Abstract
The Intensive Diet and Exercise for Arthritis (IDEA) trial was an 18-month randomized controlled trial that enrolled 454 overweight and obese older adults with symptomatic and radiographic knee osteoarthritis (OA). Participants were randomized to either exercise (E), intensive diet-induced weight loss (D), or intensive diet-induced weight loss plus exercise (D + E) interventions. We previously reported that the clinical benefits of D + E were significantly greater than with either intervention alone (e.g., greater pain reduction, and better function, mobility, and health-related quality of life). We now test the hypothesis that D + E has greater overall benefit on gait mechanics compared to either intervention alone. Knee joint loading was analyzed using inverse dynamics and musculoskeletal modeling. Analysis of covariance determined the interventions’ effects on gait. The D + E group walked significantly faster at 18-month follow-up (1.35 m s−1) than E (1.29 m s−1, p = 0.0004) and D (1.31 m s−1, p = 0.0007). Tibiofemoral compressive impulse was significantly lower (p = 0.0007) in D (1069 N s) and D + E (1054 N s) compared to E (1130 N s). D had significantly lower peak hip external rotation moment (p = 0.01), hip abduction moment (p = 0.0003), and peak hip power production (p = 0.016) compared with E. Peak ankle plantar flexion moment was significantly less (p < 0.0001) in the two diet groups compared with E. There also was a significant dose-response to weight loss; participants that lost >10% of baseline body weight had significantly (p = 0.0001) lower resultant knee forces and lower muscle (quadriceps, hamstring, and gastrocnemius) forces than participants that had less weight loss. Compared to E, D produces significant load reductions at the hip, knee, and ankle; combining D with E attenuates these reductions, but most remain significantly better than with E alone.
Keywords: Gait, Clinical trial, Diet, Exercise, Osteoarthritis, Knee
1. Introduction
In a classic review, Griffin and Guilak (2005) opined how knee osteoarthritis (OA) develops from a complex interaction of biomechanical and inflammatory disease pathways. Obesity was common to both pathways, resulting in increased mechanical joint stress and the release of proinflammatory cytokines and adipokines. The Intensive Diet and Exercise for Arthritis (IDEA) trial demonstrated that 18-months of intensive dietary weight loss, with or without exercise, affected both pathways, with the combined group exhibiting superior clinical outcomes (Messier et al., 2013a). Weight loss also affected the mechanical pathway by reducing peak knee compressive loads by more than 200 N per step relative to the exercise comparator group, even as preferred walking speed increased. This empirical evidence supported earlier studies that showed two-to-four times the reduction in peak knee compressive forces for each kg of weight loss (Aaboe et al., 2011; DeVita et al., 2016; Messier et al., 2005a). Aaboe et al. (2011) also investigated the biomechanical response to 16 weeks of intensive weight loss (mean weight loss 13.5%) in obese knee OA patients and found significantly lower (7%) knee compressive forces and internal knee abduction moments (12%). The lack of a control group and the short intervention period were limiting factors.
Weight loss outcomes in knee OA patients, however, are equivocal. Henriksen et al. (2013) found that a subset of knee OA patients actually increased joint loading after substantial weight loss, presumably due to increased walking speed and improved knee function. In contrast, others have shown significant reductions in weight leads to lower knee joint loads and faster walking speeds (DeVita et al., 2016; Messier et al., 2018). We seek to clarify the biomechanical locomotion responses to weight loss with this study, the purpose which was to examine the long-term effect of intensive dietary weight loss, with or without exercise, on hip, knee, and ankle spatiotemporal, kinematic, and kinetic variables. We hypothesized that intensive dietary weight loss combined with exercise will have the greatest overall benefit on gait mechanics in older, overweight and obese adults with knee OA compared to either intervention alone.
2. Methods
2.1. Study oversight
IDEA was conducted from July 2006 to June 2011 at Wake Forest University and the Wake Forest School of Medicine. The study was approved by the Human Subjects Committee of Wake Forest University Health Sciences and was monitored by an independent safety officer. Informed consent was obtained from all study participants.
2.2. Study design
IDEA was an assessor-blinded, single-center, 18-month, randomized controlled trial. Participants were randomized into 1 of 3 groups: exercise-only control (E), intensive diet-induced weight loss (D), or intensive diet-induced weight loss plus exercise (D + E). Personnel responsible for data collection were blinded to group assignment. A detailed description of the trial design and rationale is reported elsewhere (Messier et al., 2009).
2.3. Study sample
The study sample included 454 ambulatory, community-dwelling persons age ≥55 years with: (1) Kellgren-Lawrence (KL) grade 2 or 3 (mild to moderate) radiographic tibiofemoral OA or tibiofemoral plus patellofemoral OA of one or both knees; (2) 27.0 kg m−2 ≤ BMI ≤ 41 kg m−2; and (3) a sedentary lifestyle, defined as less than 30 min per week of formal exercise within the past 6 months. All participants maintained their usual medications including non-steroidal anti-inflammatory drugs (NSAIDs) and other analgesics. With their physician’s consent, they could adjust or discontinue medications as needed. Presented elsewhere are details of the eligibility, screening measurements, and recruitment techniques (Messier et al., 2009). Community participants were recruited between November 2006 and December 2009. A stratified-block randomization method was used to assign all eligible persons to 1 of 3 intervention arms, stratified by BMI (27–29.9, 30–34.9, 35–41 kg m−2) and gender.
2.4. Interventions
2.4.1. Intensive weight loss
Both the D + E and D groups received identical dietary interventions developed within a social cognitive framework. The weight loss goal for both was a group mean of at least 10% of baseline weight, with a desired range between 10% and 15%. The dietary plan was based on partial meal replacements, including up to 2 meal-replacement shakes per day (Lean Shake®, provided by General Nutrition Centers, Inc., Pittsburgh, PA.). All participants daily caloric intake was adjusted according to their rate of weight change between intervention visits.
