ABSTRACT
The abnormal growth of malignant plasma cells in Multiple Myeloma (MM) requires bone marrow (BM) niche consisting of proteoglycans, cytokines, etc. Versican (VCAN), a chondroitin sulphate proteoglycan promotes progression in solid tumours but there is dearth of literature in MM. Hence, we studied the involvement of VCAN in MM and its regulation by microRNAs as a therapeutic approach. Thirty MM patients and 20 controls were recruited and BM stromal cells (BMSCs) were isolated by primary culture. Molecular levels of VCAN, miR-144, miR-199 & miR-203 were determined in study subjects and cell lines. The involvement of VCAN in myeloma pathogenesis was studied using BMSCs-conditioned medium (BMSCs-CM) and VCAN-neutralizing antibody or microRNA mimics. Elevated expression of VCAN was observed in patients especially in BM stroma while microRNA expression was significantly lower and showed negative correlation with VCAN. Moreover, BMSCs-CM showed the presence of VCAN which upon supplementing to MM cells alter parameters in favour of myeloma progression, however, this effect was neutralized by VCAN antibody or miR (miR-144 and miR-199) mimics. The downstream signalling of VCAN was found to activate FAK and STAT3 which subsides by using VCAN antibody or miR mimics. The neutralization of oncogenic effect of BMSCs-CM by VCAN blockage affirms its plausible role in progression of MM. VCAN was observed as a paracrine mediator in the cross-talk of BMSCs and myeloma cells in BM microenvironment. Therefore, these findings suggest exploring VCAN as novel therapeutic target and utilization of microRNAs as a therapy to regulate VCAN for better management of MM.
KEYWORDS: Multiple myeloma, versican, microRNAs, bone marrow microenvironment, therapeutics
Introduction
Multiple myeloma (MM) is the second most common haematological malignancy after non-Hodgkin lymphoma and is manifested by uncontrolled expansion and propagation of atypical plasma cells in the bone marrow (BM) [1]. MM accounts for 1% of all neoplasms and 13% of all haematological malignancies [2]. The discovery of MM initiated more than a century ago which bought achievement when the first successful drug melphalan was identified in 1950 [3]. After this, numerous drugs have been identified for the treatment of MM. Despite the presence of various chemotherapeutic drugs, majority of myeloma cases relapse which demands identification of a better and effective treatment modality for myeloma therapeutics.
Like all cancer cells, myeloma cells require BM microenvironment (or niche) for their sustained growth and proliferation. The BM niche composed of various cells of haematopoietic or non-haematopoietic origin and extracellular matrix (ECM) consisting of certain growth factors, proteins and proteoglycans. Out of various proteoglycans, chondroitin sulphate proteoglycans formed the majority in extracellular environment [4,5]. Versican (VCAN), one of the large aggregating chondroitin sulphate proteoglycans was found to play a key role in matrix remodelling, malignant transformation and tumour progression. Alternative splicing of VCAN leads to the generation of four isoforms (V0, V1, V2 and V3) depending upon the number of glycosaminoglycans binding exons in the central domain [6,7]. The function of different isoforms of VCAN remains the same in the biological system which includes the promotion of cell proliferation, adhesion, migration, etc.; however, their expression is tissue and disease specific. For example, V0 isoform was found to be predominant in cervical cancer while both V0 and V1 were found to be secreted by acute monocytic leukaemic cells in vitro [8,9]. Further, V2 isoform was found to be highly expressed in the mature brain while V3 isoform was reported to be over-expressed in human melanoma cells [10,11] but no such reports are available in MM till date.
Earlier in our lab, we have reported the over-expression of VCAN in BM and blood of MM patients and have also shown its diagnostic significance in the malignancy [12]. There are limited studies of VCAN in MM in which authors have reported the immune-regulatory role of VCAN in myeloma niche [13–15]. Thus, we hypothesized to study the involvement of VCAN in the progression of myeloma as a novel potential therapeutic target.
Moreover, there are reports showing regulation of VCAN by certain small non-coding RNAs (i.e., microRNAs) at post-transcriptional level. microRNAs are 20–22 nucleotides small non-coding RNAs involved in the regulation of gene expression by mRNA degradation or translational repression [16]. Fang et al. reported alteration in levels of endogenous microRNAs in hepatocellular carcinoma after transfecting VCAN 3′UTR which behave as competitive endogenous RNA for microRNAs [17]. Similar results have also been reported in breast cancer by Lee et al. in which they showed modulation of certain microRNAs activities by VCAN 3′UTR fragment [18]. The regulation of VCAN by miR-203 has also been tested in melanoma cell lines wherein authors have shown the anti-cancer potential of miR-203 via targeting VCAN [19]. The downregulated expression of miR-144 and miR-203 were reported in MM patients but no report is available for miR-199 [20,21]. Further, a single report of each microRNA is available showing their myeloma-suppressing effect in vitro [21–23]. Hence, there are limited reports available showing the significance of these microRNAs in MM suggesting the need for further study of these microRNAs for myeloma therapeutics.
Thus, the involvement of VCAN and its regulation by microRNAs (miR-144, miR-199 and miR-203) in myeloma pathogenesis has not been reported till date. Hence, this maiden study aims to explore the functional involvement of VCAN in MM by mimicking biological BM microenvironment ex vivo and in vitro. To accomplish this, patient-derived BM stromal cells (BMSCs) were used as a biological mean for VCAN secretion to be studied in myeloma cells in vitro. Further, VCAN regulation by microRNAs has been studied using microRNA mimics in primary BMSCs and effect of VCAN knockdown was then investigated in myeloma cells.
Results
Patient-derived BMSCs isolation and characterization
The total of 30 biopsy-proven naive MM patients was recruited in the study along with 20 bone marrow controls. Their demographic details have been shown in Table 1. On the basis of the International Staging System (ISS), 4 patients were in Stage I, 12 patients were in Stage II and 14 patients were in Stage III. BMSCs were isolated by primary culture in representative (n = 15 each) patients and controls [24,25]. These BMSCs were seen in culture at day 6 and at around day 13–15, these cells achieved complete confluency, hence, mentioned as passage 0 cells as shown in Fig. 1A. BMSCs were then characterized at passage 2 by flow cytometry to determine their percentage purity. As per the analysis, 96%–99% cells showed dual positivity for CD90 and CD105 while dual negativity for haematopoietic cell markers (CD34 and CD45) as shown in Fig. 1B, C. Hence, this finding confirmed the purity of BMSCs which were used for further experiments. All the experiments involving BMSCs were carried out at passage 2 or 3.
Table 1.
Demographic data of multiple myeloma patients and control subjects. Values are represented as mean ± SD. Patients were categorized into stages on the basis of international staging system.
| PATIENTS | |
|---|---|
| Total number (n) | 30 |
| Male/Female | 18/12 |
| Age (Years) | 57 ± 12 |
| Range | (35-75) |
| Stage I* | 4 |
| Stage II | 12 |
| Stage III | 14 |
| Hb (g/dl) | 9.3 ± 2.1 |
| ≤10 g/dl | 21 (70.0%) |
| >10g/dl | 9 (30.0%) |
| β2 microglobulin | |
| ≤3.5 mg/L | 4 (13.3%) |
| >3.5 mg/L | 26 (86.7%) |
| Plasma cells (%) | 25.6 ± 22.9 |
| M- band (g/dL) | 2.8 ± 2.1 |
| Total protein (g/dL) | 7.4 ± 0.9 |
| Albumin (g/dL) | 3.5 ± 0.9 |
| Globulin (g/dL) | 3.8 ± 1.0 |
| Creatinine (mg/dL) | 1.2 ± 1.0 |
| Calcium (mg/dL) | 9.0 ± 1.0 |
| Bone lesions | |
| Present | 18 (60%) |
| Absent | 12 (40%) |
| CONTROLS | |
| Total number (n) | 20 |
| Male/Female | 11/9 |
| Age (Years) | 45 ± 10 |
| Range | (25-68) |
[*: staging was done on the basis of International Staging System]
Figure 1.
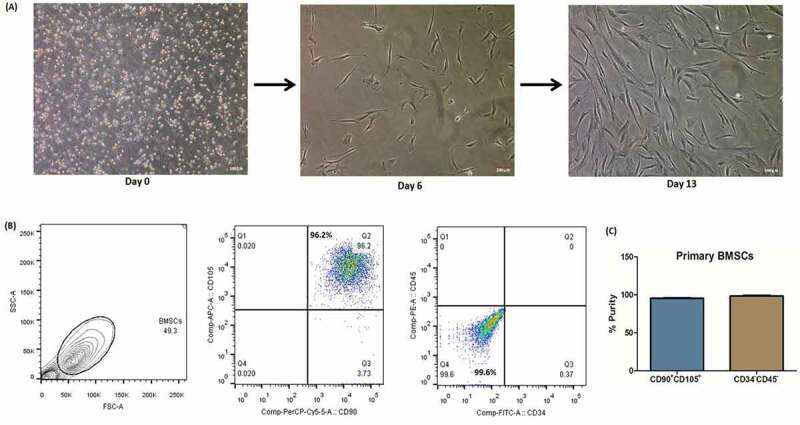
Isolation and characterization of primary bone marrow stromal cells (BMSCs). (A) BMSCs were isolated from BMMNCs which were plated in culture flasks. BMSCs have the tendency to adhere, hence, attached to the surface and proliferate with regular change of media. On day 6, some adhered cells were observed which grown further and on day 13–15, these cells attained optimum confluency (70–80%), hence, mentioned as passage 0. Scale bar represents 100 μm; (B) Flow cytometry characterization of BMSCs at passage 2 showing dual positivity for stromal cells marker (CD90 and CD105) while dual negativity for haematopoietic cell marker (CD34 and CD45); (C) percentage purity of BMSCs showing 96%-99% pure culture. [BMSCs: bone marrow stromal cells; BMMNCs: bone marrow mononuclear cells].
