Abstract
Background and study aims While several interventions may decrease risk of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis, it remains unclear whether one strategy is superior to others. The purpose of this study was to compare the effectiveness of pharmacologic and endoscopic interventions to prevent post-ERCP pancreatitis among high-risk patients.
Methods A systematic review was performed to identify randomized controlled trials from PubMed, Embase, Web of Science, and Cochrane database through May 2017. Interventions included: rectal non-steroidal anti-inflammatory drugs (NSAIDs), aggressive hydration with lactated ringerʼs (LR) solution, and pancreatic stent placement compared to placebo. Only studies with patients at high-risk for post-ERCP pancreatitis were included. Bayesian network meta-analysis was performed and relative ranking of treatments was assessed using surface under the cumulative ranking (SUCRA) probabilities.
Results We identified 29 trials, comprising 7,862 participants comparing four preventive strategies. On network meta-analysis, compared with placebo, rectal NSAIDs (B = – 0.69, 95 % CI [–1.18; – 0.21]), pancreatic stent (B = – 1.25, 95 % CI [–1.81 to –0.69]), LR (B = – 0.67, 95 % CI [–1.20 to –0.13]), and combination of LR plus rectal NSAIDs (B = – 1.58; 95 % CI [–3.0 to –0.17]), were all associated with a reduced risk of post-ERCP pancreatitis. Pancreatic stent placement had the highest SUCRA probability (0.81, 95 % CI [0.83 to 0.80]) of being ranked the best prophylactic treatment.
Conclusions Based on this network meta-analysis, pancreatic stent placement appears to be the most effective preventive strategy for post-ERCP pancreatitis in high-risk patients.
Introduction
Although endoscopic retrograde cholangiopancreatography (ERCP) is commonly performed for diagnosis and management of pancreato-biliary disease, the procedure itself may result in a host of potential adverse events (AEs) – the most common of which is post-ERCP pancreatitis 1 2 3 . While the current literature estimates incidence of post-ERCP pancreatitis to be between 3 % to 5 %, a recent systematic review including over 2000 high-risk patients who underwent ERCP demonstrated an incidence of 14.7 % with an associated 0.2 % mortality rate 4 5 6 .
Given this high incidence of post-ERCP pancreatitis in association with substantial morbidity, mortality, and healthcare costs of $ 200 million annually in the United States, it is not surprising that several preventive strategies, both pharmacologic and endoscopic, have been employed 7 . Currently, there are several studies evaluating a variety of pharmacologic prophylaxes for post-ERCP pancreatitis in high-risk patients that include administration of rectal non-steroidal anti-inflammatory drugs (NSAIDs) or aggressive hydration with lactated ringerʼs solution (LR) 8 9 10 11 12 13 . In addition, several studies have demonstrated the efficacy of prophylactic pancreatic stent placement in reducing the rate of post-ERCP pancreatitis and more importantly in reducing the risk of severe post-ERCP pancreatitis 14 15 16 .
However, due to paucity of data, it remains unclear whether pharmacologic, endoscopic, or a combination of both approaches is the preferred strategy to prevent post-ERCP pancreatitis. While previous direct pairwise meta-analyses have attempted to answer this question about prevention of post-ERCP pancreatitis, these types of studies are only able to provide partial information due to inherent design limitations. The primary aim of this study is to design a network meta-analysis, simultaneously analyzing direct and indirect evidence, to compare the effectiveness of pharmacologic and endoscopic treatment approaches to prevent post-ERCP pancreatitis.
Methods
Study design
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement outline for reporting systematic reviews and meta-analyses and was conducted following a priori established protocol 17 18 . We utilized a network meta-analysis and Bayesian framework to combine direct and indirect evidence comparing the relative efficacy of pharmacologic and endoscopic prophylaxis treatments for post-ERCP pancreatitis. This method was chosen to compare multiple interventions and synthesize evidence across a network of randomized controlled trials 19 20 . In brief, this method allows for simultaneous analysis of direct evidence from comparator trials as well as indirect evidence from different treatments compared to a common comparator (i. e., placebo or no intervention) 21 .
Selection criteria and study outcomes
Only randomized controlled trials for high-risk patients were included in this network meta-analysis. High-risk patients were defined in randomized controlled trials by procedure-related factors such as difficult cannulation, pancreatic duct injection, pancreatic sphincterotomy, and pre-cut sphincterotomy in addition to patient-associated factors including female sex, those with sphincter of Oddi dysfunction, and patients with recurrent pancreatitis or prior history of post-ERCP pancreatitis 22 23 24 . Only trials involving high-risk patients were included.
Included studies involved adult patients (age ≥ 18 years) who underwent ERCP involving use of rectal NSAIDs (i. e., indomethacin or diclofenac), aggressive hydration with LR solution (defined as ≥ 3 mL/kg/h during the procedure and post-procedure intravenous bolus or high-rate infusion), prophylactic pancreatic stent placement, or placebo. Studies evaluating these treatments alone or in combination as well as studies evaluating direct comparator studies to placebo were included. Observational studies and trials evaluating prevention or reduction of post-ERCP pancreatitis were excluded. Post-ERCP pancreatitis was defined by the consensus criteria as a clinical syndrome consistent with pancreatitis as typical epigastric abdominal pain with an amylase or lipase level at least three times 25 .
Data sources and literature search strategy
Two authors (BN and TRM) independently conducted a comprehensive search of the literature to identify articles that examined multiple electronic databases. Systematic searches of PubMed, EMBASE, Web of Science, the Cochrane Library databases, and major annual gastroenterology conference proceedings were performed from inception through May 31, 2017. Published abstract proceedings were extracted from major gastrointestinal meetings from January 2010 to May 2017. Scientific meetings included Digestive Disease Week (DDW), United European Gastroenterology Week (UEGW), and the American College of Gastroenterology (ACG) annual meeting.
