Abstract
Increasing studies have demonstrated that long noncoding RNAs (lncRNAs) play vital roles in tumor development and progression. However, the relationship between osteosarcoma and HIF1AAS2 remains unknown. The expression of HIF1AAS2 and miR-129-5p was detected in osteosarcoma cell lines and samples via qRT-PCR. Cell Counting Kit-8 (CCK-8) and invasion assays were performed to determine cell proliferation and invasion ability, and a dual luciferase reporter assay was performed to determine the interaction between HIF1AAS2 and miR-129-5p. We showed that the expression of HIF1A-AS2 was upregulated in the osteosarcoma samples compared with the expression in noncancerous samples. Moreover, patients with high HIF1A-AS2 expression had a shorter overall survival. Ectopic expression of HIF1A-AS2 enhanced osteosarcoma cell proliferation, cell cycle progression and invasion. We found that overexpression of miR-129-5p decreased the luciferase activity of wild-type (WT) HIF1A-AS2 but not mutant HIF1A-AS2. Ectopic expression of HIF1A-AS2 suppressed miR-129-5p expression in MG-63 cells. We demonstrated that miR-129-5p was downregulated in osteosarcoma and was negatively associated with HIF1A-AS2 expression. Furthermore, ectopic expression of miR-129-5p suppressed osteosarcoma cell proliferation, cell cycle progression and invasion. In addition, overexpression of HIF1A-AS2 promoted cell proliferation, cell cycle progression and invasion of osteosarcoma cells through the modulation of miR-129-5p. These results indicated that HIF1A-AS2 might be a potential therapeutic target for osteosarcoma.
Keywords: osteosarcoma, lncRNA, HIF1A-AS2, miR-129-5p
INTRODUCTION
Osteosarcoma, the major cause of tumor-related death in adolescents and children, is the most common malignant primary bone cancer [1–5]. Osteosarcoma is destructive and has high metastatic potential, mostly to the lungs [3, 6–8]. Despite treatment strategies that have been rapidly developed, the five-year survival rate of osteosarcoma is still unsatisfactory [9–11]. Until now, the molecular mechanism underlying the progression and development of osteosarcoma remained unknown [12–14]. Therefore, it is urgently necessary to identify new therapeutic factors or targets for osteosarcoma.
Long noncoding RNAs (lncRNAs) are a group of transcripts that lack protein-coding potential and that are longer than 200 nucleotides [15–18]. Increasing studies have implicated dysregulated lncRNAs in multiple tumors, such as hepatocellular carcinoma, lung cancer, colorectal cancer, bladder cancer and osteosarcoma [19–25]. LncRNAs play vital roles in tumor apoptosis, proliferation, differentiation, metastasis and invasion [26–30]. Recently, a new lncRNA, HIF1A-AS2, was found to be dysregulated in several tumors, such as colorectal cancer, breast cancer, glioblastoma, bladder cancer and gastric cancer [31–35]. It was found that HIF1A-AS2 promoted cell proliferation and invasion. However, the expression and potential role of HIF1A-AS2 in the development of osteosarcoma remain unknown.
In this study, we found that the expression of HIF1A-AS2 was upregulated in the osteosarcoma samples compared with the expression levels in noncancerous samples. Moreover, patients with high HIF1A-AS2 expression had a shorter overall survival than the low HIF1A-AS2 expression group. Furthermore, we studied the function of HIF1A-AS2 in osteosarcoma cells and indicated that ectopic expression of HIF1A-AS2 enhanced osteosarcoma cell proliferation, cell cycle progression and invasion.
RESULTS
HIF1A-AS2 was upregulated in osteosarcoma and was related to poor survival
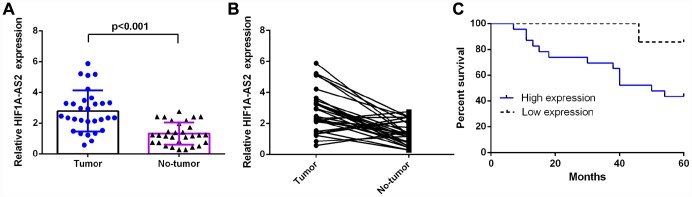
RT-qPCR was performed to measure HIF1A-AS2 expression in 30 osteosarcoma samples and paired adjacent normal tissue, which were normalized to U6. The expression of HIF1A-AS2 was significantly higher in the osteosarcoma samples compared to the expression levels in the paired normal controls (Figure 1A, mean expression in the osteosarcoma samples versus the normal tissue = 2.790 ± 0.2451 vs. 1.321 ± 0.1329, respectively; p<0.001). In addition, the expression of HIF1A-AS2 was upregulated (defined as a cutoff set at log 2.0-fold-change >1) in 23 cases (23/30; 77 %) compared to the expression in adjacent normal tissues (Figure 1B). Moreover, we showed that the median survival of osteosarcoma patients with high HIF1AAS2 expression in primary tumors was 50 months, which was shorter than those with low HIF1AAS2 expression (92 months) (Figure 1C, median overall survival, =50 vs. 92 months; log-rank p<0.01).
Figure 1.