Over-expression of VCAN in the bone marrow of myeloma patients especially in BMSCs while negligible expression in myeloma cells
The isoforms of VCAN were generated by alternative splicing, hence, differ by some exons which were exploited to design primers specific to the isoforms (Fig. 2A). The sequences of primers are given in Supplementary Table 1. The relative mRNA expression of VCAN and its isoforms (V0, V1, V2 and V3) were found significantly higher (p <0.01) in MM patients in comparison to controls in both bone marrow mononuclear cells (BMMNCs) and BMSCs. Further, the expression of VCAN and its isoforms were significantly higher in BMSCs than in BMMNCs in all the study subjects (Fig. 2B–F). Out of all isoforms, V1 was found to be predominant in the study subjects. Upon inter-stage analysis, it has been found that the expression of VCAN and its isoforms showed a trend with the severity of disease as an expression of VCAN increased as myeloma progressed to advance stage (Supplementary Fig. 1). Besides, protein expression of VCAN was observed significantly higher (p <0.01) in MM patients as compared to controls in both BMSCs and BMMNCs with significantly higher expression in BMSCs than BMMNCs (Fig. 2G).
Figure 2.
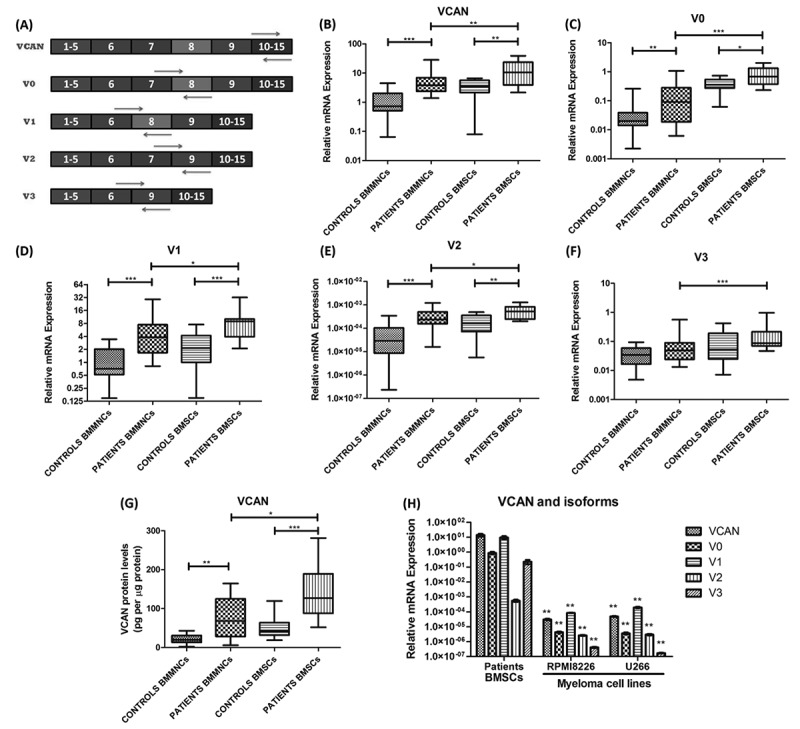
Molecular expression of VCAN and its isoforms in BMMNCs, BMSCs and cell lines. (A) Primer designing strategy for determining mRNA expression of VCAN and its four different isoforms (V0, V1, V2 and V3). These isoforms resulted by alternative splicing, hence, specific exon-exon junction were used for specific amplification; (B)-(F) box-whisker plot showing relative mRNA expression of VCAN and its four isoforms (V0, V1, V2 & V3) in BMMNCs (n = 30 patients, n = 20 controls) and BMSCs (n = 15 each) of MM patients and controls. GAPDH was used as an endogenous control for normalization; (G) box-whisker plot showing protein expression of VCAN by ELISA in BMMNCs (n = 15 each) and BMSCs (n = 15 each) of MM patients and controls. The levels of VCAN were normalized to the total protein concentration, hence, mentioned as pg per μg of total protein; (H) bar graph showing relative mRNA expression of VCAN and its four isoforms in patients BMSCs and in myeloma cell lines (RPMI8266 and U266). GAPDH was used as an endogenous control for normalization. ** represents significance with respect to patients BMSCs. Data were represented as median (range) for (B)-(G) while mean ± SD for (H). Wilcoxon rank-sum test was applied to determine significance between patients and controls. [BMMNCs: bone marrow mononuclear cells; BMSCs: bone marrow stromal cells; VCAN: versican; *p <0.05; **p <0.01; ***p <0.001].
The relative mRNA expression of VCAN was found significantly lower in MM cell lines than in the stroma of MM patients (Fig. 2H). This observation confirmed that VCAN is being produced in the stroma but might act on cancer cells to produce effects which were further tested in myeloma cells in vitro.
VCAN has pro-tumorigenic effects in myeloma cells in vitro
C-terminal domain (G3 domain) of VCAN was reported to have pro-tumorigenic property in certain solid tumours [26,27] but not in MM, hence, we cloned VCAN-G3 domain but clone always resulted in truncated protein, therefore, to study the effect of VCAN on myeloma cells, the naturally VCAN secreting phenomenon of BMSCs was exploited. The conditioned medium (CM) of patient-derived BMSCs were investigated for the presence of VCAN by ELISA and it was found that 400–500 pg/mL of VCAN was present in BMSCs-CM (Fig. 3A). Keeping in mind the fact that CM would also contain various other growth factors and proteins together with VCAN, hence, blocking strategy using VCAN antibody (Santa Cruz Biotechnology, 200 ng/mL) has been used. Therefore, in culture experiments, BMSCs-CM was added in 1:1 ratio in the culture medium of myeloma cells while in another group, BMSCs-CM with VCAN antibody was added to specifically elucidate the effect of VCAN on myeloma cells.
Figure 3.
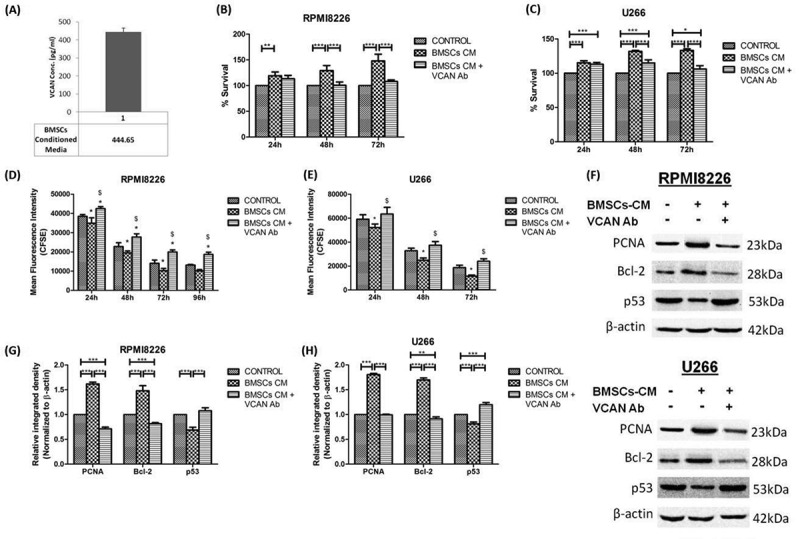
Levels of VCAN in the conditioned medium (CM) of BMSCs and the effect of VCAN on proliferation and apoptosis of myeloma cells in vitro. BMSCs-CM was supplemented in 1:1 ratio in the culture medium of myeloma cells in the presence or absence of VCAN-neutralizing antibody (200 ng/mL) for 48 h. (A) Levels of VCAN in BMSCs-CM as determined by ELISA; (B)-(E) bar graphs showing effect of BMSCs-CM alone or with VCAN-neutralizing antibody on proliferation of myeloma cells (RPMI8226 and U266) as assessed by MTT assay (B) (C) and CFSE assay (D) (E), respectively at different time points. The mean fluorescence intensity of CFSE is inversely proportional to the cell proliferation. In Figure (D) and (E), * represents significance (p <0.05) with respect to control while $ represents significance (p <0.05) with respect to BMSCs-CM; (F) western blot image showing effect of BMSCs-CM on proliferation (PCNA) and apoptosis (Bcl-2 and p53) of RPMI8226 (top) and U266 (bottom) myeloma cells which got reversed by VCAN-neutralizing antibody; (G) (H) Image J densitometry analysis of western blots showing effect of VCAN on proliferation and apoptosis in myeloma cells. β-actin was used for normalization in western blotting. Data were represented as mean ± SD and two-way ANOVA was applied to determine significance between groups. All the experiments were performed in biological triplicates. [BMSCs: bone marrow stromal cells; BMSCs-CM: bone marrow stromal cells-conditioned medium; VCAN: versican; Ab: antibody; PCNA: proliferating cell nuclear antigen; Bcl-2: B-cell lymphoma 2; *p <0.05; **p <0.01; ***p <0.001].