Search terms included: “post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis,” “rectal non-steroidal anti-inflammatory drugs (NSAIDs),” “lactated ringerʼs (LR),” “prophylactic pancreatic stent,” and “post-ERCP pancreatitis prophylaxis.” All relevant articles irrespective of language, year of publication, type of publication, or publication status were included. Titles and abstracts of all potentially relevant studies were screened for eligibility. Reference lists of studies of interest were then manually reviewed for additional articles with additional references acquired through cross-checking bibliographies of retrieved full-text papers. Any differences were resolved by mutual agreement and in consultation with the third reviewer (UN). In the case of studies with incomplete information, contact was attempted with the principal authors to obtain additional data.
Data abstraction and quality assessment
Study data were abstracted by two authors (BN and TRM) independently. Risk of bias of individual studies was evaluated and assessed in the context of the primary outcome (i. e., development of post-ERCP pancreatitis) using the Cochrane Risk of Bias assessment tool 26 . The GRADE approach to rate the quality of evidence of estimates derived from this network meta-analysis was also performed 27 28 . Direct evidence from randomized controlled trials was initially identified as high quality; however, it could be down-graded based on risk of bias, indirectness, imprecision, heterogeneity, or publication bias. Rating of indirect estimates began at the lowest rating of the two pairwise estimates that contribute as first-order loops to the indirect estimate but could be down-graded further for imprecision or intransitivity. If direct and indirect estimates were similar (i.e., coherent), then the higher of their rating was assigned to the network meta-analysis estimates.
Statistical analysis
Direct meta-analysis was performed using DerSimonian and Laird random effects model to estimate pooled odds ratio (OR) and 95 % confidence interval (CI) 29 . Multivariate random-effects meta-regression was utilized to present results using the Stata mvmeta command extension 30 . Heterogeneity was assessed using I 2 statistic with values > 50 % indicating substantial heterogeneity 31 . With regard to publication bias, funnel plot asymmetry and Egger’s regression test were performed as well 32 . Direct comparisons were performed using RevMan v5.3 (Cochrane Collaboration, Copenhagen, Denmark) 33 .
Indirect comparisons using a fixed effects Bayesian network meta-analysis was performed using a Markov chain Monte Carlo method in WinBUGS statistical analysis program version 1.4.4 (MRC Biostatistics Unit, Institute of Public Health, Cambridge, UK) and Network Plots were created in Stata SE-13 31 34 . The Bayesian meta-analysis approach offers greater flexibility for the use of more complex models and different outcome types, thereby enabling the simultaneous comparison of all treatment options 35 . Comparative effectiveness of any two treatments were modeled as a function of each treatment in relation to the reference treatment (i. e., placebo or no intervention) to assume consistency of treatment effects across all included randomized controlled trials. This thereby ensures the direct and indirect effect estimates are the same effects.
With regard to network consistency, direct and indirect estimates were evaluated using a node-splitting technique. Posterior distribution of all parameters was estimated using non-informative priors to limit inference to data derived from the trials. No assumptions about efficacy of rectal NSAIDs, aggressive resuscitation with LR, or prophylactic pancreatic stent placement from data external to the trials was included in this systematic review and network meta-analysis.
We used the Markov chain Monte Carlo method to obtain pooled effect sizes 35 . All chains were run with 50,000 simulated draws after a burn-in of 5,000 iterations. The median of the posterior distribution based upon 50,000 simulations was obtained using the 2.5 th and 97.5 th percentiles, after adjusted for multiple arms. Risk of post-ERCP pancreatitis was reported using the estimate of the beta coefficient and calculated the 95 % credible interval.
Information on the relative effects was converted into a probability that a treatment was best, (i. e., first-best, second-best, etc) and into a ranking for each treatment, called the surface under the cumulative ranking curve (SUCRA) 36 37 . A SUCRA value of 1.0 guarantees when a treatment is certainly the best and 0 when a treatment is certainly the worst. SUCRA values enable overall ranking of treatments for a particular outcome. SUCRA simplifies the information about the effect of each treatment into a single number, thereby assisting the decision-making process.
Sensitivity analysis
Sensitivity analyses were also performed to assess the robustness and generalizability of our findings. Sensitivity analysis was performed for high-quality studies as determined by GRADE assessment. Low-quality studies were excluded. Additional sensitivity analysis was performed for full-text manuscripts. Abstracts that did not result in full manuscript publication were excluded.
Results
Based on previously discussed literature search criteria, a total of 29 trials were included in this study – comprising 7,862 participants comparting four preventive strategies for post-ERCP pancreatitis. A PRISMA flow chart of search results is shown in Fig.1 . A network plot of network meta-analysis comparisons of included studies is highlighted in Fig. 2 .
Fig. 1.
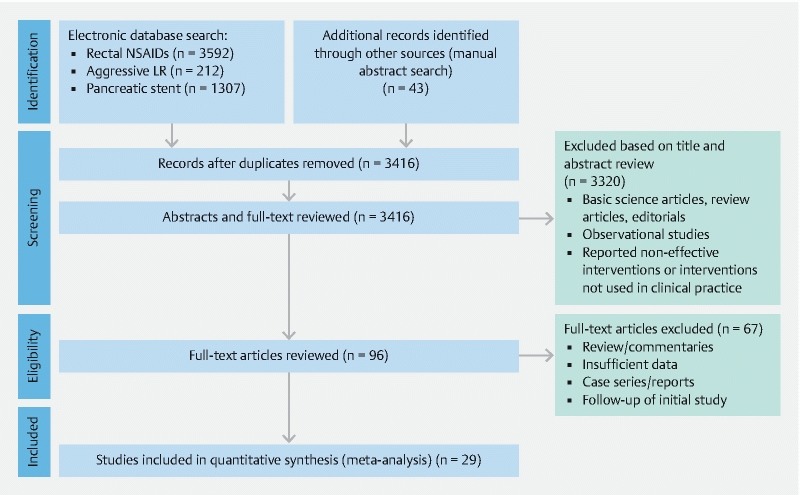
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of search results for the prevention of post-ERCP pancreatitis.
Fig. 2.

Network meta-analysis design of included studies for the prevention of post-ERCP pancreatitis.
