Abstract
Background
It has been reported that chronic inflammation may play an important role in the pathogenesis of several serious diseases and could be modulated by diet. Recently, the Dietary Inflammatory Index (DII®) was developed to assess the inflammatory potential of the overall diet. The DII has been reported as relevant to various diseases but has not been validated in Japanese. Thus, in the present study, we analyzed the relationship between DII scores and high-sensitivity C-reactive protein (hs-CRP) levels in a Japanese population.
Methods
Data of the National Integrated Project for Prospective Observation of Non-communicable Disease and its Trends in the Aged 2010 (NIPPON DATA2010), which contained 2,898 participants aged 20 years or older from the National Health and Nutrition Survey of Japan (NHNS2010), were analyzed. Nutrient intakes derived from 1-day semi-weighing dietary records were used to calculate DII scores. Energy was adjusted using the residual method. Levels of hs-CRP were evaluated using nephelometric immunoassay. Multiple linear regression analyses were performed.
Results
After adjusting for age, sex, smoking status, BMI, and physical activity, a significant association was observed between DII scores and log(CRP+1) (standard regression coefficient = 0.05, P < 0.01). Although it was not statistically significant, the positive association was consistently observed in almost all age-sex subgroups and the non-smoker subgroup.
Conclusions
The current study confirmed that DII score was positively associated with hs-CRP in Japanese.
Key words: dietary inflammatory index, inflammation, CRP, Japanese, Japanese diet
INTRODUCTION
Inflammation constitutes the body’s protective response to injury or infection and is generally beneficial to the body.1 However, when the inflammatory response proceeds disorderedly, acute inflammation can progress to chronic inflammation,2 which features sustained increased level of inflammatory cytokines, such as Interleukin 6 (IL-6), Tumor Necrosis Factor-α (TNF-α), and C-reactive protein (CRP). It has been reported that inflammation response and metabolic regulation are highly integrated and interdependent.3 Chronic inflammation, which is the dysfunction of the inflammatory response, can lead to a variety of diseases, such as diabetes, cancer, and depression, which seriously threatens health.4–6
Growing evidence has shown that diet plays a key role in the regulation of chronic inflammation. For example, the Mediterranean diet, which is rich in fish, monounsaturated fats from olive oil, fruits, vegetables, whole grains, and involves moderate alcohol consumption, has been shown to be associated with lower levels of inflammatory markers.7 In contrast, the Western diet, also known as the “obesogenic” diet, characterized by a high intake of saturated fat from red meat and dairy products, refined grains, and sugar, may promote metabolic disorders through pro-inflammatory mechanisms.8
Recently, a literature-derived, population-based diet quality assessing tool—the Dietary Inflammatory Index (DII®)—was developed for evaluating the inflammatory potential of one’s overall diet.9 The DII has been construct validated in American, European, Asian, and Australia individuals with inflammatory markers including CRP, IL-6, and TNF-α,10–14 and was reported to have associations with a variety of diseases. A recent published meta-analysis reported that there were consistent and significant positive associations between higher DII scores and cancer incidence and mortality across cancer types.15 Another review of cardiovascular diseases concluded that the DII was a useful tool for appraising the inflammatory potential of diet and for helping to explore the mechanisms between diet, inflammation, and cardio-metabolic diseases.16 A few relevant studies have been carried out in Asia; one of them was conducted in Japan.17
Japanese have enjoyed the world’s longest average life expectancy since 1985,18 which may partially be due to the Japanese traditional diet, Washoku, which was included in the United Nations Educational, Scientific and Cultural Organization list of Intangible Cultural Heritage in 2013.19,20 The Japanese diet incorporates high consumption of fish and soybean products and low consumption of animal fat and meat and has been reported as having a negative association with cardiovascular disease risks,20 psychological distress,21 and cancer.22 Whether the DII scores of the Japanese population that consumed a predominantly Japanese diet are applicable to epidemiological studies remains unclear. For this purpose, it was necessary to validate the DII using a Japanese database so that more researches could be conducted.
Therefore, we evaluated the association between DII scores and hs-CRP levels in Japanese using data from National Integrated Project for Prospective Observation of Non-Communicable Disease and Its Trends in the Aged 2010 (NIPPON DATA2010).23–25
MATERIAL AND METHODS
Study population
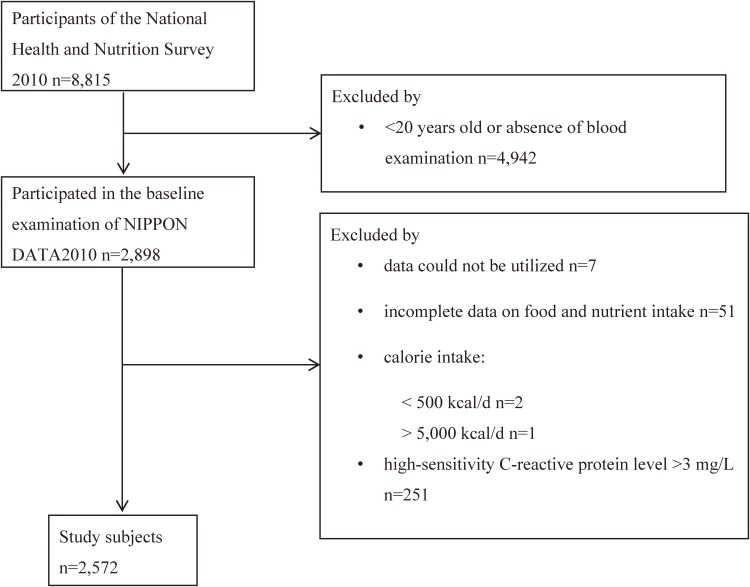
NIPPON DATA2010 was a nationally representative cohort study based on the National Health and Nutrition Survey of Japan in 2010 (NHNS2010),26 which used validated high-accuracy semi-weighing dietary records. The details of NHNS2010 and NIPPON DATA2010 have been described elsewhere.26,27 Briefly, 8,815 residents from 300 randomly selected survey areas throughout Japan participated in NHNS2010. Among them, 7,229 participants were aged 20 years or older, and 3,873 of the 7,229 completed the blood tests. Finally, 2,898 participants (1,239 men and 1,659 women, response rate: 74.6%) from the NHNS2010 agreed to be involved in the baseline survey of NIPPON DATA2010, which included electrocardiography, urinalysis, and questionnaires and was conducted in November 2010,25,27 and were subsequently recruited to the current study.
