Abstract
Background
While the clinical validity of risk-associated single nucleotide polymorphisms (SNPs) for assessment of disease susceptibility has been consistently established, risk reclassification from increasing numbers of implicated risk-associated SNPs raises concern that it is premature for clinical use. Our objective is to assess the degree and impact of risk reclassification with the increasing number of SNPs.
Methods
A total of 3,239 patients from the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial were included. Four genetic risk scores (GRSs) were calculated based on sets of sequentially discovered prostate cancer (PCa) risk-associated SNPs (17, 34, 51, and 68 SNPs).
Results
Pair-wise correlation coefficients between sets of GRSs increased as more SNPs were included in the GRS: 0.80, 0.86, and 0.95 for 17 vs. 34 SNPs, 34 vs. 51 SNPs and 51 vs. 68 SNPs, respectively. Using a GRS of 1.5 as a cutoff for higher versus lower risk, reclassification rates of PCa risk decreased: 14.11%, 12.04%, and 8.15% for 17 vs. 34 SNPs, 34 vs. 51 SNPs and 51 vs. 68 SNPs, respectively. Evolving GRSs, nevertheless, provide a tool for further refining risk assessment. When all four sequential GRSs were considered, the detection rates of PCa for men whose GRSs were consistently <1.5, reclassified, and consistently ≥1.5 were 20.8%, 29.67%, and 39.26%, respectively (P-trend=1.12×10−8). In comparison, the detection rates of PCa in men with negative or positive family history were 23.75% and 31.78%, respectively.
Conclusions
Risk assessment using currently available SNPs is justified. Multiple GRS values from evolving sets of SNPs provide a valuable tool for better refining risk.
Keywords: genetics, polymorphism, SNPs, prostate cancer
Background
Approximately 100 prostate cancer (PCa) risk-associated single nucleotide polymorphisms (SNPs) have been discovered from genome-wide association studies (GWAS) in recent years and the number of these PCa-risk associated SNPs continues to increase.[1–16] Although each of these SNPs is only modestly associated with PCa risk, their cumulative effects strongly increase PCa risk.[15–18] A potential clinical utility of these PCa risk-associated SNPs is risk stratification for targeted intervention such as PSA screening.[19]
With more PCa risk-associated SNPs identified, PCa risk estimated for an individual patient from these evolving sets of SNPs could be different. For a subset of individuals, their risk category (higher or lower based on a cutoff value) could be reclassified. The concern of this risk reclassification was raised from a recent simulation study by Krier et al. where the risk category was reclassified in 50% of men when using risk-associated SNPs available by 2007 and 2013.[20] This level of risk reclassification, if observed in empirical studies, would suggest that it is premature to use currently available risk-associated SNPs for risk assessment. The objective of this current study is to assess the degree and impact of this risk reclassification in an actual study population. We performed a reclassification analysis on four sets of sequentially identified PCa risk-associated SNPs in a well-defined prospective cohort of patients who were followed for four years for detection of PCa.
Methods
Study population
This study included 3,239 patients enrolled in the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) clinical trial who consented for further genetic studies. This trial examined the effect of Dutasteride on PCa development, and has been previously described in detail.[21] Briefly, eligible men (1) had a serum PSA between 2.5–10.0 ng/mL (50–60 years of age) or 3.0–10.0 ng/mL (61–75 years of age), and (2) had undergone one 6–12 core biopsy within 6 months of enrollment and were not diagnosed with PCa. Participants in this trial were randomized to receive Dutasteride (treatment arm) or to receive a placebo (placebo arm). We utilized the placebo arm (n=1,654) for the primary analyses, due to the effects of Dutasteride on decreasing PCa incidence. The treatment arm (n=1,585 Caucasians) was used as an independent replication cohort. Family history (FH) information was collected during the enrollment. Patients with any first degree relatives who had PCa were considered as FH positive. All patients provided informed consent, and the Institutional Review Boards at all participating institutions granted approval.
Genotyping and Quality Control
DNA samples were genotyped in the Center for Cancer Genomics at Wake Forest University using the Illumina HumanOmniExpress BeadChip, which included 729,755 SNPs. For PCa risk-associated SNPs that were not included in the GWAS array, imputation was performed using IMPUTE 2.2.2 based on the combined data of the 1000 Genomes project and HapMap3 data.[22] A posterior probability of >0.9 was applied to call imputed genotypes. More detailed description of genotyping and quality control procedures were described elsewhere.[18]
Assessment of Genetic Risk
SNP Selection
In this study, we applied stringent criteria to ensure that SNPs used in the analysis are common, independent and validated PCa risk-associated SNPs. The criteria were: 1) discovered from GWAS studies with at least 1,000 cases and 1,000 controls; 2) met the gold standard GWAS significance level of P<5×10−8; 3) SNPs with minor allele frequency>0.05 in reported studies; and 4) independent, linkage disequilibrium measurement (r2<0.2) between any pair of SNPs. As a result, 68 PCa risk-associated SNPs were selected for analysis in the study. SNPs were ordered based on the time that they were identified, and were evenly divided into 17, 34, 51 and 68, representing four sequential sets of PCa risk-associated SNPs (Supplementary Table 1).
Methods for measuring cumulative effect of SNPs
The primary method for measuring the cumulative effect of SNPs in the study was genetic risk score (GRS). It was calculated for each subject based on the genotypes of the various SNPs and weighted by their ORs and risk allele frequencies.[18,27] GRS was calculated as , where gi is the genotype of SNP i for an individual (0, 1, or 2 for individual with homozygous of non-risk allele, heterozygous or homozygous of risk allele, respectively). ORi is the OR of SNP i estimated from external studies[1–16], Wi is the average population risk of SNP i, calculated as Wi = fi2ORi2 + 2fi(1−fi)ORi + (1−fi)2, where fi is the risk allele frequency of SNP I based on the 1000 Genome Project of the CEU population. Therefore, a GRS value of 1.0 represents a population average risk. Two other methods for measuring the cumulative effect of SNPs were also used, including a simple risk allele count ( ) and a polygenic risk score (PRS), an OR-weighted risk allele count ( ).