Characteristics of included studies
The primary outcome of post-ERCP pancreatitis was reported in all studies ( Table 1 ). Thirteen studies, including 5,955 patients with rectal NSAID use for prevention of post-ERCP pancreatitis were included 8 9 10 17 38 39 40 41 42 43 44 45 46 . All but one study (abstract) were fully published manuscripts 45 . Eight studies involving 754 patients evaluated use of aggressive hydration with LR solution in the prophylaxis of post-ERCP pancreatitis 11 12 13 47 48 49 50 51 . Four studies were fully published manuscripts, three studies were major gastroenterology meeting abstracts, and a final study was a RCT identified through ClinicalTrials.gov. One of the fully published manuscripts was a double-blinded, placebo-controlled trial that included both LR solution independently, as well as in combination with rectal NSAID use (n = 96) 51 . Nine studies, including a total of 1057 participants, evaluated use of prophylactic pancreatic stent placement 14 15 16 52 53 54 55 56 57 .
Table 1. Summary characteristics of included studies for prevention of post-ERCP Pancreatitis.
| Author | Year, place of study | Place of study | Type of manuscript | Study design | Sample size | Mean age of treatment group (years) | Female gender (%) | Primary ERCP indication of bile duct stone (%) | Trial-specific treatment details |
| Rectal NSAID Therapy | |||||||||
| Murray et al. | 2003 | Scotland | Full text | Single-center comparator to placebo | 220 | 55 | 65 % | 25.45 % | Diclofenac 100 mg immediately post-ERCP |
| Sotoudehmanesh et al. | 2007 | Iran | Full text | Single-center comparator to placebo | 490 | 58.4 | 53.90 % | 53.26 % | Indomethacin 100 mg immediately pre-ERCP |
| Otsuka et al. | 2012 | Japan | Full text | Multicenter comparator to placebo | 104 | 75 | 49.04 % | 77.88 % | Diclofenac 25 – 50 mg immediately pre-ERCP |
| Elmunzer et al. | 2012 | United States | Full text | Multicenter comparator to placebo | 602 | 44.4 | 79.07 % | – | Indomethacin 100 mg immediately post-ERCP |
| Dobronte et al. | 2012 | Hungary | Full text | Single-center comparator to placebo | 228 | – | – | – | Indomethacin 100 mg 10 – 15 minutes pre-ERCP |
| Alabd and Abo | 2013 | Sudan | Abstract | Single-center comparator to placebo | 240 | – | – | – | – |
| Dobronte et al. | 2014 | Hungary | Full text | Single-center comparator to placebo | 665 | 66.8 | – | – | Indomethacin 100 mg 10 – 15 minutes pre-ERCP |
| Andrade-Davila et al. | 2015 | Mexico | Full text | Single-center comparator to placebo | 166 | 51.59 | 66.27 % | 39.76 % | Indomethacin 100 mg immediately post-ERCP |
| Patai et al. | 2015 | Hungary | Full text | Single-center comparator to placebo | 574 | 66.25 | 67.16 % | 58.63 % | Indomethacin 100 mg 60 minutes pre-ERCP |
| Lua et al. | 2015 | Malaysia | Full text | Single-center comparator to placebo | 151 | 50.3 | 59.03 % | 56.25 % | Diclofenac 100 mg immediately post-ERCP |
| Luo et al. | 2016 | China | Full text | Multicenter comparator to placebo | 2014 | 62 | 52.42 % | 77.50 % | Indomethacin 100 mg 30 minutes pre-ERCP |
| Levenick et al. | 2016 | United States | Full text | Single-center comparator to placebo | 449 | 64.9 | 52.56 % | 27.72 % | Indomethacin 50 mg × 2 during ERCP |
| Ucar et al. | 2016 | Turkey | Full text | Single-center comparator to placebo | 100 | 59 | 66.66 % | 83 % | Diclofenac 100 mg 30 – 90 minutes pre-ERCP |
| Aggressive LR solution | |||||||||
| Buxbaum et al. | 2014 | United States | Full text | Single-center comparator to standard hydration | 62 | 43 | 51.61 % | 74.20 % | IV LR solution at a rate of 3.0 mL/kg/h during ERCP, a bolus of 20 mL/kg immediately post-ERCP, followed by post-ERCP rate of 3.0 mL/kg/h for 8 h |
| Chuankrekkul et al. | 2015 | Thailand | Abstract | Single-center comparator to standard hydration | 60 | 61.9 | – | – | IV LR solution at a rate of 3.0 mL/kg/h during ERCP, a bolus of 10 mL/kg immediately post-ERCP, followed by post-ERCP rate of 3.0 mL/kg/h for 8 h |
| Shaygan-Nejad et al. | 2015 | Iran | Full text | Single-center comparator to standard hydration | 150 | 49.6 | 66 % | 95.35 % | IV LR solution at a rate of 3.0 mL/kg/h during ERCP, a bolus of 20 mL/kg immediately post-ERCP, followed by post-ERCP rate of 3.0 mL/kg/h for 8 h |
| Rosa et al. | 2016 | Portugal | Abstract | Multicenter comparator to standard hydration | 68 | – | – | – | IV LR solution at a rate of 3.0 mL/kg/h during ERCP, a bolus of 20 mL/kg immediately post-ERCP, followed by post-ERCP rate of 3.0 mL/kg/h for 8 h |