Among the 2,898 participants, 7 participants could not be included due to unusable data, and 94 were excluded for the following reasons: incomplete data of food and nutrient intake (n = 51), extreme calorie intake <500 kcal/d (n = 2) or >5,000 kcal/d (n = 1)28; and missing data on weight or height (n = 2), physical activity (n = 4), or smoking status (n = 8). Considering the extremely low level of hs-CRP in Japanese, which is approximately one third of the median value in Caucasians,29,30 and the findings of one study conducted in six Asian cities suggesting that the reference CRP interval of Japanese was from 0.04 mg/L to 2.26 mg/L,31 we excluded participants with a CRP level >3 mg/L from the analyses (n = 251). Finally, a total of 2,572 participants were included in the analysis (Figure 1).
Figure 1. Flow diagram of study population. Participants were excluded for the following reasons: 1) younger than 20 years old or absence of blood examination; 2) without informed consent; 3) data could not be utilized; 4) having incomplete data on food and nutrient intake; 5) calorie intake less than 500 kcal/d or more than 5,000 kcal/d; 6) high-sensitivity C-reactive protein level >3 mg/L; 7) physical activity unknown; 8) smoking status unknown; 9) BMI could not be calculated.
The Institutional Review Board of Shiga University of Medical Science approved this study (No. 22-29, 2010).
Dietary intake and DII
Data on dietary intake were collected from 1-day semi-weighing household dietary records. Participants were asked to weigh and record all portions of foods, beverages, and nutrient supplements consumed by each household member in a whole day. In addition, participants were asked to carry out the dietary records on a normal day for representing dietary habits. Trained dietitians visited the participants’ homes to assist with and confirm the dietary records. Nutrient intakes were estimated using the Standard Tables of Food Composition in Japan, Fifth Revised and Enlarged Edition.26,32
The DII was developed as a diet quality-assessing tool based on the inflammatory potential of the overall diet. Forty-five food or nutrient parameters were identified by their effects on six inflammatory markers (IL-1β, IL-4, IL-6, IL-10, TNF-α, and CRP), and a global standard database was created for comparing DII scores in diverse populations. A more detailed description of the DII has been provided elsewhere.9 Briefly, the DII provided an overall inflammatory effect score, a global daily mean intake, and a standard deviation for each food parameter. First, every nutrient intake was transformed to a Z-score using the standard values described above. To minimize the ‘right skewing,’ each Z-score was converted to a percentile value, which was then doubled, and 1 was subtracted from the doubled percentile value. Next, the centered value was multiplied by its respective overall inflammatory effect score. Finally, all parameter-specific DII scores were summed to achieve the overall DII score for each subject.
In the current study, 26 food or nutrient parameters, including vitamin B12, carbohydrate, cholesterol, total fat, iron (Fe), protein, saturated fat, magnesium (Mg), zinc (Zn), vitamin A, β-carotene, vitamin D, vitamin E, thiamine, riboflavin, niacin, vitamin B6, folic acid, vitamin C, monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), fiber, n-3 fatty acid, n-6 fatty acid, alcohol, and onion could be used to calculate DII scores (eTable 1). Among these, alcohol consumption was calculated from data of lifestyle surveys, and the others were derived from dietary records. Energy adjustment was performed using the residual method.33
C-reactive protein
Fasting blood samples were drawn from all participates in November 2010. Levels of hs-CRP were measured using nephelometric immunoassay at a commercial laboratory (SRL, Tokyo, Japan).
Covariates
Anthropometric measurements were performed by trained staff. Height and weight were measured and used to calculate the BMI as the ratio of weight to the square of height. Lifestyle surveys, including information on smoking (current, former, or never smoker), physical activity (Metabolic equivalents [METs]/d) and antilipidemic agent use (user or non-user), were conducted by public health nurses using a standard questionnaire.26 Information on socioeconomic status, such as marital status (married or unmarried), education (junior high school and below, high school, or university and above), and equivalent household expenditure was collected from the self-administered questionnaires. (1 Yen = 0.008989 United States dollar as of January 2018)
Statistical analysis
The characteristics of participants and food intakes across the DII quartiles were compared using chi-square test for categorical variables and ANOVA for continuous variables. Levels of hs-CRP were log-transformed due to its right-skewed distribution. To determine the association between DII scores and log-transformed (hs-CRP+1) [log(CRP+1)], Spearman’s correlation and multiple linear regression were analyzed. As potential confounders, age, sex, smoking status, BMI, and physical activity were adjusted. Moreover, analyses were further stratified by sex (men and women), age group (aged <45, 45–54, 55–64, 65–74, and ≥75 years) and smoking status (never-smoker, former-smoker, and current smoker). Additionally, we analyzed other factors as covariates, including economic status, marital status, education, and antilipidemic agent use. All statistical analyses were performed using Statistical Analysis Systems statistical software package version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
The mean DII score of the study participants was 0.82, with a SD of 1.75. Table 1 showed the characteristics of the study participants across DII score quartiles: −5.04 ≤ Q1 < −0.38; −0.38 ≤ Q2 < 0.91; 0.91 ≤ Q3 < 2.18; 2.18 ≤ Q4 ≤ 4.94. The proportion of women decreased with DII score quartiles, indicating that, compared with men, women consumed a more anti-inflammatory diet. Participants in Q4, the most pro-inflammatory diet-consuming group, were more likely to be younger, antilipidemic agent non-user, underweight or overweight, smokers, with higher physical activity, lower equivalent household expenditure, and more likely to be single than participants in other quartiles.
Table 1. Characteristics across quartiles of Dietary Inflammatory Index (DII®) scores.