HIF1A-AS2 was upregulated in osteosarcoma and was related to poor survival. (A) The expression of HIF1A-AS2 in 30 osteosarcoma samples and their noncancerous pairs was detected by qRT-PCR. U6 was used as the internal control. (B) The expression of HIF1A-AS2 was upregulated in 23 cases (23/30; 77 %) compared to the expression in adjacent tissues. (Defined as a cutoff of Log 2.0-fold-change >1) (C) The high HIF1A-AS2 expression group had a shorter overall survival than the low HIF1A-AS2 expression group (median overall survival =50 vs. 92 months, respectively; log-rank p<0.01).
HIF1A-AS2 promoted osteosarcoma cell proliferation, cell cycle progression and invasion
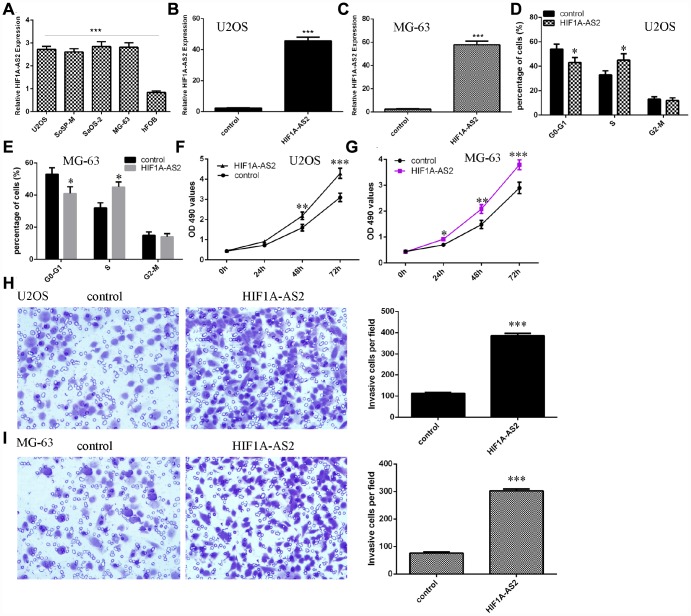
Next, we showed that the expression of HIF1A-AS2 was upregulated in osteosarcoma cell lines (U2OS, SoSP-M, SaOS-2, MG-63) compared to the expression in the osteoblast cell line (hFOB) (Figure 2A, p<0.001). Then, we confirmed that pcDNA-HIF1A-AS2 could enhance the expression of HIF1A-AS2 in U2OS (Figure 2B, p<0.001) and MG-63 (Figure 2C, p<0.001) cells by using qRT-PCR analysis. Moreover, ectopic expression of HIF1A-AS2 increased the S phase of the U2OS (Figure 2D, p<0.05) and MG-63 (Figure 2E, p<0.05) cells compared to that of the control group. The MTT analysis was conducted to measure the growth of the U2OS and MG-63 cells after ectopic expression of HIF1A-AS2. Overexpression of HIF1A-AS2 promoted U2OS (Figure 2F) and MG-63 (Figure 2G) cell growth. Ectopic expression of HIF1A-AS2 increased U2OS (Figure 2H, p<0.001) and MG-63 (Figure 2I, p<0.001) cell invasion, and the relative invasive cells are shown.
Figure 2.
HIF1A-AS2 promoted osteosarcoma cell proliferation, cell cycle progression and invasion. (A) The expression of HIF1A-AS2 in osteosarcoma cell lines (U2OS, SoSP-M, SaOS-2, MG-63) and an osteoblast cell line (hFOB) was measured by qRT-PCR. (B) The expression of HIF1A-AS2 in U2OS cells was determined by qRT-PCR. (C) The expression of HIF1A-AS2 in MG-63 cells was determined by qRT-PCR. (D) Ectopic expression of HIF1A-AS2 increased the S phase of U2OS cells. (E) Ectopic expression of HIF1A-AS2 promoted the S phase of MG-63 cells. (F) Overexpression of HIF1A-AS2 promoted U2OS cell proliferation. (G) Ectopic expression of HIF1A-AS2 promoted MG-63 cell growth. (H) Ectopic expression of HIF1A-AS2 increased U2OS cell invasion, and the relative invasive cells are shown. (I) Ectopic expression of HIF1A-AS2 increased MG-63 cell invasion, and the relative invasive cells are shown. *p<0.05, **p<0.01 and ***p<0.001.