| NCT02050048 | 2016 | United States | – | Multicenter comparator to standard hydration | 26 | 59.1 | 84.62 % | – | Initial bolus LR solution of 7.58 mL/kg pre-ERCP, IV LR solution of 5.0 mL/kg/h during ERCP, following by post-ERCP bolus of 20 mL/kg for 90 minutes |
| Chang et al. | 2016 | Thailand | Abstract | Single-center comparator to standard hydration | 171 | – | – | 50 % | IV LR solution at a rate of 150 mL/h starting 2 h pre-ERCP, and continued during and post-ERCP for 24 h |
| Choi et al. | 2016 | Korea | Full text | Multicenter comparator to standard hydration | 510 | 57.6 | 45.50 % | 53.70 % | Initial bolus LR solution of 10 mL/kg pre-ERCP, IV LR solution of 3.0 mL/kg/h during ERCP and continued 8 h post-ERCP, following by post-ERCP bolus of 10 mL/kg |
| Mok et al. | 2017 | United States | Full text | Single-center comparator with multiple therapies* | 48 | 60.25 | 60.60 % | – | Treatment arms included standard normal saline solution vs normal saline plus indomethacin versus LR versus LR plus indomethacin |
| Rectal NSAIDs + LR solution | |||||||||
| Mok et al. | 2017 | United States | Full text | Single-center comparator with multiple therapies* | 48 | 60.25 | 60.60 % | – | Treatment arms included standard normal saline solution vs normal saline plus indomethacin versus LR versus LR plus indomethacin |
| Pancreatic stent placement | |||||||||
| Smithline et al. | 1993 | United States | Full text | Single-center comparator to placebo | 99 | 46 | 78.79 % | – | 5 – 7 Fr stent, 2 – 2.5 cm in length |
| Tarnasky et al. | 1998 | United States | Full text | Single-center comparator to placebo | 80 | 46.05 | – | – | 5 – 7 Fr stent, 2 – 2.5 cm in length |
| Fazel et al. | 2003 | United States | Full text | Single-center comparator to placebo | 74 | 44.7 | 86.49 % | – | Nasopancreatic 5 Fr catheter or 5 Fr stent, 2 cm length, 2 barbed |
| Harewood et al. | 2005 | United States | Full text | Single-center comparator to placebo | 19 | 48.75 | 63.16 % | – | Straight, single flanged, polyethylene 5 Fr stent, 3 – 5 cm length |
| Sofuni et al. | 2011 | Japan | Full text | Multicenter comparator to placebo | 201 | 66.4 | 37.81 % | – | Straight, 5 Fr stent, 3 cm in length |
| Pan et al. | 2012 | China | Full text | Single-center comparator to placebo | 40 | 58.3 | 52.50 % | – | Single pigtail, 5 Fr stent |
| Lee et al. | 2012 | Korea | Full text | Single-center comparator to placebo | 101 | 57.5 | 62.38 % | 66.33 % | Single pigtail unflanged 3 Fr stent, 4 – 8 cm length |
| Kawaguchi et al. | 2012 | Japan | Full text | Single-center comparator to placebo | 120 | 67 | 56.67 % | 35.83 % | Unflanged on pancreatic duct side, 2 flanges on duodenal side 5 Fr stent, 3 cm length |
| Cha et al. | 2013 | United States | Full text | Single-center comparator to placebo | 151 | 56.6 | 58.94 % | 15.89 % | Straight or external 3/4 pigtail 5 – 7 Fr stent, 2 – 2.5 cm length |
ERCP, endoscopic retrograde cholangiopancreatography; NSAID, nonsteroidal anti-inflammatory drug; IV, intravenous; LR, lactated ringer’s
Quality assessment
GRADE assessment was performed for all included studies. These review authors’ judgements about each risk of bias item for each included study is highlighted in Supplemental Fig. 1 . A risk of bias summary graph is also available in Supplemental Fig. 2 .
Direct treatment comparisons
For direct treatment comparisons, all pharmacologic and endoscopic modalities were compared to placebo or no intervention ( Supplemental Table 1 ). Compared to placebo, use of rectal NSAID therapy significantly reduced odds of post-ERCP pancreatitis by 49 % (OR = 0.51, 95 % CI [0.33 to 0.77]) ( Fig.3 ). With regard to the effect of aggressive LR solution, this strategy was also effective in significantly reducing post-ERCP pancreatitis by 52 % (OR = 0.48, 95 % CI [0.24 to 0.97]) ( Fig. 4 ). When these two modalities were combined and compared to placebo, use of rectal NSAID and aggressive LR solution demonstrated 75 % reduced odds of developing post-ERCP pancreatitis; however, this was not statistically significant (OR = 0.25, 95 % CI [0.06 to 0.99]). Prophylactic pancreatic duct stent placement was effective in decreasing post-ERCP pancreatitis by 71 % (OR = 0.29, 95 % CI [0.17 to 0.48]) ( Fig. 5 ).
Fig. 3.
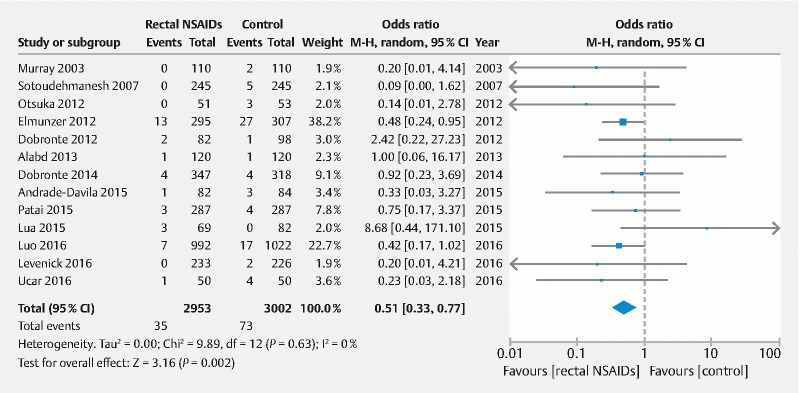
Direct treatment comparison of rectal NSAIDs to placebo for the prevention of post-ERCP pancreatitis.
Fig. 4.
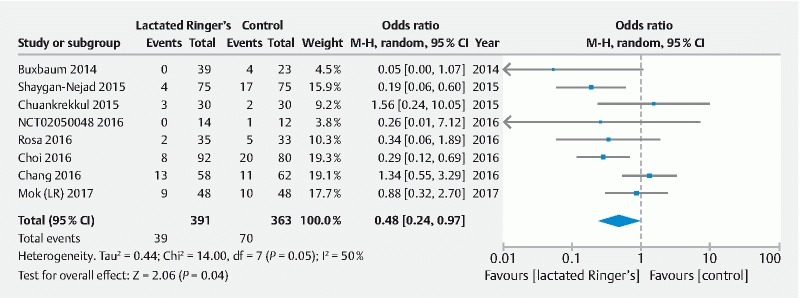
Direct treatment comparison of aggressive LR solution to placebo for the prevention of post-ERCP pancreatitis.
