Abstract
Patients infected with the fungal pathogen Cryptococcus are most effectively treated with a combination of 5-fluorocytosine (5FC) and amphotericin B. 5FC acts as a prodrug, which is converted into toxic 5-fluorouracil (5FU) upon uptake into fungal cells. However, the pathogen frequently develops resistance through unclear mechanisms. Here we show that resistance to 5FC in Cryptococcus deuterogattii is acquired more frequently in isolates with defects in DNA mismatch repair that confer an elevated mutation rate. We use whole genome sequencing of 16 independent isolates to identify mutations associated with 5FC resistance in vitro. We find mutations in known resistance genes (FUR1 and FCY2) and in a gene UXS1, previously shown to encode an enzyme that converts UDP-glucuronic acid to UDP-xylose for capsule biosynthesis, but not known to play a role in 5FC metabolism. Mutations in UXS1 lead to accumulation of UDP-glucuronic acid and alterations in nucleotide metabolism, which appear to suppress toxicity of both 5FC and its toxic derivative 5FU.
Subject terms: Antifungal agents, Antimicrobial resistance, Fungal evolution, Fungal genomics
The authors show that resistance to the antifungal 5-fluorocytosine in Cryptococcus deuterogattii is acquired more frequently in isolates with elevated mutation rate, and is associated with alterations in capsule biosynthesis and nucleotide metabolism.
Introduction
One of the key challenges of the 21st century is the emergence and reemergence of pathogens. Opportunistic fungal pathogens comprise an important component of this problem as they infect the rapidly expanding cohort of immunocompromised patients1. These pathogens are responsible for millions of infections annually, with substantial mortality. Among the most dangerous are Cryptococcus species that cause ~220,000 infections a year, with >181,000 attributable deaths2. Cryptococcosis is particularly prominent in Sub-Saharan Africa, where the HIV/AIDS epidemic has resulted in a large population of susceptible individuals. Cryptococcosis is treated most effectively using a combination of 5-fluorocytosine (5FC) and amphotericin B3,4. However, in the parts of Africa where patients are most commonly afflicted with cryptococcosis, the medical infrastructure is insufficient to allow treatment with the highly toxic amphotericin B component of this dual therapy. Instead patients are typically treated with fluconazole monotherapy, with limited success. Excitingly, recent studies have shown that 5FC can be effectively paired with fluconazole to replace amphotericin B for treatment of patients in Africa5. However, 5FC is not yet approved or available for treatment in any African countries.
5FC acts as a prodrug, which enters cells via the cytosine permease Fcy2. 5FC itself is not toxic, but upon uptake into fungal cells, it is converted into toxic 5-fluorouracil (5FU) by cytosine deaminase, an enzyme that is not present in human cells6. In Cryptococcus, and other fungi, cytosine deaminase is encoded by the FCY1 gene. 5FU is then further processed by the product of the FUR1 gene, a uracil phosphoribosyltransferase, and inhibits both DNA and protein synthesis. Resistance is well understood in other fungal pathogens, like Candida albicans, where loss of function mutations in FCY1, FCY2, and FUR1 can mediate resistance to 5FC7. In Candida lusitaniae, mutations in FUR1 can be readily distinguished from mutations in FCY1 and FCY2 because only fur1 mutations result in cross-resistance to 5FU8. Likewise, in Candida dubliniensis, natural missense fur1 mutations affect both 5FC and 5FU resistance9. However, little work has been conducted on 5FC resistance directly in Cryptococcus. One of the few early studies suggested that reductions in FUR1 activity may be linked to resistance to 5FC based on a high frequency of cross-resistance to 5FU10. However, this study took place prior to the cloning or sequencing of the FUR1 gene in Cryptococcus and attribution of resistance to FUR1 was based only on cross-resistance to 5FU. More recent studies of 5FC resistant Cryptococcus bacillisporus isolates found no mutations in FCY1, FUR1, or any of three putative FCY2 paralogs that explained drug resistance11. However, in Cryptococcus deuterogattii, deletions of FCY2 confer resistance to 5FC12.
Recent work has demonstrated one source of increased rates of resistance to antifungal drugs in Cryptococcus: defects in the DNA mismatch repair pathway13,14. Natural isolates with DNA mismatch repair defects have been identified in both an outbreak population of Cryptococcus deuterogattii13,15 and in Cryptococcus neoformans14,16. Defects in mismatch repair are also common in other human fungal pathogens, including Candida glabrata17. Depending on the population studied, multidrug resistance is sometimes linked to the hypermutator state in C. glabrata18,19. A recent study of clinical C. glabrata isolates in India found a high prevalence of msh2 mutation, but no drug resistance20. This could suggest that hypermutation is advantageous even prior to drug exposure, while also providing more rapid development of resistance when antifungal drugs or agents are encountered. Alternatively, hypermutation has also been observed in more ancient lineages of fungi not known to be pathogenic, suggesting that hypermutation may have general advantages in a broader range of settings21.
