pathogens. It is expressed on macrophages, dendritic cells, and epithelial cells (13). In pulmonary epithelial cells, dectin-1 activation by bacterial stimulation leads to cytokine signaling (14). Dectin-1 signals through an immunoreceptor tyrosine-based activation motif (ITAM), leading to nuclear factor kappa-light-chain-enhancer of activated B cells–dependent (NF-κB–dependent) cytokine production and cleavage of the dectin-1 receptor (15, 16). While dectin-1 can activate responses alone, costimulation with Toll-like receptors further augments cytokine production through nuclear factor of activated T cells (NFAT) and NF-κB transcription (17).
The rs16910526 or Y238X single-nucleotide polymorphism (SNP) in the dectin-1 gene, CLEC7A, is relatively common and leads to the presence of a premature stop codon in the carbohydrate recognition region (18). We hypothesized that lung transplant recipients with this Y238X variant would have deficient dectin-1 function resulting in increased risk for certain infections, subsequent CLAD, and impaired survival following lung transplant.
Results
Study population and polymorphism distributions.
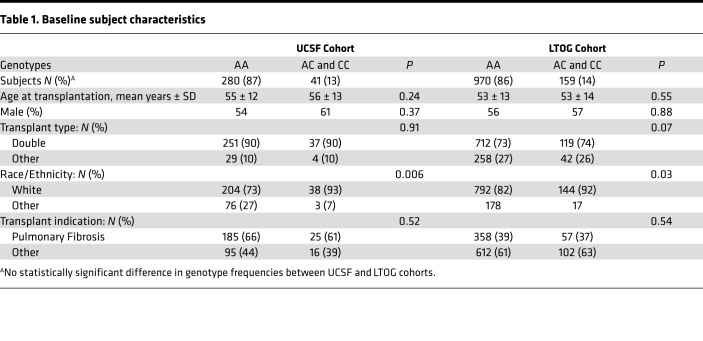
Characteristics of the included subjects are found in Table 1. In the UCSF cohort, only 1 subject was homozygous for the CLEC7A rs16910526 polymorphism (CC), and there were 40 subjects heterozygous for the Y238X variant (AC). A similar distribution of CLEC7A genotypes was found in the 1,131 subjects in the Lung Transplant Outcomes Group (LTOG) validation cohort, with 7 subjects homozygous for the CLEC7A CC genotype, and 152 subjects heterozygous for the CLEC7A Y238X variant.
Table 1. Baseline subject characteristics.
CLEC7A gene expression is decreased in lung tissue and peripheral blood cells of Y238X heterozygous subjects and decreased further after stimulation with zymosan.
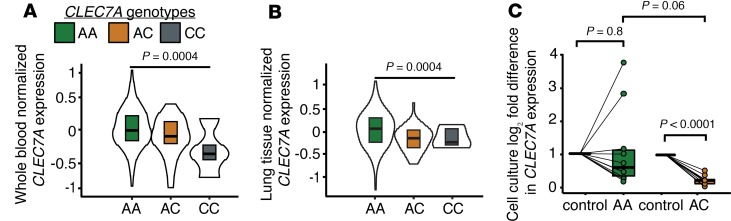
We measured baseline CLEC7A gene expression in 2 cohorts. Data from the Genome Tissue Expression (GTEx) database demonstrated that Y238X carriers (AC, n = 40; CC, n = 4) had decreased dectin-1 mRNA expression in blood compared with wild-type CLEC7A subjects (Figure 1A; AA, n = 325, P = 0.0004). A similar reduction in normalized dectin-1 mRNA expression was observed in lung tissue from subjects with the Y238X variant AC (n = 48) and CC genotypes (n = 4) compared with wild-type CLEC7A AA genotypes (Figure 1B; n = 338, P = 0.0004).
Figure 1. Decreased CLEC7A transcripts in Y238X variants.
Violin plots showing maximum and minimum values, with boxes showing 25th and 75th percentile bounds and bisecting lines showing median values for GTEx cohort data. There is decreased CLEC7A gene expression in (A) whole blood of AC genotypes (n = 40) and CC genotypes (n = 4) compared with wild-type genotypes (AA, n = 325) as well as in (B) lung tissue of AC (n = 38) and CC (n = 5) genotype subjects compared with wild-type AA (n = 330, P = 0.0004) subjects. Comparisons were made by linear regression. (C) Box-and-whisker plots depict decreased CLEC7A gene expression in Y238X heterozygous cells stimulated for 4 hours with zymosan relative to control media. RT-PCR CLEC7A mRNA and reference ACTB mRNA was quantified as 2−ΔΔCt. Cells in control media were normalized to a value of 1 and comparisons were made using ANOVA with Dunnett’s correction.
To assess differences in dectin-1 mRNA expression following dectin-1 receptor ligation, peripheral blood mononuclear cells (PBMCs) from lung transplant recipient Y238X carriers (AC, n = 9) and wild-type subjects (AA, n = 9) in the UCSF cohort were stimulated with the dectin-1 agonist zymosan or control media. While it does not reflect the true complexity of fungal cell walls, zymosan is widely used in vivo and in vitro to study receptor binding (13). We found no differences in baseline CLEC7A gene expression across genotypes within these 18 samples (P = 0.5). Figure 1B demonstrates a reduction in dectin-1 gene expression in Y238X heterozygous PBMCs stimulated with zymosan as compared with PBMCs treated with media alone (P < 0.0001). In comparison, there was no difference in dectin-1 mRNA expression for wild-type PBMCs treated with zymosan versus control media (P = 0.8). We also measured cytokines in the supernatant of these cell culture experiments but found no differences for Y238X heterozygotes compared to wild-type CLEC7A cells (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.133083DS1).