Fig. 5.
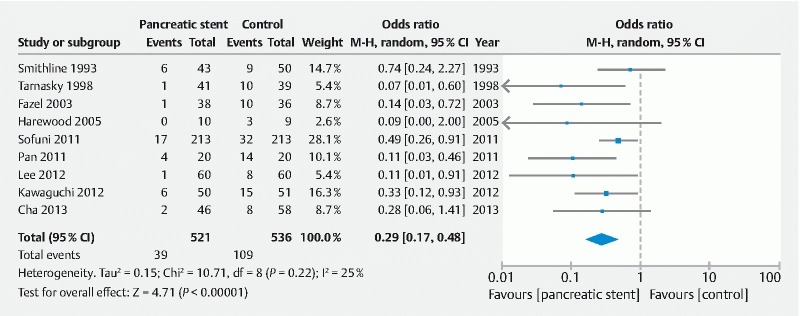
Direct treatment comparison of pancreatic stent placement to placebo for the prevention of post-ERCP pancreatitis.
Sensitivity analyses
Results from sensitivity analyses are reported in the Supplemental Table 1 . Overall, for the primary outcome, the results were largely similar to the main analysis.
Publication bias
There was no evidence of publication bias, based upon either qualitative on funnel-plot asymmetry or quantitative (i. e., Egger regression test, P > 0.05 for all comparisons) ( Supplemental Fig. 3 , Supplemental Fig. 4 , Supplement Fig. 5 ).
Network meta-analysis comparisons
With this network meta-analysis, we calculated the mixed effect estimate as a weighted average of both direct and indirect treatment effects. On direct network meta-analysis, all strategies (i. e., both pharmacologic and endoscopic treatments) were associated with a reduced risk of post-ERCP pancreatitis. In a direct network meta-analysis, compared with placebo, use of rectal NSAIDs (B = – 0.69, 95 % CI [–1.18 to – 0.21]), pancreatic stent (B = – 1.25, 95 % CI [–1.81 to – 0.69]), high-volume LR solution (B = – 0.67, 95 % CI [–1.20 to – 0.13]), and combination of LR plus rectal NSAIDs (B = – 1.58, 95 % CI [–3.0 to –0.17]), were all associated with a reduced risk of post-ERCP pancreatitis. Summary results of our network meta-analysis are summarized in Table 2 . Indirect comparisons were largely similar to those obtained in direct meta-analysis for treatment modalities compared to placebo. However, in indirect network meta-analysis, there was no statistically significant difference when treatment modalities were compared with each other. There were overlapping confidence intervals, although differences were observed in effect size.
Table 2. Summary results from network meta-analysis in the prevention of post-ERCP pancreatitis.
| Post-ERCP treatment | Beta coefficient (β) | 95 % credible interval (CI) 1 | Quality of evidence |
| Lactated ringer’s vs placebo | –0.69 | –0.77 to –2.15 | ⊕⊕⊕ Moderate 2 |
| Lactated ringer’s + rectal nsaids vs placebo | –0.82 | –1.45 to –0.19 | ⊕⊕⊕⊕ High |
| Pancreatic stent vs placebo | –1.37 | –2.98 to –0.23 | ⊕⊕⊕⊕ High |
| Rectal NSAIDs vs placebo | –1.27 | –2.30 to –0.70 | ⊕⊕⊕⊕ High |
| Lactated ringer’s + rectal NSAIDs vs lactated ringer’s | –0.55 | –2.28 to 1.17 | ⊕⊕⊕ Low 3 |
| Pancreatic stent vs lactated ringer’s | –0.45 | –1.30 to 0.39 | ⊕⊕⊕ Low 3 |
| Rectal NSAIDs vs lactated ringer’s | 0.21 | –1.32 to 1.74 | ⊕⊕⊕ Low 3 |
| Pancreatic stent vs lactated ringer’s + rectal NSAIDs | 0.10 | –1.61 to 1.80 | ⊕⊕⊕ Low 3 |
| Rectal NSAIDs vs lactated ringer’s + rectal NSAIDs | 0.76 | –0.92 to 2.44 | ⊕⊕⊕ Low 3 |
| Rectal NSAIDs vs pancreatic stent | 0.66 | –0.84 to 2.17 | ⊕⊕⊕ Low 3 |
ERCP, endoscopic retrograde cholangiopancreatography; NSAID, nonsteroidal anti-inflammatory drug
If the CI estimates are either all positive or negative (i. e., does not include a zero), it indicates that results are statistically significant.
Due to risk of bias in imprecision of summary results.
Due to risk of bias in individual studies, indirectness, and imprecision of summary results.
Ranking probability
Ranking probability based on SUCRA indicated that prophylactic pancreatic duct stent placement had the highest probability (SUCRA = 0.81, 95 % CI [0.83 to 0.80]) of being ranked the best prophylactic treatment ( Fig. 6 ). As highlighted in the SUCRA probabilities figure, a combined strategy of LR solution plus rectal NSAIDs was the second most effective treatment to prevent post-ERCP pancreatitis (SUCRA = 0.76, 95 % CI [0.79 to 0.72]), followed by aggressive infusion of LR solution alone (SUCRA = 0.51, 95 % CI [0.55 to 0.49]), and finally independent use of rectal NSAID use (SUCRA = 0.41, 95 % CI [0.44 to 0.38]). A rankogram of interventions to prevent post-ERCP pancreatitis based upon cumulative SUCRA probabilities is highlighted in Supplemental Fig. 6 .
Fig. 6.

SUCRA cumulative probability plots for the prevention of post-ERCP pancreatitis.
Discussion
In this systematic review and network meta-analysis, compared to other treatment approaches, pancreatic duct stent placement appears to be the most effective strategy for the prevention of post-ERCP pancreatitis followed by prophylaxis using aggressive LR hydration and rectal NSAIDs, followed by high-volume LR infusions alone, and finally use of rectal NSAIDs.