2.4.2. Exercise
Both the D + E and E groups received identical exercise interventions developed within a social cognitive framework. Sixty-minute sessions were conducted 3 d wk−1 for 18 months. The 3 d wk−1 program consisted of aerobic walking (15 min), strength training (20 min), a second aerobic phase (15 min), and cool-down (10 min).
2.5. Gait analysis
A 37-reflective marker set, using the Cleveland Clinic full-body configuration, and a 6-camera motion analysis system (Motion Analysis Corporation, Santa Rosa, CA) set to sample data at 60 Hz were used to collect 3-D kinematics at baseline, and follow-up tests at 6 (FU6) and 18-months (FU18). The data were collected, tracked, edited, and smoothed, using EVaRT 4.4 software, and a Butterworth low-pass filter with a cut-off frequency of 6 Hz. The processed data were compiled using OrthoTrak 6.0 β4 clinical gait analysis software.
A 6-channel force platform (Advanced Mechanical Technologies, Inc., Newton, MA) was integrated with the motion capture system for simultaneous kinetic data collection at 480 Hz. Six successful trials in which the participant walked within ± 3.5% of freely chosen speed and placed the entire foot on the force platform in a visually normal stride were collected on each participant; three were averaged to yield representative values. Relevant temporospatial and kinematic variables included walking velocity, stride length, stride rate, hip and knee flexion/extension, internal/external rotation and abduction/adduction, and ankle dorsiflexion/plantar flexion range of motion. The smoothed coordinate data, ground reaction, and gravitational and inertial forces informed an inverse dynamics model to calculate 3-D hip, knee, and ankle internal flexion/extension, abduction/adduction, internal rotation, and external rotation moments, powers, and impulses.
We used the musculoskeletal model developed by DeVita and Hortobagyi (DeVita and Hortobagyi, 2001) to calculate tibiofemoral compressive and AP shear forces and patellofemoral compressive force, as well as quadriceps, hamstrings and gastrocnemius muscle forces. The integral of each force-time curve determined force impulse. This torque-driven musculoskeletal model encompassed two basic components. The first involved calculating joint moments and reaction forces from kinematic, physiological, and force-plate data. The second used the positional kinematics, joint moments and joint reaction forces to calculate individual muscle forces and compressive and shear forces. We have used our model extensively to estimate knee-joint biomechanics (Messier et al., 2005a, 2011, 2005b, 2013a); it has been explained along with its limitations previously (Messier et al., 2011). Briefly, the principal limitation to our model is the absence of most knee ligaments. This will increase knee-muscle force predictions, since these tissues resist external loads. The model does include the lateral collateral ligament, which produces the principal non-muscular restraint during the stance phase. A second limitation was the assumption of no co-contraction by the hip flexors. This assumption may have introduced error due to missing force production in the rectus femoris in early stance when the hamstrings were active and produced force. Finally, our model determines the muscle forces of the quadriceps, hamstrings, and gastrocnemius; it does not partition these muscles into their components. Our predictions for knee muscle and joint forces compare favorably to those of other predictive models (Schipplein and Andriacchi, 1991; Winby et al., 2009) and are similar to measured forces from an instrumented knee joint prosthesis (Fregly et al., 2012; Messier et al., 2013b).
2.6. Statistical analysis
Analyses conducted with SAS v9.2 (SAS Institute, Cary, NC) included all participants in their randomly assigned groups regardless of subsequent withdrawal from the treatment or deviation from the protocol (i.e., intention-to-treat analysis). Analyses of variance and chi-square tests addressed differences in baseline characteristics among groups. Repeated measures analyses of covariance (ANCOVA) adjusted for IDEA stratification factors, baseline BMI, gender, and baseline values determined the interventions’ effects on gait kinematics. Analysis of kinetic data also included 18-month gait speed as a co-variate.
IDEA was a randomized clinical trial, hence the randomization effect removed the need for consideration of factors that may have differed across individuals at baseline such as mass and height because randomization leads to balanced groups (Evans, 2010; Zarin et al., 2019), especially with samples as large as presently used. Any differences between groups following the trial period can be attributed solely to the treatment effect (i.e., exercise and weight loss). Follow-up kinetic data were not normalized to body mass because normalization conceptually scales each participant to a “standardized” body size so that modification of a participant’s body mass due to the intervention is lost. Our adjustment of baseline values of the outcome, baseline BMI, and gender further helped guard against potential follow-up imbalances caused by differential study attrition.
All follow-up data and intervention effects were estimated at each follow-up visit. Month × intervention interaction tests determined consistency over time. When the interactions were not significant, the average effect estimates over time were tested and presented. The overall P value for the two primary outcomes (IL-6 and tibiofemoral compressive force) that were reported elsewhere was ≤ 0.025 with specific pairwise differences adjusted for 6 comparisons (p ≤ 0.008) (Messier et al., 2009, 2013a). Tibiofemoral compressive force data are restated here for completeness. When the overall FU18 p value for secondary outcomes (including all biomechanical variables with the exception of tibiofemoral compressive force) was ≤ 0.05, we calculated specific pairwise differences with the significance level adjusted for multiple comparisons (p ≤ 0.0167). Percent differences for each group were calculated as (FU18-Baseline)/Baseline.
Repeated measures ANCOVA, controlling for BMI, gender, baseline values, and group assignment assessed the dose-response relationship between weight-change category (<5%, 5–9.9%, ≥10%) and knee joint variables.
3. Results
Descriptive (mean ± SD) baseline characteristics of the study participants were age, 66 ± 6 yrs.; 72% female; weight, 93 kg; BMI, 33.6 ± 3.7 kg m−2; and KL grade, 2.56 ± 0.59. These values were similar across groups (p greater than 0.05). Additional descriptive data and a CONSORT diagram are presented elsewhere (Messier et al., 2013a). Of the 454 participants, 399 (88%) completed the study (returned for FU18). Those who did not return were not significantly different from the others at baseline in terms of age, sex, race, number of comorbidities, initial radiographic score, knee pain, or physical function. Retention did not differ significantly among the groups (E, 89%; D, 85%; D + E, 89%).