| DII quartilesa | |||||||||
| Q1 | Q2 | Q3 | Q4 | ||||||
| Characteristics | n | % | n | % | n | % | n | % | P-value |
| Median DII score | −1.38 | 0.33 | 1.55 | 2.85 | <0.01 | ||||
| Sex | |||||||||
| Men | 239 | 37.2 | 253 | 39.4 | 274 | 42.6 | 320 | 49.8 | <0.01 |
| Women | 404 | 62.8 | 390 | 60.7 | 369 | 57.4 | 323 | 50.2 | |
| Age, years, mean (SD) | 64.4 (12.3) | 61.3 (14.8) | 56.1 (16.5) | 52.3 (16.5) | <0.01 | ||||
| BMI, kg/m2 | |||||||||
| <18.5 | 38 | 5.9 | 42 | 6.5 | 32 | 5.0 | 55 | 8.6 | 0.03 |
| 18.5 to <25.0 | 448 | 69.7 | 441 | 68.6 | 430 | 66.9 | 400 | 62.2 | |
| ≥25.0 | 157 | 24.4 | 160 | 24.9 | 181 | 28.2 | 188 | 29.2 | |
| Smoking | |||||||||
| Current smoker | 51 | 7.9 | 71 | 11.0 | 110 | 17.1 | 162 | 25.2 | <0.01 |
| Former smoker | 111 | 17.3 | 131 | 20.4 | 124 | 19.3 | 132 | 20.5 | |
| Never-smoker | 481 | 74.8 | 441 | 68.6 | 409 | 63.6 | 349 | 54.3 | |
| Physical activity, METs/d | 37.3 (8.0) | 37.0 (7.9) | 37.5 (9.0) | 38.6 (9.6) | <0.01 | ||||
| Antilipidemic agentb | |||||||||
| User | 126 | 19.6 | 115 | 17.9 | 87 | 13.6 | 63 | 9.8 | <0.01 |
| Non-user | 517 | 80.4 | 528 | 82.1 | 555 | 86.5 | 580 | 90.2 | |
| Marital statusb | |||||||||
| Married | 513 | 80.0 | 511 | 79.6 | 483 | 75.4 | 456 | 71.6 | <0.01 |
| Single | 128 | 20.0 | 131 | 20.4 | 158 | 24.7 | 181 | 28.4 | |
| Educationb | |||||||||
| Middle or lower | 167 | 26.0 | 162 | 25.2 | 145 | 22.6 | 142 | 22.1 | 0.47 |
| High school | 279 | 43.4 | 267 | 41.5 | 296 | 46.1 | 288 | 44.9 | |
| University or higher | 197 | 30.6 | 214 | 33.3 | 201 | 31.3 | 212 | 33.0 | |
| Equivalent household expenditure, million Yen/month, mean (SD)b | |||||||||
| 16. (10.1) | 15.7 (14.5) | 14.6 (12.6) | 14.3 (18.7) | <0.01 | |||||
BMI, body mass index; METs, metabolic equivalents; SD, standard deviation.
aDII quartiles: −5.04 ≤ Q1 < −0.38; −0.38 ≤ Q2 < 0.91; 0.91 ≤ Q3 < 2.18; 2.18 ≤ Q4 ≤ 4.94.
bSample size: antilipidemic agent use = 2,571; marital status = 2,561; education = 2,570; equivalent household expenditure = 2,380.
Comparing the food intakes distribution across the DII quartiles, we found certain food intakes were related to the decrease or increase of DII scores. With the increase in cereal, meat, fat, and oil intake, the DII score increased. On the other hand, potato, bean, nut and seed, vegetable, fruit, mushroom, seaweed, seafood, milk, and nutrients supplementary food showed an effect of lowering DII score in the current study (Table 2).
Table 2. Food intakes across quartiles of Dietary Inflammatory Index (DII®) scoresa.
| Food item (g) | Q1 | SD | Q2 | SD | Q3 | SD | Q4 | SD |
| Cereal | 393.28 | 145.20 | 425.79 | 156.73 | 449.87 | 167.15 | 507.25 | 191.50 |
| Potato | 80.32 | 84.22 | 61.00 | 68.13 | 52.05 | 62.93 | 41.73 | 53.20 |
| Sugar and Sweeteners | 7.89 | 8.65 | 7.31 | 7.88 | 6.96 | 8.81 | 7.77 | 11.46 |
| Bean | 99.18 | 90.08 | 75.14 | 81.23 | 53.39 | 64.72 | 41.92 | 59.39 |
| Nut and seed | 4.45 | 10.23 | 2.98 | 10.01 | 1.78 | 6.84 | 1.24 | 5.06 |
| Vegetable | 459.00 | 179.31 | 329.42 | 145.18 | 258.89 | 134.71 | 179.58 | 111.20 |
| Fruit | 190.88 | 150.37 | 138.68 | 129.31 | 98.97 | 113.66 | 61.13 | 93.60 |
| Mushrooms | 28.83 | 35.24 | 22.05 | 29.48 | 15.68 | 24.44 | 11.57 | 20.95 |
| Seaweeds | 19.13 | 31.83 | 11.94 | 20.51 | 11.31 | 20.83 | 8.04 | 16.82 |
| Seafood | 107.93 | 76.29 | 89.63 | 71.39 | 79.91 | 77.64 | 57.26 | 66.25 |
| Meat | 63.20 | 57.03 | 68.38 | 59.69 | 78.28 | 67.28 | 92.12 | 80.79 |
| Egg | 33.69 | 30.66 | 37.99 | 32.36 | 36.65 | 34.39 | 34.73 | 33.49 |
| Milk | 118.36 | 122.92 | 111.36 | 127.04 | 100.23 | 132.26 | 93.13 | 125.75 |
| Fat and oil | 8.92 | 8.44 | 8.95 | 8.44 | 10.31 | 9.73 | 10.58 | 8.83 |
| Confectionery | 19.86 | 34.24 | 26.35 | 43.89 | 26.04 | 44.60 | 36.36 | 56.19 |
| Preferred beverage | 766.11 | 469.28 | 720.00 | 471.81 | 702.66 | 511.27 | 720.13 | 522.49 |
| Seasoning and Spice | 99.87 | 146.78 | 92.96 | 81.50 | 84.59 | 78.21 | 91.88 | 95.38 |
| Nutrients supplementary food | 19.03 | 58.99 | 18.39 | 61.35 | 18.22 | 69.16 | 10.63 | 62.21 |
SD, standard deviation.
aDII quartiles: −5.04 ≤ Q1 < −0.38; −0.38 ≤ Q2 < 0.91; 0.91 ≤ Q3 < 2.18; 2.18 ≤ Q4 ≤ 4.94.
We did not observe significant correlation between DII scores and log(CRP+1) when analyzing in crude (r = 0.02, P = 0.41). After adjusting for age, sex, smoking status, BMI, and physical activity, a significant relationship was observed between DII scores and log(CRP+1) (standard regression coefficient of total = 0.05, P < 0.01) (Table 3). The standardized regression coefficient of the covariates was reduced in the order of BMI (0.33), age (0.14), current smoking (0.06), physical activity (0.06), and DII score (0.05).