Pancreatic duct stents reduce post-ERCP pancreatitis by relieving pancreatic ductal hypertension that may develop as a result of transient procedure-induced stenosis 58 . The most recent European Society of Gastrointestinal Endoscopy (ESGE) guideline on prophylaxis of post-ERCP pancreatitis recommends placement of a 5 Fr prophylactic pancreatic stent in high-risk cases 59 . However, pancreatic stent placement is technically challenging. Pancreatic stent requires deep cannulation of the duct and placement of a guidewire. Attempting to place a pancreatic duct stent with subsequent failure actually increases risk of post-ERCP pancreatitis by inducing injury to the pancreatic orifice. Hence, it is these authors’ belief that pancreatic stent placement should only be attempted by providers with familiarity and expertise, in individuals deemed to be a high-risk, and in cases in which cannulation is not intentional and stent placement would be recommended in case of inadvertent cannulation. In patients in whom pancreatic stent placement is not feasible, use of alternative measures to reduce post-procedure pancreatitis should be employed.
Based on our study, aggressive hydration with LR appears to be effective when combined with rectal NSAIDs rather than using rectal NSAIDs alone or LR alone. Use of rectal NSAIDs, potent inhibitors of phospholipase A2, has been increasingly adopted in to clinical practice 59 60 . Current ESGE guidelines recommend routine rectal administration of diclofenac or indomethacin immediately before or after ERCP in all patients without contraindication 59 . Aggressive hydration with LR, which attenuates the acidosis that appears to promote zymogen activation and pancreatic inflammation, may be an effective intervention for preventing post-ERCP pancreatitis. Given the different mechanisms to prevent pancreatitis, it is not surprising that both work synergistically. Moreover, as both rectal NSAID therapy and LR infusion are inexpensive with relative few AEs, combination strategy is now has become a preferred strategy for post-ERCP pancreatitis prevention.
Our study observations are different from a previous network meta-analysis which reported that use of rectal NSAIDs when indirectly compared with pancreatic stent placement alone were associated with lower odds for developing post-ERCP pancreatitis 61 . However, that analysis included both observational studies and randomized controlled trials, which may have contributed to the divergent results. Furthermore, this previous study examined the efficacy of rectal NSAIDs and prophylactic pancreatic duct stent placement among both average-risk and high-risk cohorts. Our study specifically included only high-quality randomized controlled trials in high-risk patients and demonstrates the superiority of pancreatic stent placement as the best prophylactic measure for post-ERCP pancreatitis. Importantly, these findings do not take into account the cost-effectiveness of various therapies (i. e., rectal NSAIDs are inexpensive, easy to use, efficacious, and low risk). Despite our findings, questions still remain about whether all high-risk patients should receive a pancreatic stent – especially individuals who require multiple cannulation attempts of the biliary orifice.
It is important to note that the current systematic review and network meta-analysis is not without limitations. There were no studies comparing treatment strategies other than placebo except for one study that compared aggressive LR hydration to use of rectal NSAIDs. No currently published studies have examined the role of pancreatic duct stent versus rectal NSAIDs; although it should be pointed out there is an actively recruiting clinical trial designed to directly compare these two treatment modalities 62 . This study, with an expected enrollment of over 1400 patients, is a comparative effectiveness multicenter, non-inferiority trial of rectal indomethacin alone versus the combination of rectal indomethacin and prophylactic pancreatic stent placement for prevention of post-ERCP pancreatitis.
While we included indirect comparison in a Bayesian network meta-analysis design, we cannot discount the possibility of conceptual heterogeneity whereby differences may exist in trial design, patient population, intervention (i. e., timing of rectal NSAID administration or operator variability in pancreatic duct stent placement), and outcome assessment which may limit true comparability between included studies 19 27 . Though we performed sensitivity analyses to minimize heterogeneity and generalize our findings, it is important to understand that not all risk factors for post-ERCP pancreatitis are created equal, and combinations or risk factors are not simply additive, but multiplicative 63 64 65 66 . Additional limitations include a lack of data on follow-up – though the primary endpoint of post-ERCP pancreatitis lends itself to definitive diagnosis as defined over a discrete period of time as detailed above 25 . Treatment-related AEs were not reported in our systematic review and network meta-analysis as well, which may limit clinical adoption of these strategies, especially pancreatic duct stent placement.
Despite these limitations, our study possesses several strengths. These include the comprehensive and simultaneous assessment of the relative efficacy of four competing or in-combination strategies to prevent post-ERCP pancreatitis in a high-risk cohort. Although SUCRA probabilities may not easily substitute for clinical judgement on a patient-by-patient basis, focusing on summary effect estimates and GRADE to rate overall quality of evidence allows for better approximations 27 . In addition, while conceptual heterogeneity was present in our study, strategies to limit the effect of conceptual heterogeneity included strict inclusion and exclusion criteria and use of multiple sensitivity analyses to assess the robustness of the results.
Furthermore, although there are several available strategies available to potentially reduce incidence post-ERCP pancreatitis, our results validate the current American Society of Gastrointestinal Endoscopy (ASGE) guidelines recommending pancreatic duct stenting in high-risk individuals – strong recommendation 22 . Future trials such as the one discussed above to assess rectal indomethacin alone versus combination therapy with rectal NSAID and prophylactic pancreatic stent placement may help development of future recommendations; however, these are unlikely to occur 62 . Comparison of these different strategies alone may be of limited benefit clinically because the combination of the different treatments has already readily been adopted in clinical practice and shown to be effective with few complications.
Conclusion
In conclusion, based upon this systematic review and network meta-analysis, prophylactic pancreatic stent placement appears to be the most effective preventive strategy for post-ERCP pancreatitis in high-risk patients. Despite these findings, it is these authors’ belief that combination therapy will likely predominate clinical practice. This is in part due to the increased risk of post-ERCP pancreatitis with failed cannulation of the pancreatic duct and the ease and cost of combination therapy with rectal NSAID use and aggressive LR infusion.
Competing interests Dr. Shyam Varadarajulu is a consultant for Boston Scientific and Olympus. Dr. Udayakumar Navaneethan is a consultant for Janssen, AbbVie, Pfizer and Takeda.