Here we demonstrate that DNA mismatch repair defects also enable rapid resistance to 5FC in C. deuterogattii (previously known as C. gattii VGII22–24). We utilize whole genome Illumina sequencing, in combination with candidate-based Sanger sequencing, to identify the genetic basis for drug resistance in 16 independent isolates. We attribute resistance to mutations in FUR1 and unexpectedly, we also identify a pathway of resistance to 5FC involving mutations in the pathway responsible for producing the capsule, a core component of cryptococcal virulence.
Results
Hypermutation mediates resistance to 5FC
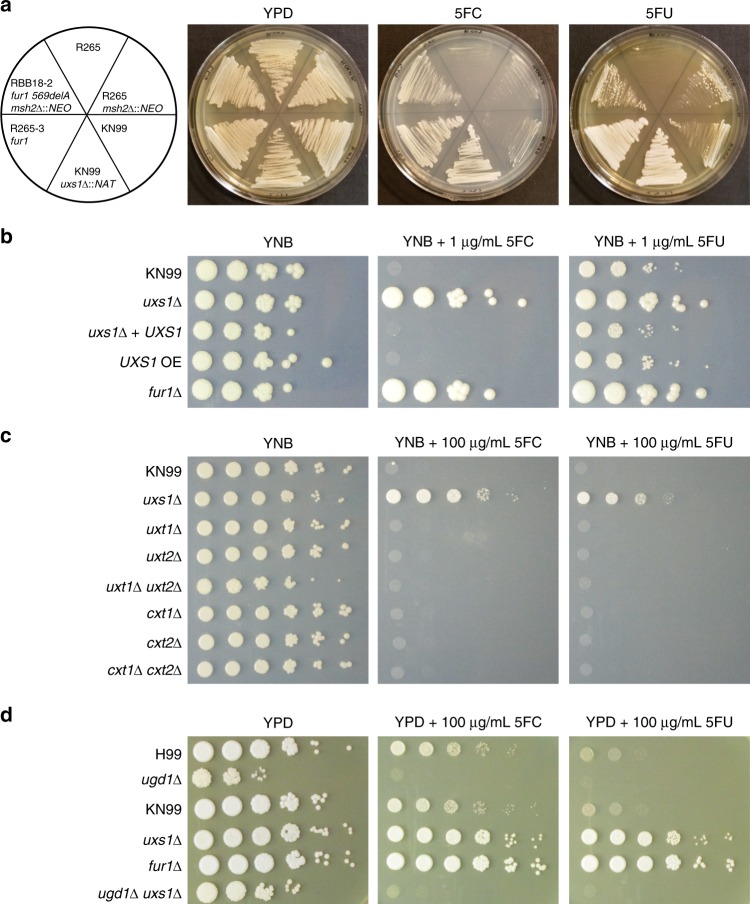
In a previous study, we demonstrated that mismatch repair mutations conferred increased rates of resistance to the antifungal drugs FK506 and rapamycin13. Because these hypermutator strains are found among both environmental and clinical isolates, here we tested if a hypermutator state could also confer resistance to one of the front-line drugs used to treat Cryptococcosis: 5-fluorocytosine (5FC). A semi-quantitative swabbing assay was first employed to demonstrate that deletions of the mismatch repair gene MSH2 in Cryptococcus deuterogattii confer an elevated rate of resistance to 5FC (Fig. 1a). This result was confirmed using a quantitative fluctuation assay approach (Fig. 1b). This assay revealed a greater than 15-fold increase in the generation of resistance to 5FC in msh2Δ mismatch repair defective mutants. Similarly, a simple spreading assay using VGIIa-like strains that had previously been found to harbor an msh2 nonsense allele13 demonstrated a much higher rate of resistance to both 5FC and 5FU than in the VGIIa non-hypermutator strains (Supplementary Fig. 1).
Fig. 1. 5FC resistance is enhanced by defects in mismatch repair.
a Swab assays were conducted using both the wildtype R265 strain and two independent msh2Δ::NEO mutants to test for the ability to generate resistance to 5FC. All three strains developed resistance; however, the mismatch repair mutants generated resistant isolates at a higher frequency. b A fluctuation assay was conducted to compare 5FC resistance quantitatively between wildtype R265 and two independent msh2Δ::NEO mutants. Mutation rate was normalized to the wildtype strain. Red is used to indicate wildtype strains while green indicates msh2 deletion strains. Both mutator strains showed a greater than 15-fold increase in the rate of resistance. Data shown are the mean of 10 replicates and error bars indicate 95% confidence intervals.