It has been observed that upon administration of BMSCs-CM to myeloma cells, percentage cell survival (assessed by MTT assay) increased significantly (p <0.001) but the effect reversed when VCAN was neutralized by antibody in a time-dependent manner (Fig. 3B, C). The cell proliferation was evaluated by CFSE assay in which myeloma cells were labelled with 1μM CFSE. The dose standardization of CFSE has been shown in Supplementary Fig. 2. Upon CFSE assay, it has been found that cell proliferation increased substantially when their culture medium was complemented with BMSCs-CM but the effect got neutralized when VCAN antibody was also included (Fig. 3D, E). The proliferation marker, PCNA has also increased 1.6 fold when BMSCs-CM was added but decreased significantly when BMSCs-CM along with VCAN antibody was added (Fig. 3F–H).
VCAN was also found to function as an anti-apoptotic molecule in myeloma cells in vitro as the inclusion of BMSCs-CM in culture medium leads to upregulation of anti-apoptotic molecule (Bcl-2) by 1.5 fold with simultaneous downregulation of pro-apoptotic molecule (p53) which got reversed by VCAN-neutralizing antibody (Fig. 3F–H). The effect of VCAN has also been investigated on migration and invasion of MM cells and it has been found that the migratory and invading ability of myeloma cells enhanced significantly (p <0.01) in the presence of BMSCs-CM but decreased upon addition of VCAN antibody (Fig. 4A, B and Supplementary Fig. 3).
Figure 4.
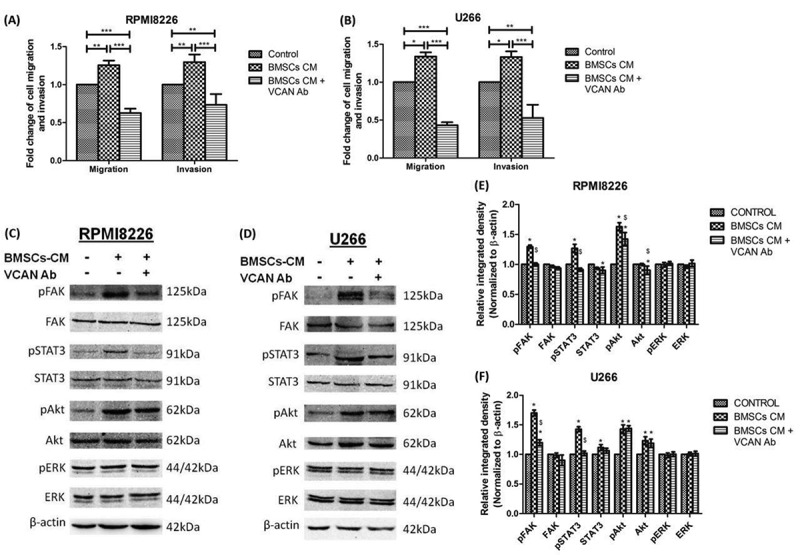
Effect of VCAN on the migration and invasion of myeloma cells in vitro along with downstream signalling cascade affected by the action of VCAN. BMSCs-CM was supplemented in 1:1 ratio in the culture medium of myeloma cells with or without VCAN-neutralizing antibody (200 ng/mL) for 48 h. (A)-(B) Bar graphs showing effect of VCAN blockage by neutralizing antibody on cell migration and invasion in RPMI8226 and U266 myeloma cells; (C)-(D) certain signalling cascades involved in myeloma pathogenesis were traced by western blotting and the effect of VCAN on FAK/STAT3 signalling was observed; (E)-(F) Image J densitometry analysis of western blots showing effect of VCAN on downstream signalling cascades in myeloma cells. * represents significance (p <0.05) with respect to control while $ represents significance (p <0.05) with respect to BMSCs-CM. β-actin was used for normalization. Data were represented as mean ± SD and two-way ANOVA was applied to determine significance. All the experiments were performed in biological triplicates. [BMSCs: bone marrow stromal cells; BMSCs-CM: bone marrow stromal cells-conditioned medium; VCAN: versican; Ab: antibody; FAK: focal adhesion kinase; STAT: signal transducer and activator of transcription; *p <0.05; **p <0.01; ***p <0.001].
Downstream signalling cascade of VCAN entails FAK/STAT3 signalling
The mechanism of myeloma promoting property of VCAN was then assessed by tracing certain signalling pathways reported to be involved in MM. To achieve this, BMSCs-CM was supplemented in a 1:1 ratio to the myeloma cells in the presence or absence of VCAN-neutralizing antibody for 48 h. It has been observed that phosphorylated (or activated form) of focal adhesion kinase (FAK) and signal transducer & activator of transcription 3 (STAT3) over-expressed in both the myeloma cells (RPMI8226 and U266) when BMSCs-CM was administered in the culture medium as identified by western blotting. However, this activation was hindered in the presence of VCAN-neutralizing antibody in BMSCs-CM confirming the involvement of VCAN in the activation of FAK and STAT3 signalling cascade in myeloma cells. The activation of Akt was also observed by BMSCs-CM but was not affected by the addition of VCAN antibody suggesting the presence of other proteins in BMSCs-CM responsible for Akt activation (Fig. 4C–F).
microRNAs regulating VCAN are downregulated in multiple myeloma and negatively correlating with VCAN
The TargetScan analysis and literature survey suggested the plausible involvement of miR-144, miR-199, miR-203 in VCAN regulation [17–19] which was not reported yet in MM, hence, firstly, we determined the expression of microRNAs in the malignancy. The relative microRNA expression of miR-144, miR-199 and miR-203 was found significantly downregulated (p <0.01) in MM patients in both BMMNCs and BMSCs (Fig. 5A). Moreover, the expression of miR-144 and miR-203 were considerably lower in BMSCs (p <0.001) as compared to BMMNCs. Upon inter-stage analysis, it has been found that microRNAs expression decreased as myeloma progressed to a higher stage, hence, showed a negative trend with the severity of disease (Supplementary Fig. 4). Furthermore, by Spearman correlation analysis, it was observed that expression of miR-144, miR-199 and miR-203 were negatively correlating with both mRNA and protein levels of VCAN in both BMMNCs and BMSCs but significant correlation (p<0.05) was mainly observed with miR-144 and miR-199 (Table 2). This finding suggests that microRNAs could be involved in the regulation of VCAN which is further tested in vitro as discussed below.
Figure 5.