Ectopic expression of HIF1A-AS2 inhibited miR-129-5p expression
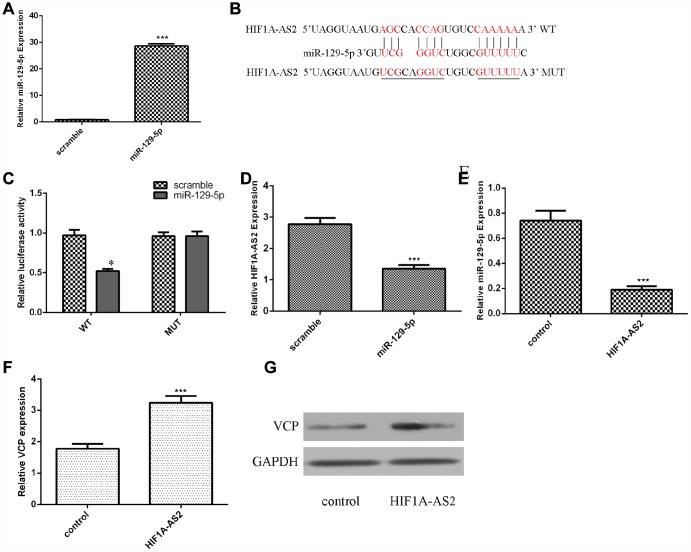
Using qRT-PCR analysis, we confirmed that the miR-129-5p mimic could enhance the expression of miR-129-5p in MG-63 cells (Figure 3A, p<0.001). The binding sites between HIF1A-AS2 and miR-129-5p were identified by using bioinformatics analysis (Figure 3B). A luciferase reporter assay was conducted to validate the binding site combinations. We showed that overexpression of miR-129-5p could decrease the luciferase activity of wild-type (WT) HIF1A-AS2 but not that of the mutant HIF1A-AS2 (Figure 3C, p<0.05). Overexpression of miR-129-5p decreased the expression of HIF1A-AS2 in MG-63 cells (Figure 3D, p<0.01). Ectopic expression of HIF1A-AS2 suppressed miR-129-5p expression in MG-63 cells (Figure 3E, p<0.01). Furthermore, we showed that overexpression of HIF1A-AS2 increased VCP expression in MG-63 cells (Figure 3F, p<0.01). We also confirmed that ectopic expression of HIF1A-AS2 promoted VCP protein expression in MG-63 cells (Figure 3G).
Figure 3.
Ectopic expression of HIF1A-AS2 inhibited miR-129-5p expression. (A) The expression of miR-129-5p in MG-63 cells was detected by using qRT-PCR analysis. (B) The binding sites between HIF1A-AS2 and miR-129-5p obtained from bioinformatics analysis are shown. (C) We showed that overexpression of miR-129-5p can decrease the luciferase activity of wild-type (WT) HIF1A-AS2 but not mutant HIF1A-AS2. (D) Overexpression of miR-129-5p decreased the expression of HIF1A-AS2 in MG-63 cells. (E) Ectopic expression of HIF1A-AS2 suppressed miR-129-5p expression in MG-63 cells. (F) Overexpression of HIF1A-AS2 increased VCP expression in MG-63 cells. (G) The protein expression of VCP was detected by Western blot. GAPDH was used as the control. *p<0.05.
MiR-129-5p was downregulated in osteosarcoma and was negatively correlated with HIF1A-AS2 expression
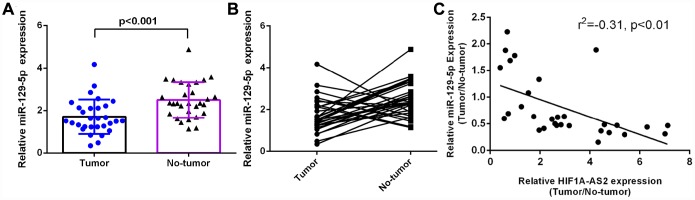
RT-qPCR was performed to analyze miR-129-5p expression in 30 osteosarcoma samples and paired adjacent normal tissues, which were normalized to U6. The expression of miR-129-5p was significantly lower in the osteosarcoma samples than in the paired normal control tissues (Figure 4A, mean expression in the osteosarcoma tissue versus the normal tissue =1.710 ± 0.1479 vs. 2.496 ± 0.1523, respectively; p<0.001). In addition, the expression of miR-129-5p was downregulated (defined as a cutoff set at Log 2.0-fold-change >1) in 22 cases (22/30; 73%) compared to the expression in adjacent normal tissues (Figure 4B). Moreover, we showed that miR-129-5p expression was negatively correlated with HIF1A-AS2 expression (Figure 4C, r2=-0.31, p<0.01) by using Pearson's correlation coefficient analysis.
Figure 4.
miR-129-5p was downregulated in osteosarcoma and was negatively related to HIF1A-AS2 expression. (A) The miR-129-5p expression in 30 osteosarcoma samples and their noncancerous pairs was determined by qRT-PCR. U6 was used as the internal control. (B) The expression of miR-129-5p was downregulated in 22 cancerous tissues (22/30; 73%) compared to the adjacent tissues. (Defined as a cutoff of Log 2.0-fold-change >1) (C) miR-129-5p expression was negatively correlated with HIF1A-AS2 expression in osteosarcoma samples by using Pearson's correlation coefficient analysis.
