Introduction
Pancreatitis is common in the United States, with a yearly incidence of 40 per 100,000 people. Pancreatitis leads to more than 300,000 inpatient admissions and 20,000 deaths annually, with costs exceeding $2.2 billion per year.1 Thirty-five to 55% of pancreatitis cases are related to gallstones.1 In patients with mild gallstone pancreatitis – characterized by the absence of organ failure, peripancreatic fluid collections or necrosis, and typical resolution within one week – evidence-based guidelines recommend cholecystectomy during index admission but do not specify further management recommendations.2 The practice of waiting for clinical and laboratory resolution of acute gallstone pancreatitis stemmed from a 1980s randomized trial that showed higher morbidity and mortality with surgery performed prior to 48 hours after admission.3 However, this practice is questioned based on recent data.4–7
Early cholecystectomy for mild gallstone pancreatitis has been reported in observational studies to be associated with reduced hospital length of stay (LOS) without increasing complications, even when performed in the subgroup of patients who have not had resolution of clinical symptoms and laboratory values.4–7 In these studies, cholecystectomy was performed within 48–72 hours of admission. A recent randomized trial also showed that cholecystectomy within 48 hours of admission regardless of clinical and laboratory resolution led to shorter hospital LOS without increasing complications; however, this trial was terminated after interim analysis at 50% enrollment once the pre-specified effect size was detected,8 and trials stopped early for benefit typically overestimate treatment effects.9
Additional randomized trials are needed to confirm the safety and generalizability of early cholecystectomy for predicted mild acute gallstone pancreatitis. Furthermore, there are no prior randomized trials evaluating laparoscopic cholecystectomy within the first 24 hours of admission or in disadvantaged populations treated at safety-net hospitals.
The objectives of this pilot randomized trial were: (1) to determine the feasibility of early cholecystectomy within 24 hours of presentation regardless of symptoms or laboratory values for patients mild gallstone pancreatitis predicted to be mild on admission, and (2) to obtain unbiased estimates of the effect of early cholecystectomy on hospital LOS, complications, and patient-reported outcomes (PROs) in order to determine the need for further evaluation. The hypothesis was that early cholecystectomy during index admission for predicted mild gallstone pancreatitis is feasible and results in shorter 30-day total hospital LOS without an increase in complications.
Methods
Design
A single-center, parallel-group randomized trial () was performed comparing timing of laparoscopic cholecystectomy with intraoperative cholangiogram (IOC) during index admission among patients with predicted mild gallstone pancreatitis.10 The trial compared cholecystectomy within 24 hours of presentation to cholecystectomy after clinical resolution on outcomes including 30-day hospital LOS, time to surgery, endoscopic retrograde cholangiopancreatography (ERCP) rates, complications, and PROs. Institutional Review Board approval was obtained.
Setting
The trial was conducted at Lyndon Baines Johnson General Hospital (LBJGH), a 207-bed safety-net hospital in Houston, Texas. LBJGH is a part of the safety-net system for Harris County, Texas, which is the third most populous county in the United States. Approximately 1300 elective and non-elective cholecystectomies are performed per year. Operative capabilities are available 24 hours a day, seven days a week. An on-call attending general surgeon is in house 24-hours a day along with a team of residents and operating room staff dedicated to perform around the clock non-elective general surgery procedures.
Study population
From June 2016 through June 2018, patients ≥ 18 years of age with predicted mild gallstone pancreatitis who were planned to undergo laparoscopic cholecystectomy prior to discharge were screened for eligibility. Gallstone pancreatitis was defined as: (a) upper abdominal pain, nausea, vomiting, (b) absence of alcohol use disorder, (c) elevated plasma lipase level above the upper limit of normal (≥370 U/L), and (d) imaging confirmation of gallstones or sludge.11 Predicted mild pancreatitis was defined as a Bedside Index of Severity in Acute Pancreatitis (BISAP) score of 0–2 and no evidence of organ failure or local or systemic complications.12, 13
A protocol change occurred in August 2017 which specified a 12-hour observational period between patient enrollment and randomization to ensure that there was no evidence of clinical deterioration from mild to more severe pancreatitis.10 This change was prompted by two patients who were initially thought to have mild gallstone pancreatitis, but progressed to severe pancreatitis requiring intensive care within the first 12 hours of hospitalization. Neither patient received cholecystectomy prior to clinical deterioration. Patients were excluded if there were two strong or one very strong indicator for choledocholithiasis based on the American Society for Gastrointestinal Endoscopy (ASGE) guidelines (Figure 1).14 Patients with any very strong predictor of choledocholithiasis on admission were excluded because of a high likelihood of requiring preoperative ERCP as laparoscopic common bile duct explorations were not routinely performed at this institution. Additional exclusion criteria included pregnancy, developmental delay, severe preexisting medical comorbidities precluding surgery, chronic pancreatitis, native language other than English and Spanish, and patient refusal.