Soluble dectin-1 protein is decreased in bronchoalveolar lavage during CLAD and among Y238X variant subjects.
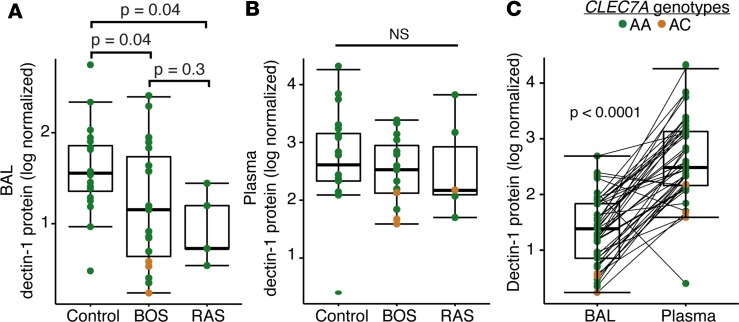
Given the observed difference in gene expression, we sought to determine if dectin-1 soluble protein concentrations differed within a nested subcohort of subjects by genotype and CLAD diagnosis (Supplemental Table 1). Dectin-1 protein differed significantly between CLAD groupings (BOS, bronchiolitis obliterans syndrome; RAS, restrictive allograft syndrome; and combined BOS and RAS subtypes), as well as across CLEC7A genotypes. Bronchoalveolar lavage (BAL) dectin-1 protein was significantly lower in subjects with BOS (P = 0.04), RAS (P = 0.04), or CLAD (BOS and RAS, P = 0.008) compared with dectin-1 protein in subjects without CLAD (Figure 2A). By contrast, plasma dectin-1 protein concentrations did not differ between subjects with CLAD (BOS and RAS) compared to those without CLAD (P = 0.3, Figure 2B). Dectin-1 soluble protein concentrations were significantly lower in BAL compared with the plasma compartment (P < 0.0001), with no correlation across paired BAL and plasma samples (Figure 2C; r2 = 0.2, P = 0.2). Compared with subjects with wild-type CLEC7A genotypes, Y238X heterozygotes had 2.8-fold less dectin-1 protein in BAL (P = 0.008) and 1.4-fold less dectin-1 protein in plasma (P = 0.04).
Figure 2. Decreased dectin-1 protein in Y238X variants and CLAD.
Soluble dectin-1 protein was measured in synchronous bronchoalveolar lavage (BAL) and plasma samples from matched subjects with CLAD (n = 20) and without CLAD (n = 25). The 2 endotypes of CLAD, BOS and RAS, are displayed separately. (A) Log-normalized soluble dectin-1 protein concentrations are decreased in the BAL of subjects with BOS (P = 0.04), RAS (P = 0.04), or CLAD (P = 0.008) compared with BAL dectin-1 protein in subjects without CLAD. (B) In plasma, log-normalized dectin-1 protein concentrations are not different between subjects with CLAD compared to those without CLAD (P = 0.3). (C) BAL dectin-1 protein concentration was significantly lower compared with plasma dectin-1 protein concentrations (P < 0.0001). Subjects with Y238X AC genotypes (orange circles) had less soluble dectin-1 protein in BAL (P = 0.008) and plasma (P = 0.04) compared with AA genotypes (green circles). Differences were assessed using ANOVA with Dunnett’s correction for multiple comparisons. BAL, bronchoalveolar lavage; BOS, bronchiolitis obliterans syndrome; CLAD, chronic lung allograft dysfunction; RAS, restrictive allograft syndrome.
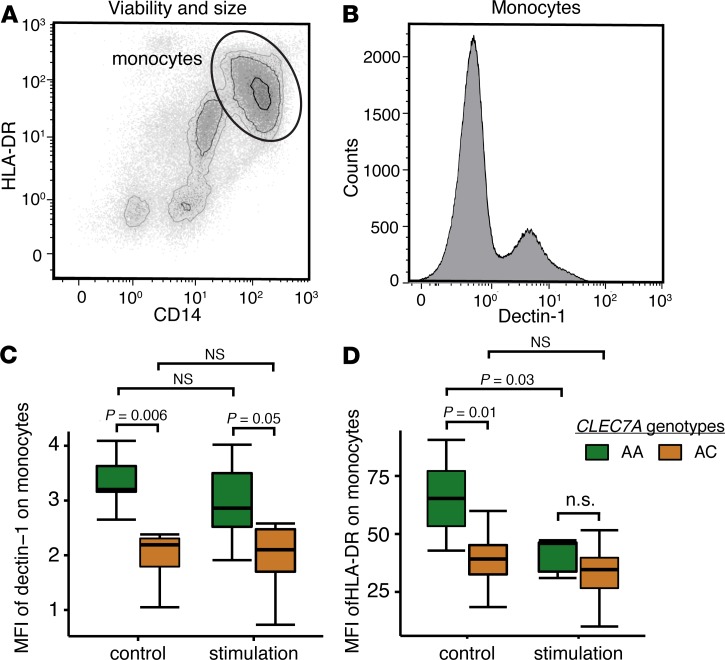
The dectin-1 receptor is decreased on monocytes from Y238X variant subjects.
We sought to characterize cell surface dectin-1 protein following receptor ligation. As shown in Figure 3, peripheral blood monocytes were analyzed following stimulation with zymosan or control media from Y238X heterozygous subjects (AC, n = 8) and wild-type subjects (AA, n = 8). In both conditions, monocytes from Y238X carriers had significantly less surface dectin-1 by mean fluorescence intensity (MFI) compared with wild-type monocytes (P ≤ 0.05, Figure 3C). Within genotypes, stimulation did not affect dectin-1 receptor levels.