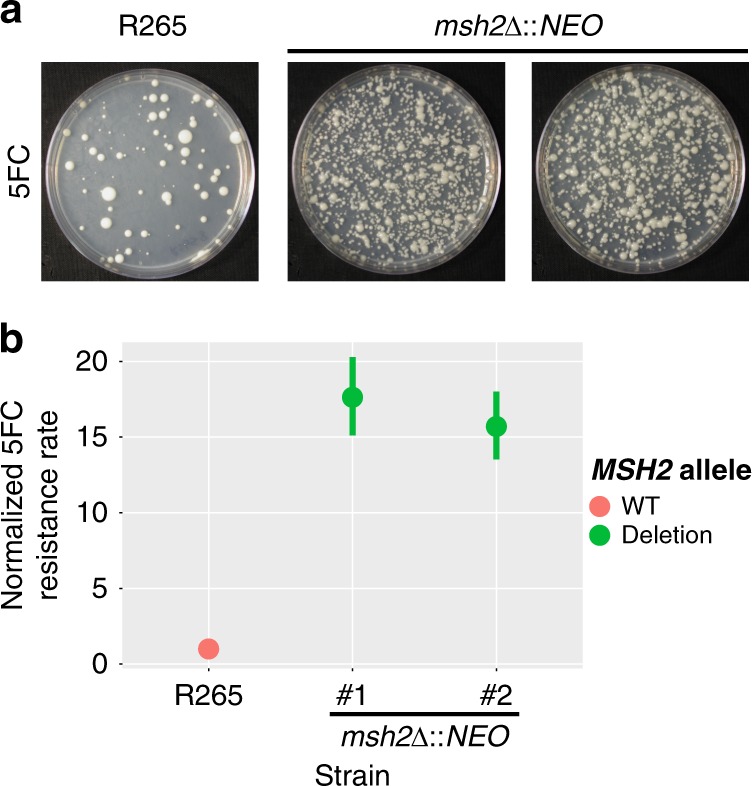
In previous studies, mutator alleles in C. deuterogattii were not found to be generally advantageous in rich media13. However, under stressful conditions, such as drug challenge with FK506 and rapamycin, mutator alleles were highly beneficial. A competitive growth experiment was utilized to test the same concept with 5FC. Mutator strains became resistant to 5FC at a higher rate and thus rapidly outcompeted wildtype strains (Fig. 2). However, in the absence of added stress, the mutator alleles showed no such advantage. This result suggests that drug challenge during infection may select for strains with elevated mutation rates that are able to acquire drug resistance more rapidly.
Fig. 2. Exposure to 5FC generates an adaptive advantage for mutator strains.
Competition experiments between a tester strain with a neomycin resistance marker and a wildtype R265 strain. (Strains used: SEC501, RBB17, RBB18). Overnight cultures were mixed 1:1 and then used to inoculate a second overnight culture in liquid YNB with and without 5FC. All three marked strains showed a slight growth defect in comparison to the unmarked strain in nonselective media but only the hypermutator strains demonstrated a dramatic growth advantage when grown in YNB + 5FC. Boxplots show minimum, first quartile, median, third quartile, and maximum values. Points represent the results from three individual replicates and are summarized by the box plot. The R265 NEOR vs wildtype competition is gray, while the two independent msh2Δ::NEO vs wildtype competitions are dark and light blue.
A subset of 5FC resistance is mediated by FUR1 mutation
In other fungi, resistance to 5FC is typically mediated by mutations in one of three genes: FCY1, FCY2, or FUR17,8,10,25. As described above, mutations in FCY1 and FCY2 are typically distinguishable from fur1 mutations because mutations in FUR1 confer resistance not only to 5FC but also to 5FU. In contrast, fcy1 and fcy2 mutations confer resistance to only 5FC. To define the mechanism underlying 5FC resistance in C. deuterogattii, 29 resistant colonies were isolated and tested, originating from the wildtype (R265, 9 colonies) and from two independent msh2Δ mutants derived in the R265 background (RBB17, 10 colonies and RBB18, 10 colonies). Cultures were started from independent colonies and a single resistant colony was selected from each culture, so that only one resistant isolate is derived from any original colony derived from the frozen stock. All of the 5FC resistant isolates (Table 1) acquired were cross-resistant to 5FU (29/29) (Fig. 3a), leading us to hypothesize that resistance to 5FC in C. deuterogattii was most commonly mediated by mutations in FUR1.