Figure 1:
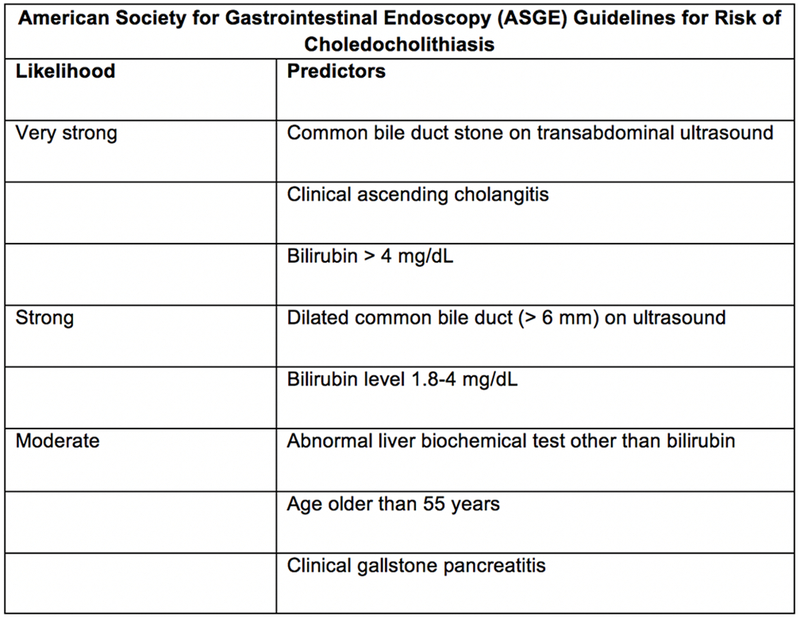
Presence of any very strong or both strong predictors suggests a high likelihood of choledocholithiasis. No predictors suggest a low likelihood, and all other patients have an intermediate likelihood.25 By definition, all patients enrolled in this trial will have at least a moderate likelihood because of the clinical diagnosis of gallstone pancreatitis.
Study protocol
CONSORT guidelines were followed.15 Patients were randomized utilizing variable permuted blocks of 4, 6, and 8 using a computer-generated random sequence. Sequentially numbered, opaque, sealed envelopes containing the randomization cards were made by a research coordinator not involved in the study and kept in a locked surgery office. Prior to the protocol change, eligible patients were enrolled and randomized concurrently after admission. After the protocol change, enrolled patients underwent delayed randomization after a 12-hour observational period if no clinical deterioration was noted. Patients were stratified based on intermediate (having only moderate predictors) versus high risk (having 1 strong predictor) for choledocholithiasis on admission using ASGE guidelines. While patients and the healthcare providers were not able to be blinded, postoperative PRO assessors and data analysts were blinded. In addition, a blinded adjudication committee reviewed the outcomes.
The intervention was laparoscopic cholecystectomy with IOC within 24 hours of presentation regardless of laboratory or clinical symptom resolution. The primary surgeon was allowed to refuse to perform cholecystectomy if the patient demonstrated worsening pancreatitis. The control was laparoscopic cholecystectomy with IOC once the patient had resolution of abdominal pain and down-trending laboratory values and was deemed appropriate for surgery by the clinical team. Patients received standardized postoperative care in both arms. Additional details of the study protocol along with a timeline of patient enrollment, randomization, interventions, and assessments have been previously published.10
Outcomes and Definitions
The primary endpoint was total 30-day hospital LOS in hours. The rationale for using 30-day LOS instead of hospital LOS was to capture any hospital readmissions within 30 days after treatment. Secondary endpoints included ERCP rates, complications (chosen a priori: unplanned transfusions, surgical site infections, pneumonia, bile duct injury, retained stone at 30 days, and bowel injury), Clavien-Dindo grading of complications,16 readmissions within 30 days, exacerbation of pancreatitis, and conversion to open cholecystectomy. Additional outcomes evaluated timeliness of treatment including time from admission to cholecystectomy, index hospital LOS, number of procedures, and nighttime cholecystectomy. PROs were assessed by a short-term change in functional health status between admission and post-operative assessment at 1 month using the Gastrointestinal Quality-of-Life Index (GIQLI).17, 18 The GIQLI is a 36-question multiple choice survey; scores range from 0 to 144, with higher scores corresponding to fewer gastrointestinal symptoms and better quality of life.
Sample size calculation and analysis
The sample size of 100 patients total was estimated based on a 1-day reduction in LOS (two-sided α =0.05, β = 0.80) from 3 to 2 with 10% non-adherence to protocol in each group. A negative binomial regression was used to compare 30-day LOS between the two groups including the stratifying variable as a covariate. Binary secondary outcomes were analyzed using chi square tests. GIQLI scores pre- and post-cholecystectomy were compared using the Wilcoxon signed-rank test for non-parametric data. The original planned analysis had included use of the Cochran-Mantel-Haenszel test for analysis of binary secondary outcomes. However complications and readmissions were rare and there were not enough occurrences per strata to perform the test. In addition to the frequentist analysis, a Bayesian analysis was also performed for the primary outcome and complication rates.18 The Bayesian approach uses preexisting data (prior probability) combined with results of the study to generate posterior probabilities for various magnitudes of effect of the intervention.19 Bayesian analyses allow for incorporation of uncertainty from what is already known or unknown based on prior studies, and reports the results as probability of observing an outcome of some magnitude or greater. For example, if the posterior probability of the intervention in this trial is 50% for the primary outcome, it would be interpreted that early cholecystectomy has a 50% probability of reducing 30-day LOS as compared to control cholecystectomy, which would suggest that the intervention and control have similar effect on the primary outcome. Given that most prior studies were observational and the early termination of Aboulian et al’s randomized trial,8 we chose a neutral prior probability distribution to estimate the posterior probability of reductions in 30-day LOS. An intention-to-treat analysis was performed.