Figure 3. Decreased dectin-1 expression on monocytes from Y238X AC individuals.
Following 4 hours of stimulation with control media or zymosan, monocytes were assessed by immunophenotyping those from CLEC7A variants (AC, n = 8) and wild-type CLEC7A (AA, n = 8). (A) Monocytes were defined by forward scatter, side scatter, size, viability, and staining for CD14 and HLA-DR. (B) Surface dectin-1 receptors were measured on monocytes by MFI. Box-and-whisker plots show dectin-1 and HLA-DR monocyte surface expression, with boxes defining the 25th and 75th percentile of data, bisecting line depicting the median value, and whiskers capturing minimum and maximum values. (C) In control media, surface dectin-1 receptor MFI was increased on wild-type monocytes (AA) relative to Y238X variants (AC, P = 0.006). Following zymosan stimulation, this difference was maintained between AA and AC genotypes (P = 0.05). There was no difference in dectin-1 receptor MFI on wild-type or variant-genotype monocytes in control media or following stimulation. (D) HLA-DR MFI was also measured. There was significantly decreased HLA-DR MFI on CLEC7A-variant monocytes (AC) compared with wild-type monocytes (AA, P = 0.03) in control media. The HLA-DR MFI decreased on AA-genotype monocytes (P = 0.01) but not on AC-genotype monocytes following stimulation. Compared to wild-type CLEC7A genotypes, there was no difference in HLA-DR MFI on Y238X variant-genotype monocytes following zymosan stimulation. Differences were assessed using 2-tailed Student’s t test and the experiment was conducted twice.
Because monocyte antigen presentation is closely tied to pathogen recognition, and decreases during infection, we measured HLA-DR expression changes following zymosan stimulation (21). At baseline, there was significantly less surface HLA-DR on Y238X-carrier monocytes compared with wild-type CLEC7A monocytes (P = 0.01, Figure 3D). With zymosan stimulation, HLA-DR significantly decreased on the surface of wild-type CLEC7A AA monocytes (P = 0.03) but not on the surface of Y238X heterozygous monocytes (P = 0.5). Thus, Y238X carriers had baseline suppression of HLA-DR expression, resembling the phenotype seen in wild-type subjects with stimulation.
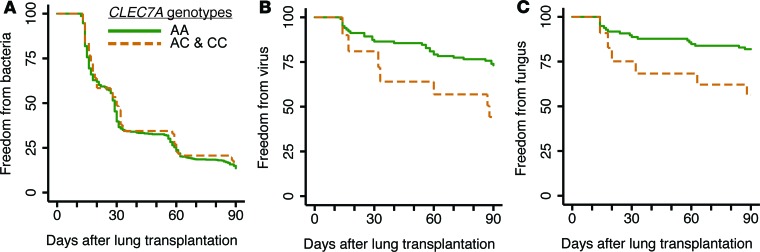
Y238X variants have increased risk for isolation of BAL fungal and viral pathogens.
We next sought to characterize the clinical impact of these genotypes on risk for pathogen isolation, graft dysfunction, and death in 2 cohorts. In the UCSF cohort, freedom from bacterial, viral, and fungal pathogen isolation in the first 90 days after transplant were assessed in subjects presenting for bronchoscopy (Supplemental Table 2). Freedom from pathogen isolation is displayed by Kaplan-Meier plot in Figure 4. There was no difference in time to positive bacterial culture for Y238X carriers (HR 0.83, CI 0.67–1.03, P = 0.09) compared to wild-type (AA) subjects. In contrast, Y238X carriers (AC and CC) had an increased HR for viral airway infection, as demonstrated by positive BAL viral PCR, compared with subjects with wild-type CLEC7A (HR 2.46, CI 1.08–5.63, P = 0.03). Similarly, fungi were identified earlier in Y238X carriers.
Figure 4. Increased hazard for early viral and fungal pathogens associated with Y238X variant genotypes.
Kaplan-Meier graphs show freedom from bacterial, viral, or fungal pathogen isolated from BAL. There were 220 subjects from the UCSF cohort included in this prospective analysis (AA genotype n = 192, AC and CC genotypes n = 29). There was no significant difference between the time to first positive bacterial culture result among CLEC7A genotypes (A) (HR 0.83, CI 0.67–1.03, P = 0.09). Y238X variants had decreased time to identification of virus in BAL by PCR (B) (HR 2.46, CI 1.08–5.63, P = 0.03) and fungus by culture (C) (HR 1.17, CI 1.01–1.37, P = 0.04). Risk for pathogen isolation was assessed by Cox proportional hazards models. BAL, bronchoalveolar lavage; PCR, polymerase chain reaction; HR, hazard ratio; CI, confidence interval.
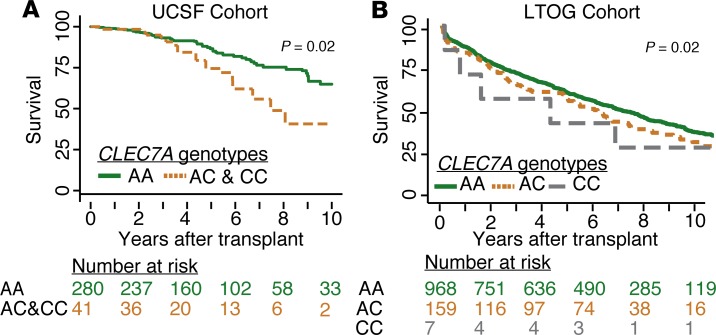
The Y238X polymorphism confers increased risk for death and graft dysfunction after lung transplantation in 2 large cohorts.