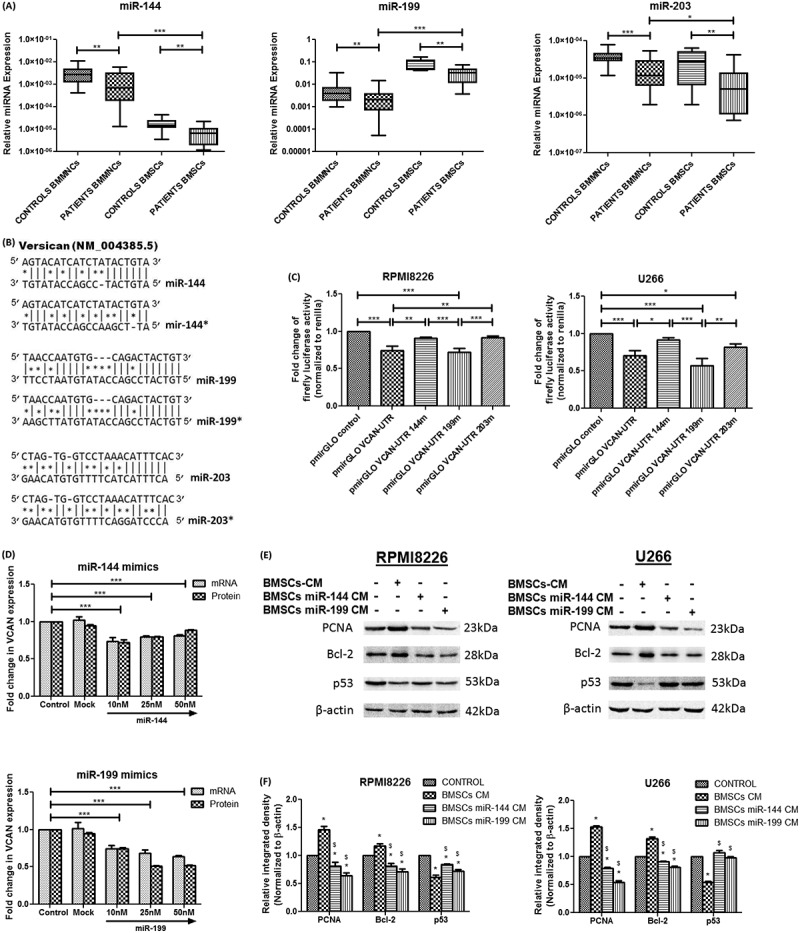
Relative microRNA expression of miR-144, miR-199 and miR-203 in study subjects followed by assessment of VCAN regulation by microRNAs and its effect on myeloma cells. (A) Box-whisker plot showing relative microRNA expression of miR-144, miR-199 and miR-203 in BMMNCs (n = 30 patients, n = 20 controls) and BMSCs (n = 15 each) of MM patients and controls. SNORD48 was used as an endogenous control to normalize microRNA expression; (B) Potential sites in VCAN 3ʹUTR targeted by miR-144, miR-144*, miR-199, miR-199*, miR-203 and miR-203* (* represents site-directed mutagenesis in the binding site of microRNAs); (C) RPMI8226 (left) and U266 (right) were transfected with pmirGLO reporter plasmid containing wild type or mutated VCAN-3′UTR. The firefly luciferase activity was measured normalized to Renilla luciferase. pmirGLO VCAN-UTR 144m/199m/203m represents mutation in particular microRNA binding site. (D) miR-144 (top) and miR-199 (bottom) mimics were transfected in patient-derived BMSCs and alteration in transcript of VCAN (by Q-PCR) along with its secretion in BMSCs-CM (by ELISA) were measured; (E)-(F) The conditioned medium of control BMSCs or microRNA mimics transfected BMSCs were supplemented in 1:1 ratio in the culture medium of myeloma cells and effect was studied after 48 h. Western blot images (E) and Image J densitometry analysis (F) showing the effect of microRNA mimics mediated knockdown of VCAN on proliferation and apoptosis of RPMI8226 (left) and U266 (right) cells. β-actin was used for normalization in western blotting. * represents significance (p <0.05) with respect to control while $ represents significance (p <0.05) with respect to BMSCs-CM. Data were represented as median (range) for (A) and Wilcoxon rank-sum test was applied to determine significance. Data were represented as mean ± SD for (C)-(F) and two-way ANOVA was applied. All the experiments were performed in biological triplicates. [BMMNCs: bone marrow mononuclear cells; BMSCs: bone marrow stromal cells; VCAN: versican; PCNA: proliferating cell nuclear antigen; Bcl-2: B-cell lymphoma 2; *p <0.05; **p <0.01; ***p <0.001].
Table 2.
Spearman correlation analysis between expression of microRNAs and VCAN at (a) mRNA and (b) protein level in BMMNCs and BMSCs of MM patients.
| r (p) |
miR-144 |
miR-199 |
miR-203 |
||||
|---|---|---|---|---|---|---|---|
| BMMNCs | BMSCs | BMMNCs | BMSCs | BMMNCs | BMSCs | ||
| (a) VCAN (mRNA levels) with microRNA | |||||||
| VCAN | BMMNCs | -0.715 | --- | -0.740 | --- | -0.569 | --- |
| (<0.001) | (<0.001) | (0.001) | |||||
| BMSCs | --- | -0.421 | --- | -0.559 | --- | -0.364 | |
| (0.118) | (0.021) | (0.182) | |||||
| V0 | BMMNCs | -0.568 | --- | -0.509 | --- | -0.533 | --- |
| (<0.001) | (0.004) | (0.002) | |||||
| BMSCs | --- | -0.343 | --- | -0.511 | --- | -0.364 | |
| (0.211) | (0.052) | (0.182) | |||||
| V1 | BMMNCs | -0.768 | --- | -0.709 | --- | -0.524 | --- |
| (<0.001) | (<0.001) | (0.003) | |||||
| BMSCs | --- | -0.571 | --- | -0.754 | --- | -0.182 | |
| (0.026) | (0.001) | (0.516) | |||||
| V2 | BMMNCs | -0.454 | --- | -0.249 | --- | -0.307 | --- |
| (0.012) | (0.184) | (0.099) | |||||
| BMSCs | --- | -0.264 | --- | -0.300 | --- | -0.293 | |
| (0.341) | (0.277) | (0.289) | |||||
| V3 | BMMNCs | -0.453 | --- | -0.309 | --- | -0.367 | --- |
| (0.012) | (0.097) | (0.046) | |||||
| BMSCs | --- | -0.596 | --- | -0.693 | --- | -0.411 | |
| (0.019) | (0.004) | (0.128) | |||||
| (b) VCAN (protein levels) with microRNA | |||||||
| VCAN | BMMNCs | -0.608 | --- | -0.511 | --- | -0.366 | --- |
| (0.016) | (0.052) | (0.179) | |||||
| BMSCs | --- | -0.529 | --- | -0.432 | --- | -0.475 | |
| (0.043) | (0.108) | (0.074) | |||||
[Values in parentheses showed p values. Bold values represent significant correlation. MM: Multiple Myeloma; VCAN: Versican; BMMNCs: bone marrow mononuclear cells; BMSCs: bone marrow stromal cells; r: Spearman correlation coefficient].
miR-144 and miR-203 directly regulate VCAN by binding to its 3′-UTR while miR-199 regulates indirectly
To functionally assess the regulation of VCAN by miR-144, miR-199 and miR-203 endogenously; wild type VCAN 3′-UTR containing putative binding sites of all three microRNAs was cloned in pmirGLO dual reporter luciferase vector (Fig. 5B and Supplementary Fig. 5) and transfected in myeloma cells. It has been observed that luciferase activity reduced significantly (p <0.01) in pmirGLO-VCAN-UTR vector as compared to control vector in myeloma cells (Fig. 5C). Keeping in mind the presence of binding sites of other microRNAs in cloned VCAN 3′-UTR sequence, site-directed mutagenesis was performed in binding sites of miR-144, miR-199 and miR-203 and mutation was confirmed by Sanger sequencing. It has been observed that pmirGLO-VCAN-UTR-144m plasmid and pmirGLO-VCAN-UTR-203m plasmid having a mutation in miR-144 and miR-199 binding site, respectively, in VCAN 3ʹ-UTR resulted in significantly higher luciferase activity (p <0.01) when compared with pmirGLO-VCAN-UTR and activity was comparable with control pmirGLO vector (Fig. 5C). However, the mutation in miR-199 binding site does not have any change in luciferase activity when compared with pmirGLO-VCAN-UTR, hence, affirmed the binding of miR-144 and miR-203 but not miR-199 to VCAN 3′-UTR site. This could suggest the direct involvement of endogenous miR-144 and miR-203 while indirect involvement of endogenous miR-199 in VCAN regulation. In previous results, miR-203 did not show significant correlation with VCAN in MM patients whereas miR-144 and miR-199 showed significant results; hence, miR-144 and miR-199 were assessed further for any effect, if any, in myeloma cells in vitro.
miR-144 and miR-199 mimics results in knockdown of VCAN in patient-derived BMSCs, hence, inhibiting myeloma pathogenesis
To study the regulation of VCAN by miR-144 and miR-199 in vitro, respective mimics were transfected in patient-derived BMSCs at different doses and it has been found that transfection of miR-144 and miR-199 resulted in significantly decreased (p<0.001) mRNA expression of VCAN in BMSCs. Further, the secretion of VCAN in conditioned medium of BMSCs was also reduced 20% and 50% (p<0.001) at 10nM and 25nM of miR-144 and miR-199, respectively (Fig. 5D); hence, these doses of miR mimics were used in further experiments.
The conditioned medium of control BMSCs or microRNA mimics (miR-144 and miR-199) transfected BMSCs were supplemented in the culture medium of myeloma cells in 1:1 ratio to observe the effect of VCAN knockdown on myeloma cells. It has been observed that proliferation increased while apoptosis decreased in the presence of BMSCs-CM as assessed by the respective markers but this effect reversed when VCAN was knockdown by miR-144 and miR-199 mimics (Fig. 5E, F). Moreover, the migratory and invading ability of myeloma cells increased significantly when BMSCs-CM was added in the culture medium but transfection of miR mimics neutralized the effect of BMSCs-CM (Fig. 6A, B and Supplementary Fig. 6).
Figure 6.