Results
Baseline Patient Characteristics
During the study period, 147 patients with gallstone pancreatitis were screened for eligibility. Of 100 consented patients (Figure 2), two were excluded prior to randomization secondary to increasing pancreatitis severity. One patient was excluded after randomization due to unrecognized mild developmental delay at the time of enrollment. A total of 49 patients (50.5%) were randomized to early cholecystectomy, while 48 patients (49.5%) were randomized to control cholecystectomy. Two patients (2%) randomized to the control group were discharged prior to receiving cholecystectomy; they both received outpatient surgery. Both patients were analyzed in their randomized group in the intention-to-treat analysis.
Figure 2 -. Patient Selection Flow Diagram:
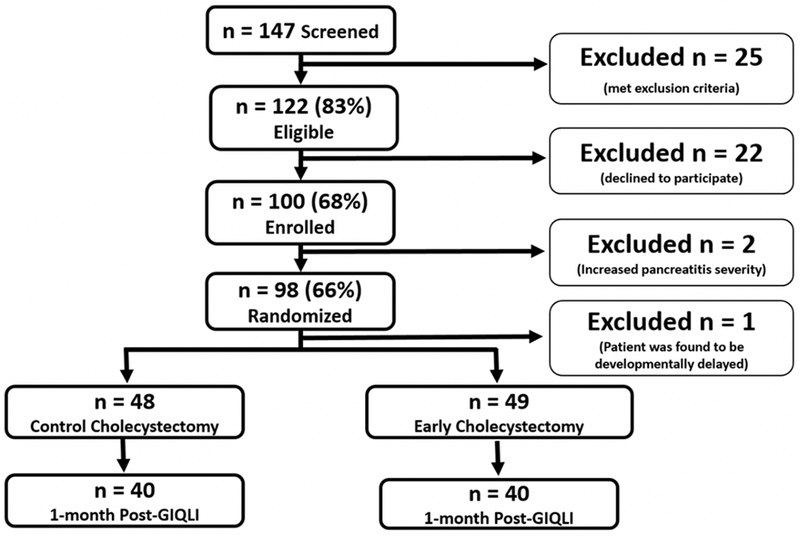
During the study period, 147 patients with gallstone pancreatitis were screened for eligibility. Forty-seven patients were excluded and 100 patients were enrolled in the study. After enrollment, 3 patients were excluded from the intention to treat analysis (2 patients excluded prior to randomization due to increasing pancreatitis severity; 1 patient excluded after randomization due to patient being developmentally delayed). A total of 48 patients were randomized to the control cholecystectomy group and 49 patients were randomized to the early cholecystectomy group. A total of 80 patients (40 from each group) were able to be reached for 1-month postoperative GIQLI assessment.
Patients were predominantly female (75%), Hispanic (89%), middle-aged (median age of 40 years), and obese (median BMI of 31) (Table 1). Patients were similar in age, race/ethnicity, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, comorbid conditions, and duration of symptoms between treatment groups. Findings on ultrasonography, BISAP scores, and admission laboratory values were also similar between groups, with the exception of lipase, which was higher in the control group.
Table 1.
Baseline Patient Characteristics
| All Patients n = 97 | Control Group, n = 48 | Early Group, n = 49 | |
|---|---|---|---|
| Age, years (median,IQR) | 40 (29 – 50) | 38 (28 – 48) | 44 (29 – 51) |
| Sex, Female (%) | 75.3 (73%) | 40 (83.3%) | 33 (67.3%) |
| Body Mass Index | 31 (27 – 36) | 33 (27 – 40) | 30 (28 – 34) |
| Race/Ethnicity, n (%) | |||
| Caucasian | 2 (2%) | 2 (4%) | 0 (0%) |
| Hispanic | 86 (89%) | 40 (83%) | 46 (94%) |
| African American | 8 (8%) | 5 (10%) | 3 (6%) |
| Other | 1 (1%) | 1 (2%) | 0 (0%) |
| ASA Classification, n (%) | |||
| 1 | 16 (16%) | 7 (15%) | 9 (18%) |
| 2 | 63 (65%) | 30 (63%) | 33 (67%) |
| 3 | 18 (19%) | 11 (23%) | 7 (14%) |
| History of Diabetes, n (%) | 23 (24%) | 8 (17%) | 15 (31%) |
| History of Hypertension, n (%) | 16 (16%) | 9 (19%) | 7 (14%) |
| History of Chronic Kidney Disease, n (%) | 1 (1%) | 1 (2%) | 0 (0%) |
| History of Liver Disease, n (%) | 2 (2%) | 2 (4%) | 0 (0%) |
| History of Immune Suppression, n (%) | 3 (3%) | 0 (0%) | 3 (6%) |
| Duration of Symptoms, days | 2 (1-3) | 2 (1 – 5) | 2 (1 – 3) |
| ASGE Risk of Choledocholithiasis, n (%) | |||
| Moderate | 65 (67%) | 32 (67%) | 33 (67%) |
| Strong | 32 (33%) | 16 (33%) | 16 (33%) |
| BISAP Score, n (%) | |||
| 0 | 70 (72%) | 34 (71%) | 36 (74%) |
| 1 | 27 (28%) | 14 (29%) | 13 (27%) |
| Abdominal Imaging Obtained, n (%) | |||
| Ultrasound only | 82 (85%) | 41 (85%) | 41 (84%) |
| Ultrasound and CT Abdomen | 13 (13%) | 6 (13%) | 7 (14%) |
| Ultrasound and MRCP | 2 (2%) | 1 (2%) | 1 (2%) |
| Ultrasound Findings, n (%) | |||
| Immobile Neck Stone | 14 (14%) | 4 (8%) | 10 (20%) |
| Peri-cholecystic Fluid | 13 (13%) | 4 (8%) | 9 (18%) |
| Adenomyomatosis | 2 (2%) | 0 (0%) | 2 (4%) |
| Polyp | 1 (1%) | 0 (0%) | 1 (2%) |
| Hepatic Granulomas | 1 (1%) | 1 (2%) | 0 (0%) |
| Gallbladder Wall Width, mm | 2.6 (2.0 – 3.0) | 2.7 (2.0 – 3.0) | 2.6 (2.0 – 3.0) |