Table 3. Multiple linear regression analysis between log-transformed hs-CRP and other variables, stratified by sexa.
| Men n = 1,086c | Women n = 1,486c | Total n = 2,572d | |||||||||||||
| Variable | Standardized β | β | 95% CI | P | Standardized β | β | 95% CI | P | Standardized β | β | 95% CI | P | |||
| DII scoreb | 0.05 | 0.01 | −0.003 | 0.02 | 0.14 | 0.06 | 0.01 | 0.001 | 0.02 | 0.02 | 0.05 | 0.01 | 0.003 | 0.02 | <0.01 |
| Ageb | 0.13 | 0.003 | 0.001 | 0.004 | <0.01 | 0.13 | 0.002 | 0.002 | 0.003 | <0.01 | 0.14 | 0.003 | 0.002 | 0.003 | <0.01 |
| BMIb | 0.27 | 0.03 | 0.02 | 0.03 | <0.01 | 0.37 | 0.03 | 0.03 | 0.04 | <0.01 | 0.33 | 0.03 | 0.026 | 0.032 | <0.01 |
| Sex (ref. women) | 0.007 | 0.004 | −0.02 | 0.03 | 0.76 | ||||||||||
| Smoking (ref. never-smokers) | |||||||||||||||
| Former smokers | 0.03 | 0.02 | −0.02 | 0.06 | 0.30 | 0.04 | 0.05 | −0.01 | 0.11 | 0.07 | 0.03 | 0.02 | −0.01 | 0.05 | 0.17 |
| Current smokers | 0.10 | 0.07 | 0.03 | 0.12 | <0.01 | −0.001 | −0.001 | −0.06 | 0.06 | 0.97 | 0.06 | 0.05 | 0.02 | 0.09 | <0.01 |
| Physical activityb | −0.06 | −0.002 | −0.003 | −0.00001 | 0.06 | −0.06 | −0.003 | −0.005 | −0.001 | 0.01 | −0.06 | −0.002 | −0.003 | −0.001 | <0.01 |
BMI, body mass index; CI, confidence interval; hs-CRP, highly sensitive C-reactive protein.
ahs-CRP, high-sensitivity C-reactive protein; DII, Dietary inflammatory index; energy was adjusted by residual method.
bContinuous variable.
cAdjusted for age, BMI, smoking status, and physical activity.
dAdjusted for age, BMI, sex, smoking status, and physical activity.
Furthermore, the results of multiple linear regression analysis stratified by sex and age group are shown in Table 4. Consistent positive associations were observed both in men (although it was not statistically significant, standardized regression coefficient = 0.05, P = 0.14) and women (standardized regression coefficient = 0.06, P = 0.02). All age groups displayed a positive association, (standardized regression coefficient<45 = 0.05, standardized regression coefficient45–54 = 0.03, standardized regression coefficient55–64 = 0.03, standardized regression coefficient65–74 = 0.05, standardized regression coefficient≥75 = 0.10). The highest standardized regression coefficient between the DII and log(CRP+1) was observed in the ≥75 years age group. As regards age-sex combined subgroups, all subgroups except for men aged <45 years and women aged 55–64 years showed positive relationships between DII scores and log(CRP+1).
Table 4. Multiple linear regression analysis between log-transformed hs-CRP and Dietary Inflammatory Index (DII®) scores, stratified by age and sex.
| Men | Women | Total | |||||||
| Age, years | N | standardized β | Pa | N | standardized β | Pa | N | standardized β | Pb |
| <45 | 212 | −0.05 | 0.42 | 361 | 0.11 | 0.02 | 573 | 0.05 | 0.21 |
| 45–54 | 135 | 0.05 | 0.53 | 202 | 0.02 | 0.75 | 337 | 0.03 | 0.51 |
| 55–64 | 255 | 0.10 | 0.12 | 336 | −0.04 | 0.50 | 591 | 0.03 | 0.43 |
| 65–74 | 309 | 0.01 | 0.91 | 369 | 0.08 | 0.11 | 678 | 0.05 | 0.19 |
| ≥75 | 175 | 0.04 | 0.61 | 218 | 0.14 | 0.04 | 393 | 0.10 | 0.05 |
| Total | 1,086 | 0.05 | 0.14 | 1,486 | 0.06 | 0.02 | 2,572 | 0.05 | <0.01 |
hs-CRP, high-sensitivity C-reactive protein.
aAdjusted for age, smoking status, BMI, and physical activity.
bAdjusted for age, sex, smoking status, BMI, and physical activity.
Additionally, we analyzed other factors as covariates, including economic status, marital status, education, and antilipidemic agent use, gaining unchanging result (standardized regression coefficient = 0.06, P < 0.01). Further, the positive association was observed in the never-smoker (standardized regression coefficient = 0.06, P = 0.01, n = 1,680) and former-smoker (standardized regression coefficient = 0.08, P = 0.07, n = 498) subgroup, but not in the current-smoker subgroup (standardized regression coefficient = −0.02, P = 0.71, n = 394), when analysis was stratified by smoking status.
DISCUSSION
In our cross-sectional study, we observed a positive association between DII scores and hs-CRP levels in participants of NIPPON DATA2010. The findings were consistent across almost all age-sex subgroups. The results suggested that the DII was applicable to the Japanese population.
Previous studies on DII scores and CRP levels
To the best of our knowledge, there have been 21 previous studies that investigated the association between DII scores and CRP levels (Table 5). Fourteen of them concurred with our conclusion that the DII scores positively associated with CRP levels. Of the other seven studies, five concluded that the DII score was associated with other inflammatory markers. To our best knowledge, ours is the first written report to correctly validate the DII in Japanese with CRP.
Table 5. Previous research on association between Dietary Inflammatory Index (DII®) and CRP.