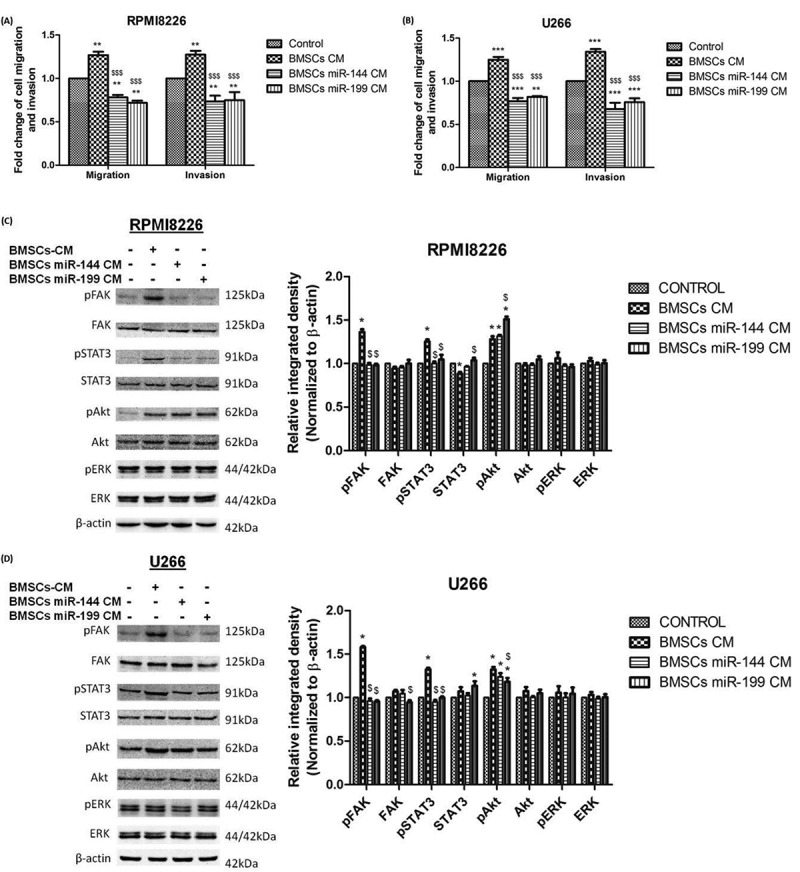
Effect of microRNA mimics mediated knockdown of VCAN on migration and invasion of myeloma cells in addition to the effect on downstream signalling cascade in MM. Control BMSCs-CM or microRNA mimics transfected BMSCs-CM was supplemented in 1:1 ratio in the culture medium of myeloma cells for 48 h. (A)-(B) bar graphs showing the effect of miR-144 and miR-199 mimics mediated knockdown of VCAN on migration and invasion of RPMI8226 and U266 myeloma cells. Data were represented as mean ± SD and two-way ANOVA was applied to determine significance between groups. * (**p <0.01; ***p <0.001) represents significance with respect to control while $ ($$$p <0.001) represents significance with respect to BMSCs-CM; (C)-(D) certain signalling cascades involved in myeloma pathogenesis were traced by western blotting (left) along with their Image J densitometry analysis (right) and the effect of VCAN knockdown on FAK/STAT3 signalling was observed in RPMI8226 (C) and U266 (D). β-actin was used for normalization in western blotting. * represents significance (p <0.05) with respect to control while $ represents significance (p <0.05) with respect to BMSCs-CM. All the experiments were performed in triplicates. [BMSCs: bone marrow stromal cells; BMSCs-CM: bone marrow stromal cells-conditioned medium; FAK: focal adhesion kinase; STAT: signal transducer and activator of transcription; *p <0.05; **p <0.01; ***p <0.001].
Furthermore, the downstream FAK/STAT3 signalling cascade activated by VCAN has also found to be abrogated by VCAN knockdown by mimics of miR-144 and miR-199 as shown in Fig. 6C, D. Hence, miR-144 and miR-199 possess the ability to regulate VCAN and inhibit its paracrine effect on myeloma cells to diminish the progression of multiple myeloma.
Discussion
Despite the availability of numerous chemotherapeutics for the treatment of MM, a vast majority of myeloma cases relapse generating an urge to identify some better and effective treatment modality for the malignancy. The abnormal growth of malignant plasma cells in the BM of MM patients makes the BM microenvironment (or niche) an interesting topic for research with the aim to identify an effective target. BM microenvironment consists of growth factors, proteins and proteoglycans and it has been reported that out of various proteoglycans, chondroitin sulphate proteoglycans are found in the majority in tumour niche [5]. Earlier our group has observed significant upregulation of one such chondroitin sulphate proteoglycan, VCAN in MM patients and also reported its diagnostic significance in the disease [12] which prompted us to further explore its functional role in myeloma pathogenesis in vitro. This study, therefore, aimed to investigate the involvement of VCAN in MM using patient-derived BMSCs along with its regulation by microRNAs for the first time.
VCAN, being a proteoglycan of tumour microenvironment would be secreted naturally by stromal cells in the BM, hence, we isolated BMSCs by primary culture [24], characterized by flow cytometry using positive (CD90 and CD105) and negative markers (CD34 and CD45) and observed 96% purity. The relative mRNA expression of VCAN and its four isoforms along with protein expression of VCAN were found significantly higher in MM patients in both BMMNCs and BMSCs. Out of all the isoforms of VCAN, V1 was found to be predominantly expressed in patients. In addition, expression of VCAN positively correlated with the severity of disease. Higher expression of VCAN has also been reported earlier in patients of colon and thyroid cancer [28–30]. Further, we have observed a significantly higher expression of VCAN in BMSCs as compared to BMMNCs. This observation is in concordance with the findings of Gorter et al. and Takahashi et al. in which they reported the predominant localization of VCAN in the stroma of cervical and breast cancer, respectively [8,31] but no such reports are available for the BM microenvironment of MM. The conditioned medium of primary BMSCs was also analysed in this study and observed to contain a significant amount of VCAN. Ricciardelli et al. had also reported the secretion of VCAN in CM of mammary fibroblasts in breast cancer [32] while no such studies have been described in MM. Further, we investigated the expression of VCAN in myeloma cell lines and observed negligible expression. Corral et al. have also shown that levels of VCAN downregulated in sorted malignant plasma cells in patients with MM [33]. These findings suggest that VCAN is mainly produced in the BM microenvironment and BMSCs is a natural source for VCAN secretion in the BM.
The effect of VCAN was then assessed on myeloma cells in vitro by supplementing BMSCs-CM with or without VCAN-neutralizing antibody in the culture medium of myeloma cells. It has been observed that the addition of BMSCs-CM enhanced myeloma cell proliferation, decreased cell apoptosis, increased cell migration and invasion. However, the addition of VCAN antibody along with BMSCs-CM abrogated the pro-myeloma effect of BMSCs-CM. Hence, it could be stated that VCAN plays a significant role in the pathogenesis of MM. Related studies have been performed in MM in which authors have shown that CM of either stromal cell line or patient-derived BMSCs promoted myeloma proliferation via IL-6 [34,35]. Roccaro et al. have also reported that exosomes derived from myeloma patients BMSCs consisting of various proteins and growth factors facilitate the progression of MM [36]. These studies are in concordance with our findings but the involvement of VCAN in the cross-talk of BMSCs and myeloma cells has been discussed for the first time in this study.
Furthermore, this study showed that VCAN promotes myeloma progression by activating downstream cascade of FAK & STAT3 signalling. Our findings are in concordance with the previous studies in which the authors reported the interaction of VCAN with β1 integrin via its C-terminal domain leading to the activation of FAK signalling [37,38]. Also, studies have shown the activation of JAK/STAT signalling due to the over-expression of VCAN [39] but no such reports are available in MM. In addition, we also observed the stimulation of Akt signalling in the presence of BMSCs-CM but this up-regulation was not affected by treatment with VCAN antibody suggesting the presence of other factors in BMSCs-CM responsible for Akt activation. This finding could be supported by the studies in which the authors have reported that BMSCs-CM promoted the tumour progression via PI-3K/Akt signalling in acute myeloid leukaemia and head & neck cancer [40,41]. Altogether, these results suggest that VCAN behaves as a paracrine pro-myeloma regulator secreted by BMSCs in the BM microenvironment which interacts with myeloma cells activating FAK/STAT3 signalling further leading to myeloma progression.
Subsequently, after identifying the tumorigenic potential of VCAN and discussed it as one of the important targets in MM, the question arises for its regulation to inhibit VCAN. To fulfil this objective, certain microRNAs (miR-144, miR-199 and miR-203) involved in regulation of VCAN were scrutinized from TargetScan and literature search [17–19]. Their expression was determined in MM patients of different stages and found these microRNAs at a significantly lower level in both BMMNCs and BMSCs of MM patients as compared to controls. The expression of microRNAs was also decreased significantly as myeloma progresses to advance stage. As per the published reports, down-regulation of miR-144, miR-199 and miR-203 has been reported in various solid tumours such as hepatocellular carcinoma, liposarcoma and few reports are available in haematological malignancies [42–46]. Recently, we have reported the downregulation of all these microRNAs in the circulation milieu of BM and blood in MM patients and hence, discussed the diagnostic potential of miR-203 in the disease [47]. There is another report of miR-144 in MM in which its down-regulated expression has been described in plasma samples of MM patients [21]. miR-203 has also shown to be hyper-methylated in myeloma, thus, contributing to its downregulation in the malignancy [20] but no report is available for miR-199 in myeloma patients. Besides, the expression of these microRNAs has not been studied in BMSCs earlier. Moreover, we have observed for the first time the negative correlation of these microRNAs (miR-144, miR-199 and miR-203) with transcript as well as protein levels of VCAN in MM patients proposing that upregulation of VCAN might be due to decrease in levels of microRNAs but this needs further validation.