| Common Bile Duct Width, mm | 4.0 (3.0 – 5.8) | 4.3 (2.5 – 6.0) | 4.0 (3.0 – 5.4) |
| White Blood Cell Count, 109/L | 10.1 (8.4 – 13.1) | 10.3 (8.7 – 13.6) | 4.0 (3.0 – 5.4) |
| Total Bilirubin, mg/dL | 0.8 (0.5 – 1.3) | 0.9 (0.5 – 1.2) | 0.8 (0.5 – 1.4) |
| Lipase, IU/L | 3581 (713-13282) | 5032 (760 – 17621) | 2066 (695 – 10065) |
ASA: American Society of Anesthesiologists; ASGE: American Society for Gastrointestinal Endoscopy; BISAP: Bedside Index of Severity in Acute Pancreatiti;, CT: Computed Tomography; MRCP: Magentic Resonance Cholangiopancreatography
Procedural Characteristics
Of the 97 randomized patients, all received cholecystectomy. There were no conversions to open surgery. IOC was performed or attempted in 91% of cases in the early group and 83% of cases in the control group. Reasons for failure to perform IOC were not recorded. Patients who did and did not receive IOC had similar preoperative median total bilirubin (0.9, IQR 0.5 – 1.3 mg/dL in IOC versus 0.7, IQR 0.4 – 1.0 mg/dL in no IOC, p = 0.3) and similar median common bile duct diameter (4, IQR 3 – 5.8 mm in IOC versus 5, IQR 3.6 – 5.4 mm in no IOC, p = 0.3). Additionally, there were no differences in IOC rates in patients whose total bilirubin down trended or remained the same. ERCP was performed in 7 (15%) patients in the early group and 14 (30%) patients in the control group, with preoperative ERCP being performed in 1 (1%) case in the early group and 6 (6%) cases in the control group due to worsening laboratory values suggestive of choledocholithiasis. Overall, the early group had fewer ERCPs as compared to the control group (15% versus 30%, p = 0.038). There were no differences in rates of stone extraction during ERCP between groups. Five (38%) stones were retrieved in the intervention arm versus 8 (62%) stones retrieved in the control arm (p = 0.35). Two (25%) patients had only biliary sludge removed in the intervention arm versus 6 (75%) patients in the control arm (p = 0.131). Bayesian analysis showed that early cholecystectomy has a 93% probability of decreased ERCP use as compared to control cholecystectomy. Rates of intraoperative drain placement and operative duration were similar between groups (Table 2).
Table 2.
Procedural Characteristics
| Control Group, n = 48 | Early Group, n = 49 | p-value | |
|---|---|---|---|
| Intraoperative Cholangiogram, n (%) | |||
| Negative | 30 (64%) | 27 (57%) | |
| Positive, Stones Extracted | 0 (0%) | 3 (6%) | 0.055 |
| Positive, Stones Unable to be Extracted | 8 (17%) | 6 (13%) | |
| Attempted/Failed | 1 (2%) | 7 (15%) | |
| Not Performed | 8 (17%) | 4 (9%) | |
| ERCP, n (%) | |||
| Not Indicated | 34 (71%) | 42 (86%) | |
| Preoperative | 6 (13%) | 0 (0%) | 0.038 |
| Postoperative | 8 (17%) | 7 (15%) | |
| ERCP with Stone Extraction, n (%) | 8 (17%) | 5 (10%) | 0.349 |
| ERCP with Sludge Only, n (%) | 6 (13%) | 2 (4%) | 0.131 |
| Gallbladder Fossa Drain Placement, n (%) | 3 (6%) | 1 (2%) | 0.362 |
| Conversion to Open Cholecystectomy, n (%) | 0 (0%) | 0 (0%) | 1.000 |
| Nighttime Cholecystectomy, n (%) | 17 (35%) | 26 (53%) | 0.103 |
| Operative Time, minutes (median, IQR) | 72 (58 – 92) | 76 (64 – 92) | 0.489 |
ERCP: Endoscopic Retrograde Cholangiopancreatography
Outcomes
Total 30-day LOS was significantly shorter in the early group (median LOS 50 hours, IQR 27 – 82 hours) when compared with the control group (median LOS 77 hours, IQR 52 – 111 hours), with IRR of 0.68, 95% CI 0.65 – 0.71, p < 0.005 (Table 3). Readmission rates were low and similar between groups. Causes of readmission were related to the index procedure: 1) persistent right upper quadrant pain, 2) recurrent pancreatitis, and 3) cystic stump leak. Bayesian analysis showed that early cholecystectomy has a 99% probability of reducing 30-day LOS as compared to control cholecystectomy.