Drs. Njei and McCarty: These authors contributed equally.
Supplementary material :
References
- 1.Peery A F, Dellon E S, Lund Jet al. Burden of gastrointestinal disease in the United States: 2012 update Gastroenterology 20121431179–1187.e1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman S. ERCP and endoscopic sphincterotomy-induced pancreatitis. Am J Gastroenterol. 1994;89:303–305. [PubMed] [Google Scholar]
- 3.Silviera M L, Seamon M J, Porshinsky B et al. Complications related to endoscopic retrograde cholangiopancreatography: a comprehensive clinical review. J Gastrointestin Liver Dis. 2009;18:73–82. [PubMed] [Google Scholar]
- 4.Freeman M L, Nelson D B, Sherman S et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 5.Glomsaker T, Hoff G, Kvaloy J T et al. Patterns and predictive factors of complications after endoscopic retrograde cholangiopancreatography. Br J Surg. 2013;100:373–380. doi: 10.1002/bjs.8992. [DOI] [PubMed] [Google Scholar]
- 6.Kochar B, Akshintala V S, Afghani E et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143–149 e9. doi: 10.1016/j.gie.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Arata S, Takada T, Hirata K et al. Post-ERCP pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:70–78. doi: 10.1007/s00534-009-0220-5. [DOI] [PubMed] [Google Scholar]
- 8.Murray B, Carter R, Imrie C et al. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786–1791. doi: 10.1016/s0016-5085(03)00384-6. [DOI] [PubMed] [Google Scholar]
- 9.Otsuka T, Kawazoe S, Nakashita S et al. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol. 2012;47:912–917. doi: 10.1007/s00535-012-0554-7. [DOI] [PubMed] [Google Scholar]
- 10.Sotoudehmanesh R, Khatibian M, Kolahdoozan S et al. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978–983. doi: 10.1111/j.1572-0241.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang A S, Pausawasdi N, Charatcharoenwitthaya P et al. A randomized, controlled trial of aggressive fluid hydration for the prevention of post-ERCP pancreatitis. Gastroenterol. 2016;150:S209. [Google Scholar]
- 12.Buxbaum J, Yan A, Yeh K et al. Aggressive hydration with lactated Ringer’s solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2014;12:303–307 e1. doi: 10.1016/j.cgh.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J H, Kim H J, Lee B U et al. Vigorous periprocedural hydration with lactated ringer’s solution reduces the risk of pancreatitis after retrograde cholangiopancreatography in hospitalized patients. Clin Gastroenterol Hepatol. 2017;15:86–92 e1. doi: 10.1016/j.cgh.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Fazel A, Quadri A, Catalano M F et al. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291–294. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 15.Smithline A, Silverman W, Rogers D et al. Effect of prophylactic main pancreatic duct stenting on the incidence of biliary endoscopic sphincterotomy-induced pancreatitis in high-risk patients. Gastrointest Endosc. 1993;39:652–657. doi: 10.1016/s0016-5107(93)70217-5. [DOI] [PubMed] [Google Scholar]
- 16.Tarnasky P R, Palesch Y Y, Cunningham J T et al. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–1524. doi: 10.1016/s0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 17.Dobronte Z, Szepes Z, Izbeki F et al. Is rectal indomethacin effective in preventing of post-endoscopic retrograde cholangiopancreatography pancreatitis? World J Gastroenterol. 2014;20:10151–10157. doi: 10.3748/wjg.v20.i29.10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutton B, Salanti G, Chaimani A et al. The quality of reporting methods and results in network meta-analyses: an overview of reviews and suggestions for improvement. PLoS One. 2014;9:e92508. doi: 10.1371/journal.pone.0092508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipriani A, Higgins J P, Geddes J R et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159:130–137. doi: 10.7326/0003-4819-159-2-201307160-00008. [DOI] [PubMed] [Google Scholar]
- 20.Mills E J, Ioannidis J P, Thorlund K et al. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. 2012;308:1246–1253. doi: 10.1001/2012.jama.11228. [DOI] [PubMed] [Google Scholar]
- 21.Lu G, Ades A E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 22.ASGE Standards of Practice Committee . Chandrasekhara V, Khashab M A et al. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32–47. doi: 10.1016/j.gie.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 23.Testoni P A, Mariani A, Giussani A et al. Risk factors for post-ERCP pancreatitis in high- and low-volume centers and among expert and non-expert operators: a prospective multicenter study. Am J Gastroenterol. 2010;105:1753–1761. doi: 10.1038/ajg.2010.136. [DOI] [PubMed] [Google Scholar]
- 24.Chen J J, Wang X M, Liu X Q et al. Risk factors for post-ERCP pancreatitis: a systematic review of clinical trials with a large sample size in the past 10 years. Eur J Med Res. 2014;19:26. doi: 10.1186/2047-783X-19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotton P B, Eisen G M, Aabakken L et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J PT, Altman D G, Sterne J AC.Cochrane Handbook for Systematic Reviews of InterventionsIn: Higgins JPT, ed.The Cochrane Collaboration; 2011. Available fromhttp://www.cochranehandbook.org
- 27.Puhan M A, Schunemann H J, Murad M H et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt G, Oxman A D, Sultan S et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151–157. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.White I R. Multivariate random-effects meta-regression: Updates to mvmeta. The Stata Journal. 2011;11:255–270. [Google Scholar]
- 31.Higgins J P, Thompson S G, Deeks J J et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Review Manager (RevMan), ver. 5.3 The Cochrane Collaboration; (ed.)2014
- 34.Lu G, Ades A E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 35.Caldwell D M, Ades A E, Higgins J P. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaimani A, Higgins J P, Mavridis D et al. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salanti G, Ades A E, Ioannidis J P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Dobronte Z, Toldy E, Mark L et al. [Effects of rectal indomethacin in the prevention of post-ERCP acute pancreatitis] Orv Hetil. 2012;153:990–996. doi: 10.1556/OH.2012.29403. [DOI] [PubMed] [Google Scholar]