As previously reported (Messier et al., 2013a) both diet groups lost significantly (p < 0.0001) more weight relative to the E group. The D group lost an average of 9.5% (8.9 kg), the D + E group lost11.4% (10.6 kg), and the E group lost 2.2% (1.8 kg) of their baseline body weight (Messier et al., 2013a). P values for significant pairwise comparisons (i.e., p ≤ 0.0167) are noted in the succeeding sections of the results.
3.1. Spatiotemporal and kinematics
At FU18 the D + E group walked significantly faster than the D (p = 0.0007) or E (p = 0.0004) groups. D + E also had a significantly (p = 0.0001) longer stride length than E and a faster cadence than D (p = 0.008) (Table 1). The within-group changes were 6–11% for gait speed, 3–6% for stride length, and 1–3% for cadence.
Table 1.
Intervention effects on spatiotemporal and kinematic gait data. Mean (95% CI) values adjusted for baseline values, baseline BMI, and gender.
| Overall Baseline | Exercise | Diet | Diet + Exercise | |||||
|---|---|---|---|---|---|---|---|---|
| FU6 | FU18 | FU6 | FU18 | FU6 | FU18 | #P | ||
| Stride Length (cm) | 129.8 (128.3, 131.4) |
136.1 (134.6, 137.6) |
134.7 (133.2, 136.3) |
135.6 (134.0, 137.1) |
136.9 (135.3, 138.4) |
138.8 (137.3, 140.3) |
138.9 (137.3, 140.4) |
0.001† |
| Cadence (steps min−1) | 111.0 (110.2, 111.9) |
114.6 (113.6, 115.7) |
113.4 (112.3, 114.3) |
111.5 (110.4, 112.6) |
112.3 (111.2, 113.4) |
114.7 (113.6, 115.7) |
114.3 (113.3, 115.4) |
0.03!! |
| Gait speed (m s−1) | 1.20 (1.19, 1.22) |
1.32(1.29, 1.34) | 1.29 (1.27, 1.32) |
1.28 (1.26, 1.30) |
1.31 (1.28, 1.33) |
1.34 (1.32, 1.36) |
1.35 (1.33, 1.37) |
0.001* |
| Knee Flexion (deg) | 16.5 (15.9, 17.2) |
18.9 (17.9, 19.9) |
16.7 (15.7, 17.8) |
16.3 (15.3, 17.4) |
15.8 (14.8, 16.8) |
17.7 (16.8, 18.7) |
16.4 (15.4, 17.4) |
0.41 |
| Varus Thrust (deg) | 4.3 (4.2, 4.5) |
4.3 (4.0, 4.6) |
4.0 (3.6, 4.3) |
4.1 (3.8, 4.4) |
4.1 (3.8, 4.4) |
4.1 (3.8, 4.4) |
4.0 (3.7, 4.3) |
0.85 |
| Hip ROM (deg) | 42.0 (41.5, 42.6) |
447 (43.9, 45.4) |
44.0 (43.2, 44.7) |
43.4 (42.6, 44.2) |
44.1 (43.4, 44.9) |
44.7 (44.0, 45.4) |
45.1 (44.4, 45.9) |
0.07 |
| Hip adduction (deg) | 7.9 (7.5, 8.3) |
7.0 (6.4, 7.6) |
7.1 (6.5, 7.8) |
6.8 (6.1, 7.4) |
7.2 (6.6, 7.8) |
6.3 (5.6, 6.9) |
7.0 (6.4, 7.7) |
0.92 |
| Hip rotation ROM (deg) | 4.3 (4.1, 4.6) |
4.1 (3.7, 4.6) |
4.1 (3.6, 4.5) |
4.1 (3.6, 4.5) |
4.1 (3.7, 4.5) |
4.7 (4.3, 5.1) |
4.7 (4.3, 5.2) |
0.05 |
| Ankle ROM (deg) | 22.8 (22.5, 23.2) |
22.0 (21.5, 22.4) |
22.1 (21.7, 22.6) |
21.9 (21.4, 22.3) |
21.8 (21.4, 22.3) |
21.5 (21.1, 22.0) |
21.6 (21.2, 22.1) |
0.25 |
P adjusted for baseline values, baseline BMI, and gender. Significant (P ≤ 0.0167) pairwise comparisons.
D + E > D, E.
D + E > E.
D + E > D.
Pairwise comparisons revealed no statistically significant sagittal or transverse plane kinematic between group differences at FU18 (Table 1). Analysis of frontal plane knee kinematics revealed varus thrust was not significantly different between the groups at FU18 (Table 1).
3.2. Kinetics
Since the intent of the interventions was to improve mobility (i.e., gait speed), co-variates were limited to baseline values, baseline BMI, and gender. We also present significance values that accounted for differences in gait speed at FU18.
Peak vertical ground reaction forces were greater in E than in D (p < 0.0001) and D + E (p < 0.0001), with and without adjusting for gait speed (Table 2). Loading rate (N s−1), however, was not significantly different between the groups (p = 0.30). Peak anterior braking ground reaction forces were less in D + E than E (p = 0.003). After adjusting for gait speed, both D and D + E had lower values than E (p ≤ 0.009).
Table 2.