Table 3.
Negative Binomial Regression for Length of Stay and Number of Procedures
| Control Group, n = 48 | Early Group, n = 49 | IRR | 95% CI | P Value | |
|---|---|---|---|---|---|
| Preoperative LOS, hours | 43 (34 - 63) | 16 (13 - 21) | 0.34 | 0.32 – 0.37 | <0.005 |
| Postoperative LOS, hours | 19 (11 - 43) | 23 (10 - 54) | 0.99 | 0.93 – 1.05 | 0.641 |
| Index Hospital LOS, hours | 77 (52 - 111) | 45 (26 - 72) | 0.67 | 0.64 – 0.70 | <0.005 |
| Number of Procedures at 30 Days, n | 1 (1 – 1) | 1 (1 – 2) | 0.82 | 0.58 – 1.17 | 0.282 |
| Total 30 Day Length of Stay, hours | 77 (52 - 111) | 50 (27 - 82) | 0.68 | 0.65 – 0.71 | <0.005 |
Adjusted for American Society for Gastrointestinal Endoscopy Risk of Choledocholithiasis classification
Patients in the early cholecystectomy group had significantly shorter time from admission to surgery. The adjusted preoperative median LOS was 66% shorter in the early group (median LOS 16 hours, IQR 13 – 21 hours) than in the control group (median LOS 43 hours, IQR 34 – 63 hours). Median time to surgery was shorter within the early cholecystectomy group pre-protocol change as compared to post-protocol change, with median time to surgery being 13 (IQR 11 – 17) hours pre-protocol change versus 18 (IQR 15 – 22) hours post-protocol change (p = 0.003). Median time to surgery was similar pre- and post-protocol change in the control group, with median time to surgery being 43 (IQR 36 – 57) hours pre-protocol change versus 42 (IQR 33 – 66) hours post-protocol change (p = 1.0). No patients exhibited worsening pancreatitis during the 12 to 24-hour observational period after the protocol change. Postoperative LOS was similar between groups. Index hospital LOS was likewise shorter in the early group; the adjusted index hospital LOS was 33% shorter in the early group (median LOS 23 hours, IQR 10 – 54 hours) than in the control group (median LOS 19 hours, IQR 11 – 43 hours).
Complication rates were low in both groups (6% versus 2%, p = 0.617). Complications included recurrence/progression of pancreatitis (2 early versus 1 control) and one cystic duct stump leak in the early group. There were no differences in grades of Clavien-Dindo complications, with 10 (21%) patients having any Clavien-Dindo in the early group versus 4 (8%) patients in the control group (p = 0.09). There were no Clavien-Dindo grades higher than 3a, which only occurred in 1 (2%) patient in the early group (Table 4). On Bayesian analysis, early cholecystectomy had a 72% probability of increased minor complications as compared to control cholecystectomy.
Table 4.
Complications After Control versus Early Cholecystectomy
| Control Group, N = 48 | Early Group, N = 49 | P Value | |
|---|---|---|---|
| Any Complication, n (%) | 1 (2%) | 3 (6%) | 0.617 |
| Complications, n (%) | |||
| Common Bile Duct Injury | 0 (0%) | 0 (0%) | |
| Biloma or Bile Leak | 0 (0%) | 1 (2%) | 0.513 |
| Retained Stone | 0 (0%) | 0 (0%) | |
| Surgical Site Infection | 0 (0%) | 0 (0%) | |
| Exacerbation of Pancreatitis | 1 (2%) | 2 (4%) | |
| Bleeding Requiring Transfusion | 0 (0%) | 0 (0%) | |
| Bowel Injury | 0 (0%) | 0 (0%) | |
| Pneumonia | 0 (0%) | 0 (0%) | |
| Clavien-Dindo Classification, n (%) | |||
| 0 | 43 (91%) | 38 (79%) | |
| 1 | 3 (6%) | 7 (15%) | 0.357 |
| 2 | 1 (2%) | 2 (4%) | |
| 3a | 0 (0%) | 1 (2%) | |
| Readmissions, n (%) | 1 (2%) | 3 (6%) | 0.617 |
Change in Functional Health Status
Preoperative GIQLI scores were obtained in all patients, and 81 patients (84%) completed a postoperative GIQLI survey at one-month post-operative. Preoperative median GIQLI scores were 74 points (IQR 63 – 96) in early group versus 83 points (IQR 64 – 102) in the control group (p = 0.284). At one-month postoperative follow-up, GIQLI scores were similar between treatment groups (108 points in early group versus 109 points in control group, p = 0.869). There was a significant improvement in GIQLI scores post-cholecystectomy at 1-month follow-up, as compared to pre-cholecystectomy [median GIQLI score 78 (IQR 63 – 97) pre-cholecystectomy versus 108 (IQR 95 – 117) post-cholecystectomy, p < 0.001]. The majority of patients had improvement in GIQLI scores after cholecystectomy, and degrees of improvement were comparable between treatment groups (29 point increase in early group versus 26 point increase in control group) (Table 5).