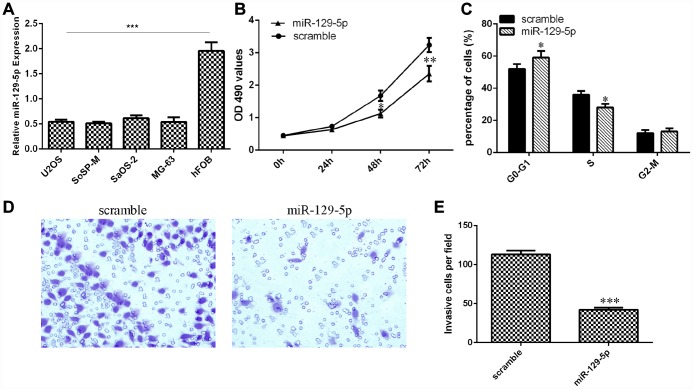
miR-129-5p suppressed osteosarcoma cell proliferation, cell cycle progression and invasion
Next, we showed that the expression of miR-129-5p was downregulated in osteosarcoma cell lines (U2OS, SoSP-M, SaOS-2, MG-63) compared to the expression in the osteoblast cell line (hFOB) (Figure 5A, p<0.001). The MTT analysis was conducted to measure the growth of MG-63 cells after ectopic expression of miR-129-5p. Overexpression of miR-129-5p suppressed MG-63 cell growth (Figure 5B). Ectopic expression of miR-129-5p decreased the S phase of the MG-63 cells compared to that of the control group (Figure 5C, p<0.05). Ectopic expression of miR-129-5p decreased MG-63 cell invasion, and the relative invasive cells are shown (Figure 5D and 5E, p<0.001).
Figure 5.
miR-129-5p suppressed osteosarcoma cell proliferation, cell cycle progression and invasion. (A) The expression of miR-129-5p in osteosarcoma cell lines (U2OS, SoSP-M, SaOS-2, MG-63) and an osteoblast cell line (hFOB) was measured by qRT-PCR. (B) Overexpression of miR-129-5p suppressed MG-63 cell growth. (C) Ectopic expression of miR-129-5p decreased the S phase of MG-63 cells compared to that of the scramble group. (D) Ectopic expression of miR-129-5p decreased MG-63 cell invasion. (E) The relative invasive cells are shown. *p<0.05, **p<0.01 and ***p<0.001.
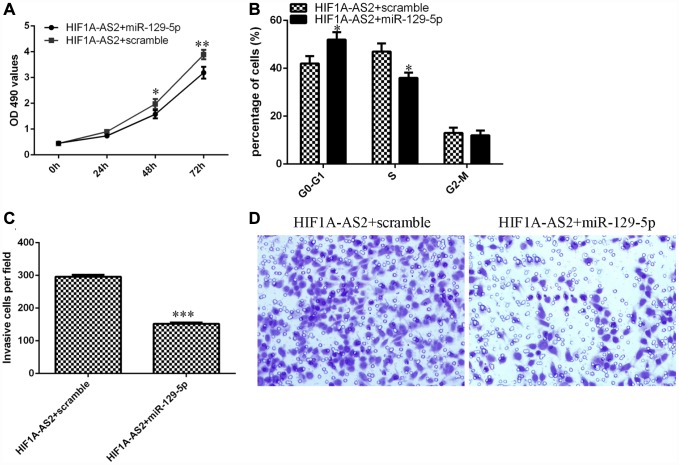
HIF1A-AS2 regulated cell proliferation, cell cycle progression and invasion of osteosarcoma cells through the modulation of miR-129-5p
We aimed to determine whether HIF1A-AS2 acted by silencing miR-129-5p expression. The MTT assay indicated that the miR-129-5p mimic could inhibit the promotion of proliferation induced by HIF1A-AS2 overexpression (Figure 6A). Ectopic expression of miR-129-5p decreased the S phase of the HIF1A-AS2-overexpressing MG-63 cells compared to that of the scrambled group (Figure 6B, p<0.05). In addition, elevated expression of miR-129-5p suppressed the invasion of HIF1A-AS2-overexpressing MG-63 cells compared to that of the scramble group (Figure 6C and 6D, p<0.001).
Figure 6.
HIF1A-AS2 regulates cell proliferation, cell cycle progression and invasion of osteosarcoma cells through the modulation of miR-129-5p. (A) The cell proliferation of MG-63 cells was determined by MTT analysis. (B) Ectopic expression of miR-129-5p decreased the S phase of HIF1A-AS2-overexpressing MG-63 cells compared to that of the scrambled group. (C) Elevated expression of miR-129-5p suppressed the invasion of HIF1A-AS2-overexpressing MG-63 cells compared to the invasion of the scrambled group. (D) The relative invasive cells are shown. *p<0.05, **p<0.01 and ***p<0.001.
DISCUSSION
Increasing studies have suggested that lncRNAs perform critical functions in the modulation of genes that regulate cancer growth, migration, and apoptosis, which has increased our understanding of biological behaviors of several diseases, especially tumors including osteosarcoma [22, 28, 36–39]. Moreover, previous evidence has shown that lncRNAs are therapeutic targets and valuable biomarkers [40, 41]. A previous study suggested that a new lncRNA, HIF1A-AS2, located on chromosome 14q23.2 was upregulated in several cancers, such as neuroblastoma, chronic myeloid leukemia, colorectal cancer and bladder cancer [33, 35]. HIF1A-AS2 was overexpressed in gastric cancer, and HIF1A-AS2 knockdown could suppress gastric cancer cell proliferation and tumorigenesis [31]. However, the relationship between osteosarcoma and HIF1AAS2 remains unknown.