- 39.Andrade-Davila V F, Chavez-Tostado M, Davalos-Cobian C et al. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol. 2015;15:85. doi: 10.1186/s12876-015-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patai A, Solymosi N, Patai A V. Effect of rectal indomethacin for preventing post-ERCP pancreatitis depends on difficulties of cannulation: results from a randomized study with sequential biliary intubation. J Clin Gastroenterol. 2015;49:429–437. doi: 10.1097/MCG.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 41.Lua G W, Muthukaruppan R, Menon J. Can rectal diclofenac prevent post endoscopic retrograde cholangiopancreatography pancreatitis? Dig Dis Sci. 2015;60:3118–3123. doi: 10.1007/s10620-015-3609-9. [DOI] [PubMed] [Google Scholar]
- 42.Luo H, Zhao L, Leung J et al. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet. 2016;387:2293–2301. doi: 10.1016/S0140-6736(16)30310-5. [DOI] [PubMed] [Google Scholar]
- 43.Levenick J M, Gordon S R, Fadden L Let al. Rectal indomethacin does not prevent post-ERCP pancreatitis in consecutive patients Gastroenterology 2016150911–917.; quiz e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ucar R, Biyik M, Ucar E et al. Rectal or intramuscular diclofenac reduces the incidence of pancreatitis afterendoscopic retrograde cholangiopancreatography. Turk J Med Sci. 2016;46:1059–1063. doi: 10.3906/sag-1502-104. [DOI] [PubMed] [Google Scholar]
- 45.Alabd M, Abdo A. Role of rectal NSAIDs in the prevention of post-ERCP pancreatitis. J Gastroenterol Hepatol. 2013;28:495–496. [Google Scholar]
- 46.Elmunzer B J, Scheiman J M, Lehman G A et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chuankrerkkul P. Aggressive lactated Ringer’s solution for prevention of post ERCP pancreatitis (preliminary data of a prospective randomized trial) Gastrointest Endosc. 2015;81:AB410–AB411. [Google Scholar]
- 48.Rosa B, Carvalho P B, De Castro F D et al. Impact of intensive hydration on the incidence of post-ERCP pancreatitis: doubleblinded randomized controlled trial. Gastrointest Endosc. 2016;83:AB250. [Google Scholar]
- 49.Shaygan-Nejad A, Masjedizadeh A R, Ghavidel A et al. Aggressive hydration with Lactated Ringer’s solution as the prophylactic intervention for postendoscopic retrograde cholangiopancreatography pancreatitis: A randomized controlled double-blind clinical trial. J Res Med Sci. 2015;20:838–843. doi: 10.4103/1735-1995.170597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NorthShore University Health System, Medical College of Wisconsin et al. High volume lactated Ringer’s solution and pancreatitis National Library of Medicine (US)2016. Available at:https://clinicaltrials.gov/show/NCT02050048[Accessed December 21, 2016.]
- 51.Mok S RS, Ho H C, Shah P et al. Lactated Ringer’s solution in combination with rectal indomethacin for prevention of post-ERCP pancreatitis and readmission: a prospective randomized, double-blinded, placebo-controlled trial. Gastrointest Endosc. 2017;85:1005–1013. doi: 10.1016/j.gie.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 52.Harewood G C, Pochron N L, Gostout C J. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367–370. doi: 10.1016/j.gie.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 53.Sofuni A, Maguchi H, Itoi T et al. Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clin Gastroenterol Hepatol. 2007;5:1339–1346. doi: 10.1016/j.cgh.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Pan X P, Dang T, Meng X M et al. Clinical study on the prevention of post-ERCP pancreatitis by pancreatic duct stenting. Cell Biochem Biophys. 2011;61:473–479. doi: 10.1007/s12013-011-9230-4. [DOI] [PubMed] [Google Scholar]
- 55.Lee T H, Moon J H, Choi H J et al. Prophylactic temporary 3F pancreatic duct stent to prevent post-ERCP pancreatitis in patients with a difficult biliary cannulation: a multicenter, prospective, randomized study. Gastrointest Endosc. 2012;76:578–585. doi: 10.1016/j.gie.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Kawaguchi Y, Ogawa M, Omata F et al. Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18:1635–1641. doi: 10.3748/wjg.v18.i14.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cha S W, Leung W D, Lehman G A et al. Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized, prospective study. Gastrointest Endosc. 2013;77:209–216. doi: 10.1016/j.gie.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Olsson G, Lubbe J, Arnelo U et al. The impact of prophylactic pancreatic stenting on post-ERCP pancreatitis: A nationwide, register-based study. United European Gastroenterol J. 2017;5:111–1118. doi: 10.1177/2050640616645434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dumonceau J M, Andriulli A, Elmunzer B J et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799–815. doi: 10.1055/s-0034-1377875. [DOI] [PubMed] [Google Scholar]
- 60.Yokoe M, Takada T, Mayumi T et al. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22:405–432. doi: 10.1002/jhbp.259. [DOI] [PubMed] [Google Scholar]
- 61.Akbar A, Abu Dayyeh B K et al. Rectal nonsteroidal anti-inflammatory drugs are superior to pancreatic duct stents in preventing pancreatitis after endoscopic retrograde cholangiopancreatography: a network meta-analysis. Clin Gastroenterol Hepatol. 2013;11:778–783. doi: 10.1016/j.cgh.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 62.Medical University of South Carolina Stent vs. indomethacin for preventing post-ERCP pancreatitis (SVI) National Library of Medicine (US)2018. Available at:https://clinicaltrials.gov/ct2/show/NCT02476279?cond=stent+vs.+indomethacin+for+preventing+post-ercp+pancreatitis&rank=1[Accessed March 21, 2018.]
- 63.Cote G A. The end of prophylactic pancreatic duct stents? Proceed with caution and courage. Clin Gastroenterol Hepatol. 2014;12:528. doi: 10.1016/j.cgh.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 64.Cheng C L, Sherman S, Watkins J L et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139–147. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 65.Cotton P B, Garrow D A, Gallagher J et al. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80–88. doi: 10.1016/j.gie.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 66.Freeman M L, DiSario J A, Nelson D B et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