UCSF subjects with Y238X genotypes had a median time to CLAD or death of 3.7 ± 0.4 years compared with 6.3 ± 0.2 years in subjects with wild-type CLEC7A genotypes (adjusted HR 1.8, CI 1.2–2.8, P = 0.008). CLAD-free survival risk was also examined within time intervals where the relative hazard was observed to be proportional. With this model, Y238X carriers had an increased risk of CLAD or death after 3 years (adjusted HR 3.9, CI 2.2–6.9, P < 0.001) compared with wild-type CLEC7A subjects (AA), but there was no CLAD-free survival difference during the first 3 years following lung transplantation (adjusted HR 0.9, CI 0.4–1.8, P = 0.8).
Figure 5 shows Kaplan-Meier survival graphs demonstrating overall survival stratified by CLEC7A genotype in the UCSF (panel A) and LTOG (panel B) cohorts. Within the UCSF cohort, there was an increased risk of death among Y238X carriers, with median survival of 7.5 ± 0.9 years compared with 11.8 ± 1 years in wild-type CLEC7A subjects (adjusted HR 1.8, CI 1.1–3.8, P = 0.02). A similar phenomenon was found among the 1,129 subjects included in the LTOG validation cohort, with freedom from death displayed by Kaplan-Meier plot in Figure 4B. LTOG subjects with Y238X loss-of-function AC and CC polymorphisms had a median survival of 6.3 ± 1.1 years compared with 7.4 ± 0.7 years in wild-type CLEC7A subjects (adjusted HR 1.3, CI 1.1–1.6, P = 0.02).
Figure 5. Impaired survival in lung transplant recipients with Y238X variant genotypes in derivation and validation cohorts.
(A) Kaplan-Meier graph demonstrating survival in years after transplant in UCSF cohort subjects with loss-of-function CLEC7A polymorphisms compared with those with wild-type dectin-1 genotypes (multivariable HR 1.8, CI 1.1–3.8, P = 0.02). (B) In the LTOG cohort, overall survival in subjects stratified by CLEC7A genotype was also decreased in subjects with CLEC7A AC and CC variants compared with wild-type CLEC7A genotypes (multivariable HR 1.3, CI 1.1–1.6, P = 0.02). Risk for death was determined using Cox proportional hazards models adjusted for subject characteristics established as important for lung transplant outcomes.
While lung function and CLAD outcomes were not available within the LTOG cohort, 39 subjects underwent retransplantation. Graft survival, defined as freedom from retransplantation or death, was significantly decreased in Y238X carriers (AC and CC, 6 ± 0.6 years) compared with wild-type CLEC7A genotypes (AA, 7 ± 0.3 years, multivariable HR 1.3, CI 1.0–1.6, P = 0.02). Similarly, in the UCSF cohort, there was a 60%-increased risk of graft failure, defined as retransplantation or death, in Y238X carriers compared with wild-type CLEC7A subjects (multivariable HR 1.6, CI 1.0–2.6, P = 0.05).
Discussion
Our results in 2 large lung transplant cohorts show that the Y238X loss-of-function polymorphism in the CLEC7A gene for dectin-1, a key innate immune receptor, was associated with increased risk of retransplantation, or death. Within a single cohort, this polymorphism was also associated with increased risk for CLAD or death and the isolation of fungus or virus in BAL. These findings suggest that intact dectin-1 can be protective of chronic rejection or death by decreasing inflammation subsequent to infection that may lead to bystander activation of allospecific immune responses.
The Y238X variant results in a premature stop codon in the last 10 amino acids of the dectin-1 carbohydrate recognition domain (18). In patients homozygous for this variant, it has been shown to result in impaired recognition of fungal β-glucan motifs, and, subsequently, decreased cytokine production (18–20). However, our study focused primarily on heterozygous individuals, for whom we did not observe differences in cytokine production at rest or following stimulation in the presence of calcineurin inhibition. Rather, Y238X carriers had impaired dectin-1 transcription, decreased monocyte receptor expression, and decreased soluble protein levels. One possible explanation for the finding of decreased dectin-1 expression is that this variant decreases the nuclear export or stability of transcripts through conserved mechanisms of addressing transcripts with premature stop codons (21). This mechanism would be consistent with our observation that ligation of dectin-1 with zymosan, which accelerates dectin-1 shedding and turnover, resulted in further decreases in dectin-1 transcript in heterozygous individuals. At the same time, baseline monocyte HLA-DR expression was decreased in Y238X carriers. While HLA-DR is subject to negative feedback regulation, baseline HLA-DR expression is also positively correlated with innate immune function (22). Together, these findings suggest that pressure of intense immunosuppression associated with lung transplantation appears to evoke a skewed phenotype of impaired innate immunity.
These findings may be applicable to other immunosuppressed patients. While heterozygosity in this SNP is a risk factor for benign mucocutaneous candidiasis in otherwise immunocompetent individuals, it has also been linked to invasive aspergillosis in hematopoietic cell transplantation recipients (18, 23, 24). The finding of decreased time to virus identified by multiplex PCR in lung transplant recipients with dectin-1 polymorphisms is potentially novel. However, several studies have shown an association between viral and fungal infection and CLAD (10, 25, 26). Our finding of decreased CLAD-free survival may be partially explained by an increased risk for these pathogens. Consequently, the immunomodulatory effects of β-glucans, the agonists of dectin-1, are well established; β-glucan therapy increases mucosal immunity, decreases surgical infections, and reduces recurrent respiratory viruses in children (27–31). Mouse studies have shown dectin-1 to be crucial in activating the inflammasome and reactive oxygen species necessary for mitigating infection of parasites and fungus (32).Our findings may motivate investigation as to whether oral or intravenous β-glucan therapy could ameliorate the risk of graft infection or inflammation in Y238X carriers.