| Author | Year | Country or race |
Number of food parameters |
Inflammatory markers |
Risk estimate |
| Vahid F47 | 2018 | Iran | 31 | TNF-αa IL-4a IL-10a IL-1βa CRPa IL-6a |
Partial correlation coefficient |
| CRP (mg/L) 0.328 P > 0.001 | |||||
| TNF-α (pg/ml) 0.373 P > 0.001 | |||||
| IL-6 (pg/ml) 0.337 P > 0.001 | |||||
| IL-1β (pg/ml) 0.326 P > 0.001 | |||||
| IL-4 (pg/ml) 0.046 P = 0.544 | |||||
| IL-10 (pg/ml) −0.333 P > 0.001 | |||||
| Phillips CM48 | 2018 | Ireland | 26 | Inflammatory score C3a CRP IL-6 TNF-α Adiponectin Leptin Resistin WBCa Neutrophils Lymphocytes Monocytes Eosinophils Basophils Neutrophil to lymphocyte ratio |
Mean of < Median E-DII vs > Median E-DII Inflammatory score 7.74 ± 0.12 vs 8.29 ± 0.10 P < 0.001 C3 (mg/dL) 134.31 ± 0.78 vs 136.90 ± 0.76 P = 0.04 CRP (mg/L) 2.19 ± 0.12 vs 2.45 ± 0.11 P = 0.03 IL-6 (pg/mL) 2.72 ± 0.14 vs 3.02 ± 0.15 P < 0.001 TNF-α (pg/mL) 6.23 ± 0.08 vs 6.51 ± 0.09 P = 0.001 Adiponectin (ng/mL) 6.05 ± 0.13 vs 5.41 ± 0.13 P < 0.001 Leptin (ng/mL) 2.85 ± 0.12 vs 2.78 ± 0.10 P = 0.11 Resistin (ng/mL) 5.64 ± 0.10 vs 5.78 ± 0.11 P = 0.50 WBC (109/L) 5.85 ± 0.07 vs 6.14 ± 0.06 P = 0.001 Neutrophils (109/L) 3.23 ± 0.04 vs 3.48 ± 0.04 P = <0.001 Lymphocytes (109/L) 1.83 ± 0.02 vs 1.86 ± 0.03 P = 0.37 Monocytes (109/L) 0.51 ± 0.005 vs 0.54 ± 0.01 P < 0.001 Eosinophils (109/L) 0.20 ± 0.004 vs 0.21 ± 0.005 P = 0.06 Basophils (109/L) 0.031 ± 0.001 vs 0.033 ± 0.001 P = 0.03 Neutrophil to lymphocyte ratio 1.89 ± 0.03 vs 2.04 ± 0.03 P < 0.001 |
| Shivappa N49 | 2018 | USA | 26 | CRP | OR (95%CI) DII continuous (age adjusted) 1.13 (1.07, 1.20) DII continuous (multivariable) 1.12 (1.05, 1.19) |
| Shivappa N45 | 2018 | Germany | Not found | CRP | r = 0.12 |
| Farhangi MA50 | 2018 | Iran | 28 | CRP IL-6 |
Beta estimate (95%CI) for the association Q4vsQ1 Men 0.97 (0.89, 1.06) Women 0.93 (0.67, 1.30) |
| Almeida-de-Souza J51 | 2017 | Portugal | 31 | CRP IL-6 C3 C4a Overall score |
OR (95%CI) T3vsT1 CRPa 2.33 (0.88, 6.20) IL-6 3.38 (1.24, 9.20) C3a 1.71 (0.63, 4.66) C4a 3.12 (1.21, 8.10) Overall 5.61 (2.00, 15.78) |
| Tabung FK52 | 2017 | USA | 38 | CRP IL-6 TNFαR2a Adiponectin |
Percentage change (95%CI) Q5vsQ1 NHS-IIa: CRPa +49% (+25%, +77%) IL-6 +21% (+9%, +33%) TNFαR2 +4% (+1%, +8%) Adiponectin −10% (−10%, −4%) Q5vsQ1 HPFSa: CRPa +29% (+15%, +44%) IL-6 +24% (+12%, +38%) TNFαR2 +5% (+1%, +8%) Adiponectin −4% (−9%, +2%) |
| Wirth MD11 | 2017 | African Americans |
31 | CRP IL-6 |
Percentile regression (95%CI) CRPa β 0.75: 3.95 (1.71, 6.19) β 0.90: 6.83 (1.11, 12.55) |
| Vahid F13 | 2017 | Iran | 31 | CRP IL-6 |
Beta estimates (95%CI) CRPa 0.04 (−0.09, 0.18) IL-6 0.16 (0.02, 0.30) |
| Julia C53 | 2017 | France | 36 | CRP | OR (95%CI) T3vsT1 1.32 (0.89, 1.95) |
| Shivappa N54 | 2017 | European | 25 | CRP TNF-α IL-6, 1,2,4,10, IFN-γa sICAMa sVCAMa |
Beta estimates (95%CI) T3vsT1 CRPa 0.09 (−0.18, 0.36) TNF-α 0.13 (0.007, 0.26) IL-6 0.09 (−0.22, 0.40) IL-1 0.30 (0.02, 0.58) IL-2 0.42 (0.04, 0.79) IL-4 0.17 (−0.25, 0.59) IL-10 0.09 (−0.17, 0.35) INF-γ 0.58 (0.09, 1.06) ICAMa 0.02 (−0.08, 0.11) VCAMa 0.07 (0.01, 0.13) |
| Shivappa N55 | 2017 | USA | 27 | CRP | OR (95%CI) Q4vsQ1 1.53 (1.20, 1.95) |
| Bodén S56 | 2017 | Sweden | 30 | CRP IL-6 |
Beta coefficients (95%CI) Q4vsQ1 CRPa 0.41 (0.16, 0.67) IL-6 0.26 (0.06, 0.46) |
| Kizil M57 | 2016 | Turkey | 25 | CRP | r = 0.35 |
| Sarbattama Sen58 | 2016 | USA | 28 | CRP WBC |
Beta coefficients (95%CI) CRPa Continuous 0.08 (0.02, 0.14) Q4vsQ1 0.25 (−0.01, 0.50) WBCa Continuous −0.03 (−0.11, 0.05) Q4vsQ1 −0.14 (−0.45, 0.17) |
| Akbaraly T37 | 2016 | UK | 27 | CRP IL-6 |
CRPa T1 −0.13 ± 1.3 T2 0.02 ± 1.3 T3 0.03 ± 1.3 IL-6 T1 −0.12 ± 1.3 T2 0.002 ± 1.3 T3 0.04 ± 1.3 |
| Tabung FK12 | 2015 | USA | 32 | IL-6 CRP TNFα-R2 Overall score |
Beta coefficients (95%CI) Q5vsQ1 IL-6 1.26 (1.15, 1.38) CRPa 1.07 (0.95, 1.2) TNFα-R2 81.43 (19.15, 143.71) Overall 0.26 (0.12, 0.40) OR Q5vsQ1 (95%CI) CRPa NSAIDsa non-user 1.67 (1.09, 2.55) NSAIDsa user 0.99 (0.65, 1.52) |
| Shivappa N10 | 2015 | Belgians | 17 | CRP Leucocyte count Fibrinogen Homocysteine IL-6 |
OR (95%CI) CRPa 1.03 (0.86, 1.17) IL-6 1.19 (1.04, 1.36) Homocysteine 1.56 (1.25, 1.94) Fibrinogen 1.08 (0.78, 1.48) |
| Alkerwi A59 | 2014 | Luxembourg | 24 | CRP | P for trend = 0.39 |
| Wirth MD60 | 2014 | USA | Not found | CRP IL-6 TNF-α |
OR (95%CI) Q4vQ1 1.57 (0.85, 2.88) |
| Shivappa N34 | 2013 | USA | 44 (24-hour dietary recalls) 28 (7-day dietary recalls) |
CRP | OR (95%CI) T3vsT1 24 hour dietary recalls: 1.47 (1.03, 2.12) 7-day dietary recall: 1.61 (1.15, 2.27) |
C3, complement C3; C4, complement C4; CRP, C-reactive protein; DII, Dietary inflammatory index; HPFS, Health Professionals Follow-Up Study; IL, Interleukin; NHS-II, Nurses’ Health Study II; NSAIDs, non-steroidal anti-inflammatory drugs; sICAM, soluble intercellular cell adhesion molecule; sVCAM, soluble vascular cell adhesion molecule; TNF-α, Tumor Necrosis Factor-α; TNF-αR2, Tumor Necrosis Factor-α Receptor 2; WBC, white blood cell.