Statistical analysis
In this study, a GRS cutoff value of 1.5 was used for risk stratification; men with GRS <1.5 or ≥1.5 were classified as lower or higher risk, respectively. This cutoff value was chosen because it confers a risk of PCa similar to that of having positive FH (odds ratio of FH for PCa diagnosis was 1.5 in the placebo arm of the REDUCE study). The Pearson correlation coefficient was used to evaluate the linear correlation between each pair of GRS values. T-tests were used to test the differences in means of normally distributed variables between two groups. For variables that were not normally distributed, two tests were performed; (1) a nonparametric method using the Wilcoxon rank sum test, and (2) t-tests for different means between two groups after log-transformation. Differences in binary variables were tested using chi-square tests. Area under the receiver operating characteristic curve (AUC) was used to evaluate the performance in discriminating two groups of subjects. The difference between two AUCs was tested using Delong’s test.[23] Cochran-Armitage trend test was used to test the difference of detection rates across risk categories.
Results
The baseline demographic and clinical characteristics of men included in this study, stratified by placebo and treatment arms, are shown (Table 1). Univariate analyses demonstrated that men with PCa differed significantly (P<0.05) from men without PCa for all baseline clinical and demographic variables, with the exception of digital rectal examination. Men diagnosed with PCa had a significantly higher proportion of positive FH of PCa in the placebo arm (17% vs. 12%, P=0.01), but not in the treatment arm (16% vs. 14%, P=0.36).
Table 1.
Baseline clinical and demographic variables of the subjects in the REDUCE study
| Variables Baseline clinical variables |
Placebo group | Drug group | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| PCa (N=410) | Non-PCa (N=1244) | P | PCa(N=304) | Non-PCa(N=1281) | P | |
| Age | ||||||
| Mean(SD), year | 63.52(5.99) | 62.22(6.01) | 0.0001 | 63.88(5.87) | 62.78(5.97) | 0.0037 |
| Median(range), year | 63(50–76) | 62(49–76) | 64(51–75) | 63(49–75) | ||
| No.(%) with positive DRE | 20(5) | 47(4) | 0.33 | 12(4) | 55(4) | 0.78 |
| Prostate volume | ||||||
| Mean(SD), mL* | 44.2(21.4) | 46.76(16.13) | 0.03 | 40.27(1.47) | 44.93(1.44) | 3.63×10−6 |
| Median(range), mL | 41.61(9.01–257) | 45.46(3.66–127) | 40.69(12.46–108.4) | 45.62(11.97–257.17) | ||
| Total PSA levels | ||||||
| Mean(SD), ng/mL* | 5.78(1.37) | 5.52(1.4) | 0.01 | 5.77(1.4) | 5.55(1.39) | 0.058 |
| Median(range), ng/mL | 5.7(2.5–10.2) | 5.7(1.8–14.2) | 5.8(2–15.6) | 5.6(1.9–11.1) | ||
| PSA density(PSA/PV) | ||||||
| Mean(SD), ng/mL2* | 0.14(1.67) | 0.14(0.07) | 4.67×10−6 | 0.14(1.62) | 0.12(1.55) | 1.10×10−6 |
| Median(range), ng/mL2 | 0.14(0.02–0.63) | 0.12(0.03–0.58) | 0.14(0.04–0.56) | 0.12(0.02–0.61) | ||
| Free-to-total PSA ratio | ||||||
| Mean(SD) | 0.16(0.06) | 0.17(0.06) | 0.02 | 1.17(1.06) | 1.19(1.06) | 7.73×10−6 |
| Median(range) | 0.16(0.03–0.47) | 0.16(0.03–0.47) | 1.16(1.04–1.56) | 1.18(1.02–1.86) | ||
| No. of cores sampled at baseline biopsy | ||||||
| Mean(SD) | 8.21(2.27) | 8.58(2.39) | 0.004 | 8.08(2.19) | 8.65(2.40) | 0.0002 |
| Median(range) | 8(3–19) | 8(2–22) | 8(4–16) | 8(2–20) | ||
| No.(%) with positive family history | 68(17) | 146(12) | 0.01 | 48(16) | 176(14) | 0.36 |
P-value were analyzed after taking natural logarithm of the variables.
DRE: digital rectal exam; PCa: prostate cancer; PSA: prostate-specific antigen; PV: prostate volume; SD: standard deviation
Although not every individual SNP was significantly associated with PCa risk (data not shown), combinations of these SNPs, measured by GRS, were strongly associated with PCa susceptibility. In the placebo group, the associations between GRS values calculated from each of the four SNP sets and the detection of PCa were highly significant (all P values <10−7) (Table 2). The performance (measured by AUC) of these increasing numbers of SNPs in discriminating biopsy outcomes (PCa from non-PCa) increased from 0.58 to 0.61, 0.60, and 0.60 for the 17, 34, 51 and 68 SNPs, respectively. A plateau effect of increasing AUC with the increased number of SNPs was observed with the 51 SNP group. The AUC values of all SNP sets were significantly higher than that of FH (AUC=0.52), all P values <0.05. Similar findings were observed for the treatment group (Supplementary Table 2).
Table 2.
Predictive performance of FH and GRS for prostate cancer in the placebo arm.