While we postulate that increased pathogen burden secondary to impaired immunity as the mechanism linking dectin-1 impairment with CLAD, other mechanisms could also contribute. Decreased surface dectin-1 on Y238X monocytes could potentially drive the airway fibrosis more directly. For example, intact macrophage dectin-1 has been shown to promote adaptive immune suppression and tolerance of pancreatic adenocarcinoma through the ligation of the lectin galectin 9. Our subjects with decreased monocyte dectin-1 could therefore have reduced allograft tolerance. We additionally found that wild-type CLEC7A monocytes had decreased surface dectin-1 after zymosan stimulation (33). Decreased dectin-1 in BAL of CLAD subjects was not solely dependent on CLEC7A genotypes and thus may reflect dectin-1 downregulation during fungal colonization and support this mechanism of reduced tolerance and increased alloreactivity leading to chronic injury (33). It is possible that other variants in dectin-1 might result in differences in baseline function or that fibrosis or chronic infection could result in dectin-1 activation and subsequent transcriptional inhibition. A number of genetic variants in other genes of innate immunity have also been associated with CLAD risk, further exemplifying how the stress of lung transplantation can evoke phenotypes that might otherwise be silent (34–38).
A strength of this study was the inclusion of 2 genotyped cohorts of lung transplant patients, representing the largest genetic association study in this field. Further, some of the 321 genotyped subjects in the UCSF cohort had additional samples to allow nested mechanistic studies. Nonetheless, the study has limitations; for example, some clinical data were not available for part of the cohort, limiting statistical power for clinical analyses. This study is largely associative due to limitations in the ability to perform mechanistic studies by the scarcity of subject samples. Results should be interpreted in the setting of this reduced power. While the increased risk for CLAD or mortality among CLEC7A AC and CC genotypes in the UCSF cohort increased 3 years after transplant, this polymorphism was associated with a more constant risk in the LTOG cohort. This difference may reflect antifungal prophylaxis practices, which are relatively aggressive at UCSF. Variation in posttransplant protocols across LTOG centers may bias our associations to the mean. Along the same lines, our modeling of genotype effect on CLAD and survival may be incomplete, as we were unable to control for some known mediators such as gastroesophageal reflux disease, induction regimen, or graft infections.
In summary, we describe an association between a common polymorphism in the innate immune receptor dectin-1 and increased risk of lung transplant allograft failure and recipient mortality. Dectin-1 dysfunction likely contributes to CLAD risk through increased pathogen susceptibility, which is augmented by the intense immunosuppression of lung transplantation. CLEC7A genotyping of lung transplant recipients may help to inform personalized surveillance and antimicrobial treatment strategies.
Methods
All mechanistic studies were performed on samples from subjects in the UCSF cohort. Quantitative tissue gene expression was available from the GTEx database (19).
Study populations and clinical data.
Genotype analysis was performed in the UCSF and LTOG cohorts. For the UCSF cohort, adults who received single-lung, bilateral lung, or heart-lung allografts at UCSF between 2000 and 2016 were included if they had DNA available for genotyping, survived to the first follow-up lung function study (approximately 14 days), and provided informed consent. Supplemental Figure 2 depicts the inclusion and exclusion criteria for the analyses performed as part of this study.
BAL was performed using 5–8 aliquots of 20 mL saline, typically in the right middle lobe, followed by transbronchial biopsies as per institutional clinical management protocols. The first aliquot was sent for culture. Between August 1, 2012 and January 1, 2017, bronchoscopy was scheduled for routine surveillance at 0.5, 1, 2, 3, 6, 12, 18, and 24 months after transplantation. Additional bronchoscopy procedures were performed when clinically indicated for suspicion of acute infection or rejection.
BAL cell count and microbiologic culture results were obtained as part of routine clinical care. Data abstracted from the medical charts included information on demographics, transplant indication, bronchoscopy indication, symptoms, acute and chronic rejection, clinical culture results, management changes after bronchoscopy, and survival. Acute cellular rejection was assessed and graded in clinical transbronchial biopsies by 1 of 2 experienced thoracic pathologists using standard nomenclature (39). CLAD was defined according to established criteria as an unresolving 20% decline in forced expiratory volume in 1 second (FEV1) or forced vital capacity (FVC) lasting over 30 days (40) and adjudicated as previously described (41, 42). Graft failure was defined as retransplantation or death. BOS and RAS CLAD endotypes were defined according to international criteria as a 20% decrease in posttransplant baseline values for FEV1 or FVC, respectively (40, 43, 44). CLAD-free survival was quantified as years of freedom from CLAD or death.
Standard posttransplant induction regimens for all subjects included methylprednisolone and basiliximab. Anti-thymocyte globulin was used for combined heart and lung transplant recipients. Initial maintenance immunosuppressant therapy included tacrolimus, prednisone, and mycophenolate mofetil. Antifungal prophylaxis with posaconazole or voriconazole and inhaled amphotericin was initiated on postoperative day 0 and stopped after 90 days of treatment if BAL surveillance fungal cultures were negative. Identification of pathogenic fungus on subsequent surveillance bronchoscopy in the absence of allograft dysfunction was treated with inhaled amphotericin.
A validation genotype analysis was performed in the multicenter LTOG. These data were prospectively collected, as previously described (45). Study subjects provided informed consent for enrollment and study protocols were approved by the institutional review boards at each of the 12 participating sites. Subjects were included in this study if complete survival data and genotyping data were available. A total of 1,129 subjects from the LTOG cohort transplanted between July 2002 and January 2018 met inclusion criteria for the validation analysis.