In order to validate this fact, VCAN 3′-UTR was cloned into pmirGLO reporter plasmid downstream to the luciferase gene and transfected into myeloma cells. It has been observed that luciferase activity reduced significantly in the presence of VCAN 3′-UTR suggesting the binding of endogenous microRNAs at 3′-UTR site and hence, leading to regulation of gene upstream to the binding site. Further, the involvement of miR-144, miR-199 and miR-203 in the regulation of VCAN was assessed by mutating the putative binding site of these microRNAs using site-directed mutagenesis. In comparison to wild type VCAN 3′-UTR, mutated binding site of miR-144 and miR-203 resulted in gain in luciferase activity, however, mutation of miR-199 binding site does not alter luciferase activity. The similar results have been previously reported in hepatocellular carcinoma cells [17]. Thus, our findings showed that miR-144 and miR-203 could regulate VCAN by binding to VCAN 3′-UTR but miR-199 might have some indirect mechanism involved in the regulation of VCAN.
The correlation of miR-203 with VCAN was non-significant in clinical samples, hence, functional regulation of VCAN was assessed using miR-144 and miR-199 mimics. We observed that transcript levels of VCAN along with its secretion in CM decreased significantly in BMSCs upon transfection. This could suggest the transcript degradation or translational repression of VCAN via microRNAs. Moreover, the effects caused by BMSCs-CM in myeloma cells were reversed by the action of microRNA mimics (miR-144 and miR-199). The proliferation decreased and apoptosis increased followed by the decrease in the migration and invasion ability of myeloma cells upon supplementing CM of microRNA mimics transfected BMSCs. Furthermore, decrease in FAK/STAT3 downstream signalling pathways activated by VCAN was also observed confirming the regulation of VCAN by miR-144 and miR-199. These results are in concordance with the reports of Fang et al. in which the authors showed that transfection of VCAN 3′-UTR acted as a competitive endogenous RNA for miR-144 and miR-199, hence, downregulating these microRNAs followed by upregulation of VCAN in hepatocellular carcinoma [17]. The similar results have also been reported for miR-203 in fibroblasts and melanoma cells [19,48]. Thus, these observations suggest that microRNAs could be the potential mean for targeting tumour promoting VCAN in MM.
Taken together, it could be stated that VCAN is an important molecule as far as its therapeutic potential is concerned in MM. It is found to be upregulated in myeloma patients especially in the BM stroma and also observed in the secretion of BMSCs. It is identified to be the paracrine stimulator of myeloma cells involved in the progression of MM as evidenced by favouring various cancer hallmarks. microRNAs were observed to functionally regulate VCAN expression and neutralized its pro-oncogenic ability in myeloma cells either directly or indirectly. This maiden attempt is indeed the first step in studying the translational significance of VCAN and microRNAs in MM with microRNAs as a mean for targeting VCAN (Fig. 7). This study opens up a new avenue for the employment of microRNAs as an effective therapy targeting VCAN for better management of MM in clinical settings in future.
Figure 7.
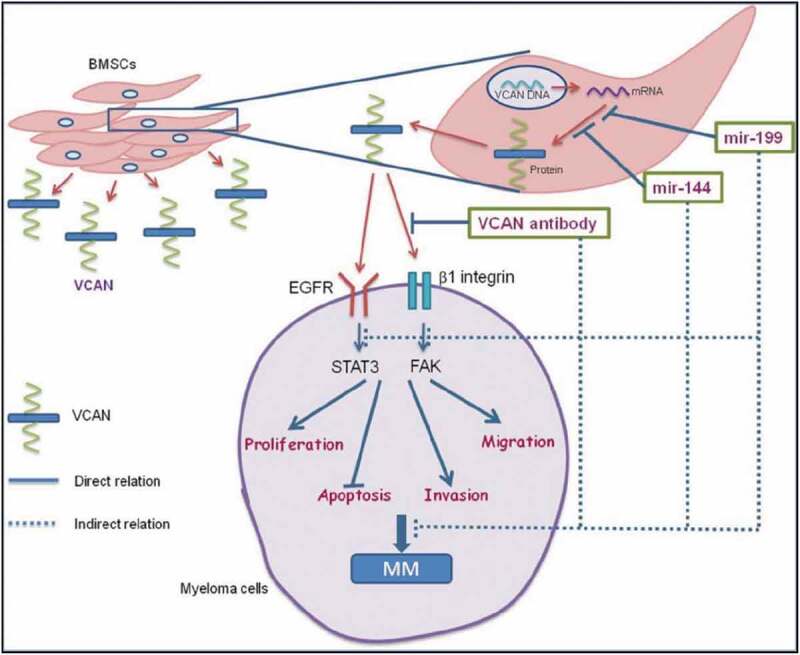
Graphical abstract showing paracrine regulatory mechanism of VCAN in the cross-talk of BMSCs and myeloma cells. VCAN is secreted by BMSCs in the bone marrow which then interacts with myeloma cells activating FAK & STAT3 signalling cascade favouring myeloma progression. The regulation of VCAN could be achieved at various steps by different sources as shown. microRNAs (miR-144 and miR-199) could inhibit the translation of VCAN protein from mRNA either by transcript degradation or translational repression. Secondly, VCAN-neutralizing antibody could inhibit the interaction of VCAN with the myeloma cells. These inhibitory approaches indirectly impede the growth and proliferation of myeloma cells. Therefore, targeting of VCAN via microRNAs could be employed as a therapy to inhibit myeloma progression. Continuous lines indicate direct relation while dashed lines indicate indirect relation. [MM: multiple myeloma; BMSCs: bone marrow stromal cells; VCAN: versican; FAK: focal adhesion kinase; STAT: signal transducer and activator of transcription; EGFR: Epidermal growth factor receptor].
Materials and methods
Clinical samples
This study has been approved by institute’s ethics committee (IESC/T-240/05.05.2015) and informed written consent was obtained from all the study subjects. Thirty naive MM patients registered at the Department of Hematology, AIIMS were included. In addition, 20 patients undergone for bone grafting procedure or spine surgery registered at the Department of Orthopedics, AIIMS were recruited as BM controls for precise comparison with patients. Five milliliter BM aspirate was taken from the study subjects and collected in EDTA vials at the time of their diagnostic or therapeutic procedure. BM aspirate was used to isolate BM mononuclear cells (BMMNCs) and BM stromal cells (BMSCs) for various experiments discussed below. The patients were categorized into different stages on the basis of international staging system (ISS).
Cell lines
MM cell lines (RPMI8226 and U266) were purchased from ATCC and have also been characterized by short tandem repeat polymorphism (STR) typing (DNA Forensics). Cells were grown in RPMI-1640 medium (Himedia) containing 2.0mM L-glutamine, 10.0mM HEPES, 1.0mM sodium pyruvate, 4.5g/L glucose, and 1.5g/L sodium bicarbonate at 37°C humidified incubator with 5% CO2. Media was supplemented with 1% penicillin-streptomycin (Thermo Fisher Scientific) and 10% or 15% foetal bovine serum (FBS, Thermo Fisher Scientific) for RPMI8226 and U266, respectively. The cell line experiments were carried out between 4 and 10 passages after revival.
Bone marrow mononuclear cells (BMMNCs) isolation
BMMNCs were isolated from BM aspirate by ficoll density gradient centrifugation method in sterile environment in which BM aspirate was diluted in 1:1 ratio with 1× PBS and then layered on histopaque ficoll (Himedia) in 2:1 ratio followed by centrifugation at 300g for 20 min. The buffy coat containing BMMNCs was collected and centrifuged at 250 g for 10 min. The cells obtained were then treated with RBCs lysis solution, if required followed by washing with 1× PBS. The cells were used for RNA isolation, cell lysate preparation and BM stromal cells (BMSCs) isolation by primary culture.
Primary BMSCs isolation and characterization by flow cytometry
4–5 × 106 BMMNCs were resuspended in 1× DMEM low-glucose media (Himedia) containing 2.0 mM L-glutamine, 10.0 mM HEPES, 1.0 mM sodium pyruvate, 1.0 g/L glucose, and 1.5 g/L sodium bicarbonate supplemented with 15% FBS and 1% penicillin-streptomycin. The cells were plated in T-25 cm2 culture flasks and kept at 37°C-5% CO2 incubator for 72 h. Then, non-adhered cells were removed with the change of media and further incubated. The media was changed after every 2–3 days and when cells reached passage 2, their purity was assessed by flow cytometry using positive markers (CD90 and CD105) (Biolegend) and negative markers (CD34 and CD45) (Invitrogen) [24,25]. RNA isolation, cell lysate preparation and certain experiments were performed from BMSCs at passage 2 or 3 as discussed below.
Patient-derived primary BMSCs-conditioned medium preparation
1.0–1.5 × 105 patient-derived BMSCs were plated in 6-well plate for 2–3 days. The cells began to grow in monolayer and when they reached 70–80% confluency, these were starved in 1× serum-free DMEM low-glucose media. Media was then collected after 72 h and filtered using 0.4 μm syringe filter and stored at −20°C till further use. The levels of VCAN in BMSCs-CM were determined by ELISA. Further experiments were carried out with BMSCs-CM in which myeloma cells (RPMI8226 and U266) were supplemented with BMSCs-CM in 1:1 ratio and effect was observed after 48 h.