Mean (95%CI) intervention effects on kinetic data.
| Overall Baseline | Exercise | Diet | Diet + Exercise | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FU6 | FU18 | FU6 | FU18 | FU6 | FU18 | #P | +P | ||
| Peak Vertical Impact Peak (N) | 971 (956, 986) |
1005 (990, 1019) |
991 (977, 1006) |
921 (906, 935) |
921 (906, 935) |
931 (917, 945) |
921 (906, 935) |
<0.0001$ | <0.0001$ |
| Peak Braking AP Force (N) | 151 (147, 155) |
167 (161, 172) |
161 (156, 167) |
151 (145, 156) |
152 (146, 158) |
158 (153, 163) |
149 (144, 155) |
0.007£ | <0.009$ |
| Peak Propelling AP Force (N) | −142 (−146, −138) |
−162 (−168, −157) |
−157 (−163, −152) |
−147 (−153, −141) |
−150 (−156, −144) |
−157 (−162, −151) |
−151 (−157, −145) |
0.13 | 0.003§ |
| Loading Rate (N s−1) | 4322 (4136, 4508) |
5377 (5088, 5667) |
5109 (4810, 5408) |
4798 (4502, 5095) |
4804 (4504, 5105) |
5050 (4766, 5335) |
5051 (4759, 5344) |
0.30 | 0.27 |
| TF Compressive Force (N) | 2683 (2597, 2770) |
3020 (2918, 3123) |
2727 (2621, 2833) |
2607 (2501, 2713) |
2525 (2417, 2633) |
2755 (2654, 2856) |
2589 (2484, 2694) |
0.02‡ | 0.01$ |
| Resultant Knee Force (N) | 2712 (2625, 2799) |
3051 (2949, 3154) |
2759 (2653, 2865) |
2637 (2531, 2742) |
2555 (2547, 2763) |
2787 (2686, 2889) |
2621 (2516, 2726) |
0.02‡ | 0.01‡ |
| TF Shear Force (N) | 397 (383, 410) |
447 (428, 466) |
435 (416, 455) |
396 (377, 416) |
406 (387, 426) |
432 (414, 451) |
419 (400, 438) |
0.11 | 0.09 |
| PF Comp Force (N) | 431 (398, 464) |
556 (514, 598) |
467 (424, 511) |
427 (384, 471) |
398 (354, 443) |
484 (442, 526) |
422 (379, 466) |
0.07 | 0.08 |
| TF Compressive Impulse (Ns) | 1185 (1154, 1216) |
1191 (1161, 1220) | 1130 (1099, 1160) |
1099 (1068, 1130) |
1069 (1037, 1100) |
1094 (1064, 1123) |
1054 (1024, 1084) |
0.0007$ | 0.002$ |
| Quadriceps Force (N) | 1246 (1189, 1303) |
1479 (1412, 1547) |
1334 (1264, 1403) |
1265 (1195, 1335) |
1244 (1173, 1315) |
1374 (1307, 1440) |
1279 (1210, 1348) |
0.18 | 0.19 |
| Hamstring Force (N) | 700 (668, 732) |
831 (787, 875) |
718 (673, 764) |
675 (630, 721) |
624 (577, 670) |
719 (676, 763) |
682 (637, 727) |
0.01$ | 0.007‡ |
| Gastrocnemius Force (N) | 707 (692, 721) |
729 (716, 742) |
711 (698, 725) |
670 (656, 684) |
674 (661, 688) |
678 (665, 691) |
669 (656, 683) |
<0.0001$ | <0.0001$ |
D + E > D, E.
P adjusted for baseline values, baseline BMI, and gender.
P adjusted for baseline values, baseline BMI, gender and follow-up gait speed. Mean values not shown Significant (P 0.0167) pairwise comparisons.
E > D, D + E.
D < E.
D + E > E.
E > D + E.
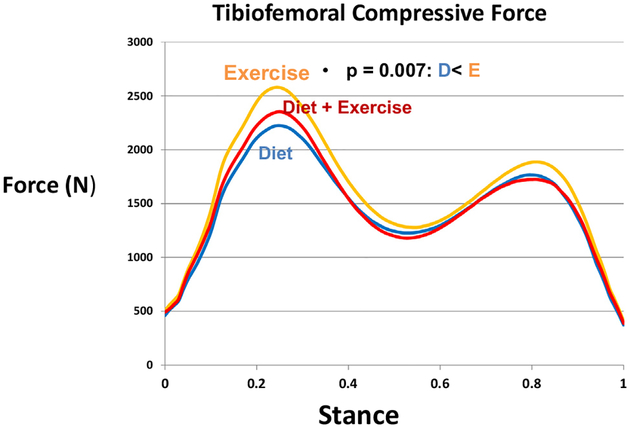
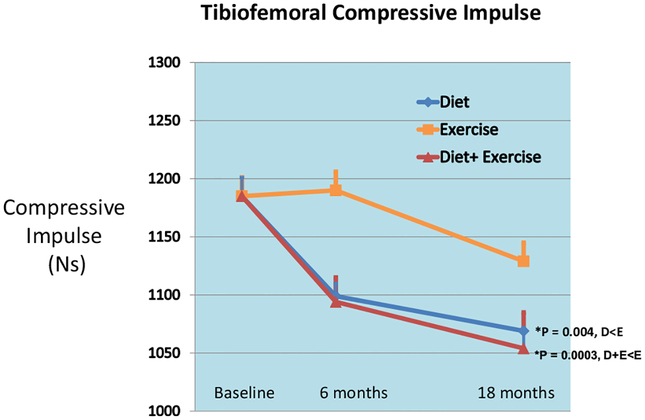
Peak bone-on-bone tibiofemoral compressive force was significantly less in D (p = 0.007) compared to E. When adjusted for gait speed at FU18, this force remained significantly less in D (p = 0.007); D + E was not significant (p = 0.0175) relative to E. Tibiofemoral compressive impulse, a marker of total knee compressive load per step, was significantly different among the groups (p = 0.0007); D (p = 0.004) and D + E (p = 0.0003) were significantly less than E (Table 2, Figs. 1–2). Knee shear and patellofemoral forces at FU18 were not different between the groups, and adjusting for gait speed did not change these outcomes.
Fig. 1.
Mean knee bone-on-bone compressive forces at FU18 for the exercise, diet, and diet plus exercise groups.
Fig. 2.
Mean knee compressive impulse (mean + SE) for the diet, exercise, and diet plus exercise groups adjusted for gender, baseline BMI, and baseline values.