This is the first study to explore the function of HIF1A-AS2 in osteosarcoma. In our research, we detected the mean expression levels and clinical importance of HIF1A-AS2 in osteosarcoma and further investigated its cellular function in osteosarcoma cell lines. Our study demonstrated that the expression of HIF1A-AS2 was upregulated in osteosarcoma samples compared with the expression in noncancerous samples. Moreover, patients with high HIF1A-AS2 expression had a shorter overall survival than the low HIF1A-AS2 expression group. Furthermore, we studied the function of HIF1A-AS2 in osteosarcoma cells and demonstrated that ectopic expression of HIF1A-AS2 enhanced osteosarcoma cell proliferation, cell cycle progression and invasion. Suppressing HIF1A-AS2 expression may present one therapeutic strategy for curing osteosarcoma in the clinical setting. More work is required to explain the molecular mechanisms of HIF1A-AS2 in the development of osteosarcoma.
Previous studies have demonstrated that lncRNAs exert crucial functions in regulating biological cell processes by acting as ‘sponges’ for miRNAs [25, 42]. Lin et al showed that HIF1A-AS2 promoted colorectal cancer progression and epithelial-mesenchymal transition by suppressing miR-129-5p expression. To study the downstream genes of HIF1A-AS2, we used bioinformatics to find a potential miRNA with complementary binding at the HIF1A-AS2 3’-UTR. Furthermore, we performed a luciferase reporter assay and found that overexpression of miR-129-5p could decrease the luciferase activity of wild-type (WT) HIF1A-AS2 but not mutant HIF1A-AS2. Ectopic expression of HIF1A-AS2 suppressed miR-129-5p expression in MG-63 cells. Several studies have suggested that miR-129-5p plays important roles in the development of tumors such as hepatocellular carcinoma, colon cancer, breast cancer, lung cancer, gastric cancer and osteosarcoma [43–48]. Long et al [48] showed that miR-129-5p expression might be regulated by demethylation and that miR-129-5p suppressed osteosarcoma cell invasion and migration by targeting valosin-containing protein (VCP) in osteosarcoma. In addition, Liu et al [49] indicated that lncRNA MALAT1 increased osteosarcoma progression by regulating HMGB1 expression via miR-129-5p and miR-142-3p. In our study, we observed that miR-129-5p was downregulated in osteosarcoma and was negatively related to HIF1A-AS2 expression. Furthermore, ectopic expression of miR-129-5p suppressed osteosarcoma cell proliferation, cell cycle progression and invasion. In addition, overexpression of HIF1A-AS2 promoted cell proliferation, the cell cycle progression and invasion of osteosarcoma cells through the modulation of miR-129-5p.
In conclusion, the data from our study suggested that upregulated HIF1A-AS2 acted as an oncogene in osteosarcoma and induced the tumorigenesis of osteosarcoma by regulating miR-129-5p expression, indicating that HIF1A-AS2 might be a potential therapeutic target for osteosarcoma.
MATERIALS AND METHODS
Tissue samples
Thirty paired osteosarcoma samples and matched adjacent normal bone samples from osteosarcoma patients who underwent surgery were collected from the No. 2 Affiliated Hospital of Qingdao University. The surgically removed tissues were quickly stored in liquid nitrogen until they were used. This research was approved by the local clinical ethics committee of the No. 2 Affiliated Hospital of Qingdao University, and our study was performed following the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Cell culture and transfection
Human osteosarcoma cell lines (U2OS, SoSP-M, SaOS-2, MG-63) and an osteoblast cell line (hFOB 1.19) were purchased from the American Type Culture Collection (ATCC) (Rockville, MD). These cell lines were maintained in DMEM (Dulbecco’s modified Eagle’s medium) (Invitrogen, Carlsbad, CA, USA) supplemented with FBS (fetal bovine serum) and streptomycin/penicillin. miR-129-5p mimic and scramble, pcDNA-HIF1A-AS2 and pcDNA-control were purchased from Ambion. Cells were transfected with these vectors using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
RNA isolation and real-time PCR
Total RNA from the samples or cell lines was separated using a TRIzol kit (Invitrogen, Carlsbad, USA). The expression of miRNA, lncRNA and mRNA was determined using SYBR Green (TaKaRa) with the Applied Biosystems 7900 system according to the manufacturer’s recommendations. The qRT-PCR data were normalized using the 2-ddCt method. The expression of miRNA and lncRNA was normalized to U6. The expression of mRNA was normalized to GAPDH. The primer sequences of these genes were as follows: U6 forward, 5ʹ-CGCTAGCACATATCGGC TA-3ʹ and reverse, 5ʹ-TTCTGCGACGAATTTGTCAT-3ʹ; HIF1A-AS2 forward, 5ʹ-TCTGTGGCTCAGTTCCT TTTGT-3ʹ and reverse, 5ʹ-ATGTAGGAAGTGCCA GAGCC-3ʹ; GAPDH forward, 5ʹ-CGCTCTCTGCTCC TCCTGTTC-3ʹ and reverse, 5ʹ-ATCCGTTGACTCC GACCTTCAC-3ʹ.