Table 1.
5FC-resistant isolates whole genome sequenced or successfully genotyped by Sanger sequencing.
| Strain Name | Original Genotype | Putative Resistance Allele |
|---|---|---|
| R265-1 | Wildtype | fur1 1134delT |
| R265-2 | Wildtype | ~18.5 kb deletion spanning fur1 |
| R265-3 | Wildtype | fur1 1003delT, mutation detected via Sanger |
| R265-4 | Wildtype | fur1 1136delT, mutation detected via Sanger |
| R265-5 | Wildtype | uxs1 1520delT |
| R265-6 | Wildtype | fur1 1440delA, mutation detected via Sanger |
| R265-7 | Wildtype | ~14.7 kb deletion spanning fur1 |
| R265-8 | Wildtype | ~26.7 kb deletion spanning fur1 |
| RBB17-1 | msh2Δ::NEO | |
| RBB17-2 | msh2Δ::NEO | |
| RBB17-3 | msh2Δ::NEO | |
| RBB17-4 | msh2Δ::NEO | |
| RBB17-5 | msh2Δ::NEO | fur1 1358delA in 6 base homopolymer |
| RBB17-6 | msh2Δ::NEO | |
| RBB17-7 | msh2Δ::NEO | |
| RBB17-8 | msh2Δ::NEO | fur1 G448A (splice acceptor) |
| RBB18-1 | msh2Δ::NEO | |
| RBB18-2 | msh2Δ::NEO | fur1 1358delA in 6 base homopolymer |
| RBB18-3 | msh2Δ::NEO | |
| RBB18-4 | msh2Δ::NEO |
fcy2 Trp167Stop uxs1 Asp306Gly |
| RBB18-5 | msh2Δ::NEO | fur1 1358delA in 6 base homopolymer |
| RBB18-6 | msh2Δ::NEO | fur1 1027delT in 5 base homopolymer |
| RBB18-7 | msh2Δ::NEO | |
| RBB18-8 | msh2Δ::NEO | uxs1 1182insC in 7 base homopolymer |
| RBB18-9 | msh2Δ::NEO | uxs1 Tyr217Cys |
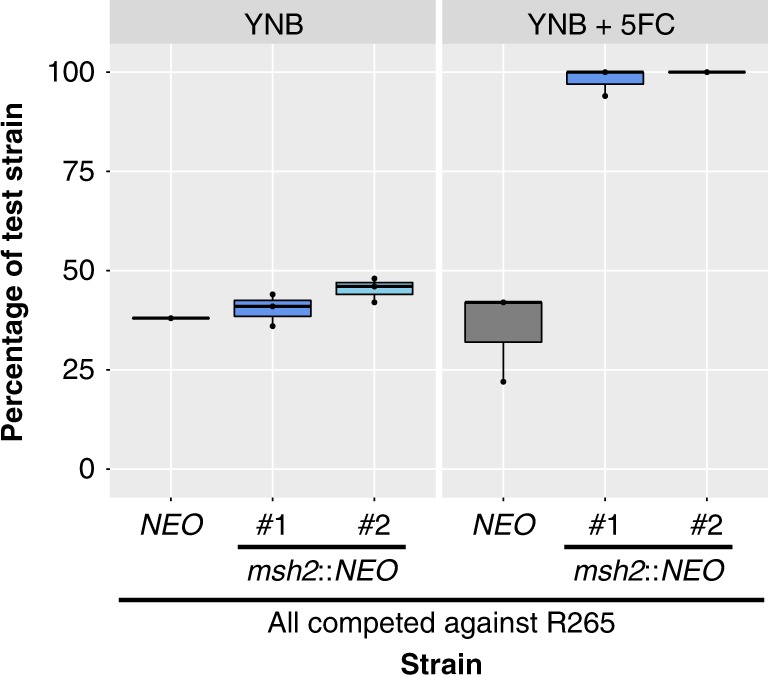
Fig. 3. 5FC resistant mutants are cross-resistant to 5FU.
a Isolates that were selected based on growth on 5FC media were patched to YNB, YNB plus 5FC, and YNB plus 5FU. Each plate has parental and fur1 mutant controls in the top row. Hypermutator controls have occasional resistant colonies that emerged in the growth patch. Sanger and Illumina sequencing revealed that 12 of 29 isolates had sustained mutations in FUR1. b Schematic showing the predicted domains encoded by the UXS1 gene, as well as the location and number of mutations identified. Frame-shift alleles are shown in red and missense are shown in blue.