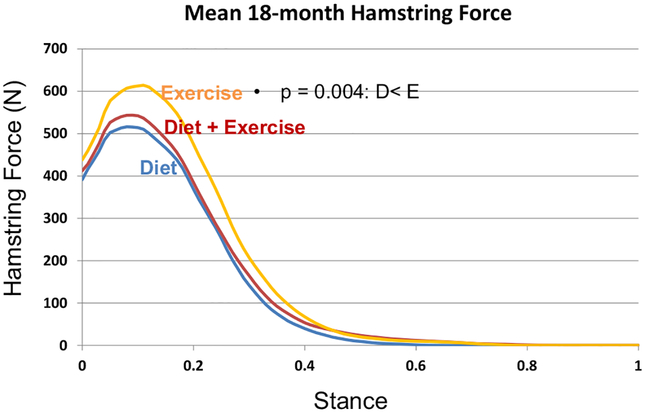
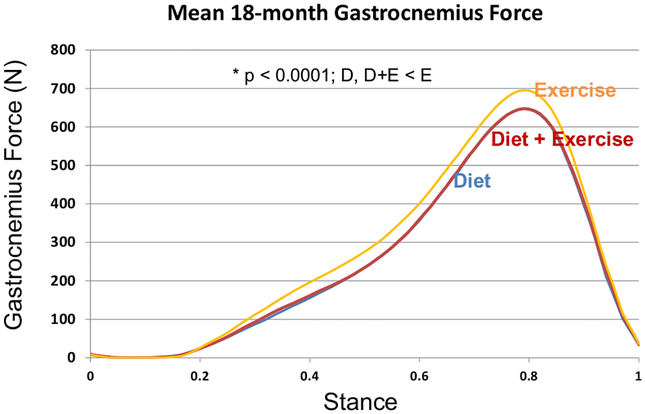
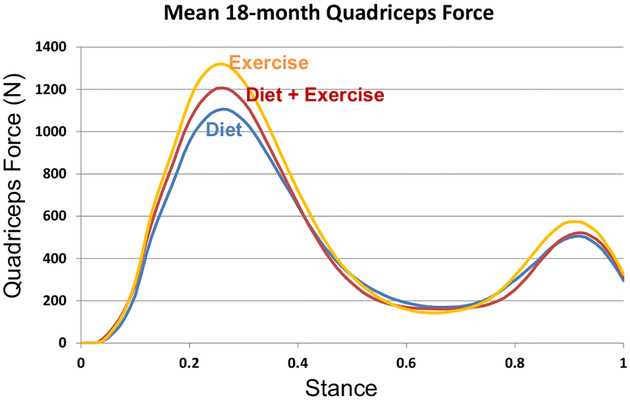
Compared to E, the hamstring forces were significantly less in the D (p = 0.004) group; the gastrocnemius forces were significantly less in D (p = 0.0001) and D + E (p < 0.0001); and quadriceps forces were similar between groups at FU18 (Table 2, Figs. 3–5). Adjusting for gait speed did not alter these results.
Fig. 3.
Mean hamstring muscle forces at FU18 for the exercise, diet, and diet plus exercise groups.
Fig. 5.
Mean gastrocnemius muscle forces at FU18 for the exercise, diet, and diet plus exercise groups.
The D group displayed a significantly lower mean peak hip extension moment than E (p = 0.002); peak hip adduction moments in both D (p = 0.005) and D + E (p = 0.0002) were less than in E; and peak hip power production (p = 0.004) in D was lower than in E. D + E lowered hip extension moment by 12% and D lowered hip power production by 20% relative to E. When adjusted for 18-month gait speed, the results were similar and significant (Table 3).
Table 3.
Mean (95% CI) intervention effects on lower extremity moments, power, and knee stiffness.
| Overall Baseline | Exercise | Diet | Diet + Exercise | #P | +P | ||||
|---|---|---|---|---|---|---|---|---|---|
| FU6 | FU18 | FU6 | FU18 | FU6 | FU18 | ||||
| Hip Extension Moment (Nm) | 67.1 64.2, 70.0 |
80.7 76.6, 84.8 |
70.2 66.0, 74.5 |
65.9 61.7, 70.1 |
61.1 56.8, 65.3 |
69.9 65.8, 73.9 |
67.0 62.9, 71.1 |
0.008‡ | 0.005‡ |
| Hip Adduction Moment (Nm) | 82.5 80.6, 84.5 |
83.7 80.5, 86.8 |
80.4 77.1, 83.6 |
74.5 71.2, 77.7 |
73.8 70.5, 77.1 |
75.1 72.0, 78.2 |
71.7 68.5, 74.9 |
0.0005$ | 0.0005$ |
| Hip Flexion Moment (Nm) | −66.9 −69.8, −63.9 |
−74.4 −79.1, −70.0 |
−77.2 −82.0, −72.3 |
−70.0 −74.8, −65.2 |
−72.7 −77.6, −67.9 |
−70.7 −75.2, −66.1 |
−76.3 −81.0, −71.6 |
0.39 | 0.53 |
| Hip Power Production (W) | 93.0 87.5, 98.4 |
107.9 99.2, 116.7 |
89.9 80.8, 99.0 |
81.4 72.3, 90.4 |
71.2 62.1, 80.3 |
89.9 81.3, 98.6 |
83.3 74.4, 92.2 |
0.013‡ | 0.015‡ |
| Hip Power Absorption (W) | −57.5 −60.7, −54.3 |
−69.9 −75.6, −64.2 |
−74.5 −80.5, −68.6 |
−66.8 −72.7, −60.9 |
−70.4 −76.4, −64.5 |
−69.6 −75.2, −64.0 |
−76.1 −81.9, −70.3 |
0.37 | 0.64 |
| Knee Extension Moment (Nm) | 35.6 33.7, 37.4 |
42.5 40.1, 44.9 |
40.7 38.2, 43.2 |
37.2 34.7, 40.0 |
37.1 34.6, 39.6 |
41.0 38.7, 43.4 |
39.6 37.1, 42.0 |
0.11 | 0.20 |
| Knee Abduction Moment (Nm) | 28.1 26.8, 29.5 |
29.0 27.2, 30.8 |
29.0 27.1, 30.8 |
29.7 27.9, 31.6 |
28.6 26.8, 30.5 |
30.8 29.0, 32.6 |
29.3 27.5, 31.1 |
0.88 | 0.90 |
| Knee Flexion Moment (Nm) | −27.4 −28.4, −26.4 |
−30.5 −31.9, −29.1 |
−29.3 −30.8, −27.9 |
−26.8 −28.3, −25.4 |
−27.5 −28.9, −26.0 |
−27.4 −28.7, −26.0 |
−28.1 −29.5, −26.6 |
0.17 | 0.015£ |
| Knee Stiffness (Nm/rad) | 218 208, 229 |
234 218, 250 |
231 214, 247 |
202 186, 219 |
227 210, 244 |
213 197, 229 |
227 211, 244 |
0.95 | 0.91 |
| Knee Power Absorption (W) | −29.9 −33.0, −26.7 |
−44.3 −50.9, −37.7 |
−41.5 −48.4, −34.7 |
−40.9 −47.7, −34.1 |
−37.8 −44.7, −30.9 |
−51.1 −57.6, −44.6 |
−41.3 −48.0, −34.6 |
0.69 | 0.81 |
| Knee Power Production (W) | 49.7 47.0, 52.4 |
65.9 57.4, 74.4 |
61.9 53.0, 70.8 |
54.5 45.7, 63.3 |
55.4 46.5, 64.3 |
68.6 60.2, 76.9 |
59.2 50.5, 67.9 |
0.58 | 0.59 |
| Ankle Plantar Flexion Moment (Nm) | 108.5 106.3, 110.7 |
111.6 109.6, 113.5 |
109.1 107.1, 111.1 |
102.8 100.8, 104.8 |
103.5 101.5, 105.6 |
104.0 102.1, 105.9 |
102.9 100.9, 104.8 |
<0.0001$ | <0.0001$ |
| Ankle Power (W) | 166 160, 171 |
181 174, 187 |
171 164, 178 |
158 152, 165 |
163 156, 169 |
167 161, 174 |
163 156, 169 |
0.11 | 0.005‡ |
D + E > D, E.
P adjusted for baseline values, baseline BMI, and gender.
P adjusted for baseline values, baseline BMI, gender and follow-up gait speed. Mean values not shown Significant (P ≤ 0.0167) pairwise comparisons.
E > D, D + E.
D < E.
E > D + E.