Cell growth and invasion assay
Cell growth was determined using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). Osteosarcoma cells were seeded in a 96-well plate, and 10 μL of MTT was added to each well. After three hours, the absorbance value at 490 nm was measured. For cell invasion, cells were cultured in the top of a Matrigel-coated chamber with serum-free DMEM. Medium containing FBS was added to the lower chamber. After 48 hours, cells that invaded the lower well were fixed, stained with hematoxylin and counted. For the cell cycle assay, flow cytometric analysis was used. The cells were fixed with ethanol (70 %) and incubated with RNase A. Subsequently, these cells were incubated with propidium iodide (PI) (Becton–Dickinson, CA, USA) and analyzed on the FACScan flow cytometer (San Jose, USA).
Western blotting
Protein from cells or tissues was extracted using RIPA buffer (Pierce) in accordance with the manufacturer’s recommendations. The protein concentration was quantified using a BCA kit. Equal amounts of protein were separated with SDS–acrylamide gel and transferred into a PVDF membrane (Millipore). After blocking with nonfat milk, the membrane was incubated with anti-VCR and anti-GAPDH antibodies. The membrane was then incubated with secondary antibody at room temperature for half an hour. The signal was determined by enhanced chemiluminescence (ECL). GAPDH was used as the control.
Luciferase reporter assay
MG-63 cells were cultured in a 24-well plate. The binding sites of miR-129-5p and the HIF1A-AS2 3’UTR and the wild-type 3’UTR were subcloned into the pGL3 luciferase promoter vector (Promega, USA). Cells were cotransfected with 3’UTR and wild-type 3’UTR HIF1A-AS2 or miR-129-5p mimics and luciferase reporter plasmids (Promega, USA) using Lipofectamine 2000 following the manufacturer’s protocol. Luciferase activity was detected using the Dual Luciferase Reporter Assay kit (Promega).
Statistical analysis
Data are presented as the mean ± standard deviation (SD) and were measured by SPSS 17.0 (IBM, Chicago, USA). The two-tailed Student’s t test and ANOVA were performed to assess the significance differences. The overall survival analysis of these osteosarcoma patients was performed by log-rank test, and the correlation between HIF1A-AS2 or miR-129-5p was determined by Pearson's correlation coefficient analysis. P<0.05 was accepted as statistically significant.
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interests
FUNDING: This study was supported by the Shandong Province College Science and Technology Project (J15LL10, ZR2015050013) and the Shandong Natural Research Foundation (ZR2016HM31).
REFERENCES
- 1.Li Z, Shen J, Chan MT, Wu WK. MicroRNA-379 suppresses osteosarcoma progression by targeting PDK1. J Cell Mol Med. 2017; 21:315–23. 10.1111/jcmm.12966 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Li Z, Yu X, Shen J. Long non-coding RNAs: emerging players in osteosarcoma. Tumour Biol. 2016; 37:2811–16. 10.1007/s13277-015-4749-4 [DOI] [PubMed] [Google Scholar]
- 3.Tang J, Shen L, Yang Q, Zhang C. Overexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transition. Cell Prolif. 2014; 47:427–34. 10.1111/cpr.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei H, Jin Z, Chen S, Sun X, Yu J, Guo W. MiR-135b promotes proliferation and invasion of osteosarcoma cells via targeting FOXO1. Mol Cell Biochem. 2015; 400:245–52. 10.1007/s11010-014-2281-2 [DOI] [PubMed] [Google Scholar]
- 5.Delebinski CI, Georgi S, Kleinsimon S, Twardziok M, Kopp B, Melzig MF, Seifert G. Analysis of proliferation and apoptotic induction by 20 steroid glycosides in 143B osteosarcoma cells in vitro. Cell Prolif. 2015; 48:600–10. 10.1111/cpr.12208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes DP. How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat Res. 2009; 152:479–96. 10.1007/978-1-4419-0284-9_28 [DOI] [PubMed] [Google Scholar]
- 7.Huang G, Nishimoto K, Zhou Z, Hughes D, Kleinerman ES. miR-20a encoded by the miR-17-92 cluster increases the metastatic potential of osteosarcoma cells by regulating Fas expression. Cancer Res. 2012; 72:908–16. 10.1158/0008-5472.CAN-11-1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji F, Zhang H, Wang Y, Li M, Xu W, Kang Y, Wang Z, Wang Z, Cheng P, Tong D, Li C, Tang H. MicroRNA-133a, downregulated in osteosarcoma, suppresses proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone. 2013; 56:220–26. 10.1016/j.bone.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Jia LS, Yuan W, Wu Z, Wang HB, Xu T, Sun JC, Cheng KF, Shi JG. Low miR-34a and miR-192 are associated with unfavorable prognosis in patients suffering from osteosarcoma. Am J Transl Res. 2015; 7:111–19. [PMC free article] [PubMed] [Google Scholar]