However, when the FUR1 gene was Sanger sequenced in this set of 5FC/5FU resistant isolates, unexpectedly, only three out of 29 isolates (10.3%) were found to have sustained mutations in FUR1 (R265-3, R265-4, and R265-6) (Table 1), although PCR amplification of the FUR1 locus failed for another 3 isolates (R265-2, R265-7, R265-8), suggesting a possible large deletion or insertion event. Because fur1 mutations were the only known cause of 5FC/5FU cross-resistance, we performed whole genome Illumina sequencing on a subset of the remaining isolates (22 isolates) to identify unknown genes underlying resistance. We sequenced the whole genomes of 5 additional R265 isolates, 8 additional RBB17 isolates, and 9 additional RBB18 isolates, for a total of 22 5FC and 5FU resistant isolates.
From the sequenced genomes, reads were aligned to the R265 reference genome and SNPs and indels were identified. This analysis revealed that one pair of the presumed independent isolates were in fact siblings (RBB17-3 and RBB17-4), resulting in a total of 5 independent R265 genomes, 7 independent RBB17 genomes, and 9 independent RBB18 genomes (21 total independent isolates).
Of these 21 independent genome sequences, six contained unambiguous mutations in FUR1 that were not detected by Sanger sequencing. The first fur1 mutation discovered by whole genome sequencing was a single base deletion that introduced a frameshift (R265-1). Two sets of homopolymer shifts were also identified in FUR1: a single base deletion in a 6xA homopolymer run at position 1358 found in three independent isolates (RBB17-5, RBB18-2, and RBB18-5) and a single base deletion in a 5xT homopolymer run at position 1027. Finally, a mutation within a splice acceptor (G to A) was identified at position 448 (RBB17-8).
For three 5FC resistant R265 strains (R265-2, R265-7, R265-8), PCR amplification of the FUR1 locus failed and subsequent whole genome sequencing revealed regional deletions consistent with these failed PCRs. For two strains, break points were clearly identifiable. R265-7 sustained a deletion of bases 189022-203758 (14,736 bp) surrounding FUR1, while R265-8 sustained a deletion of bases 190136-216860 (26,724 bp), also including FUR1. For R265-2, one end of the deletion lies within FUR1, eliminating one of the primer binding sites and consistent with the failed PCR. The other end of the ~18.5 kb deletion fell within a sequencing gap of the annotated V2 R265 reference genome. To identify the precise location of this second breakpoint, reads from R265-2 were mapped to a recent Nanopore and Illumina hybrid assembly of the R265 strain26. Interestingly, the second breakpoint was found within a gene encoding a weak paralog of FUR1 (5 × 10−10 protein BLAST e-value). This paralog (CNBG_4055) is also present in C. neoformans (CNAG_2344), suggesting that if it arose via duplication, it was before the last common ancestor to both species. Given that deletion of FUR1 confers resistance to 5FC and 5FU, it is unlikely that this paralog performs the same function as Fur1 (Fig. 3a). Despite the protein similarity, no obvious nucleotide homology was found that may have mediated this large deletion conferring 5FC resistance. In fact, the FUR1 paralog is inverted relative to FUR1, reducing the likelihood that remnant homology may have generated a region susceptible to frequent homology-mediated deletion of FUR1 that would yield the type of regional deletion observed here.
A Trp167STOP mutation in FCY2 (CNBG_3227) was also detected in the sequenced set (RBB18-4). Mutations in FCY2 were unexpected because in other fungi they do not confer resistance to 5FU and because there are 2 additional paralogs with substantial similarity to FCY2 present in the Cryptococcus genome. Because this fcy2 strain also contains a second mutation in a gene that plays a role in 5FC and 5FU resistance (discussed below), the fcy2 mutation may be unrelated to drug resistance or may enhance resistance in the presence of the second mutation. We attempted to test the ortholog of FCY2 from Cryptococcus neoformans using a deletion collection strain but found that the mutant in the collection retained a functional copy of the FCY2 gene. However, fcy2 deletion has recently been reported to confer resistance to 5FC in C. deuterogattii12.
In total, out of 29 original 5FC resistant strains (Table 1), twelve independent fur1 mutations were identified using Sanger and Illumina sequencing. One independent fcy2 mutation was identified by Illumina sequencing. We did not identify any fcy1 mutations, although fcy1 mutations confer resistance to 5FC in C. neoformans (Supplementary Fig. 2).