Knee extension, abduction, and internal rotation moments were not significantly different among the groups with and without adjusting for 18-month gait speed. Peak extension moments tended to increase between 5 and 10% in the three groups. Peak knee power absorption and production values did not differ between the groups at FU18; all groups increased these values from baseline. Knee stiffness, defined as the change in the knee extension moment/change in the knee flexion angle, was similar between the groups.
Peak ankle plantar flexion moment was significantly less (5%) in the two diet groups compared with E (p < 0.0001). When adjusted for 18-month gait speed, the plantar flexion moment remained significantly lower in the two diet groups.
3.3. Dose response to weight loss
Tests for significance (p < 0.05) between categories (<5%, 5–9.9%, ≥ 10%) revealed a dose response to weight loss such that greater weight loss was related to lower resultant knee joint forces (p < 0.0001) (Table 4). The decline in resultant knee force from the lowest to the highest weight loss categories was 319 N per step, a 13% decline. The reduction in knee forces was due, in large part, to a reduction in muscle forces with greater weight loss. Participants that lost greater than 10% of their baseline weight had significantly reduced quadriceps, hamstring, and gastrocnemius muscle forces compared to those that lost less weight (Table 4).
Table.4.
Dose response of knee joint and muscle forces to weight loss independent of group assignment (M, 95%CI).
| Force (N) | Weight Loss Category | P value | ||
|---|---|---|---|---|
| ≥10% | 5–10% | < 5% | ||
| Resultant Joint Force | 2443 2335, 2550 |
2661 2542, 2780 |
2761 2672, 2851 |
<0.0001† |
| Quadriceps Force | 1280 1197, 1363 |
1405 1315, 1496 |
1404 1335, 1473 |
0.03# |
| Hamstring Force | 633 586, 680 |
679 623, 735 |
735 696, 775 |
0.003† |
| Gastrocnemius Force | 675 658, 693 |
728 710, 747 |
755 740, 770 |
<0.0001# |
≥10% group less than < 5% group.
≥10% group<5–10% and < 5% groups.
4. Discussion
IDEA was the first randomized clinical trial to study the long-term effects of intensive dietary weight loss and exercise, alone and in combination, on lower extremity gait biomechanics in older, overweight and obese adults with knee OA. Significantly longer stride length and faster cadence in D + E resulted in significantly improved gait speed compared to either intervention alone. This has potentially important long-term health implications as epidemiologic data indicate that increased gait speed is associated with increased life expectancy; every 0.1 m/s increase in gait speed at age 65 is associated with a 2.5 year increase in life expectancy (Studenski et al., 2011). D + E improved gait speed by 0.15 m/s from baseline.
An analysis of lower extremity motion revealed no significant differences between groups in hip, knee, or ankle range of motion. This was due, in part, to the use of an exercise-only group as the comparison group. For example, maximum hip extension angle that occurred prior to toe-off increased 66% and 72% from baseline in the D and D + E groups, respectively; an increase of 51% in the E group, however, resulted in no between-group difference.
The kinetic data reported here support our OA disease model that suggests intensive weight loss and exercise significantly affect the biomechanical OA disease pathway (Messier et al., 2009). Specifically, vertical and anterior braking ground reaction forces and tibiofemoral compressive impulse were significantly lower in the D and D + E groups compared to E. In contrast, the sharp rise in tibiofemoral and patellofemoral compressive and shear forces at 6 months in the E group mimicked the increase in gait speed. From 7-to-18 months, however, these loads decreased, moving closer to baseline values, suggesting that the E group adapted biomechanically and perhaps physiologically to the new gait speed. The adaptations included decreased stride length, gait velocity, and knee flexion, the combined effect that helped reduce knee loads (Tables 1–2). Hence, in the short term, exercise therapy without intensive dietary weight loss in overweight and obese adults with knee OA has some potentially negative biomechanical consequences.