In the current study, 18 items of 45 food parameters were unavailable for DII score calculation, which were caffeine, eugenol, garlic, ginger, saffron, selenium, trans fat, turmeric, green/black tea, flavan-3-ol, flavones, flavonols, flavonones, anthocyanidins, isoflavones, pepper, thyme/oregano, and rosemary. However, in previous studies, the number of food parameters used was between 17 and 44. Furthermore, a construct validation study using two different diet record methods, 24-hour dietary recalls and 7-day dietary recalls, reported that the reduction of available food parameters would not lead to a large drop-off in the predictive ability of DII.34 Thus, the 26 food parameters we used might be sufficient for validation.
International comparison of DII scores
The mean DII score of this study’s participants was 0.82 (standard deviation, 1.75). The Japanese diet is characterized by lower fat intake and higher soy and fish consumption.35 Therefore, we expected that the mean DII score in our study would be lower than that reported for western populations. However, our results did not bear out this expectation. For instance, a study on the association between the DII score and memory function using a population-based national sample of elderly Americans reported a mean DII score of −0.25 (standard error, 0.07).36 The mean DII score of the Whitehall II study, which was carried out in the United Kingdom, was −0.03 (standard deviation, 1.3).37 We reasoned that it may be due to the different food parameters used. Although DII score is calculated based on the global standard database, it cannot be used to compare the inflammatory potential of diets of different countries directly without using a unified set of food parameters.
Factors relevant to elevated CRP levels
The multiple linear regression analysis suggested that ageing, smoking, and being overweight were positively associated with CRP levels, while physical activity was inversely related. We could not determine the causality through the cross-sectional studies; however, it is unlikely that an increased CRP level leads to smoking. Moreover, many previous studies reported similar results that CRP levels were higher among current smokers.38–40 According to our analysis, the effect of smoking on CRP levels was similar to the effect of DII scores (standardized regression coefficient = 0.06, P < 0.01).
BMI and physical activity had inverse effects on CRP levels. Our results are in accordance with several previous studies. A systematic review and a reciprocal Mendelian randomization study suggested that obesity was correlated with elevated levels of CRP.41,42 Moreover, increasing evidence points to the negative association between physical activity and inflammatory biomarker levels.43,44 Given the health benefits in metabolic regulation from physical activity, we propose that, besides diet, weight control, smoking cessation, and increasing physical activity may contribute to lower CRP levels.
We found a positive association between DII scores and CRP levels in almost all age-sex subgroups, but not in a few young men and women aged 55–64 years. This was likely due to that, in the current study, participants in the youngest male subgroup had the highest smoking rate (40.09% current smoker and 22.17% former smoker). According to previous researches, smoking was an important confounder due to its relatively strong inflammatory effect. The strong inflammatory effect might cover the effect bought by diet.45 As described in the results, only the current-smoker subgroup did not show the positive association. The smaller sample size of current-smoker may be partially responsible; however, we still believed that smoking could be considered as a reason of the negative association in this subgroup of young men. Moreover, women in the 55–64 year age group were possibly in menopause, which has been confirmed to associate with increases in CRP levels.46 The effect of menopause might modify the association between DII scores and CRP levels. Further study investigating DII scores and CRP levels in this age-sex group might be required.
Strengths and limitations
Our study has several strengths. To our best knowledge, this is the first study of the inflammatory potential of the world-renowned Japanese diet and validation of the DII among Japanese. In addition, the participants of NIPPON DATA2010 were collected from all over Japan, with a large age span, ensuring a good representation of the Japanese population. This allowed the relatively detailed analysis of the association between DII scores and CRP levels in different sex and age groups.
Certain limitations should be mentioned. It was difficult to infer the temporal association between DII scores and CRP levels with the cross-sectional study design. However, it was almost impossible that participants changed their diets due to a high CRP level. Another limitation was the lack of information on anti-inflammatory medication use. The effect of diet on inflammation might partially be masked by using medicine12 that could lead to underestimation, which might partially explain why only weak associations were observed in the current study. Future studies should stratify analysis of the association between DII and CRP by anti-inflammatory medication.
In conclusion, we confirmed that a positive association between DII scores and CRP levels was observed in the Japanese population. The findings were consistent for almost all age-sex subgroups and the never-smoker subgroup.
ACKNOWLEDGMENTS
We deeply appreciate the Japanese Association of Public Health Center Directors and the Japan Medical Association for their support with NIPPON DATA2010’s baseline and follow-up survey. We also appreciate Shionogi Co. Ltd. for their support measuring brain natriuretic peptide. The authors thank Japanese public health centers and medical examination institutions listed in the Appendix of the reference (24) for their support with NIPPON DATA2010’s baseline survey.
Funding sources: This study was supported by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare, Japan (Comprehensive Research on Life-Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus [H22-Junkankitou-Seishuu-Sitei-017, H25-Junkankitou-Seishuu-Sitei-022, H30-Junkankitou-Sitei-002]).
Conflicts of interest: None declared.
Author Contributions: KM, AO, TO, HU: study concept and design. AK, TO, NO: acquisition of data. YY, AH, MK, AN, TH, TN, NT, NN, TN: analysis and interpretation of data. YY, MK: drafting article. AH: final content.
Statement: All authors, including YY, AH, MK, AN, TH, TN, NT, NN, TN, NO, AK, TO, TO, HU, AO and KM, have read and approved the final article, and the article is not being considered for publication elsewhere.