Genotyping and nomenclature.
In accordance with the Human Genome Organization Nomenclature Committee, the dectin-1 gene is denoted by CLEC7A (46). SNPs for subjects in both cohorts were determined using the Affymetrix TxArray designed by the iGeneTRAiN consortium to target solid organ transplant–specific loci, including the CLEC7A SNP rs16910526 (47). Less than 1% of samples were excluded based on low call rates, mismatches between imputed and reported HLA-typing, and sex. Given the low frequency of the CC dectin-1 genotype, we dichotomized subjects as either CLEC7A homozygous wild type (AA) or as carriers of the CLEC7A variant polymorphism (AC or CC) conferring decreased function.
Soluble protein analysis and nomenclature.
Synchronous plasma and BAL supernatant samples were selected from a nested subcohort of 20 subjects with CLAD and 25 subjects without CLAD matched by age and time after transplant in the UCSF cohort. Soluble dectin-1 protein (Uniprot ID: Q9BXN2) was measured using a proximity extension assay within a panel of 92 immune-related proteins (Immune Response panel, Olink Proteomics). Protein abundance was quantified using real-time PCR cycle values and the protein quantity was expressed in normalized units on a log2 scale (48).
Quantitative RT-PCR.
Zymosan is a glucan ligand found on the cell surface of fungi, shown to be a potent agonist of the dectin-1 receptor (17). It is an important sterile agonist used in cell culture experiments of dectin-1 (18). Following 4 hours of stimulation with zymosan (10 μg/mL, Sigma-Aldrich, 58856-93-2) or control (R10 media), total RNA was extracted from PBMCs of dectin-1 heterozygous subjects (AC) and wild-type dectin-1 subjects (AA) using QIAzol (Qiagen) and mRNeasy kits (Qiagen, 74104). cDNA was generated and Taqman qRT-PCR (Life Technologies) was performed to quantify dectin-1 and ACTB mRNA as previously described (49). ΔCt and ΔΔCt were calculated for CLEC7A and ACTB gene expression. CLEC7A gene expression data were also available from the GTEx database (19).
Monocyte immunophenotyping.
We phenotyped monocytes by flow cytometry in cryopreserved PBMC samples following 4 hours of stimulation with heat-pretreated zymosan (10 μg/mL, InvivoGen, tlrl-zyd) to block TLR2 binding or R10 media alone. Samples were pretreated with saturating concentrations of human aggregated immunoglobulin to prevent nonspecific binding and then labeled with the following reagent and antibody combinations to define monocytes: viability dye (eBioscience, 65-0863-18), fluorescein isothiocyanate–conjugated (FITC-conjugated) anti-CD14 (clone HCD14, VWR International, 325604), allophycocyanin-cyanine 7 (APC-Cy7)–conjugated anti–HLA-DR (clone L243, VWR International, 307617), R-phycoerythrin (PE)–conjugated anti–dectin-1 (clone 259931, Thermo Fisher Scientific, FAB1859P025). Monocytes were identified by forward scatter, side scatter, and size. Viable monocytes were identified by staining for CD14 and HLA-DR. After washing, samples were acquired with a Beckman Coulter cytometer and data were analyzed with Kaluza software (Beckman Coulter), using the gating strategy illustrated in Figure 3. MFI was measured for dectin-1 and HLA-DR, as HLA class II is important for pathogen recognition (50). Samples with less than 1,000 viable monocytes were excluded from statistical analysis.
Statistics.
Paired whole-genome and tissue-specific RNA sequencing data were obtained from the GTEx project portal (https://www.gtexportal.org/home/) on June 5, 2019. Genotype effects on lung and PBMC dectin-1 gene expression were compared using FastQTL with nominal P values generated by testing the alternative hypothesis that the slope of a linear regression model between genotype and expression deviates from 0 (51, 52). Comparisons between groups within mechanistic studies of dectin-1 protein, gene expression, and monocyte surface markers were made using a 2-tailed Student’s t test for pairwise comparisons or 1-way ANOVA with Dunnett’s correction for studies with multiple comparisons, defining P values less than 0.05 as significant.
Subject characteristics, genotypes, and cell culture results were compared using 2-tailed Student’s t and χ2 tests for continuous and categorical variables, respectively. We used Cox proportional hazards models to determine survival and CLAD-free survival HRs as a function of genotype, which included covariates for subject characteristics frequently associated with poor transplant outcomes or genetic variability: age at transplantation, recipient sex, recipient-reported ethnicity, transplant diagnosis, and transplant type (single, double, or heart-lung). Validation analyses were also adjusted for individual LTOG center by inclusion as a covariate. The primary 5 principle components were included as a covariate to address LTOG population admixture. Violations of proportional hazards were assessed visually and with the Schoenfeld test. In the UCSF cohort, we also modeled the HR of these outcomes as a step function by splitting time after transplant into 2 sequential periods (0–3 years, and 3–10 years), as the proportionality of CLAD-free survival and overall survival risk varied with time. Cox proportional hazards models in the UCSF group were also used to determine risk for positive culture result stratified by genotype. BAL culture analyses were limited to the first 90 days after transplant to avoid the confounding effect of azole antifungal treatment, and subjects were censored once an organism was identified on culture. We visualized time to these events by Kaplan-Meier plots.
Statistical analyses were performed in R (R Foundation for Statistical Computing) using the survminer, plyr, gee, ggplot, survival, stringr, multcomp, and ggpubr packages.
Study approval.
The UCSF institutional review board approved the single-center study components. LTOG study subjects provided informed consent for enrollment and study protocols were approved by the institutional review boards at each of the 12 participating sites.