Both the D and D + E interventions resulted in significantly lower joint loads compared to the E intervention. At FU18, D + E walked at a mean speed (1.35 m/s) that is commonly associated with healthy, middle-aged men and women (Perry, 1992). Hence, weight loss combined with exercise provided the positive consequences of higher preferred gait speeds with the benefits of lower knee joint loads. After controlling for gait speed the knee joint loads in D and D + E remained significantly lower than in E, indicating the powerful effect a 10% weight loss has on joint loading, independent of exercise.
The external knee adduction moment, equivalent in magnitude to our internal abduction moment, is a surrogate measure of medial knee joint loading (Hurwitz et al., 2002) and is useful in distinguishing the effects that frontal plane knee alignment has on knee loading (Messier et al., 2014). The lack of a between group difference in knee abduction moment is consistent with our previous work that showed that bone-on-bone tibiofemoral compressive forces are more sensitive to changes in BMI than the internal abduction moment (Messier et al., 2011, 2014). Indeed, D decreased tibiofemoral compressive force and D and D + E decreased tibiofemoral compressive impulses whereas the abduction moment was unaffected by these treatments. This lack of response in the abduction moment may be due to a narrower step width after weight loss that would increase the lever arm of the smaller GRF (Jegede et al., 2017).
The peak knee extension moment that occurs during loading after heel strike was not different between the groups at 18-month follow-up. Reduced knee extension moments in knee OA patients indicates a quadriceps avoidance pattern as the patient attempts to reduce the load on the painful knee (Berchuck et al., 1990). The magnitude of peak knee extension moments in healthy adults is approximately 60 Nm (DeVita and Hortobagyi, 2000; Winter, 1980). All three groups increased this value from baseline but remained well below normal at FU18 with a mean value of 40 Nm. Furthermore, peak quadriceps muscle force relative to body weight and peak knee extension moment relative to body mass increased across the three groups, from means of 1.37 to 1.53 BW and from 0.38 to 0.46 Nm/kg, respectively. Taken together, these data indicate that the interventions partially attenuated any avoidance tendency at baseline and support the idea that exercise and weight loss, alone or in combination, can reduce neural inhibition of the quadriceps in knee OA patients (Hurley and Scott, 1998).
The hip represents the junction between the lower extremity and the head, arms and trunk with the primary role of the hip musculature during stance being to stabilize the mass of the superimposed trunk (Perry, 2010) and perform positive work to accelerate this mass (DeVita et al., 2007). This task becomes more difficult when upper body mass is excessive, acting as a wobbling mass that may be difficult to control (Liu and Nigg, 2000). As body weight decreased over the 18 months in the D and D + E groups, hamstring force, hip extension and abduction moments, and hip power production decreased as the demand on the hip extensors, primarily the hamstrings and gluteus maximus, decreased. Hence weight loss dramatically reduced all hip kinetics values, indicating less force is required at heel strike to control and then accelerate a much lighter upper body mass despite the increase in gait speed (Hortobagyi et al., 2011).
The ankle plantar flexors, primarily the gastrocnemius and soleus, act eccentrically to control the forward motion of the leg throughout stance, to stabilize body mass, and to assist in propulsion in late stance. After weight loss, both diet groups had significantly lower gastrocnemius muscle forces and plantar flexion moments that tended to reduce the power required to control and propel the lighter body weight. Interestingly, all of the changes in kinetics at the hip and ankle in the diet groups were accomplished with virtually no between-group differences in kinematics. Moreover, these hip and ankle differences were not accompanied by lower knee moments. Hortobagyi et al. (2011) found similar results in patients that had massive weight loss after bariatric surgery.
The beneficial effects of weight loss on the knee in our patients were more apparent in the bone-on-bone compressive forces and impulses, which were a consequence of lower hamstring and gastrocnemius forces. Hence, significant weight loss results in significantly lower loads (moments and/or forces) on all lower extremity joints while concurrently increasing gait speed, cadence, and stride length. Previously, we reported that this significant weight loss resulted in only a slight reduction in bone mineral density in this overweight and obese population (Messier et al., 2018).
Muscle weakness is a well-accepted impairment in knee OA; yet excessive muscle forces around the knee may cause abnormal joint loading (Bennell et al., 2009; Henriksen et al., 2013). This contradiction, muscle weakness as a risk factor for knee OA yet excessive muscle forces may exacerbate symptoms (Sharma et al., 2001), suggests there is a range of muscle forces that produces physiologic optimal joint loads. We speculate that joint loads within this range may initiate healthy, rather than unhealthy responses in joint tissues. Mild-to-moderate exercise did not influence muscle forces, yet weight loss resulted in significantly lower gastrocnemius and hamstring forces compared to E, suggesting less co-contraction about the knee. These results emphasize the importance of at least a 10% weight loss as an initial goal for overweight and obese adults with knee OA (Messier et al., 2013a).
We hypothesized dietary weight loss combined with exercise would have the greatest overall benefit on gait mechanics compared to either intervention alone. However, it was dietary weight loss that produced the most significant load reductions at the hip, knee, and ankle; combining diet with exercise attenuated these reductions, but most remained significantly better than with exercise alone. Of the three interventions, our previous results clearly indicated that intensive dietary weight loss plus exercise produced the best clinical outcomes (Messier et al., 2013a). Biomechanically, however, there appears to be a slight advantage for dietary weight loss-only. Given the influence of obesity on OA pathogenesis, intervening on this modifiable risk factor with a dietary weight loss intervention, alone or in combination with exercise, will lead to improved clinical and biomechanical outcomes compared to exercise alone.
Fig. 4.
Mean quadriceps muscle forces at FU18 for the exercise, diet, and diet plus exercise groups.
Acknowledgement
We would like to thank the IDEA research staff and the IDEA participants for their important contributions. Support for this study was provided by grants from the National Institutes of Health: R01 AR052528-01 from NIAMS, P30 AG21332 from NIA, M01-RR00211 from NCRR, and General Nutrition Centers, Inc, USA.
Footnotes
Declaration of Competing Interests
The authors declare that there is no conflict of interest regarding the content of this article.