APPENDIX A. SUPPLEMENTARY DATA
The following is the supplementary data related to this article:
eTable 1. Food parameters of Dietary Inflammatory Index (DII®) used in the current study
REFERENCES
- 1.Janeway CA Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. [Google Scholar]
- 2.Wärnberg J, Gomez-Martinez S, Romeo J, Díaz LE, Marcos A. Nutrition, inflammation, and cognitive function. Ann N Y Acad Sci. 2009;1153:164–175. 10.1111/j.1749-6632.2008.03985.x [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- 4.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363(9415):1139–1146. 10.1016/S0140-6736(04)15900-X [DOI] [PubMed] [Google Scholar]
- 5.Friedrich MJ. Research on psychiatric disorders targets inflammation. JAMA. 2014;312(5):474–476. 10.1001/jama.2014.8276 [DOI] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 7.Bonaccio M, Cerletti C, Iacoviello L, de Gaetano G. Mediterranean diet and low-grade subclinical inflammation: the Moli-sani study. Endocr Metab Immune Disord Drug Targets. 2015;15(1):18–24. 10.2174/1871530314666141020112146 [DOI] [PubMed] [Google Scholar]
- 8.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40(6):833–842. 10.1016/j.immuni.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 9.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. 10.1017/S1368980013002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivappa N, Hébert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113(4):665–671. 10.1017/S000711451400395X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth MD, Shivappa N, Davis L, et al. Construct validation of the Dietary Inflammatory Index among African Americans. J Nutr Health Aging. 2017;21(5):487–491. 10.1007/s12603-016-0775-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabung FK, Steck SE, Zhang J, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25(6):398–405. 10.1016/j.annepidem.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahid F, Shivappa N, Hekmatdoost A, Hebert JR, Davoodi SH, Sadeghi M. Association between Maternal Dietary Inflammatory Index (DII) and abortion in Iranian women and validation of DII with serum concentration of inflammatory factors: case-control study. Appl Physiol Nutr Metab. 2017;42(5):511–516. 10.1139/apnm-2016-0274 [DOI] [PubMed] [Google Scholar]
- 14.Mayr HL, Itsiopoulos C, Tierney AC, et al. Improvement in dietary inflammatory index score after 6-month dietary intervention is associated with reduction in interleukin-6 in patients with coronary heart disease: the AUSMED heart trial. Nutr Res. 2018;55:108–121. 10.1016/j.nutres.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 15.Fowler ME, Akinyemiju TF. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int J Cancer. 2017;141(11):2215–2227. 10.1002/ijc.30922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Canela M, Bes-Rastrollo M, Martínez-González MA. The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int J Mol Sci. 2016;17(8):1265. 10.3390/ijms17081265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe M, Shivappa N, Ito H, et al. Dietary inflammatory index and risk of upper aerodigestive tract cancer in Japanese adults. Oncotarget. 2018;9(35):24028–24040. 10.18632/oncotarget.25288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Organization WH. World Health Organization, World Health Statistics 2016: Monitoring health for the SDGs. 2016.
- 19.Gabriel AS, Ninomiya K, Uneyama H. The Role of the Japanese traditional diet in healthy and sustainable dietary patterns around the world. Nutrients. 2018;10(2):173. 10.3390/nu10020173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamori Y, Sagara M, Arai Y, et al. Soy and fish as features of the Japanese diet and cardiovascular disease risks. PLoS One. 2017;12(4):e0176039. 10.1371/journal.pone.0176039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hozawa A, Kuriyama S, Nakaya N, et al. Green tea consumption is associated with lower psychological distress in a general population: the Ohsaki Cohort 2006 Study. Am J Clin Nutr. 2009;90(5):1390–1396. 10.3945/ajcn.2009.28214 [DOI] [PubMed] [Google Scholar]
- 22.Sonoda T, Nagata Y, Mori M, et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004;95(3):238–242. 10.1111/j.1349-7006.2004.tb02209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiyoshi N, Arima H, Satoh A, et al. ; NIPPON DATA2010 Research Group . Associations between Socioeconomic Status and the Prevalence and Treatment of Hypercholesterolemia in a General Japanese Population: NIPPON DATA2010. J Atheroscler Thromb. 2018;25(7):606–620. 10.5551/jat.42531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoh A, Arima H, Ohkubo T, et al. ; NIPPON DATA2010 Research Group . Associations of socioeconomic status with prevalence, awareness, treatment, and control of hypertension in a general Japanese population: NIPPON DATA2010. J Hypertens. 2017;35(2):401–408. 10.1097/HJH.0000000000001169 [DOI] [PubMed] [Google Scholar]
- 25.Kogure M, Tsuchiya N, Hozawa A, et al. Does the flushing response modify the relationship between alcohol intake and hypertension in the Japanese population? NIPPON DATA2010. Hypertens Res. 2016;39(9):670–679. 10.1038/hr.2016.46 [DOI] [PubMed] [Google Scholar]
- 26.Ministry of Health LaW, Japan. The National Health and Nutrition Survey in Japan 2010. Tokyo: Office for Life-style Related Diseases Control GAD, Health Service Bureau, Ministry of Health, Labour and Welfare; ed2013.