| Risk factors | OR | P-value | AUC | P-value for comparison | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| FH | 17 SNPs | 34 SNPs | 51 SNPs | 68 SNPs | ||||
| FH | 1.50 | 0.012 | 0.52 | - | 0.002 | 3.30×10−5 | 7.32×10−5 | 4.06×10−5 |
| 17 SNPs | 1.70 | 8.77×10−7 | 0.58 | - | - | 0.051 | 0.21 | 0.17 |
| 34 SNPs | 1.76 | 5.37×10−10 | 0.61 | - | - | - | 0.52 | 0.77 |
| 51 SNPs | 1.57 | 3.16×10−8 | 0.60 | - | - | - | - | 0.68 |
| 68 SNPs | 1.54 | 2.18×10−8 | 0.60 | - | - | - | - | - |
AUC: area under the curve; FH: family history; GRS: genetic risk score; OR: odds ratio; SNP: single nucleotide polymorphism;
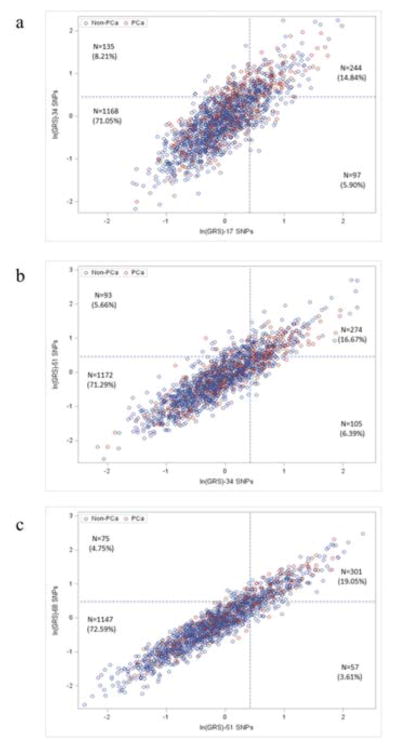
On a per individual subject level, variability of GRS values calculated from 17, 34, 51, and 68 SNPs was documented, but these GRS values were highly correlated. Scatter-plots of GRS values between two sequential SNP sets are shown in Figure 1 for the placebo arm. The shape of scatter-plots became tighter with an increasing number of risk-associated SNPs: 17 vs. 34 SNPs (a), 34 vs. 51 SNPs (b), and 51 vs. 68 SNPs (c). Correspondingly, the pair-wise correlation coefficient (r) of GRS values increased from 0.80 to 0.86 to 0.95 for 17 vs. 34 SNPs, 34 vs. 51 SNPs and 51 vs. 68 SNPs, respectively. Using GRS values <1.5 and ≥1.5 to define lower and higher risk, respectively, reclassification of PCa risk was observed. However, the rates of reclassification decreased with each sequential SNP set; 14.11%, 12.04%, and 8.15% for 17 vs. 34 SNPs, 34 vs. 51 SNPs and 51 vs. 68 SNPs, respectively (Figure 1 and Table 3). Most risk reclassification occurred in subjects whose GRS values were near the 1.5 cutoff value; 81.03%, 80.81%, and 92.54% of reclassified subjects were between 1.0 and 2.0 for 17 vs. 34 SNPs, 34 vs. 51 SNPs and 51 vs. 68 SNPs, respectively. Similar findings were observed for the treatment arm (Supplementary Figure 1 and Supplementary Table 3).
Figure 1.
Scatter plots of GRS values from two consecutive SNP sets in the placebo arm: (a) 17 vs. 34 SNPs, (b) 34 vs. 51 SNPs, and (c) 51 SNPs vs. 68 SNPs. Blue and red circle indicates patients with or without a diagnosis of PCa, respectively. Two dotted lines indicate cutoff value (1.5) to classify higher or lower risk.
Table 3.
Risk categories based on evolving SNP sets and PCa detection rate in the placebo arm using GRS cutoff value of 1.5.
| Set of SNPs | No. (%) of subjects
|
% of PCa
|
% of high-grade PCa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Remain lower | Reclassified | Remain higher | Remain lower | Reclassified | Remain higher | P-trend | Remain lower | Reclassified | Remain higher | P-trend | |
| 17 vs. 34 SNPs | 1168 (71.05%) | 232(14.11%) | 244 (14.84%) | 20.98 | 34.91 | 34.43 | 7.80×10−8 | 5.91 | 7.76 | 8.61 | 0.086 |
| 34 vs. 51 SNPs | 1172 (71.29%) | 198 (12.04%) | 274 (16.67%) | 21.42 | 27.27 | 38.32 | 5.58×10−9 | 5.63 | 8.59 | 9.12 | 0.019 |
| 51 vs. 68 SNPs | 1213 (73.78%) | 134 (8.15%) | 297 (18.07%) | 21.93 | 27.61 | 36.03 | 3.84×10−7 | 5.61 | 10.45 | 8.75 | 0.020 |
| 17, 34, 51, and 68 SNPsa | 1053 (64.05%) | 428 (26.03%) | 163 (9.91%) | 20.8 | 29.67 | 39.26 | 1.12×10−8 | 5.60 | 7.71 | 9.82 | 0.021 |
PCa: prostate cancer; SNP: single nucleotide polymorphism.
Reclassification of patient risk based on any GRS. For example, 1053 (64.05%) patients had lower risk for all of the four GRSs, 428 (26.03%) had reclassification for any of the GRSs, and 163 (9.91%) had higher risk for all of the four GRSs.
Multiple GRS values from evolving sets of SNPs, nevertheless, offered a tool for further refining risk prediction. In the placebo arm, when all four sequential GRS values were considered, risk reclassification occurred in 26% of men. The observed detection rate of PCa in the 4-year study period were 20.80%, 29.67%, and 39.26% for men whose GRS values were consistently <1.5, changed between <1.5 and ≥1.5 (reclassified), and were consistently ≥1.5, respectively, Ptrend=1.12×10−8 (Table 3). In comparison, the detection rates of PCa in men with negative or positive FH were 23.75% and 31.78%, respectively. Comparable findings were observed for the treatment arm (Supplementary Figure 1 and Supplementary Table 3).
The performance of multiple GRS values from sequentially discovered SNP sets in refining risk assessment was also supported by the observed detection rate of high-grade PCa (Gleason score ≥7) in the 4-year study period. For example, in the placebo arm, the detection rate of high-grade PCa were 5.60%, 7.71%, and 9.82% for men whose GRS values were consistently <1.5, reclassified (changed between 1.5 and ≥1.5), and were consistently ≥1.5, respectively, Ptrend=0.02 (Table 3). In comparison, the observed detection rate of high-grade PCa was 6.04% and 9.81% for men with a negative or positive FH, respectively. Again, similar findings were observed for the treatment arm (Supplementary Table 3).
Parallel results were found using simple risk allele count (sRAC) and PRS for measuring the cumulative effect of multiple risk-associated SNPs. Mean PRS and sRAC of each SNP set in PCa patients were significantly higher than that of non-PCa patients for each set of SNPs (all P values <0.05). For discriminating cases from controls at a population level, the performance (AUC) of PRS and GRS were the same and were both better than sRAC for each set of SNPs (Supplementary Table 4). At an individual level, scores of two sequential SNP sets for both sRAC and PRS were highly correlated and the scatter plots became tighter with evolving SNP sets (Supplementary Figures 2 and 3).