Enzyme-linked immunosorbent assay (ELISA)
The levels of Versican (VCAN) in lysates of BMMNCs, BMSCs and supernatant of cultured cells were estimated using high-sensitive commercially available ELISA kit supplied by USCN Cloud Clone Corporation (Houston, USA). This kit was based on the principle of sandwich ELISA in which a reference curve was obtained by plotting different concentrations of standards versus absorbance and levels of VCAN in unknown samples were calculated by the standard plot.
Quantitative PCR (Q-PCR)
The relative mRNA expression of VCAN and its isoforms (V0, V1, V2 and V3) along with relative microRNA expression of miR-144, miR-199 and miR-203 were determined in BMMNCs, BMSCs and cell lines using SYBR Green chemistry in Biorad CFX96TM real-time system. Total RNA including microRNA was isolated using miRNeasy mini kit (Qiagen) as per the manufacturer’s instructions and stored at −80°C till further use. One microgram DNase-treated RNA was used to synthesize cDNA using miscript II RT kit (Qiagen) which reverse transcribed both mRNA and microRNA into cDNA which was then used as a template in Q-PCR. The primers specific to VCAN and its isoforms were made by Primer 3 software using strategy as shown in Fig. 2A. The primers were synthesized by IDT and their sequences are shown in Supplementary Table 1 while primers for microRNAs were commercially synthesized by Qiagen. GAPDH and SNORD48 were used as endogenous controls for mRNA and microRNAs, respectively. The data were expressed as 2−δCt method, where Ct values of the molecules were normalized to that of housekeeping gene and compared with their respective controls.
Western blotting
Cell lysate was prepared using RIPA lysis buffer (150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris pH 8.0) containing protease inhibitor cocktail. Protein quantification was done by Bradford assay. 20–30 µg of total protein was resolved in SDS-PAGE and transferred onto a nitrocellulose membrane. Membrane was blocked in 5% BSA-TBST (Tris-buffered saline, 0.1% tween 20, pH 7.4) for 1 h followed by incubation with primary antibody for overnight at 4°C. The antibodies used for western blotting have been described in Supplementary Table 2. Horseradish peroxidase (HRP) conjugated secondary antibody was then added for 2 h at room temperature. Chemiluminescence detection reagent (Thermo Scientific) was used to develop blots and band images were acquired with Fluorchem E (Cell Biosciences) followed by quantification using Image J analyzer software.
Cell survival (MTT) assay
RPMI8226 and U266 cells were seeded in triplicates in 96-well plate at a density of 10,000 cells/well and 15,000 cells/well, respectively. The cells were then supplemented with BMSCs-CM in 1:1 ratio with or without VCAN antibody (200 ng/mL, Santa Cruz Biotechnology) for 24, 48 and 72 h duration. After respective incubation, 10.0 μL of 5mg/mL MTT stock (Calbiochem) was added and incubated at 37°C for 4 h. The crystals formed were then dissolved in 100 μL Di-methyl sulphoxide (DMSO, Himedia) and kept at orbital shaker for 20 min. The plate was then read at 570 nm wavelength and normalized by 630 nm reference wavelength to calculate % survival.
Cell proliferation (CFSE) assay
5 × 105 myeloma cells were labelled with 1–10 μM carboxy-fluorescein succinimidyl ester (CFSE, Sigma) for 15 min in dark at 37°C. After incubation, complete media was added to quench the reaction and incubated for 5 min at room temperature. CFSE-labelled cells were then washed twice with media followed by plating in 6-well plate. The respective treatment was given to CFSE-labelled cells for desired time points. The cells were then harvested and acquired in green filter in BD FACSCanto system.
Transwell migration and matrigel invasion assay
1.5 × 105 MM cells were resuspended in 150 μl serum-free RPMI-1640 media and seeded into the upper compartment of 8 μm polyethylene terephthalate 24-well transwell boyden chamber (SPL Life Sciences) while 500 μL 1× RPMI-1640 complete media was added into the lower compartment. For matrigel invasion assay, 50μl BD MatrigelTM of 2μg/μl concentration was added into the upper compartment of boyden chamber and kept in 37°C-5% CO2 incubator for 3 h to allow solidification of matrigel. Then, cells were seeded into upper chamber in 100 μL serum-free media. The chamber system was incubated at 37°C-5% CO2 for overnight (18–20 h). Following incubation, cells that had migrated or invaded through membrane to the lower compartment were collected by centrifugation and counted in neubauer chamber.
VCAN 3′-UTR cloning
The 3′-UTR of VCAN containing putative target sites for miR-144, miR-199 and miR-203 was PCR amplified using primers: forward (NheI 5ʹCACTGAGCTAGCCCTCATCAGCAAAGGACAATTC3ʹ) and reverse (XbaI 5ʹCGATAGTCTAGAAGCAGCCGAACCAATGATTA3ʹ) and cloned into pmirGLO-dual luciferase vector (Promega). The respective microRNA target sites were then mutated by overlap PCR based site-directed mutagenesis method using primers: VCAN 3ʹ UTR-144m (forward: NheI 5ʹ TCCTAATGTAAGCTTGCCTACTGTACTA3ʹ; reverse XbaI 5ʹCAGTAGGCAAGCTTACATTAGGAAATGG3ʹ); VCAN 3ʹ UTR-199m (forward: NheI 5ʹGCAGTCCATAAGCTTATGTATACCAGCCT3ʹ; reverse: XbaI 5ʹGGTATACATAAGCTTATGGACTGCACTGG3ʹ); VCAN 3ʹ UTR-203m (forward: NheI 5ʹGTGTTTTCAGGATCCCAGCCAAAGTCCTAAC3ʹ; reverse: XbaI 5ʹCTTTGGCTGGGATCCTGAAAACACATGTTC3ʹ)
Dual reporter luciferase assay
5.0 × 105 RPMI8226 and U266 cells were plated in 6-well plate, starved for 2–3 h and transfected with control and test pmirGLO vector using polyethyleneimine (PEI, Polysciences) and Fugene®HD (Promega) respectively. The cells were harvested after 48 h for luciferase assay using Dual-Luciferase® Reporter Assay System (Promega). For this, cell lysate was prepared by resuspending the cells in lysis buffer and given one freeze-thaw cycle followed by centrifugation on high speed at 4°C. The firefly luciferase activity was measured in cell lysates using luminometer (Berthold Detection Systems) and normalized by renilla luciferase. All the experiments were carried out in triplicates.
Transfection of BMSCs
Patient-derived BMSCs were transfected using Lipofectamine 3000 reagent (Invitrogen) as per the manufacturer’s protocol. Briefly, 2.0 × 105 cells in logarithmic growth phase were plated in 6-well plate and kept overnight in 37°C-5% CO2 for adherence. The cells were starved for 1–2 h and transfected with 10 nM-50 nM each of miR-144 and miR-199 mimics (Thermo Fisher Scientific) and miR negative control (Thermo Fisher Scientific) was used as mock. 2× DMEM low-glucose media was added 5 h after transfection. After 48 h of transfection, cells were harvested for RNA isolation. Simultaneously, one set of cells were starved in serum-free 1× DMEM low-glucose media for another 48 h to collect BMSCs-CM to determine changes in secretion of VCAN by ELISA. All the experiments were performed in triplicates.
Statistical analysis
GraphPad Prism 5.0 was used for statistical assessment. Data were presented as Median (Range) for non-parametric data while Mean ± SD for parametric data. Wilcoxon rank-sum test and two-way ANOVA were applied to statistically analyse differences between groups for non-parametric and parametric data, respectively. Spearman’s correlation analysis was performed to identify the correlation between VCAN and microRNAs in MM patients. A p value of <0.05 with 95% confidence interval was considered statistically significant.
Funding Statement
This work was supported by the All India Institute of Medical Sciences [A-489]; Council of Scientific and Industrial Research [09/006(0451)/2015-EMR-I].
