Background:
There are many treatment modalities for benign cystic lesions of bone, but all methods, except for total resection, are plagued with varying rates of recurrence1. Thorough curettage with the use of a high-speed burr, however, has demonstrated a low recurrence rate of 12% and has been suggested to be the best method for the treatment of benign cystic lesions. Approximately 90% of aneurysmal bone cysts can be controlled adequately with this treatment alone2.
Description:
Treatment of benign cystic lesions of bone with the use of excisional curettage requires careful preoperative planning and patient positioning before the initial incision is made. The initial incision must be carefully planned to expose the entire lesion without violating multiple compartments unnecessarily. A sizeable cortical window must then be made using a high-speed burr followed by evacuation of all cystic contents via curettage. The cavity is copiously irrigated before an adjuvant is used, and the lesion is stabilized, if necessary, before closing.
Alternatives:
There are many types of alternatives to curettage, such as wide resection, radiation, and embolization of feeding vessels.
Rationale:
In orthopaedics, as in all medical specialties, many interventions and techniques have been rendered obsolete and, ultimately, replaced by newer, safer, and more efficient ones. The appeal of curettage has remained because of its procedural simplicity and adaptability in the management of a plethora of diseases such as benign cystic lesions of bone. Additionally, curettage, unlike wide resection, radiation, and embolization of feeding vessels, is minimally invasive and often definitive in nature when used as a treatment modality2. Lastly, curettage grants the performing surgeon the ability to maintain a contained cavity that can be treated with a variety of adjuvant therapies3-17. These reasons listed above make curettage a viable option for the surgical treatment of benign cystic lesions such as giant cell tumors of bone, aneurysmal bone cysts, unicameral bone cysts, chondromyxoid fibromas, and symptomatic nonossifying fibromas.
Introductory Statement
Excisional curettage can be used to treat a vast range of benign cystic conditions, such as giant cell tumors of bone (GCTs), aneurysmal bone cysts (ABCs), unicameral bone cysts, chondromyxoid fibromas, and symptomatic nonossifying fibromas, more definitively and with less morbidity and lower health-care costs than other treatment modalities.
Indications & Contraindications
Indications
Treatment of benign cystic lesions such as GCTs, ABCs, unicameral bone cysts, chondromyxoid fibromas, and symptomatic nonossifying fibromas. Many osseous lesions fall in this category, and a biopsy can be performed to confirm a benign and cystic nature if imaging modalities are equivocal.
Revision curettage for previously insufficient curettage that yielded recurrence of original cystic pathology.
Contraindications
Malignant tumors.
Step-by-Step Description of Procedure (Video 1)
Video 1.
Curettage.
Step 1: Preoperative Planning
Thoroughly plan prior to the operation.
Generate a differential diagnosis and final diagnosis on the basis of the patient history, physical examination, and radiographs.
Perform a biopsy, if necessary. An image-guided or incisional biopsy can be performed before excision.
Before the procedure, secure all necessary equipment including a radiolucent table, high-speed burr, variable straight and angled curets, a surgical spoon, and a pulse lavage.
Secure any adjuvants that may be required. We use an argon beam and polymethylmethacrylate (PMMA). Other adjuvants that can be used include phenol, ethanol, hydrogen peroxide, and cryosurgery with liquid nitrogen3,5,7,9,11,17.
Secure appropriate implants because larger lesions or pathological fractures sustained during the procedure may require fixation.
Plan the initial incision. The initial incision should longitudinally cover the lesion but should not violate multiple compartments unnecessarily.
Step 2: Patient Positioning
Position the patient depending on which extremity is being operatively treated and allowing for appropriate radiographic evaluation and maneuvering of the extremity.
Make sure that as much of the entire extremity as possible is exposed to allow appropriate radiographic evaluation of the entire lesion.
Pad all osseous and soft-tissue prominences.
Step 3: Incision and Cortical Window
Create a cortical window, which can be accomplished in many ways.
Perform an incisional biopsy prior to curettage if a biopsy specimen was not obtained preoperatively (Fig. 1).
Create the window directly with the use of a high-speed burr through the cortex.
Alternatively, create a cortical window by making multiple drill-holes in a circular manner and connecting them with the use of an osteotome.
Use a burr to further enlarge the cavity to make sure that the cortical window is large enough to allow access to the lesion by all instruments necessary (Fig. 2).
Fig. 1.