Mutations in UXS1 also confer resistance to 5FC
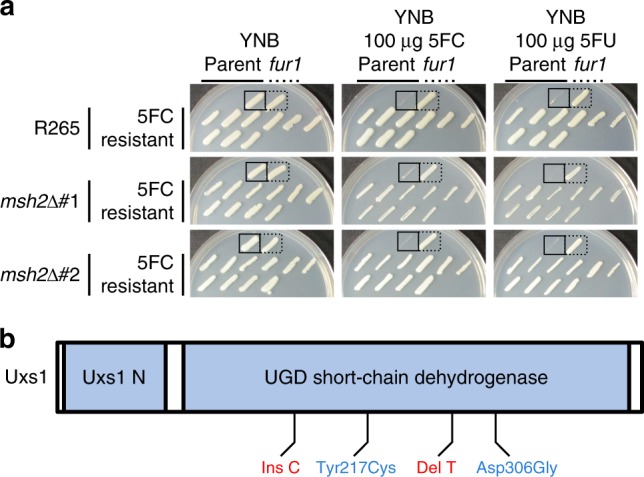
In total, 11 sequenced genomes representing 10 independent isolates remained with no mutations in any genes previously described to have a role in 5FC or 5FU resistance. These genomes were examined to identify candidate mutations. To distinguish causal variants from background mutations, candidate genes were required to be mutated in at least two different independent isolates. Variant impact was also scored using SNPeff27 and mutations were not considered if predicted to have low impact (i.e., synonymous, intronic, or non-coding variants). Mutations of moderate or higher impact were identified at a total of 128 sites (Supplementary Data 1). To further prioritize, we specifically focused on mutations that were present in isolates from more than one of the parental backgrounds. We identified UXS1, which sustained four distinct mutations in four isolates from two parental backgrounds (Fig. 3b).
UXS1 encodes the enzyme that converts UDP-glucuronic acid to UDP-xylose28. This pathway is critical for the formation of the capsule, a core virulence trait of Cryptococcus, and for synthesis of other glycoconjugates. There is no UXS1 ortholog in either Saccharomyces cerevisiae or Candida albicans, where many of the resistance mechanisms for 5FC were elucidated. The mutations in UXS1 included a single base deletion in a 3xT homopolymer (R265-5), a single base insertion in a 7xC homopolymer (RBB18-8), and a missense mutation (Tyr217Cys, RBB18-9) (Fig. 3b, Table 1). Finally, a uxs1 mutation (Asp306Gly) was identified in the isolate previously identified to have an fcy2 mutation (RBB18-4). In sum, 9 sequenced genomes representing 8 independent isolates remained for which we were unable to identify a mutation that conferred resistance to 5FC and 5FU, all derived from msh2 mutant isolates.
To confirm the role of uxs1 mutation in resistance to 5FC and 5FU, a uxs1 deletion available from a C. neoformans deletion collection was employed (Fig. 4a). This uxs1Δ strain was completely resistant to both drugs, suggesting that all four alleles isolated were likely loss of function mutations because they shared a drug resistance phenotype with the null mutant. We tested the MIC of 5FC for uxs1 and fur1 mutants in both YPD and YNB using a broth microdilution assay. Both uxs1 and fur1 mutants were resistant to 5FC above the limits of our assays (MIC > 400 µg/mL in YPD and > 4 µg/mL in YNB) while the wildtype parent strains were sensitive at 200 µg/mL in YPD and 0.5 µg/mL in YNB (Table 2).
Fig. 4. uxs1 mutants mediate 5FC resistance through a xylosylation-independent mechanism.
a KN99 deletion strains from the C. neoformans deletion collection show that deletion of UXS1 confers resistance to 5FC and 5FU. The RBB18-2 strain carrying a fur1 mutation is resistant to 5FC and 5FU although more weakly to 5FU. The R265-3 strain carrying a fur1 mutation is completely resistant to both drugs. b Spot dilution assay on YNB, YNB plus 5FC, and YNB plus 5FU demonstrating overexpression of UXS1 driven by the actin promoter does not confer increased sensitivity to 5FC or 5FU. c Spot dilution assays on YNB, YNB plus 5FC, and YNB plus 5FU demonstrating that mutants deficient in UDP-xylose transport (uxt1Δ, uxt2Δ, uxt1Δ uxt2Δ) and xylose transferase mutants (cxt1Δ, cxt2Δ, cxt1Δ cxt2Δ) show no change in 5FC and 5FU sensitivity. d Spot dilution assay on YPD, YPD plus 5FC, and YPD plus 5FU showing that ugd1 mutants are viable on rich YPD media but retain sensitivity to 5FC and 5FU. In addition, ugd1 uxs1 double mutants retain sensitivity to 5FC and 5FU like a ugd1 single mutant rather than gain resistance like the uxs1 single mutant.
Table 2.