Abbreviations:
- Bcl-2
B-cell lymphoma
- BM
Bone marrow
- BMMNCs
Bone marrow mononuclear cells
- BMSCs
Bone marrow stromal cells
- BMSCs-CM
Bone marrow stromal cells-conditioned medium
- ECM
Extracellular matrix
- FAK
Focal adhesion kinase
- MM
Multiple myeloma
- PCNA
Proliferating cell nuclear antigen
- STAT
Signal transducer and activator of transcription
- VCAN
Versican
Acknowledgments
The author’s research fellowship was supported by the Council of Scientific and Industrial Research (CSIR), India (Grant Number: 09/006(0451)/2015-EMR-I). This work was also supported by the All India Institute of Medical Sciences (AIIMS), New Delhi, India (Grant Number: A-489).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
References
- [1].Palumbo A, Anderson K.. Multiple myeloma. N Engl J Med. 2011. March 17;364(11):1046–1060. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. November;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [3].Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008. March 15;111(6):2962–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ghobrial IM. Myeloma as a model for the process of metastasis: implications for therapy. Blood. 2012. July 5;120(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rahmani M, Wong BW, Ang L, et al. Versican: signaling to transcriptional control pathways. Can J Physiol Pharmacol. 2006. January;84(1):77–92. [DOI] [PubMed] [Google Scholar]
- [6].Dours-Zimmermann MT, Zimmermann DR. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem. 1994. December 30;269(52):32992–32998. [PubMed] [Google Scholar]
- [7].Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. Embo J. 1989. October;8(10):2975–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gorter A, Zijlmans HJ, van Gent H, et al. Versican expression is associated with tumor-infiltrating CD8-positive T cells and infiltration depth in cervical cancer. Mod Pathol. 2010. December;23(12):1605–1615. [DOI] [PubMed] [Google Scholar]
- [9].Makatsori E, Lamari FN, Theocharis AD, et al. Large matrix proteoglycans, versican and perlecan, are expressed and secreted by human leukemic monocytes. Anticancer Res. 2003. August;23(4):3303–3309. [PubMed] [Google Scholar]
- [10].Yang W, Yee AJ. Versican V2 isoform enhances angiogenesis by regulating endothelial cell activities and fibronectin expression. FEBS Lett. 2013. January 16;587(2):185–192. [DOI] [PubMed] [Google Scholar]
- [11].Miquel-Serra L, Serra M, Hernández D, et al. V3 versican isoform expression has a dual role in human melanoma tumor growth and metastasis. Lab Invest. 2006. September;86(9):889–901. [DOI] [PubMed] [Google Scholar]
- [12].Gupta N, Khan R, Kumar R, et al. Versican and its associated molecules: potential diagnostic markers for multiple myeloma. Clin Chim Acta. 2015. March;10(442):119–124. [DOI] [PubMed] [Google Scholar]
- [13].Hope C, Ollar SJ, Heninger E, et al. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood. 2014. May 22;123(21):3305–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hope C, Foulcer S, Jagodinsky J, et al. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016. April;128(5):680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gupta N, Kumar R, Sharma A. Versikine, a proteolysis product of Versican: novel therapeutics for multiple myeloma. Transl Cancer Res. 2016. December 29;5(7):S1437-S1439–S1439. [Google Scholar]
- [16].Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006. December 1;108(12):3646–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fang L, Du WW, Yang X, et al. Versican 3ʹ-untranslated region (3ʹ-UTR) functions as a ceRNA in inducing the development of hepatocellular carcinoma by regulating miRNA activity. Faseb J. 2013. March;27(3):907–919. [DOI] [PubMed] [Google Scholar]
- [18].Lee DY, Jeyapalan Z, Fang L, et al. Expression of versican 3ʹ-untranslated region modulates endogenous microRNA functions. PLoS ONE. 2010. October 25;5(10):e13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bu P, Yang P. MicroRNA-203 inhibits malignant melanoma cell migration by targeting versican. Exp Ther Med. 2014. July;8(1):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [20].Wong K-Y, Liang R, So -C-C, et al. Epigenetic silencing of MIR203 in multiple myeloma. Br J Haematol. 2011. September;154(5):569–578. [DOI] [PubMed] [Google Scholar]
- [21].Zhao Y, Xie Z, Lin J, et al. MiR-144-3p inhibits cell proliferation and induces apoptosis in multiple myeloma by targeting c-Met. Am J Transl Res. 2017;9(5):2437–2446. [PMC free article] [PubMed] [Google Scholar]
- [22].Raimondi L, Amodio N, Di Martino MT, et al. Targeting of multiple myeloma-related angiogenesis by miR-199a-5p mimics: in vitro and in vivo anti-tumor activity. Oncotarget. 2014. May 30;5(10):3039–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu S-Q, Niu W-Y, Li Y-P, et al. miR-203 inhibits cell growth and regulates G1/S transition by targeting Bmi-1 in myeloma cells. Mol Med Rep. 2016. November;14(5):4795–4801. [DOI] [PubMed] [Google Scholar]
- [24].Petrini M, Pacini S, Trombi L, et al. Identification and purification of mesodermal progenitor cells from human adult bone marrow. Stem Cells Dev. 2009. August;18(6):857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hung S-C, Chen N-J, Hsieh S-L, et al. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20(3):249–258. [DOI] [PubMed] [Google Scholar]
- [26].Du WW, Yang BB, Yang BL, et al. Versican G3 domain modulates breast cancer cell apoptosis: a mechanism for breast cancer cell response to chemotherapy and EGFR therapy. PLoS ONE. 2011;6(11):e26396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Du WW, Fang L, Yang X, et al. The role of versican in modulating breast cancer cell self-renewal. Mol Cancer Res. 2013. May;11(5):443–455. [DOI] [PubMed] [Google Scholar]
- [28].Suhovskih AV, Aidagulova SV, Kashuba VI, et al. Proteoglycans as potential microenvironmental biomarkers for colon cancer. Cell Tissue Res. 2015. September;361(3):833–844. [DOI] [PubMed] [Google Scholar]
- [29].Liu G, Wu K, Sheng Y. Elucidation of the molecular mechanisms of anaplastic thyroid carcinoma by integrated miRNA and mRNA analysis. Oncol Rep. 2016. November;36(5):3005–3013. [DOI] [PubMed] [Google Scholar]
- [30].Kischel P, Waltregny D, Dumont B, et al. Versican overexpression in human breast cancer lesions: known and new isoforms for stromal tumor targeting. Int J Cancer. 2010. February 1;126(3):640–650. [DOI] [PubMed] [Google Scholar]
- [31].Takahashi Y, Kuwabara H, Yoneda M, et al. Versican G1 and G3 domains are upregulated and latent transforming growth factor-β binding protein-4 is downregulated in breast cancer stroma. Breast Cancer. 2012. January;19(1):46–53. [DOI] [PubMed] [Google Scholar]
- [32].Ricciardelli C, Brooks JH, Suwiwat S, et al. Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer. Clin Cancer Res. 2002. April;8(4):1054–1060. [PubMed] [Google Scholar]
- [33].López-Corral L, Corchete LA, Sarasquete ME, et al. Transcriptome analysis reveals molecular profiles associated with evolving steps of monoclonal gammopathies. Haematologica. 2014. August;99(8):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Furukawa M, Ohkawara H, Ogawa K, et al. Autocrine and paracrine interactions between multiple myeloma cells and bone marrow stromal cells by growth arrest-specific gene 6 cross-talk with interleukin-6. J Biol Chem. 2017. October;292(10):4280–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gunn WG, Conley A, Deininger L, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006. April;24(4):986–991. [DOI] [PubMed] [Google Scholar]
- [36].Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013. April;123(4):1542–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu Y, Chen L, Zheng P-S, et al. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002. April 5;277(14):12294–12301. [DOI] [PubMed] [Google Scholar]
- [38].Carthy JM, Meredith AJ, Boroomand S, et al. Versican V1 overexpression induces a myofibroblast-like phenotype in cultured fibroblasts. PLoS ONE. 2015;10(7):e0133056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Akinfenwa PY, Bond WS, Ildefonso CJ, et al. Versican G1 domain enhances adenoviral-mediated transgene expression and can be modulated by inhibitors of the Janus kinase (JAK)/STAT and Src family kinase pathways. J Biol Chem. 2017. January;292(35):14381–14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu C, Feng X, Wang B, et al. Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci. 2018. March;109(3):688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cheng J, Li Y, Liu S, et al. CXCL8 derived from mesenchymal stromal cells supports survival and proliferation of acute myeloid leukemia cells through the PI3K/AKT pathway. Faseb J. 2019 Apr;33(4):4755–4764. [DOI] [PubMed] [Google Scholar]
- [42].Sun X, Liu D, Xue Y, et al. Enforced miR-144-3p expression as a Non-invasive biomarker for the acute myeloid leukemia patients mainly by targeting NRF2. Clin Lab. 2017. April 1;63(4):679–687. [DOI] [PubMed] [Google Scholar]
- [43].Gits CMM, van Kuijk PF, Jonkers MBE, et al. MicroRNA expression profiles distinguish liposarcoma subtypes and implicate miR-145 and miR-451 as tumor suppressors. Int J Cancer. 2014. July 15;135(2):348–361. [DOI] [PubMed] [Google Scholar]
- [44].Lou Z, Gong Y-Q, Zhou X, et al. Low expression of miR-199 in hepatocellular carcinoma contributes to tumor cell hyper-proliferation by negatively suppressing XBP1. Oncol Lett. 2018. November;16(5):6531–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chim CS, Wong KY, Leung CY, et al. Epigenetic inactivation of the hsa-miR-203 in haematological malignancies. J Cell Mol Med. 2011. December;15(12):2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fu Q, Zhang J, Xu X, et al. miR-203 is a predictive biomarker for colorectal cancer and its expression is associated with BIRC5. Tumour Biol. 2016. October 6. [DOI] [PubMed] [Google Scholar]
- [47].Gupta N, Kumar R, Seth T, et al. Clinical significance of circulatory microRNA-203 in serum as novel potential diagnostic marker for multiple myeloma. J Cancer Res Clin Oncol. 2019. June;145(6):1601–1611. [DOI] [PubMed] [Google Scholar]
- [48].Yang W, Yee AJM. Versican 3ʹ-untranslated region (3ʹUTR) promotes dermal wound repair and fibroblast migration by regulating miRNA activity. Biochim Biophys Acta. 2014. July;1843(7):1373–1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