Table 5.
One Month Outcomes, Early versus Control Operation
| Control Group, N = 48 | Early Group, N = 49 | P Value | |
|---|---|---|---|
| Completed One Month Follow Up, n (%) | 41 (85%) | 40 (82%) | 0.616 |
| Preoperative GIQLI Score | 83 (64 – 102) | 74 (63 – 96) | 0.284 |
| Postoperative GIQLI Score | 109 (93 – 118) | 108 (96 – 117) | 0.869 |
| Change in GIQLI Score | 26 (10 – 40) | 29 (11 – 47) | 0.337 |
| Pre-Cholecystectomy N = 92 | Post-Cholecystectomy N = 80 | P Value | |
| GIQLI Score (median, IQR) | 78 (63 – 97) | 108 (95 – 117) | <0.001 |
GIQLI: Gastrointestinal Quality of Life Index (given in median and interquartile range). Higher GIQLI scores indicate better quality of life.
Discussion
This is the first completed randomized trial that compared the timing of laparoscopic cholecystectomy before 24 hours of admission to later within the same admission for patients with predicted mild gallstone pancreatitis. The trial demonstrated that early laparoscopic cholecystectomy between 12 to 24 hours of admission is feasible at a busy safety-net hospital and decreased 30-day hospital LOS. Early cholecystectomy also decreased index hospital LOS, need for ERCP, and had similar improvements in patient reported quality of life after surgery as compared to control cholecystectomy. The trial’s findings are similar to those of Aboulian et al’s randomized trial which reported decreased hospital LOS with early cholecystectomy within 48 hours of admission regardless of symptoms or laboratory values.8 However, by narrowing the window for early cholecystectomy to within 24 hours, the mean time to cholecystectomy in the early group (17.8 versus 35.1 hours) and index LOS (2.5 versus 3.5 days) were shorter in the present trial.
In addition to decreasing LOS, early cholecystectomy has the potential to improve patient-centered outcomes. An unpublished qualitative study performed at LBJGH suggested that effective and timely resolution of symptoms is important amongst patients with gallstone disease such as acute cholecystitis.20 However, despite an increasing interest in PROs after surgery, PROs after biliary surgery are rarely measured,21 particularly after acute pancreatitis. The Gallstone PANC trial utilized the GIQLI which was used to measure PROs after acute pancreatitis in one of 16 studies included in a systematic review and meta-analysis.22 The study showed that the GIQLI did demonstrate differences between patients with acute cholecystitis as compared to age-matched controls (mean GIQLI score 104±3 after pancreatitis versus 126±1 in controls).23 In the present study, both groups showed a significant improvement in one-month post-cholecystectomy GIQLI scores as compared to pre-cholecystectomy, and the one-year postoperative GIQLI scores are in the process of being collected. Further evaluation is required of other patient-centered outcomes that may be improved by early cholecystectomy and shorter hospital LOS.
Despite evidence-based recommendations, opponents to early cholecystectomy, even when defined as within index admission, cite concern for increased risk of surgical complications due to severity of inflammation or to unrecognized pancreatic necrosis. Although worsened outcomes with unrecognized necrosis at the time of same admission cholecystectomy have been reported,24 the literature is limited to small retrospective cohort studies that are subject to selection bias. However, a high complication rate is not supported by higher quality evidence. Based on systematic reviews of randomized trials of same admission versus post-discharge cholecystectomy for mild gallstone pancreatitis, same admission cholecystectomy results in an approximately 7% complication rate.25, 26 In the present trial, the complication rates were 2% and 6% in the control and early groups, respectively. Furthermore there were no major complications (i.e., Clavien-Dindo IV or V) in either group. From a Bayesian perspective, there is a 72% probability that early cholecystectomy led to increased complications, the majority of which were minor complications (Clavien-Dindo grade 1 or 2). Thus, larger trials are necessary to evaluate the risks of post-operative cholecystectomy and to validate the benefits across a broad range of hospitals.