MIC values for deletion mutants of genes identified in this study.
| Genetic Background | 5FC in YPD | 5FC in YNB |
|---|---|---|
| Wildtype | 200 µg/mL | 0.5 µg/mL |
| fur1 | > 400 µg/mL | > 4 µg/mL |
| uxs1 | > 400 µg/mL | > 4 µg/mL |
Accumulation of UDP-glucuronic acid mediates 5FC resistance
We next sought to genetically define the mechanism by which drug resistance may be mediated by loss of uxs1 function. Multiple models were considered to explain why 5FC/5FU toxicity would require Uxs1. The first was that Uxs1 directly converts 5FU into a toxic product. If so, Uxs1 and Fur1 would function in the same pathway, as either mutant independently confers drug resistance. This hypothesis was tested using an overexpression allele of UXS1 that is driven by the actin promoter29. If this hypothesis were correct, we would expect to observe additional sensitivity conferred by the overexpression allele compared to wildtype. By reducing the amount of 5FU used to only 1 µg/mL, wildtype strains were only partially inhibited. However, introduction of an overexpression allele of UXS1 did not increase sensitivity (Fig. 4b). This suggests that Uxs1 does not act by converting 5FU or a 5FU derivative into a toxic product.
We next tested whether 5FC resistance in uxs1 mutants may occur through an indirect effect of the role of Uxs1 in synthesis of UDP-xylose. UDP-xylose is the donor molecule for xylose addition to glycans, a process that primarily occurs in the secretory compartment. If xylosylation of an unknown glycoconjugate is required to mediate 5FC toxicity, mutation of UXS1 would indirectly confer drug resistance. To test this, deletion mutants lacking transporters that move UDP-xylose into the secretory compartment (uxt1, uxt2, and a uxt1 uxt2 double mutant30) or that lack Golgi xylosyl-transferases that act in protein, glycolipid, and polysaccharide synthesis (cxt131, cxt2, and a cxt1 cxt2 double mutant) were analyzed. None of these mutants demonstrated any change in sensitivity to 5FC or 5FU (Fig. 4c). However, these data did not rule out a requirement for a (previously undescribed) cytoplasmic xylosyl protein modification. To test this hypothesis, a mutant that cannot generate UDP-glucuronic acid, the immediate precursor for UDP-xylose synthesis was used. This mutant (ugd1) is somewhat growth impaired relative to wildtype and cannot grow on YNB media. However, it does grow, albeit poorly, on rich YPD media, where it clearly exhibited sensitivity to 5FC. This result demonstrated that xylose modification, in any cellular compartment, is not required for 5FC toxicity (Fig. 4d).
The previous models ruled out the lack of UDP-xylose for synthetic processes as an explanation for 5FC resistance. Another result of the loss of UXS1 function is the accumulation of UDP-glucuronic acid, the immediate precursor in the production of UDP-xylose. Past studies have shown that UDP-glucuronic acid accumulates to extremely high levels in uxs1 mutant cells, while it is undetectable in ugd1 mutants32. To test whether this mediates resistance, we generated a uxs1 ugd1 double mutant, which should produce neither UDP-glucuronic acid nor UDP-xylose32. While the uxs1 ugd1 mutant was growth impaired, like the ugd1 single mutant, it was clearly sensitive to 5FC (Fig. 4d). That uxs1 mutants are 5FC resistant, whereas uxs1 ugd1 double mutants are restored to 5FC sensitivity suggests that accumulation of UDP-glucuronic acid in uxs1 mutants mediates resistance to 5FC and 5FU (Fig. 5).
Fig. 5. Model of inhibition of 5FC/5FU toxicity by uxs1 mutation.
Potential mechanisms by which uxs1 mutations may confer resistance to both 5FC and 5FU. Mutation of uxs1 causes an accumulation of UDP-glucuronic acid, the product of Ugd1, which either impairs production of toxic fluoridated molecules or rescues inhibition of the targets of those fluoridated molecules, such as thymidylate synthase. Protein names are in red for those where mutations were found in this study.
Discussion
Treating fungal diseases is complicated both by the limited number of drugs that effectively treat infection without harming the patient and by the rapid rate at which fungi develop resistance to the few drugs that are effective. 5FC is a particularly emblematic example of this issue, as it is highly efficacious with limited toxicity. Human cells lack the ability to convert 5FC to 5FU and toxicity is conferred only by the conversion of 5FC to the chemotherapeutic 5FU by a patient’s microbiota33. However, 5FC is ineffective when used for solo treatment because fungal resistance rapidly emerges. Here, we demonstrate that DNA mismatch repair mutants exhibit accelerated acquisition of resistance to 5FC. Evolutionary theory predicts that hypermutators should be rare in eukaryotic microbes because sex unlinks mutator alleles from the mutations they generate, eliminating the advantage of an elevated mutation rate and leaving only the general decrease in fitness from introduced mutations34. This result lends further support to the recent appreciation that mismatch repair mutants may be common in pathogenic fungi in part because treatment with antifungal drugs increases selection for mutations that generate resistance13,14,16,17.