- 27.Kadota A, Okuda N, Ohkubo T, et al. The National Integrated Project for Prospective Observation of Non-communicable Disease and its Trends in the Aged 2010 (NIPPON DATA2010): objectives, design, and population characteristics. J Epidemiol. 2018;28(Suppl 3):S2–S9. 10.2188/jea.JE20170240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuda N, Miura K, Yoshita K, et al. ; NIPPON DATA80/90 Research Group . Integration of data from NIPPON DATA80/90 and National Nutrition Survey in Japan: for cohort studies of representative Japanese on nutrition. J Epidemiol. 2010;20(Suppl 3):S506–S514. 10.2188/jea.JE20090218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito I, Sato S, Nakamura M, et al. A low level of C-reactive protein in Japanese adults and its association with cardiovascular risk factors: the Japan NCVC-Collaborative Inflammation Cohort (JNIC) study. Atherosclerosis. 2007;194(1):238–244. 10.1016/j.atherosclerosis.2006.07.032 [DOI] [PubMed] [Google Scholar]
- 30.Saito I, Maruyama K, Eguchi E. C-reactive protein and cardiovascular disease in East asians: a systematic review. Clin Med Insights Cardiol. 2014;8(Suppl 3):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichihara K, Itoh Y, Min WK, et al. ; Committee on Plasma Proteins, International Federation of Clinical Chemistry and Laboratory Medicine . Diagnostic and epidemiological implications of regional differences in serum concentrations of proteins observed in six Asian cities. Clin Chem Lab Med. 2004;42(7):800–809. 10.1515/CCLM.2004.133 [DOI] [PubMed] [Google Scholar]
- 32.Agency SaT. Standard tables of food composition in Japan. Fifth revised and enlarged edition ed. Tokyo, Japan: Printing Bureau of the Ministry of Finance; 2015. [Google Scholar]
- 33.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. 10.1093/oxfordjournals.aje.a114366 [DOI] [PubMed] [Google Scholar]
- 34.Shivappa N, Steck SE, Hurley TG, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014;17(8):1825–1833. 10.1017/S1368980013002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogce F, Ceber E, Ekti R, Oran NT. Comparison of mediterranean, Western and Japanese diets and some recommendations. Asian Pac J Cancer Prev. 2008;9(2):351–356. [PubMed] [Google Scholar]
- 36.Frith E, Shivappa N, Mann JR, Hébert JR, Wirth MD, Loprinzi PD. Dietary inflammatory index and memory function: population-based national sample of elderly Americans. Br J Nutr. 2018;119(5):552–558. 10.1017/S0007114517003804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbaraly T, Kerlau C, Wyart M, et al. Dietary inflammatory index and recurrence of depressive symptoms: Results from the Whitehall II Study. Clin Psychol Sci. 2016;4(6):1125–1134. 10.1177/2167702616645777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasue H, Hirai N, Mizuno Y, et al. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ J. 2006;70(1):8–13. 10.1253/circj.70.8 [DOI] [PubMed] [Google Scholar]
- 39.Hastie CE, Haw S, Pell JP. Impact of smoking cessation and lifetime exposure on C-reactive protein. Nicotine Tob Res. 2008;10(4):637–642. 10.1080/14622200801978722 [DOI] [PubMed] [Google Scholar]
- 40.Ohsawa M, Okayama A, Nakamura M, et al. CRP levels are elevated in smokers but unrelated to the number of cigarettes and are decreased by long-term smoking cessation in male smokers. Prev Med. 2005;41(2):651–656. 10.1016/j.ypmed.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 41.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14(3):232–244. 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- 42.Timpson NJ, Nordestgaard BG, Harbord RM, et al. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes. 2011;35(2):300–308. 10.1038/ijo.2010.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers—A systematic review. J Am Coll Cardiol. 2005;45(10):1563–1569. 10.1016/j.jacc.2004.12.077 [DOI] [PubMed] [Google Scholar]
- 44.Phillips CM, Dillon CB, Perry IJ. Does replacing sedentary behaviour with light or moderate to vigorous physical activity modulate inflammatory status in adults? Int J Behav Nutr Phy. 2017;14:138 10.1186/s12966-017-0594-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shivappa N, Schneider A, Hébert JR, Koenig W, Peters A, Thorand B. Association between dietary inflammatory index, and cause-specific mortality in the MONICA/KORA Augsburg Cohort Study. Eur J Public Health. 2018;28(1):167–172. 10.1093/eurpub/ckx060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens. 2011;24(7):740–749. 10.1038/ajh.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vahid F, Shivappa N, Faghfoori Z, et al. Validation of a Dietary Inflammatory Index (DII) and association with risk of gastric cancer: a case-control study. Asian Pac J Cancer Prev. 2018;19(6):1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips CM, Shivappa N, Hébert JR, Perry IJ. Dietary Inflammatory Index and Biomarkers of Lipoprotein Metabolism, Inflammation and Glucose Homeostasis in Adults. Nutrients. 2018;10(8):1033. 10.3390/nu10081033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shivappa N, Wirth MD, Murphy EA, Hurley TG, Hébert JR. Association between the Dietary Inflammatory Index (DII) and urinary enterolignans and C-reactive protein from the National Health and Nutrition Examination Survey-2003–2008. Eur J Nutr. 2018. 10.1007/s00394-018-1690-5 [DOI] [PubMed] [Google Scholar]
- 50.Farhangi MA, Najafi M. Dietary inflammatory index: a potent association with cardiovascular risk factors among patients candidate for coronary artery bypass grafting (CABG) surgery. Nutr J. 2018;17(1):20. 10.1186/s12937-018-0325-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Almeida-de-Souza J, Santos R, Barros R, et al. Dietary inflammatory index and inflammatory biomarkers in adolescents from LabMed physical activity study. Eur J Clin Nutr. 2018;72(5):710–719. 10.1038/s41430-017-0013-x [DOI] [PubMed] [Google Scholar]
- 52.Tabung FK, Smith-Warner SA, Chavarro JE, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr. 2017;147(8):1567–1577. 10.3945/jn.117.248377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Julia C, Assmann KE, Shivappa N, et al. Long-term associations between inflammatory dietary scores in relation to long-term C-reactive protein status measured 12 years later: findings from the Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) cohort. Br J Nutr. 2017;117(2):306–314. 10.1017/S0007114517000034 [DOI] [PubMed] [Google Scholar]
- 54.Shivappa N, Hebert JR, Marcos A, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. 2017;61(6):1600707. 10.1002/mnfr.201600707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivappa N, Wirth MD, Hurley TG, Hébert JR. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999–2002. Mol Nutr Food Res. 2017;61(4):1600630. 10.1002/mnfr.201600630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodén S, Wennberg M, Van Guelpen B, et al. Dietary inflammatory index and risk of first myocardial infarction; a prospective population-based study. Nutr J. 2017;16(1):21. 10.1186/s12937-017-0243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kizil M, Tengilimoglu-Metin MM, Gumus D, Sevim S, Turkoglu İ, Mandiroglu F. Dietary inflammatory index is associated with serum C-reactive protein and protein energy wasting in hemodialysis patients: a cross-sectional study. Nutr Res Pract. 2016;10(4):404–410. 10.4162/nrp.2016.10.4.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sen S, Rifas-Shiman SL, Shivappa N, et al. Dietary inflammatory potential during pregnancy is associated with lower fetal growth and breastfeeding failure: results from Project Viva. J Nutr. 2016;146(4):728–736. 10.3945/jn.115.225581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alkerwi A, Shivappa N, Crichton G, Hébert JR. No significant independent relationships with cardiometabolic biomarkers were detected in the Observation of Cardiovascular Risk Factors in Luxembourg study population. Nutr Res. 2014;34(12):1058–1065. 10.1016/j.nutres.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wirth MD, Burch J, Shivappa N, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56(9):986–989. 10.1097/JOM.0000000000000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.