Another potential barrier to early cholecystectomy is the difficulty in accurately predicting the severity of pancreatitis, as evidenced by the need to change the protocol to allow for 12 hours of observation prior to randomization. Several studies have compared both clinical and radiologic scoring systems for the prediction of severity of pancreatitis.27–30 Comparators included Ranson’s criteria, Acute Physiology and Chronic Health Evaluation (APACHE) II, and Modified CT Severity Index (MCTSI). These studies all suggested that BISAP is accurate for risk stratification. In addition, it is easy to use and correlates with mortality and ICU admission.27, 28 A 2015 systematic review and meta-analysis of ten studies suggested that a BISAP score ≥ 3 had a sensitivity of 51% and a specificity of 91% for severe acute pancreatitis,31 suggesting that the BISAP score is useful in ruling in severe pancreatitis but not in ruling it out. Thus, despite the advantages of the BISAP score, there is still significant possibility of inaccurately predicting severity of acute pancreatitis. Unfortunately, no current scoring system is entirely accurate in ruling out severe acute pancreatitis at admission, which is one of the reasons that the trial protocol was changed to include a 12-hour observation window. Furthermore, the grading of severity of acute pancreatitis at admission is not granular enough to identify predictors of complications with early cholecystectomy. Therefore, more research is needed to determine which patients would truly benefit from cholecystectomy within 24-hours for mild acute gallstone pancreatitis.
Limitations
This trial has several limitations. First, results may not be generalizable to other hospitals. The population consists primarily of low socioeconomic status and racial/ethnic minority patients, namely Hispanic patients who are known to present at an earlier age and with milder disease than non-Hispanic patients.32 Additionally, LBJGH is capable of performing cholecystectomies 24 hours a day, 7 days a week, due to the presence of in-house faculty, which may not be possible in other hospitals across the country. However, hospitals with an acute care surgery model may have similar capacity. Second, the trial did not incorporate laparoscopic common bile duct exploration which is commonly performed at other institutions and which may have an effect on the results. Third, as noted, the inability to accurately predict the severity of acute pancreatitis soon after admission may limit implementation, particularly given the possibility of increased complications noted on Bayesian analysis. These limitations are best addressed by planning a future multi-center trial to confirm the findings of decreased LOS, to provide more precision in determining the risk of complications, and to ascertain generalizability to other centers. Such a trial could include less strict timing criteria (i.e., early cholecystectomy within 12–36 hours), enroll patients at centers that routinely practice laparoscopic common bile duct explorations, and prospectively track patients who worsen after enrollment to identify better predictive criteria.
Conclusions
In patients with predicted mild gallstone pancreatitis, cholecystectomy within 24 hours of admission significantly reduced rate of ERCPs, time to surgery, and 30-day length-of-stay. However, early cholecystectomy may increase the risk of Clavien-Dindo Grade I-III complications suggesting caution should be employed in applying the results of this trial. Identification of patients with mild gallstone pancreatitis in whom early cholecystectomy is safe given limitations in current prediction models of disease severity warrants further investigation.
Acknowledgments
Richard Escamilla BA, Debbie Lew MPH, Akshita Kumar MD MS, and Louis Carrillo MD provided assistance with screening, consent, and enrollment of patients. The residents and faculty of the Department of Surgery at the McGovern Medical School at the University of Texas Health Science Center at Houston assisted with screening of patients. Faculty of the Center for Clinical Research and Evidence-Based Medicine and the Center for Surgical Trials and Evidence-based Practice critiqued the study design. All authors have no conflicts of interest to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. All authors made substantial contributions to the conception and design of the project, the acquisition, analysis, and interpretation of data, drafting and revising of the content, and have given final approval of the version to be published.
The trial was approved by the Institutional Review Board on December 2, 2015. The trial was registered on ClinicalTrials.gov on June 13, 2016. The first patient was randomized on June 27, 2016. The trial was stopped from May 18, 2017 to August 16, 2017 for analysis by the DSMB and modifications to the protocol (including on ClinicalTrials.gov). Enrollment resumed in August 2017, and was completed June 28, 2018. The ClinicalTrials.gov identifier for the trial is . All patients gave informed consent to participate in the study. The trial was approved by the Institutional Review Boards of the McGovern Medical School at the University of Texas Health Science Center and Harris Health System. (HSC-MS-15-0719).
Conflicts of Interest and Sources of Funding:
SW is supported by a T32 fellowship (grant no 5T32GM008792) from NIGMS. The remaining authors have no sources of funding to declare. The authors declare no conflict of interest.