Author contributions
DRC, JRG, SRH, JAG, and JK designed the study. DRC, PW, TC, DD, and JH performed experiments. DRC, JRG, JPS, and DT analyzed results. LTOG investigators, DRC, JRG, and JDC provided vital data and interpretation of results. DRC and JRG prepared the manuscript with input from all authors.
Supplementary Material
Acknowledgments
See Supplemental Acknowledgments for LTOG consortium details. Project funding came from the UCSF Nina Ireland Program for Lung Health (NIPLH) Innovative Grant program, the Clinical Sciences Research & Development Service of the VA Office of Research and Development (award number IK2CX001034), and the Joel D. Cooper Award from the International Society for Heart and Lung Transplantation (ISHLT). We are thankful for the cooperation of Donor Network West, and for all of the organ and tissue donors, and their families for giving gifts of life and knowledge with their generous donation.
Version 1. 10/15/2019
In-Press Preview
Version 2. 11/14/2019
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(22):e133083.https://doi.org/10.1172/jci.insight.133083.
Contributor Information
Daniel R. Calabrese, Email: daniel.calabrese@ucsf.edu.
Ping Wang, Email: ping.wang2@ucsf.edu.
Tiffany Chong, Email: t.chong1994@gmail.com.
Jonathan Hoover, Email: jonathan.hoover@ucsf.edu.
Jonathan P. Singer, Email: Jon.singer@ucsf.edu.
Dara Torgerson, Email: dara.torgerson@me.com.
Steven R. Hays, Email: Steven.Hays@ucsf.edu.
Jeffrey A. Golden, Email: Jeff.Golden@ucsf.edu.
Jasleen Kukreja, Email: Joy.Chen@ucsf.edu.
Daniel Dugger, Email: daniel.dugger@ucsf.edu.
Jason D. Christie, Email: jchristi@upenn.edu.
LTOG investigators, Email: jason.christie@pennmedicine.upenn.edu.
John R. Greenland, Email: john.greenland@ucsf.edu.
References
- 1.[No author listed] OPTN/SRTR 2017 Annual Data Report: Introduction. Am J Transplant. 2019;19 Suppl 2:11–18. doi: 10.1111/ajt.15273. [DOI] [PubMed] [Google Scholar]
- 2.Verleden SE, Vos R, Vanaudenaerde BM, Verleden GM. Chronic lung allograft dysfunction phenotypes and treatment. J Thorac Dis. 2017;9(8):2650–2659. doi: 10.21037/jtd.2017.07.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer KC, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44(6):1479–1503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 4.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol. 1993;142(6):1945–1951. [PMC free article] [PubMed] [Google Scholar]
- 5.Greenland JR, et al. HLA mismatching favoring host-versus-graft NK cell activity via KIR3DL1 is associated with improved outcomes following lung transplantation. Am J Transplant. 2017;17(8):2192–2199. doi: 10.1111/ajt.14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes D, et al. Influence of human leukocyte antigen mismatching on bronchiolitis obliterans syndrome in lung transplantation. J Heart Lung Transplant. 2016;35(2):186–194. doi: 10.1016/j.healun.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Le Pavec J, et al. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. 2016;35(9):1067–1077. doi: 10.1016/j.healun.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Tikkanen JM, et al. De novo DQ donor-specific antibodies are associated with chronic lung allograft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2016;194(5):596–606. doi: 10.1164/rccm.201509-1857OC. [DOI] [PubMed] [Google Scholar]
- 9.Burton CM, et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant. 2009;28(9):888–893. doi: 10.1016/j.healun.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Weigt SS, et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: a two-center validation study. Am J Transplant. 2013;13(4):919–927. doi: 10.1111/ajt.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigt SS, et al. Gene expression profiling of bronchoalveolar lavage cells during aspergillus colonization of the lung allograft. Transplantation. 2018;102(6):986–993. doi: 10.1097/TP.0000000000002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreisel D, Goldstein DR. Innate immunity and organ transplantation: focus on lung transplantation. Transpl Int. 2013;26(1):2–10. doi: 10.1111/j.1432-2277.2012.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 14.Heyl KA, et al. Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. MBio. 2014;5(5):e01492–e01414. doi: 10.1128/mBio.01492-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingeter LM, Lin X. C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9(2):105–112. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths JS, et al. Differential susceptibility of dectin-1 isoforms to functional inactivation by neutrophil and fungal proteases. FASEB J. 2018;32(6):3385–3397. doi: 10.1096/fj.201701145R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178(5):3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 18.Ferwerda B, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361(18):1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai LY, et al. The Y238X stop codon polymorphism in the human β-glucan receptor dectin-1 and susceptibility to invasive aspergillosis. J Infect Dis. 2011;203(5):736–743. doi: 10.1093/infdis/jiq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi M, et al. Premature termination codons are recognized in the nucleus in a reading-frame dependent manner. Cell Discov. 2015;1:15001. doi: 10.1038/celldisc.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faivre V, Lukaszewicz AC, Payen D. Downregulation of blood monocyte HLA-DR in ICU patients is also present in bone marrow cells. PLoS ONE. 2016;11(11):e0164489. doi: 10.1371/journal.pone.0164489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher CE, et al. Validation of single nucleotide polymorphisms in invasive aspergillosis following hematopoietic cell transplantation. Blood. 2017;129(19):2693–2701. doi: 10.1182/blood-2016-10-743294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sainz J, et al. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS ONE. 2012;7(2):e32273. doi: 10.1371/journal.pone.0032273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah PD, McDyer JF. Viral infections in lung transplant recipients. Semin Respir Crit Care Med. 2010;31(2):243–254. doi: 10.1055/s-0030-1249120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paraskeva M, et al. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11(10):2190–2196. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- 27.Babineau TJ, Marcello P, Swails W, Kenler A, Bistrian B, Forse RA. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann Surg. 1994;220(5):601–609. doi: 10.1097/00000658-199411000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dellinger EP, et al. Effect of PGG-glucan on the rate of serious postoperative infection or death observed after high-risk gastrointestinal operations. Betafectin Gastrointestinal Study Group. Arch Surg. 1999;134(9):977–983. doi: 10.1001/archsurg.134.9.977. [DOI] [PubMed] [Google Scholar]