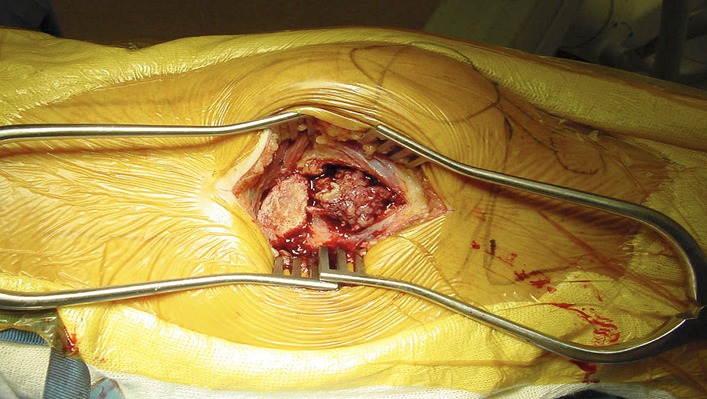
Cortical window created, allowing for biopsy.
Fig. 2.
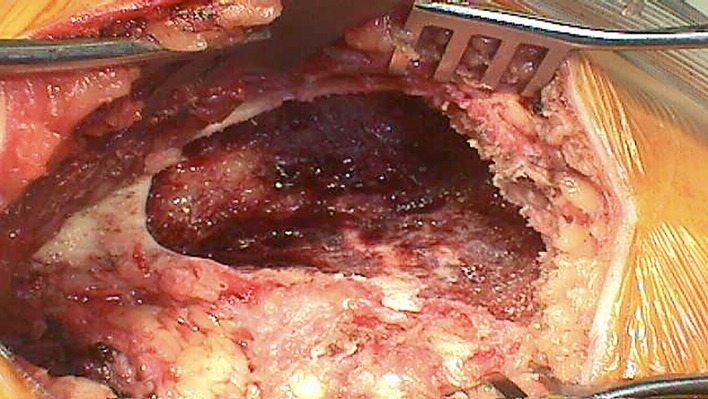
Enlargement of the cavity to an appropriate size to allow for visualization and appropriate evacuation.
Step 4: Evacuate Contents
Evacuate the contents of the cavity using any instrument that can reach into and empty the cavity; however, if the contents of the cavity are solid, use sturdier instruments and suction the liquid cystic contents.
Use curets, rongeurs, or spoons to remove all contents from the cavity (Fig. 3).
Irrigate and suction until the entire cavity can be clearly visualized.
Perform curettage of the cavity and ensure that the entire cavity can be accessed with the curet.
For many lesions, it is difficult to know whether all neoplastic cells have been evacuated; thus, it is advisable to use visual aids such as intraoperative fluoroscopy to be certain that the instruments are able to reach the entire lesion. These images can be compared with the preoperative imaging as well.
Fig. 3.
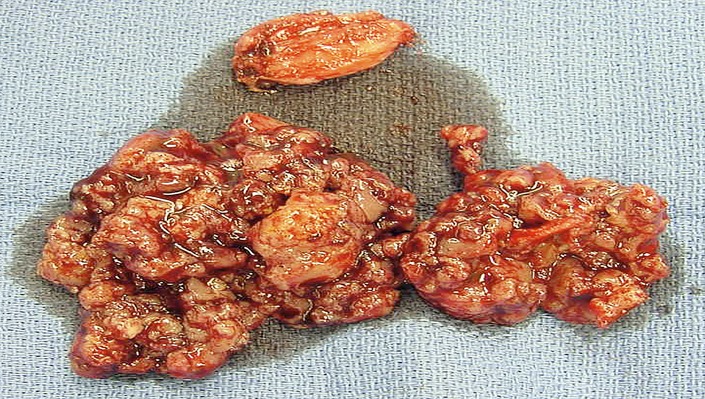
Evacuated GCT contents.
Step 5: Use a High-Speed Burr and/or an Adjuvant
Use a high-speed burr in a methodical manner until normal bone is reached in all parts of the lesion, as this allows for additional improvement of the margin following curettage.
Reach an approximate depth of 5 mm when possible.
Create a uniform cavity by making multiple burr holes of the same size and then use the burr to connect the holes created. Perform this technique throughout the lesion where possible (Fig. 4).
Copiously irrigate the cavity.
Use an argon beam coagulator to paint the freshly irrigated cavity black. We prefer the use of a noncontact electrode tip with the setting at 100. This allows for an additional layer of damage to any pathological cells.
Fig. 4.
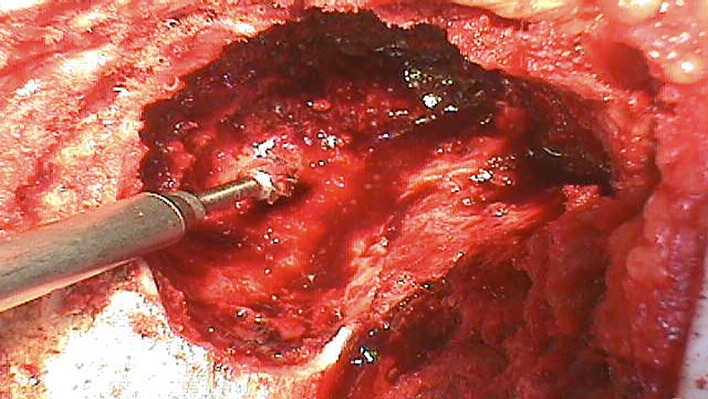
Use of a high-speed burr to create a uniform cavity.