Abbreviations:
- APACHE
Acute Physiology and Chronic Health Evaluation
- ASA
American Society of Anesthesiologists
- ASGE
American Society of Gastrointestinal Endoscopy
- BISAP
Bedside Index of Severity in Acute Pancreatitis
- BMI
Body Mass Index
- CONSORT
Consolidated Standards of Reporting Trials
- CT
Computed Tomography
- DSMB
Data Safety Monitoring Board
- ERCP
Endoscopic Retrograde Cholangiopancreatography
- GIQLI
Gastroinstestinal quality of life index
- HRQoL
Health-related quality of life
- IOC
Intraoperative Cholangiogram
- LOS
Length of Stay
- MCTSI
Modified CT Severity Index
- MRCP
Magnetic Resonance Cholangiopancreatography
- PRO
Patient-reported outcome
- QOL
Quality of life
- RCT
Randomized controlled trial
- VAS
Visual analogue scale
References
- 1.Cucher D, Kulvatunyou N, Green DJ, et al. Gallstone pancreatitis: a review. Surg Clin North Am. 2014;94(2):257–280. [DOI] [PubMed] [Google Scholar]
- 2.Working Group IAPAPAAPG. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–15. [DOI] [PubMed] [Google Scholar]
- 3.Kelly TR, Wagner DS. Gallstone pancreatitis: a prospective randomized trial of the timing of surgery. Surgery. 1988;104(4):600–605. [PubMed] [Google Scholar]
- 4.Rosing DK, de Virgilio C, Yaghoubian A, et al. Early cholecystectomy for mild to moderate gallstone pancreatitis shortens hospital stay. J Am Coll Surg. 2007;205(6):762–766. [DOI] [PubMed] [Google Scholar]
- 5.Taylor E, Wong C. The optimal timing of laparoscopic cholecystectomy in mild gallstone pancreatitis. Am Surg. 2004;70(11):971–975. [PubMed] [Google Scholar]
- 6.Falor AE, de Virgilio C, Stabile BE, et al. Early laparoscopic cholecystectomy for mild gallstone pancreatitis: time for a paradigm shift. Arch Surg. 2012;147(11):1031–1035. [DOI] [PubMed] [Google Scholar]
- 7.Dubina ED, de Virgilio C, Simms ER, et al. Association of Early vs Delayed Cholecystectomy for Mild Gallstone Pancreatitis With Perioperative Outcomes. JAMA Surg. 2018;153(11):1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg. 2010;251(4):615–619. [DOI] [PubMed] [Google Scholar]
- 9.Bassler D, Briel M, Montori VM, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303(12):1180–1187. [DOI] [PubMed] [Google Scholar]
- 10.Mueck KM, Wei S, Liang MK, et al. Protocol for a randomized trial of the effect of timing of cholecystectomy during initial admission for predicted mild gallstone pancreatitis at a safety-net hospital. Trauma Surg Acute Care Open. 2018;3(1):e000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toouli J, Brooke-Smith M, Bassi C, et al. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17 Suppl:S15–39. [DOI] [PubMed] [Google Scholar]
- 12.Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57(12):1698–1703. [DOI] [PubMed] [Google Scholar]
- 13.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis−-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. [DOI] [PubMed] [Google Scholar]
- 14.Committee ASoP Maple JT, Ben-Menachem T, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71(1):1–9. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carraro A, Mazloum DE, Bihl F. Health-related quality of life outcomes after cholecystectomy. World J Gastroenterol. 2011;17(45):4945–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82(2):216–222. [DOI] [PubMed] [Google Scholar]
- 19.Wijeysundera DN, Austin PC, Hux JE, et al. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol. 2009;62(1):13–21 e15. [DOI] [PubMed] [Google Scholar]
- 20.Hatton GE MK, Leal IM, Wei S, Ko TC, Kao LS. Timely Care is Patient-Centered Care for Patients with Acute Cholecystitis at a Safety-Net Hospital. Unpublished Manuscript. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueck KM, Cherla DV, Taylor A, et al. Randomized Controlled Trials Evaluating Patient-Reported Outcomes after Cholecystectomy: A Systematic Review. J Am Coll Surg. 2018;226(2):183–193 e185. [DOI] [PubMed] [Google Scholar]
- 22.Pendharkar SA, Salt K, Plank LD, et al. Quality of life after acute pancreatitis: a systematic review and meta-analysis. Pancreas. 2014;43(8):1194–1200. [DOI] [PubMed] [Google Scholar]
- 23.Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP. 2006;7(5):447–453. [PubMed] [Google Scholar]
- 24.Kwong WT, Vege SS. Unrecognized necrosis at same admission cholecystectomy for pancreatitis increases organ failure and infected necrosis. Pancreatology. 2017;17(1):41–44. [DOI] [PubMed] [Google Scholar]
- 25.Lyu YX, Cheng YX, Jin HF, et al. Same-admission versus delayed cholecystectomy for mild acute biliary pancreatitis: a systematic review and meta-analysis. BMC Surg. 2018;18(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang DJ, Lu HM, Guo Q, et al. Timing of Laparoscopic Cholecystectomy After Mild Biliary Pancreatitis: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2018;28(4):379–388. [DOI] [PubMed] [Google Scholar]
- 27.Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105(2):435–441; quiz 442. [DOI] [PubMed] [Google Scholar]
- 28.Valverde-Lopez F, Matas-Cobos AM, Alegria-Motte C, et al. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol. 2017;32(9):1649–1656. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Liu J, Xing Y, et al. Comparison of BISAP, Ranson, MCTSI, and APACHE II in Predicting Severity and Prognoses of Hyperlipidemic Acute Pancreatitis in Chinese Patients. Gastroenterol Res Pract. 2016;2016:1834256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Shahbaz M, Fang R, et al. Comparison of the BISAP scores for predicting the severity of acute pancreatitis in Chinese patients according to the latest Atlanta classification. J Hepatobiliary Pancreat Sci. 2014;21(9):689–694. [DOI] [PubMed] [Google Scholar]
- 31.Gao W, Yang HX, Ma CE. The Value of BISAP Score for Predicting Mortality and Severity in Acute Pancreatitis: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(6):e0130412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaghoubian A, De Virgilio C, El-Masry M, et al. Gallstone pancreatitis: a benign disease in Hispanics. Am Surg. 2007;73(10):1071–1074. [PubMed] [Google Scholar]