Discussion
Utilizing data from the prospective REDUCE study where all subjects were monitored for the development of PCa, we assessed the degree and impact of risk reclassification from increasing numbers of risk-associated SNPs on PCa diagnosis. We found that GRS values calculated from each of the four sequential sets of PCa risk-associated SNPs (17, 34, 51, and 68 SNPs) were significantly associated with PCa risk and had a better performance in discriminating PCa from non-PCa than FH. Although there was variability in GRS values for individual patients when using these four sequential sets of risk-associated SNPs, they were highly correlated, and their correlations increased with evolving SNP sets.
Using GRS values ≥1.5 to define higher PCa risk, reclassification of risk categories among different SNP sets was observed. However, the reclassification rate continued to decrease with evolving SNP sets and was only 8.15% between the two latest SNP sets (51 vs. 68 SNPs). More importantly, multiple GRS values from evolving SNP sets actually provide a valuable tool for refining risk for all subjects. Risk reclassification effectively captures men with GRS values in a grey zone (near 1.5) that are at intermediate risk, and men who have consistently lower (<1.5) or higher (≥1.5) GRS values from multiple SNP sets are further assured of their low or high genetic risk, respectively. Taken together, the present study suggests that 1) on the basis of comparative effectiveness principle, GRS from currently available risk-associated SNPs should be used to stratify PCa risk, and 2) newly discovered SNPs should be used to calculate new GRS values where a combination of new and previous GRS values should be considered together to further refine risk.
Although there were similarities between the results of this study and Krier et al., several differences were noted.[20] The reclassification rate of PCa risk was higher in the study of Krier et al. (50%) than ours (26%).[20] This difference is likely due to a combination of factors, including differences in the SNP sets for risk assessment, estimates of ORs and allele frequencies of SNPs used in calculations, and cutoff values (2.0 vs. 1.5). A GRS cutoff value of 1.5 was chosen in our study because it confers a risk of PCa similar to that of having positive FH (odds ratio of FH for PCa diagnosis was 1.5 in the placebo arm of the REDUCE study). However, similar findings were observed when a GRS cutoff value of 2.0 was implemented (Supplementary Table 5). The most important difference between the two studies is the availability of follow-up data on observed PCa detection. With the prospective design of our study, we are able to evaluate the impact of risk reclassification and demonstrate that multiple SNP sets do not pose a challenge, but rather provide an effective tool for further refining risk.
Most of the analyses described in this study were performed separately in the two trial arms. The decision to perform these analyses separately was based on the consideration that PCa detection rate was significantly lower in patients treated with dutasteride.[8] Despite these differences, the key findings from the treatment group were consistent with that of the placebo group.
Several methods are commonly used to measure the cumulative effect of multiple risk-associated SNPs, including sRAC, PRS, and GRS.[18,24–26] Although the same conclusion of the study can be made from any of these methods, there are important differences between them. sRAC simply counts the number of risk allele without considering OR of each SNP. In PRS, the risk allele count is weighted by the OR of each SNP. For GRS, the risk allele count is first weighted by the OR of each SNP and then standardized by population average risk. At a population level, all three methods perform similarly in predicting PCa risk; the score of sRAC, PRS, and GRS are significantly higher in cases than in controls. PRS and GRS have the same performance in discriminating cases and controls and both have a better performance than sRAC.[27] At an individual level, scores from GRS are easiest to interpret because values higher or lower than 1.0 indicate higher or lower risk than population average, respectively, regardless of the number of SNPs used for the calculation. Scores from PRS and sRAC are difficult for risk assessment because different values from different SNP sets are needed to indicate higher or lower risk. This limitation of PRS and sRAC also makes it difficult to compare multiple risk scores of the same individual from evolving SNP sets. Therefore, GRS is a better choice for risk assessment at an individual level risk assessment.
Important limitations of the study should be noted. First, considering that all subjects in the REDUCE study were men with initially negative biopsies, caution should be made when we attempt to generalize the current results to the general population. Second, the study was restricted to Caucasian men due to the composition of the REDUCE cohort and the fact that most PCa risk-associated SNPs were discovered and confirmed in this racial group. We hypothesize that major findings derived from Caucasians in the study can be replicated in other racial groups, though this needs to be further tested. Third, using 1.5 (or 2.0) as cutoff value of GRS might be subjective. As GRS provides population standardized risk assessment, GRS=1.5 (or 2.0) represents a 1.5-fold (or 2-fold) increase of risk while GRS=0.5 represents a 0.5-fold decrease of risk, comparing to average risk of PCa in general population. [27] These cutoffs were easier to understand and were comparable to FH. On the other hand, our results were consistent at both cutoffs (1.5 and 2.0), suggesting that our conclusions could be applied to a wider range. Fourth, the GRSs did not include rare mutations in high-penetrance genes. DNA sequencing analysis is required to detect these mutations, but was not performed in the REDUCE study. Future studies on assessment of inherited risk should include common PCa risk-associated SNPs, high-penetrance genes associated with PCa risk, as well as FH.
Conclusions
Risk assessment using currently available SNPs is justified when compared with FH. Multiple GRS values from evolving sets of SNPs provide a valuable tool for better refining risk.
Supplementary Material
Scatter plots of GRS values from two consecutive SNP sets in the treatment arm: (a) 17 vs. 34 SNPs, (b) 34 vs. 51 SNPs, and (c) 51 SNPs vs. 68 SNPs. Blue and red circle indicates patients with or without a diagnosis of PCa, respectively. Two dotted lines indicate cutoff value (1.5) to classify higher or lower risk.
Scatter plots of sRAC values from two consecutive SNP sets in the placebo arm: (a) 17 vs. 34 SNPs, (b) 34 vs. 51 SNPs, and (c) 51 SNPs vs. 68 SNPs. Blue and red circle indicates patients with or without a diagnosis of PCa, respectively.