References
- Aaboe J, Bliddal H, Messier SP, Alkjaer T, Henriksen M, 2011. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartil. 19, 822–828. [DOI] [PubMed] [Google Scholar]
- Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS, 2009. Muscle and exercise in the prevention and management of knee osteoarthritis: an internal medicine specialist’s guide. Med. Clin. North Am 93 (161–177), xii. [DOI] [PubMed] [Google Scholar]
- Berchuck M, Andriacchi TP, Bach BR, Reider B, 1990. Gait adaptations by patients who have a deficient anterior cruciate ligament. J. Bone Joint Surg. Am 72, 871–877. [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T, 2000. Age causes a redistribution of joint torques and powers during gait. J. Appl. Physiol 88, 1804–1811. [DOI] [PubMed] [Google Scholar]
- Devita P, Hortobagyi T, 2001. Functional knee brace alters predicted muscle and joint forces in people with ACL reconstruction during walking. J. Appl. Biomech 17, 297–311. [Google Scholar]
- DeVita P, Helseth J, Hortobagyi T, 2007. Muscles do more positive than negative work in human locomotion. J. Exp. Biol 210, 3361–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVita P, Rider P, Hortobágyi T, 2016. Reductions in knee joint forces with weight loss are attenuated by gait adaptations in class III obesity. Gait Posture 45, 25–30. [DOI] [PubMed] [Google Scholar]
- Evans SR, 2010. Fundamentals of clinical trial design. J. Exp. Stroke Transl. Med 3, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly BJ, Besier TF, Lloyd DG, Delp SL, Banks SA, Pandy MG, D’Lima DD, 2012. Grand challenge competition to predict in vivo knee loads. J. Orthop. Res 30, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin TM, Guilak F, 2005. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport Sci. Rev 33, 195–200. [DOI] [PubMed] [Google Scholar]
- Henriksen M, Hunter DJ, Dam EB, Messier SP, Andriacchi TP, Lohmander LS, Aaboe J, Boesen M, Gudbergsen H, Bliddal H, et al. , 2013. Is increased joint loading detrimental to obese patients with knee osteoarthritis? A secondary data analysis from a randomized trial. Osteoarthritis Cartil. 21, 1865–1875. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Herring C, Pories WJ, Rider P, Devita P, 2011. Massive weight loss-induced mechanical plasticity in obese gait. J. Appl. Physiol 111, 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MV, Scott DL, 1998. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. Br. J. Rheumatol 37, 1181–1187. [DOI] [PubMed] [Google Scholar]
- Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP, 2002. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J. Orthop. Res 20, 101–107. [DOI] [PubMed] [Google Scholar]
- Jegede JA, Adegoke BOA, Olagbegi OM, 2017. Effects of a Twelve-Week Weight Reduction Exercise Programme on Selected Spatiotemporal Gait Parameters of Obese Individuals. J. Obes 2017, 4193256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Nigg BM, 2000. A mechanical model to determine the influence of masses and mass distribution on the impact force during running. J. Biomech 33, 219–224. [DOI] [PubMed] [Google Scholar]
- Messier S, Devita P, Cowan R, Seay J, Young H, Marsh A, 2005a. Do older adults with knee osteoarthritis place greater loads on the knee during gait? a preliminary study. Arch. Phys. Med. Rehabil 86, 703–709. [DOI] [PubMed] [Google Scholar]
- Messier S, Legault C, Loeser RF, VanArsdale SJ, Davis C, Ettinger WH, Devita P, 2011. Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking?. Osteoarthritis Cartil. 19, 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Gutekunst DJ, Davis C, Devita P, 2005b. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 52, 2026–2032. [DOI] [PubMed] [Google Scholar]
- Messier SP, Legault C, Mihalko S, Miller GD, Loeser RF, Devita P, Lyles M, Eckstein F, Hunter DJ, Williamson JD, et al. , 2009. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. BMC. Musculoskelet. Disord 10, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, Devita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, et al. , 2013a. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 310, 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Mihalko SL, Beavers DP, Nicklas BJ, Devita P, Carr JJ, Hunter DJ, Williamson JD, Bennell KL, Guermazi A, et al. , 2013b. Strength Training for Arthritis Trial (START): design and rationale. BMC. Musculoskelet. Disord 14, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Pater M, Beavers DP, Legault C, Loeser RF, Hunter DJ, DeVita P, 2014. Influences of alignment and obesity on knee joint loading in osteoarthritic gait. Osteoarthritis and Cartilage/OARS, Osteoarthritis Research Society 22, 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Resnik AE, Beavers DP, Mihalko SL, Miller GD, Nicklas BJ, DeVita P, Hunter DJ, Lyles MF, Eckstein F, et al. , 2018. Intentional Weight Loss for Overweight and Obese Knee Osteoarthritis Patients: Is More Better?. Arthritis Care Res (Hoboken). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J, 1992. Gait Analysis: Normal and Pathological Function. Slack Inc., Thorofare, NJ. [Google Scholar]
- Perry JB, J.M. (2010). Gait analysis: Normal and pathological function (Thorofare, NJ: Slack Inc.). [Google Scholar]
- Schipplein OD, Andriacchi TP, 1991. Interaction between active and passive knee stabilizers during level walking. J. Orthop. Res 9, 113–119. [DOI] [PubMed] [Google Scholar]
- Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD, 2001. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 286, 188–195. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, et al. , 2011. Gait speed and survival in older adults. JAMA 305, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winby CR, Lloyd DG, Besier TF, Kirk TB, 2009. Muscle and external load contribution to knee joint contact loads during normal gait. J. Biomech 42, 2294–2300. [DOI] [PubMed] [Google Scholar]
- Winter DA, 1980. Overall principle of lower limb support during stance phase of gait. J. Biomech 13, 923–927. [DOI] [PubMed] [Google Scholar]
- Zarin DA, Goodman SN, and Kimmelman J (2019). Harms From Uninformative Clinical Trials. JAMA. [DOI] [PubMed] [Google Scholar]