Step 6: Irrigate the Cavity
Irrigate the cavity until clear.
Use several liters of normal saline solution during irrigation to fully clear the cavity.
Step 7: Fill the Defect with Adjuvant
Following the procedure, fill the cavity with an adjuvant.
Use an adjuvant to fill the cavity; the choice of adjuvant is often dependent on the surgeon. Bone cement affords an additional thermal component that helps to improve the margin and provides added stability. Other bone substitutes have the benefit of being resorbed and encouraging bone formation within the cavity.
Fill with bone cement, bone graft, or bone substitutes.
Stabilize as needed (Fig. 5).
Fig. 5.
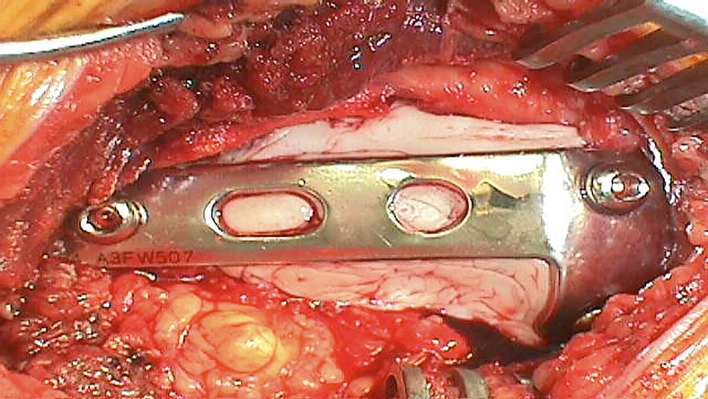
Cement augmentation and stabilization.
Step 8: Perform Fixation if Appropriate
Perform fixation if it is appropriate, e.g., if the defect is large and in a region of high torsional stress or if the patient has a high level of activity.
Perform fixation if the defect is large and in a region of high torsional stress or impact. Various patient-related factors such as activity level and ability to follow postoperative restrictions should be included in this decision-making. The fixation implant of choice is often a plate, especially in periarticular regions. Stainless steel plates are likely to limit visualization in settings when surveillance would be performed using magnetic resonance imaging. In such situations, titanium or carbon fiber implants should be considered.
Ensure that the fixation spans the lesion and has purchase in normal bone tissue.
Obtain postoperative images following fixation (Fig. 6). These will serve as a means of comparison during the follow-up period.
Fig. 6.
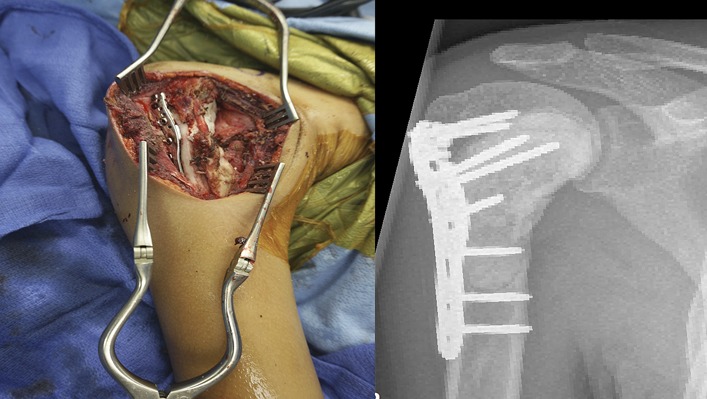
Postoperative imaging showing fixation after excisional curettage of a lesion in the shoulder.
Results
Many treatment modalities for benign cystic lesions of bone exist; however, all of them, except for total excision, have non-negligible rates of recurrence of the initial pathology1,18. Curettage is one of the most commonly employed techniques for the treatment of benign cystic lesions of bone, and rates of recurrence after curettage in ABCs have been reported to be between 18.75% and 64%18. The high rate of recurrence following curettage in this group has been found to be the result of inadequate cortical window formation19 and insufficient curettage allowing remnants of the cyst to repropagate18. It has been shown that proper cortical window formation and use of a high-speed burr can decrease recurrence to 12%2. Moreover, even if the lesion recurs following curettage, repeated curettage has been shown to eradicate all pathological growths in all patients during the revision procedure18. Thus, utilization of this simple and easily amenable technique can be used as an effective treatment modality for a variety of benign cystic lesions of bone.
Pitfalls & Challenges
Inadequately sized cortical windows. When incising the cortical window, make sure that the incision is large enough to allow access to the entire cavity of the lesion. Failure to create a sizeable cortical window will lead to decreased visualization and, possibly, residual cyst cells and recurrence19.