- 10.Huang G, Nishimoto K, Yang Y, Kleinerman ES. Participation of the Fas/FasL signaling pathway and the lung microenvironment in the development of osteosarcoma lung metastases. Adv Exp Med Biol. 2014; 804:203–17. 10.1007/978-3-319-04843-7_11 [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Luo LH, Li S, Yang C. miR-17 inhibitor suppressed osteosarcoma tumor growth and metastasis via increasing PTEN expression. Biochem Biophys Res Commun. 2014; 444:230–34. 10.1016/j.bbrc.2014.01.061 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Duan G, Feng S. MicroRNA-301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha 1. Biochem Biophys Res Commun. 2015; 459:367–73. 10.1016/j.bbrc.2015.02.101 [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013; 25:398–406. 10.1097/CCO.0b013e3283622c1b [DOI] [PubMed] [Google Scholar]
- 14.Kelly AD, Haibe-Kains B, Janeway KA, Hill KE, Howe E, Goldsmith J, Kurek K, Perez-Atayde AR, Francoeur N, Fan JB, April C, Schneider H, Gebhardt MC, et al. MicroRNA paraffin-based studies in osteosarcoma reveal reproducible independent prognostic profiles at 14q32. Genome Med. 2013; 5:2. 10.1186/gm406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Xue WJ, Feng Y, Mao QS. Long non-coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016; 8:3522–29. [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y, Jiang X. LncRNA-ATB: an indispensable cancer-related long noncoding RNA. Cell Prolif. 2017; 50:50. 10.1111/cpr.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Lian Y, Yan C, Cai Z, Ding J, Ma Z, Peng P, Wang K. Long non-coding RNA FOXP4-AS1 is an unfavourable prognostic factor and regulates proliferation and apoptosis in colorectal cancer. Cell Prolif. 2017; 50:50. 10.1111/cpr.12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Shen J, Chan MT, Wu WK. TUG1: a pivotal oncogenic long non-coding RNA of human cancers. Cell Prolif. 2016; 49:471–75. 10.1111/cpr.12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin Y, Li Z, Shen J, Chan MT, Wu WK. CCAT1: a pivotal oncogenic long non-coding RNA in human cancers. Cell Prolif. 2016; 49:255–60. 10.1111/cpr.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Jin Y, Ren H, Ma X, Wang B, Wang Y. Downregulation of the long non-coding RNA TUSC7 promotes NSCLC cell proliferation and correlates with poor prognosis. Am J Transl Res. 2016; 8:680–87. [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Lin J. Long noncoding RNA ZEB1-AS1 acts as an oncogene in osteosarcoma by epigenetically activating ZEB1. Am J Transl Res. 2016; 8:4095–105. [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Liu S, Wang H, Zhang Z, Yang Q, Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016; 8:5025–34. [PMC free article] [PubMed] [Google Scholar]
- 23.Dou J, Ni Y, He X, Wu D, Li M, Wu S, Zhang R, Guo M, Zhao F. Decreasing lncRNA HOTAIR expression inhibits human colorectal cancer stem cells. Am J Transl Res. 2016; 8:98–108. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu HQ, Zhou X, Chang H, Li HG, Liu FF, Ma CQ, Lu J. Aberrant expression of ccat1 regulated by c-myc predicts the prognosis of hepatocellular carcinoma. Asian Pacific journal of cancer prevention. Asian Pac J Cancer Prev. 2015; 16:5181–85. 10.7314/APJCP.2015.16.13.5181 [DOI] [PubMed] [Google Scholar]
- 25.Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015; 34:18. 10.1186/s13046-015-0136-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, Yang J, Li Q, Xu B, Lian Y, Miao L. LINC00152: A pivotal oncogenic long non-coding RNA in human cancers. Cell Prolif. 2017; 50:50. 10.1111/cpr.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H, Zhou X, Chang H, Li H, Liu F, Ma C, Lu J. CCAT1 promotes hepatocellular carcinoma cell proliferation and invasion. Int J Clin Exp Pathol. 2015; 8:5427–34. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Yao T, Wang Y, Yu J, Liu Y, Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther. 2016; 17:104–13. 10.1080/15384047.2015.1108496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H, Li X, Song Y, Zhang P, Xiao Y, Xing Y. Long non-coding RNA ANRIL is up-regulated in bladder cancer and regulates bladder cancer cell proliferation and apoptosis through the intrinsic pathway. Biochem Biophys Res Commun. 2015; 467:223–28. 10.1016/j.bbrc.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 30.Bashari D, Jerbi A, Feldstein O, Ginsberg D. The long non-coding rna anril is a direct transcriptional target of e2f1 that mediates e2f1-induced proliferation. Eur J Cancer. 2012; 48:S55–55. 10.1016/S0959-8049(12)70920-6 [DOI] [PubMed] [Google Scholar]
- 31.Chen WM, Huang MD, Kong R, Xu TP, Zhang EB, Xia R, Sun M, De W, Shu YQ. Antisense long noncoding rna hif1a-as2 is upregulated in gastric cancer and associated with poor prognosis. Dig Dis Sci. 2015; 60:1655–62. 10.1007/s10620-015-3524-0 [DOI] [PubMed] [Google Scholar]