Scatter plots of PRS values from two consecutive SNP sets in the placebo arm: (a) 17 vs. 34 SNPs, (b) 34 vs. 51 SNPs, and (c) 51 SNPs vs. 68 SNPs. Blue and red circle indicates patients with or without a diagnosis of PCa, respectively.
Acknowledgments
Funding
This work was partially supported by National Cancer Institute at the National Institutes of Health grant CA148463, National Key Basic Research Program Grant 973 of China (2012CB518301), and the Key Project of the National Natural Science Foundation of China (81130047), National Natural Science Foundation of China (81402339) and Shanghai Outstanding Reward for Youth Doctor (to Rong Na).
We thank the REDUCE team and all study subjects included in this study. We also thank the support of the Ellrodt-Schweighauser Family Chair of Cancer Genomic Research of NorthShore University HealthSystem to Xu.
List of abbreviations
- AUC
Area under the receiver operating characteristic curve
- FH
family history
- GRS
genetic risk scores
- GWAS
genome-wide association studies
- PCa
prostate cancer
- PRS
polygenic risk score
- REDUCE
the Reduction by Dutasteride of Prostate Cancer Events trial
- SNP
single nucleotide polymorphisms
- sRAC
simple risk allele count
Footnotes
Declarations
Ethics approval and consent to participate:
All patients provided informed consent, and the Institutional Review Boards at all participating institutions granted approval.
Consent for publication:
All patients provided informed consent for publication
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
Two patent applications were filed by Wake Forest School of Medicine related to the prostate cancer risk assessment using SNPs.
Authors’ contributions
HC contributed to data acquisition, statistical analysis and manuscript drafting; RN contributed to acquisition of data and manuscript drafting; VTP contributed to data acquisition; CAC contributed to manuscript drafting; DJ contributed to statistical analysis; ST and SLZ contributed to technical and material support; HY and XL contributed to data analysis and interpretation; Charles B. Brendler contributed to critical revision of the manuscript; BTH and JX contributed to study design and supervision.
References
- 1.Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni JF, Jr, Hoover R, Hunter DJ, Chanock SJ, Thomas G. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39(5):645–649. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- 2.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39(8):977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 3.Gudmundsson J, Sulem P, Rafnar T, Bergthorsson JT, Manolescu A, Gudbjartsson D, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Blondal T, Jakobsdottir M, Stacey SN, Kostic J, Kristinsson KT, Birgisdottir B, Ghosh S, Magnusdottir DN, Thorlacius S, Thorleifsson G, Zheng SL, Sun J, Chang BL, Elmore JB, Breyer JP, McReynolds KM, Bradley KM, Yaspan BL, Wiklund F, Stattin P, Lindstrom S, Adami HO, McDonnell SK, Schaid DJ, Cunningham JM, Wang L, Cerhan JR, St Sauver JL, Isaacs SD, Wiley KE, Partin AW, Walsh PC, Polo S, Ruiz-Echarri M, Navarrete S, Fuertes F, Saez B, Godino J, Weijerman PC, Swinkels DW, Aben KK, Witjes JA, Suarez BK, Helfand BT, Frigge ML, Kristjansson K, Ober C, Jonsson E, Einarsson GV, Xu J, Gronberg H, Smith JR, Thibodeau SN, Isaacs WB, Catalona WJ, Mayordomo JI, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet. 2008;40(3):281–283. doi: 10.1038/ng.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J, Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cussenot O, Valeri A, Andriole GL, Crawford ED, Tucker M, Gerhard DS, Fraumeni JF, Jr, Hoover R, Hayes RB, Hunter DJ, Chanock SJ. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40(3):310–315. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 5.Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, Field HI, Southey MC, Severi G, Donovan JL, Hamdy FC, Dearnaley DP, Muir KR, Smith C, Bagnato M, Ardern-Jones AT, Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Cox A, Lewis S, Brown PM, Jhavar SG, Tymrakiewicz M, Lophatananon A, Bryant SL, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Fisher C, Jamieson C, Cooper CS, English DR, Hopper JL, Neal DE, Easton DF Collaborators UKGPCS, British Association of Urological Surgeons’ Section of O, Collaborators UKPS. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40(3):316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 6.Yeager M, Chatterjee N, Ciampa J, Jacobs KB, Gonzalez-Bosquet J, Hayes RB, Kraft P, Wacholder S, Orr N, Berndt S, Yu K, Hutchinson A, Wang Z, Amundadottir L, Feigelson HS, Thun MJ, Diver WR, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Crawford ED, Haiman CA, Henderson B, Kolonel L, Le Marchand L, Siddiq A, Riboli E, Key TJ, Kaaks R, Isaacs W, Isaacs S, Wiley KE, Gronberg H, Wiklund F, Stattin P, Xu J, Zheng SL, Sun J, Vatten LJ, Hveem K, Kumle M, Tucker M, Gerhard DS, Hoover RN, Fraumeni JF, Jr, Hunter DJ, Thomas G, Chanock SJ. Identification of a new prostate cancer susceptibility locus on chromosome 8q24. Nat Genet. 