- 29.Richter J, Svozil V, Král V, Rajnohová Dobiášová L, Stiborová I, Vetvicka V. Clinical trials of yeast-derived β-(1,3) glucan in children: effects on innate immunity. Ann Transl Med. 2014;2(2):15. doi: 10.3978/j.issn.2305-5839.2014.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bashir KMI, Choi JS. Clinical and physiological perspectives of β-glucans: The past, present, and future. Int J Mol Sci. 2017;18(9):E1906. doi: 10.3390/ijms18091906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jesenak M, Urbancikova I, Banovcin P. Respiratory tract infections and the role of biologically active polysaccharides in their management and prevention. Nutrients. 2017;9(7):E779. doi: 10.3390/nu9070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lima-Junior DS, Mineo TWP, Calich VLG, Zamboni DS. Dectin-1 Activation during Leishmania amazonensis phagocytosis prompts Syk-dependent reactive oxygen species production to trigger inflammasome assembly and restriction of parasite replication. J Immunol. 2017;199(6):2055–2068. doi: 10.4049/jimmunol.1700258. [DOI] [PubMed] [Google Scholar]
- 33.Daley D, et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med. 2017;23(5):556–567. doi: 10.1038/nm.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kastelijn EA, van Moorsel CH, Ruven HJ, Lammers JW, Grutters JC. Genetic polymorphisms and bronchiolitis obliterans syndrome after lung transplantation: promising results and recommendations for the future. Transplantation. 2012;93(2):127–135. doi: 10.1097/TP.0b013e31823915d5. [DOI] [PubMed] [Google Scholar]
- 35.Palmer SM, et al. Genetic regulation of rejection and survival following human lung transplantation by the innate immune receptor CD14. Am J Transplant. 2007;7(3):693–699. doi: 10.1111/j.1600-6143.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- 36.Palmer SM, et al. Innate immunity influences long-term outcomes after human lung transplant. Am J Respir Crit Care Med. 2005;171(7):780–785. doi: 10.1164/rccm.200408-1129OC. [DOI] [PubMed] [Google Scholar]
- 37.Kwakkel-van Erp JM, et al. The killer immunoglobulin-like receptor (KIR) group A haplotype is associated with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2008;27(9):995–1001. doi: 10.1016/j.healun.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Calabrese DR, et al. NKG2C natural killer cells in bronchoalveolar lavage are associated with cytomegalovirus viremia and poor outcomes in lung allograft recipients. Transplantation. 2019;103(3):493–501. doi: 10.1097/TP.0000000000002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart S, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33(2):127–133. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Greenland JR, et al. Association of large-airway lymphocytic bronchitis with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2013;187(4):417–423. doi: 10.1164/rccm.201206-1025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenland JR, et al. Bronchoalveolar lavage cell immunophenotyping facilitates diagnosis of lung allograft rejection. Am J Transplant. 2014;14(4):831–840. doi: 10.1111/ajt.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato M, Hwang DM, Waddell TK, Singer LG, Keshavjee S. Progression pattern of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant. 2013;32(1):23–30. doi: 10.1016/j.healun.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Glanville AR, et al. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38(5):483–492. doi: 10.1016/j.healun.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Diamond JM, et al. Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2012;186(6):546–552. doi: 10.1164/rccm.201204-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wain HM, Bruford EA, Lovering RC, Lush MJ, Wright MW, Povey S. Guidelines for human gene nomenclature. Genomics. 2002;79(4):464–470. doi: 10.1006/geno.2002.6748. [DOI] [PubMed] [Google Scholar]
- 47.International Genetics & Translational Research in Transplantation Network (iGeneTRAiN) Design and implementation of the International Genetics and Translational Research in Transplantation Network. Transplantation. 2015;99(11):2401–2412. doi: 10.1097/TP.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith JG, Gerszten RE. Emerging affinity-based proteomic technologies for large-scale plasma profiling in cardiovascular disease. Circulation. 2017;135(17):1651–1664. doi: 10.1161/CIRCULATIONAHA.116.025446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dugger DT, Gerriets JE, Miller LA. Attenuated airway epithelial cell interleukin-22R1 expression in the infant nonhuman primate lung. Am J Respir Cell Mol Biol. 2015;53(6):761–768. doi: 10.1165/rcmb.2014-0452RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrillo-Meléndrez H, Ortega-Hernández E, Granados J, Arroyo S, Barquera R, Arenas R. Role of HLA-DR alleles to increase genetic susceptibility to onychomycosis in nail psoriasis. Skin Appendage Disord. 2016;2(1–2):22–25. doi: 10.1159/000446444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ongen H, Buil A, Brown AA, Dermitzakis ET, Delaneau O. Fast and efficient QTL mapper for thousands of molecular phenotypes. Bioinformatics. 2016;32(10):1479–1485. doi: 10.1093/bioinformatics/btv722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carithers LJ, et al. A novel approach to high-quality postmortem tissue procurement: The GTEx project. Biopreserv Biobank. 2015;13(5):311–319. doi: 10.1089/bio.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.