- 32.Mineo M, Ricklefs F, Lyons SS, Ivanov P, Chiocca EA, Godlewski J, Bronisz A. The role of long noncoding rna hif1a-as2 in hypoxic environment of glioblastoma. Cancer Res. 2016; 76 10.1158/1538-7445.nonrna15-pr04 [DOI] [Google Scholar]
- 33.Chen M, Zhuang C, Liu Y, Li J, Dai F, Xia M, Zhan Y, Lin J, Chen Z, He A, Xu W, Zhao G, Guo Y, et al. Tetracycline-inducible shRNA targeting antisense long non-coding RNA HIF1A-AS2 represses the malignant phenotypes of bladder cancer. Cancer Lett. 2016; 376:155–64. 10.1016/j.canlet.2016.03.037 [DOI] [PubMed] [Google Scholar]
- 34.Li L, Wang M, Mei Z, Cao W, Yang Y, Wang Y, Wen A. lncRNAs HIF1A-AS2 facilitates the up-regulation of HIF-1α by sponging to miR-153-3p, whereby promoting angiogenesis in HUVECs in hypoxia. Biomed Pharmacother. 2017; 96:165–72. 10.1016/j.biopha.2017.09.113 [DOI] [PubMed] [Google Scholar]
- 35.Lin J, Shi Z, Yu Z, He Z. LncRNA HIF1A-AS2 positively affects the progression and EMT formation of colorectal cancer through regulating miR-129-5p and DNMT3A. Biomed Pharmacother. 2018; 98:433–39. 10.1016/j.biopha.2017.12.058 [DOI] [PubMed] [Google Scholar]
- 36.Li C, Lei B, Huang S, Zheng M, Liu Z, Li Z, Deng Y. H19 derived microRNA-675 regulates cell proliferation and migration through CDK6 in glioma. Am J Transl Res. 2015; 7:1747–64. [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Lin Y, Meng H, Liu C, Xue J, Zhang Q, Li C, Zhang P, Cui F, Chen W, Jiang A. Long non-coding RNA LOC283070 mediates the transition of LNCaP cells into androgen-independent cells possibly via CAMK1D. Am J Transl Res. 2016; 8:5219–34. [PMC free article] [PubMed] [Google Scholar]
- 38.Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, Quan ZW. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015; 6:e1583. 10.1038/cddis.2014.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng ZQ, Lu RB, Xiao DM, Xiao ZM. Increased expression of the lncRNA BANCR and its prognostic significance in human osteosarcoma. Genet Mol Res. 2016; 15. 10.4238/gmr.15017480 [DOI] [PubMed] [Google Scholar]
- 40.Tian ZZ, Guo XJ, Zhao YM, Fang Y. Decreased expression of long non-coding RNA MEG3 acts as a potential predictor biomarker in progression and poor prognosis of osteosarcoma. Int J Clin Exp Pathol. 2015; 8:15138–42. [PMC free article] [PubMed] [Google Scholar]
- 41.Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH, Guo RH. Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biol. 2015; 36:4851–59. 10.1007/s13277-015-3139-2 [DOI] [PubMed] [Google Scholar]
- 42.Wang G, Pan J, Zhang L, Wei Y, Wang C. Long non-coding RNA CRNDE sponges miR-384 to promote proliferation and metastasis of pancreatic cancer cells through upregulating IRS1. Cell Prolif. 2017; 50:50. 10.1111/cpr.12389 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Xu C, Shao Y, Xia T, Yang Y, Dai J, Luo L, Zhang X, Sun W, Song H, Xiao B, Guo J. lncRNA-AC130710 targeting by miR-129-5p is upregulated in gastric cancer and associates with poor prognosis. Tumour Biol. 2014; 35:9701–06. 10.1007/s13277-014-2274-5 [DOI] [PubMed] [Google Scholar]
- 44.You BR, Park WH. Suberoylanilide hydroxamic acid induces thioredoxin1-mediated apoptosis in lung cancer cells via up-regulation of miR-129-5p. Mol Carcinog. 2017; 56:2566–77. 10.1002/mc.22701 [DOI] [PubMed] [Google Scholar]
- 45.Zuo Y, Li Y, Zhou Z, Ma M, Fu K. Long non-coding RNA MALAT1 promotes proliferation and invasion via targeting miR-129-5p in triple-negative breast cancer. Biomed Pharmacother. 2017; 95:922–28. 10.1016/j.biopha.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, Cao J, Zhong Q, Zeng L, Cai C, Lei L, Zhang W, Liu F. Long noncoding RNA PCAT-1 promotes invasion and metastasis via the miR-129-5p-HMGB1 signaling pathway in hepatocellular carcinoma. Biomed Pharmacother. 2017; 95:1187–93. 10.1016/j.biopha.2017.09.045 [DOI] [PubMed] [Google Scholar]
- 47.Fu L, Chen Q, Yao T, Li T, Ying S, Hu Y, Guo J. Hsa_circ_0005986 inhibits carcinogenesis by acting as a miR-129-5p sponge and is used as a novel biomarker for hepatocellular carcinoma. Oncotarget. 2017; 8:43878–88. 10.18632/oncotarget.16709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long XH, Zhou YF, Peng AF, Zhang ZH, Chen XY, Chen WZ, Liu JM, Huang SH, Liu ZL. Demethylation-mediated miR-129-5p up-regulation inhibits malignant phenotype of osteogenic osteosarcoma by targeting Homo sapiens valosin-containing protein (VCP). Tumour Biol. 2015; 36:3799–806. 10.1007/s13277-014-3021-7 [DOI] [PubMed] [Google Scholar]
- 49.Liu K, Huang J, Ni J, Song D, Ding M, Wang J, Huang X, Li W. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle. 2017; 16:578–87. 10.1080/15384101.2017.1288324 [DOI] [PMC free article] [PubMed] [Google Scholar]