2009;41(10):1055–1057. doi: 10.1038/ng.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, Muir K, Hopper JL, Henderson BE, Haiman CA, Schleutker J, Hamdy FC, Neal DE, Donovan JL, Stanford JL, Ostrander EA, Ingles SA, John EM, Thibodeau SN, Schaid D, Park JY, Spurdle A, Clements J, Dickinson JL, Maier C, Vogel W, Dork T, Rebbeck TR, Cooney KA, Cannon-Albright L, Chappuis PO, Hutter P, Zeegers M, Kaneva R, Zhang HW, Lu YJ, Foulkes WD, English DR, Leongamornlert DA, Tymrakiewicz M, Morrison J, Ardern-Jones AT, Hall AL, O’Brien LT, Wilkinson RA, Saunders EJ, Page EC, Sawyer EJ, Edwards SM, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As N, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper CS, Southey MC, Lophatananon A, Liu JF, Kolonel LN, Le Marchand L, Wahlfors T, Tammela TL, Auvinen A, Lewis SJ, Cox A, FitzGerald LM, Koopmeiners JS, Karyadi DM, Kwon EM, Stern MC, Corral R, Joshi AD, Shahabi A, McDonnell SK, Sellers TA, Pow-Sang J, Chambers S, Aitken J, Gardiner RA, Batra J, Kedda MA, Lose F, Polanowski A, Patterson B, Serth J, Meyer A, Luedeke M, Stefflova K, Ray AM, Lange EM, Farnham J, Khan H, Slavov C, Mitkova A, Cao G, Easton DF Oncology UKGPCSCBAoUSSo, Collaborators UKPS, Consortium P. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009;41(10):1116–1121. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudmundsson J, Sulem P, Gudbjartsson DF, Blondal T, Gylfason A, Agnarsson BA, Benediktsdottir KR, Magnusdottir DN, Orlygsdottir G, Jakobsdottir M, Stacey SN, Sigurdsson A, Wahlfors T, Tammela T, Breyer JP, McReynolds KM, Bradley KM, Saez B, Godino J, Navarrete S, Fuertes F, Murillo L, Polo E, Aben KK, van Oort IM, Suarez BK, Helfand BT, Kan D, Zanon C, Frigge ML, Kristjansson K, Gulcher JR, Einarsson GV, Jonsson E, Catalona WJ, Mayordomo JI, Kiemeney LA, Smith JR, Schleutker J, Barkardottir RB, Kong A, Thorsteinsdottir U, Rafnar T, Stefansson K. Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat Genet. 2009;41(10):1122–1126. doi: 10.1038/ng.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takata R, Akamatsu S, Kubo M, Takahashi A, Hosono N, Kawaguchi T, Tsunoda T, Inazawa J, Kamatani N, Ogawa O, Fujioka T, Nakamura Y, Nakagawa H. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet. 2010;42(9):751–754. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- 10.Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, Kittles RA, Rybicki BA, Isaacs WB, Ingles SA, Stanford JL, Diver WR, Witte JS, Hsing AW, Nemesure B, Rebbeck TR, Cooney KA, Xu J, Kibel AS, Hu JJ, John EM, Gueye SM, Watya S, Signorello LB, Hayes RB, Wang Z, Yeboah E, Tettey Y, Cai Q, Kolb S, Ostrander EA, Zeigler-Johnson C, Yamamura Y, Neslund-Dudas C, Haslag-Minoff J, Wu W, Thomas V, Allen GO, Murphy A, Chang BL, Zheng SL, Leske MC, Wu SY, Ray AM, Hennis AJ, Thun MJ, Carpten J, Casey G, Carter EN, Duarte ER, Xia LY, Sheng X, Wan P, Pooler LC, Cheng I, Monroe KR, Schumacher F, Le Marchand L, Kolonel LN, Chanock SJ, Van Den Berg D, Stram DO, Henderson BE. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet. 2011;43(6):570–573. doi: 10.1038/ng.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kote-Jarai Z, Olama AA, Giles GG, Severi G, Schleutker J, Weischer M, Campa D, Riboli E, Key T, Gronberg H, Hunter DJ, Kraft P, Thun MJ, Ingles S, Chanock S, Albanes D, Hayes RB, Neal DE, Hamdy FC, Donovan JL, Pharoah P, Schumacher F, Henderson BE, Stanford JL, Ostrander EA, Sorensen KD, Dork T, Andriole G, Dickinson JL, Cybulski C, Lubinski J, Spurdle A, Clements JA, Chambers S, Aitken J, Gardiner RA, Thibodeau SN, Schaid D, John EM, Maier C, Vogel W, Cooney KA, Park JY, Cannon-Albright L, Brenner H, Habuchi T, Zhang HW, Lu YJ, Kaneva R, Muir K, Benlloch S, Leongamornlert DA, Saunders EJ, Tymrakiewicz M, Mahmud N, Guy M, O’Brien LT, Wilkinson RA, Hall AL, Sawyer EJ, Dadaev T, Morrison J, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As N, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper CS, Lophatonanon A, Southey MC, Hopper JL, English DR, Wahlfors T, Tammela TL, Klarskov P, Nordestgaard BG, Roder MA, Tybjaerg-Hansen A, Bojesen SE, Travis R, Canzian F, Kaaks R, Wiklund F, Aly M, Lindstrom S, Diver WR, Gapstur S, Stern MC, Corral R, Virtamo J, Cox A, Haiman CA, Le Marchand L, Fitzgerald L, Kolb S, Kwon EM, Karyadi DM, Orntoft TF, Borre M, Meyer A, Serth J, Yeager M, Berndt SI, Marthick JR, Patterson B, Wokolorczyk D, Batra J, Lose F, McDonnell SK, Joshi AD, Shahabi A, Rinckleb AE, Ray A, Sellers TA, Lin HY, Stephenson RA, Farnham J, Muller H, Rothenbacher D, Tsuchiya N, Narita S, Cao GW, Slavov C, Mitev V, Easton DF, Eeles RA Oncology UKGPCSCBAoUSSo, Uk ProtecT Study Collaborators TAPCB, Consortium P. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet. 2011;43(8):785–791. doi: 10.1038/ng.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akamatsu S, Takata R, Haiman CA, Takahashi A, Inoue T, Kubo M, Furihata M, Kamatani N, Inazawa J, Chen GK, Le Marchand L, Kolonel LN, Katoh T, Yamano Y, Yamakado M, Takahashi H, Yamada H, Egawa S, Fujioka T, Henderson BE, Habuchi T, Ogawa O, Nakamura Y, Nakagawa H. Common variants at 11q12, 10q26 and 3p11.2 are associated with prostate cancer susceptibility in Japanese. Nat Genet. 