Drilling through the PMMA cement when it has completely hardened can prove to be a challenge. If possible, drill while the cement is still soft as it can help to avoid this problem. When drilling through hardened cement, it is helpful to do so a slow and steady manner and to apply irrigation to cool the drill during the process.
Cortical breach or fracture. It is paramount to remember that certain areas surrounding the cyst are likely to have been pathologically thinned by the advancement of the cyst—thinned areas are easily subject to fracture and should be stabilized.
Published outcomes of this procedure can be found at: Pan Afr Med J. 2016 Aug 16;24:311.
Investigation performed at the Department of Orthopaedics and Rehabilitation, Yale University School of Medicine, New Haven, Connecticut
Disclosure: The authors indicated that no external funding was received for any aspect of this work. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSEST/A255).
References
- 1.Herring JA, editor. Tachdjian’s pediatric orthopaedics. 4th ed. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 2.Gibbs CP, Jr, Hefele MC, Peabody TD, Montag AG, Aithal V, Simon MA. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am. 1999. December;81(12):1671-8. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki T, Hillmann A, Lindner N, Winkelmann W. Cementation of primary aneurysmal bone cysts. Clin Orthop Relat Res. 1997. April;337:240-8. [DOI] [PubMed] [Google Scholar]
- 4.Marcove RC, Sheth DS, Takemoto S, Healey JH. The treatment of aneurysmal bone cyst. Clin Orthop Relat Res. 1995. February;311:157-63. [PubMed] [Google Scholar]
- 5.Capanna R, Sudanese A, Baldini N, Campanacci M. Phenol as an adjuvant in the control of local recurrence of benign neoplasms of bone treated by curettage. Ital J Orthop Traumatol. 1985. September;11(3):381-8. [PubMed] [Google Scholar]
- 6.Malawer MM, Bickels J, Meller I, Buch RG, Henshaw RM, Kollender Y. Cryosurgery in the treatment of giant cell tumor. A long-term followup study. Clin Orthop Relat Res. 1999. February;359:176-88. [DOI] [PubMed] [Google Scholar]
- 7.Marcove RC. A 17-year review of cryosurgery in the treatment of bone tumors. Clin Orthop Relat Res. 1982. March;163:231-4. [PubMed] [Google Scholar]
- 8.Pritsch T, Bickels J, Wu CC, Squires HM, Malawer MM. The risk for fractures after curettage and cryosurgery around the knee. Clin Orthop Relat Res. 2007. May;458:159-67. [DOI] [PubMed] [Google Scholar]
- 9.Dürr HR, Maier M, Jansson V, Baur A, Refior HJ. Phenol as an adjuvant for local control in the treatment of giant cell tumour of the bone. Eur J Surg Oncol. 1999. December;25(6):610-8. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson NC, Ramp WK, Kneisl JS, Kaysinger KK. Hydrogen peroxide inhibits giant cell tumor and osteoblast metabolism in vitro. Clin Orthop Relat Res. 1998. February;347:250-60. [PubMed] [Google Scholar]
- 11.Jones KB, DeYoung BR, Morcuende JA, Buckwalter JA. Ethanol as a local adjuvant for giant cell tumor of bone. Iowa Orthop J. 2006;26:69-76. [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994. December;76(12):1827-33. [DOI] [PubMed] [Google Scholar]
- 13.Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res. 2005. June;435:211-8. [DOI] [PubMed] [Google Scholar]
- 14.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G, Davis AM; Canadian Sarcoma Group. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res. 2002. April;397:248-58. [DOI] [PubMed] [Google Scholar]
- 15.Ward WG, Sr, Li G., 3rd Customized treatment algorithm for giant cell tumor of bone: report of a series. Clin Orthop Relat Res. 2002. April;397:259-70. [DOI] [PubMed] [Google Scholar]
- 16.Cooper IS. Cryogenic surgery—a new method of destruction or extirpation of benign or malignant tissues. N Engl J Med. 1963;268:743-9. [DOI] [PubMed] [Google Scholar]
- 17.DiCaprio MR, Boyle J, Gibbs CP. Use of the argon beam coagulator as an adjuvant for treating bone tumors. Tech Orthop. 2007;22(2):110-15. [Google Scholar]
- 18.Çelik S, Uludağ A, Tosun HB, Serbest S, Gürger M, Kılıç S. Unicameral (simple) and aneurysmal bone cysts: the effect of insufficient curettage on recurrence. Pan Afr Med J. 2016. August 16;24:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole WG. Treatment of aneurysmal bone cysts in childhood. J Pediatr Orthop. 1986. May-Jun;6(3):326-9. [DOI] [PubMed] [Google Scholar]