2012;44(4):426–429. S421. doi: 10.1038/ng.1104. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Mo Z, Ye D, Wang M, Liu F, Jin G, Xu C, Wang X, Shao Q, Chen Z, Tao Z, Qi J, Zhou F, Wang Z, Fu Y, He D, Wei Q, Guo J, Wu D, Gao X, Yuan J, Wang G, Xu Y, Wang G, Yao H, Dong P, Jiao Y, Shen M, Yang J, Ou-Yang J, Jiang H, Zhu Y, Ren S, Zhang Z, Yin C, Gao X, Dai B, Hu Z, Yang Y, Wu Q, Chen H, Peng P, Zheng Y, Zheng X, Xiang Y, Long J, Gong J, Na R, Lin X, Yu H, Wang Z, Tao S, Feng J, Sun J, Liu W, Hsing A, Rao J, Ding Q, Wiklund F, Gronberg H, Shu XO, Zheng W, Shen H, Jin L, Shi R, Lu D, Zhang X, Sun J, Zheng SL, Sun Y. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat Genet. 2012;44(11):1231–1235. doi: 10.1038/ng.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, Dadaev T, Neal DE, Hamdy FC, Donovan JL, Muir K, Giles GG, Severi G, Wiklund F, Gronberg H, Haiman CA, Schumacher F, Henderson BE, Le Marchand L, Lindstrom S, Kraft P, Hunter DJ, Gapstur S, Chanock SJ, Berndt SI, Albanes D, Andriole G, Schleutker J, Weischer M, Canzian F, Riboli E, Key TJ, Travis RC, Campa D, Ingles SA, John EM, Hayes RB, Pharoah PD, Pashayan N, Khaw KT, Stanford JL, Ostrander EA, Signorello LB, Thibodeau SN, Schaid D, Maier C, Vogel W, Kibel AS, Cybulski C, Lubinski J, Cannon-Albright L, Brenner H, Park JY, Kaneva R, Batra J, Spurdle AB, Clements JA, Teixeira MR, Dicks E, Lee A, Dunning AM, Baynes C, Conroy D, Maranian MJ, Ahmed S, Govindasami K, Guy M, Wilkinson RA, Sawyer EJ, Morgan A, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As NJ, Woodhouse CJ, Thompson A, Dudderidge T, Ogden C, Cooper CS, Lophatananon A, Cox A, Southey MC, Hopper JL, English DR, Aly M, Adolfsson J, Xu J, Zheng SL, Yeager M, Kaaks R, Diver WR, Gaudet MM, Stern MC, Corral R, Joshi AD, Shahabi A, Wahlfors T, Tammela TL, Auvinen A, Virtamo J, Klarskov P, Nordestgaard BG, Roder MA, Nielsen SF, Bojesen SE, Siddiq A, Fitzgerald LM, Kolb S, Kwon EM, Karyadi DM, Blot WJ, Zheng W, Cai Q, McDonnell SK, Rinckleb AE, Drake B, Colditz G, Wokolorczyk D, Stephenson RA, Teerlink C, Muller H, Rothenbacher D, Sellers TA, Lin HY, Slavov C, Mitev V, Lose F, Srinivasan S, Maia S, Paulo P, Lange E, Cooney KA, Antoniou AC, Vincent D, Bacot F, Tessier DC, Kote-Jarai Z, Easton DF Initiative CO-CRUG-E, Australian Prostate Cancer B, Oncology UKGPCSCBAoUSSo, Collaborators UKPS, Consortium P. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45(4):385–391. 391e381–382. doi: 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, Benlloch S, Hazelett DJ, Wang Z, Saunders E, Leongamornlert D, Lindstrom S, Jugurnauth-Little S, Dadaev T, Tymrakiewicz M, Stram DO, Rand K, Wan P, Stram A, Sheng X, Pooler LC, Park K, Xia L, Tyrer J, Kolonel LN, Le Marchand L, Hoover RN, Machiela MJ, Yeager M, Burdette L, Chung CC, Hutchinson A, Yu K, Goh C, Ahmed M, Govindasami K, Guy M, Tammela TL, Auvinen A, Wahlfors T, Schleutker J, Visakorpi T, Leinonen KA, Xu J, Aly M, Donovan J, Travis RC, Key TJ, Siddiq A, Canzian F, Khaw KT, Takahashi A, Kubo M, Pharoah P, Pashayan N, Weischer M, Nordestgaard BG, Nielsen SF, Klarskov P, Roder MA, Iversen P, Thibodeau SN, McDonnell SK, Schaid DJ, Stanford JL, Kolb S, Holt S, Knudsen B, Coll AH, Gapstur SM, Diver WR, Stevens VL, Maier C, Luedeke M, Herkommer K, Rinckleb AE, Strom SS, Pettaway C, Yeboah ED, Tettey Y, Biritwum RB, Adjei AA, Tay E, Truelove A, Niwa S, Chokkalingam AP, Cannon-Albright L, Cybulski C, Wokolorczyk D, Kluzniak W, Park J, Sellers T, Lin HY, Isaacs WB, Partin AW, Brenner H, Dieffenbach AK, Stegmaier C, Chen C, Giovannucci EL, Ma J, Stampfer M, Penney KL, Mucci L, John EM, Ingles SA, Kittles RA, Murphy AB, Pandha H, Michael A, Kierzek AM, Blot W, Signorello LB, Zheng W, Albanes D, Virtamo J, Weinstein S, Nemesure B, Carpten J, Leske C, Wu SY, Hennis A, Kibel AS, Rybicki BA, Neslund-Dudas C, Hsing AW, Chu L, Goodman PJ, Klein EA, Zheng SL, Batra J, Clements J, Spurdle A, Teixeira MR, Paulo P, Maia S, Slavov C, Kaneva R, Mitev V, Witte JS, Casey G, Gillanders EM, Seminara D, Riboli E, Hamdy FC, Coetzee GA, Li Q, Freedman ML, Hunter DJ, Muir K, Gronberg H, Neal DE, Southey M, Giles GG, Severi G, Cook MB, Nakagawa H, Wiklund F, Kraft P, Chanock SJ, Henderson BE, Easton DF, Eeles RA, Haiman CA Breast, Prostate Cancer Cohort C, Consortium P, Consortium C, Consortium G-OE. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46(10):1103–1109. doi: 10.1038/ng.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann TJ, Van Den Eeden SK, Sakoda LC, Jorgenson E, Habel LA, Graff RE, Passarelli MN, Cario CL, Emami NC, Chao CR, Ghai NR, Shan J, Ranatunga DK, Quesenberry CP, Aaronson D, Presti J, Wang Z, Berndt SI, Chanock SJ, McDonnell SK, French AJ, Schaid DJ, Thibodeau SN, Li Q, Freedman ML, Penney KL, Mucci LA, Haiman CA, Henderson BE, Seminara D, Kvale MN, Kwok PY, Schaefer C, Risch N, Witte JS. A large multiethnic genome-wide association study of prostate cancer identifies novel risk variants and substantial ethnic differences. Cancer Discov. 2015;5(8):878–891. doi: 10.1158/2159-8290.CD-15-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng SL, Sun J, Wiklund F, Smith S, Stattin P, Li G, Adami HO, Hsu FC, Zhu Y, Balter K, Kader AK, Turner AR, Liu W, Bleecker ER, Meyers DA, Duggan D, Carpten JD, Chang BL, Isaacs WB, Xu J, Gronberg H. Cumulative association of five genetic variants with prostate cancer. N Engl J Med. 2008;358(9):910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 18.Kader AK, Sun J, Reck BH, Newcombe PJ, Kim ST, Hsu FC, D’Agostino RB, Jr, Tao S, Zhang Z, Turner AR, Platek GT, Spraggs CF, Whittaker JC, Lane BR, Isaacs WB, Meyers DA, Bleecker ER, Torti FM, Trent JM, McConnell JD, Zheng SL, Condreay LD, Rittmaster RS, Xu J. Potential impact of adding genetic markers to clinical parameters in predicting prostate biopsy outcomes in men following an initial negative biopsy: findings from the REDUCE trial. Eur Urol. 2012;62(6):953–961. doi: 10.1016/j.eururo.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conran CA, Brendler CB, Xu J. Personalized prostate cancer care: From screening to treatment. Asian J Androl. 2016 doi: 10.4103/1008-682X.179529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krier J, Barfield R, Green RC, Kraft P. Reclassification of genetic-based risk predictions as GWAS data accumulate. Genome Med. 2016;8(1):20. doi: 10.1186/s13073-016-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS, Group RS. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 22.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39(7):906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 24.Szulkin R, Whitington T, Eklund M, Aly M, Eeles RA, Easton D, Kote-Jarai ZS, Amin Al Olama A, Benlloch S, Muir K, Giles GG, Southey MC, Fitzgerald LM, Henderson BE, Schumacher F, Haiman CA, Schleutker J, Wahlfors T, Tammela TL, Nordestgaard BG, Key TJ, Travis RC, Neal DE, Donovan JL, Hamdy FC, Pharoah P, Pashayan N, Khaw KT, Stanford JL, Thibodeau SN, McDonnell SK, Schaid DJ, Maier C, Vogel W, Luedeke M, Herkommer K, Kibel AS, Cybulski C, Lubinski J, Kluzniak W, Cannon-Albright L, Brenner H, Butterbach K, Stegmaier C, Park JY, Sellers T, Lin HY, Slavov C, Kaneva R, Mitev V, Batra J, Clements JA, Spurdle A, Teixeira MR, Paulo P, Maia S, Pandha H, Michael A, Kierzek A, Practical C, Gronberg H, Wiklund F Australian Prostate Cancer B. Prediction of individual genetic risk to prostate cancer using a polygenic score. Prostate. 2015;75(13):1467–1474. doi: 10.1002/pros.23037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin Al Olama A, Benlloch S, Antoniou AC, Giles GG, Severi G, Neal DE, Hamdy FC, Donovan JL, Muir K, Schleutker J, Henderson BE, Haiman CA, Schumacher FR, Pashayan N, Pharoah PD, Ostrander EA, Stanford JL, Batra J, Clements JA, Chambers SK, Weischer M, Nordestgaard BG, Ingles SA, Sorensen KD, Orntoft TF, Park JY, Cybulski C, Maier C, Doerk T, Dickinson JL, Cannon-Albright L, Brenner H, Rebbeck TR, Zeigler-Johnson C, Habuchi T, Thibodeau SN, Cooney KA, Chappuis PO, Hutter P, Kaneva RP, Foulkes WD, Zeegers MP, Lu YJ, Zhang HW, Stephenson R, Cox A, Southey MC, Spurdle AB, FitzGerald L, Leongamornlert D, Saunders E, Tymrakiewicz M, Guy M, Dadaev T, Little SJ, Govindasami K, Sawyer E, Wilkinson R, Herkommer K, Hopper JL, Lophatonanon A, Rinckleb AE, Kote-Jarai Z, Eeles RA, Easton DF Oncology UKGPCSCBAoUSSo, Collaborators UKPS, Consortium P. Risk Analysis of Prostate Cancer in PRACTICAL, a Multinational Consortium, Using 25 Known Prostate Cancer Susceptibility Loci. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1121–1129. doi: 10.1158/1055-9965.EPI-14-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pashayan N, Duffy SW, Neal DE, Hamdy FC, Donovan JL, Martin RM, Harrington P, Benlloch S, Amin Al Olama A, Shah M, Kote-Jarai Z, Easton DF, Eeles R, Pharoah PD. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med. 2015;17(10):789–795. doi: 10.1038/gim.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conran CA, Na R, Chen H, Jiang D, Lin X, Zheng SL, Brendler CB, Xu J. Population-standardized genetic risk score: the SNP-based method of choice for inherited risk assessment of prostate cancer. Asian J Androl. 2016 doi: 10.4103/1008-682X.179527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plots of GRS values from two consecutive SNP sets in the treatment arm: (a) 17 vs. 34 SNPs, (b) 34 vs. 51 SNPs, and (c) 51 SNPs vs. 68 SNPs. Blue and red circle indicates patients with or without a diagnosis of PCa, respectively. Two dotted lines indicate cutoff value (1.5) to classify higher or lower risk.
Scatter plots of sRAC values from two consecutive SNP sets in the placebo arm: (a) 17 vs. 34 SNPs, (b) 34 vs. 51 SNPs, and (c) 51 SNPs vs. 68 SNPs. Blue and red circle indicates patients with or without a diagnosis of PCa, respectively.
Scatter plots of PRS values from two consecutive SNP sets in the placebo arm: (a) 17 vs. 34 SNPs, (b) 34 vs. 51 SNPs, and (c) 51 SNPs vs. 68 SNPs. Blue and red circle indicates patients with or without a diagnosis of PCa, respectively.