We explored the underlying genetic and genomic basis of 5FC resistance. The resistant mutants in C. deuterogattii selected here were cross-resistant to 5FU. Sanger and whole genome Illumina sequencing identified a presumptive genetic basis for drug resistance in 16 independent isolates. Analysis of resistance loci from whole genome data was relatively facile in wildtype strains (5/5 strains assigned a causative mutation), where an average of 1.2 coding mutations (range 0–3) were identified by whole genome sequencing, including the putative resistance mutation, relative to the reference. However, this analysis was substantially more difficult in mutator strains (8/17) where an average of 11.47 coding mutations were found per strain (range 2–25), with numerous additional noncoding or synonymous mutations. For the purposes of identifying the genetic basis of a trait that occurs at a high rate in wildtype, future studies would be advised to avoid mutations that increase mutation rate, as they contribute to background noise.
We identified multiple mutations in the FUR1 locus (12 of the 16 identified causative mutations). fur1 mutations occurred through multiple mechanisms, including regional deletions, homopolymer tract length changes that introduced frameshift mutations, and a splice site acceptor point mutation. Surprisingly, we did not identify mutations in FCY1 or mutations in FCY2 that were unaccompanied by a second resistance mutation. Although we selected with only 5FC, all drug resistant isolates were cross-resistant to 5FU as well. One possible explanation is that selection with 100 µg/mL of 5FC may be above the MIC for fcy1 or fcy2 mutants in C. deuterogattii, although fcy1 mutants in C. neoformans are resistant to 100 µg/mL of 5FC (Supplementary Fig. 2). Further experiments will be necessary to test this hypothesis, which could provide guidance into treatment levels for 5FC. Further experiments based on this hypothesis could provide insight into the function of the other FCY2 paralogs, perhaps as lower affinity transporters of 5FC that confer toxicity at higher concentrations of 5FC.
Mutations in UXS1 are particularly interesting as a mechanism of resistance in Cryptococcus because Uxs1 catalyzes the production of UDP-xylose, the donor molecule for essential components of Cryptococcal capsule polysaccharides. Strains lacking UXS1 are hypocapsular with altered capsule structure32. In addition, uxs1 mutants are avirulent in a murine tail-vein injection disseminated infection model35. This suggests that uxs1 mutants might be unlikely to emerge during exposure to 5FC in vivo, even though they represent a substantial proportion of the resistant isolates observed in this study. Likewise, regional deletions including FUR1 affected multiple neighboring genes as well, including the direct neighboring gene GIS2. Gis2 has previously been described to play a role in stress tolerance, including fluconazole and oxidative stress tolerance36. Like uxs1 mutants, these regional deletion mutants may be less likely to emerge in vivo. It is important to note that in vitro resistance to 5FC is not necessarily associated with clinical treatment failure and does not prevent synergy of combination treatment with Amphotericin B and flucytosine37. Continued selection by 5FC treatment of a deleterious resistance allele like a uxs1 mutation or a collateral gis2 deletion might explain the maintenance of synergy. Future studies examining the mechanisms of resistance during treatment with 5FC in vivo will provide further insights into the possible contribution of each of these mechanisms to resistance in patients.
This study also illustrates the importance of examining drug resistance in the context of the pathogen being treated. Previous work in C. albicans and S. cerevisiae suggested that resistance would occur through mutations in FUR1, but both species are evolutionarily distant from Cryptococcus and lack a UXS1 ortholog. While these previous studies provided substantial insight into 5FC toxicity, studies in the pathogen of interest are essential. Surprisingly, one strain (RBB18-4) that was cross resistant to 5FU had a mutation in the FCY2 gene (CNBG_3227), which in other species confers resistance to 5FC but not 5FU. Mutation of FCY2 is known to result in resistance to 5FC in C. deuterogattii, but cross-resistance to 5FU has not been tested12. Unexpected cross-resistance between 5FC and fluconazole has been previously observed in fcy2 mutants of Candida lusitaniae but is proposed to occur through competitive inhibition of fluconazole uptake by 5FC that can no longer enter through Fcy2-mediated transport8,38,39. C. lusitaniae fcy2 mutants are not resistant to fluconazole without the addition of 5FC. In addition, multiple resistant strains were not assigned a presumptive causative mutation here and lacked mutations in any genes known to cause 5FC resistance from this or previous work (FUR1, FCY1, FCY2, and UXS1). Presumably unknown mechanisms are responsible for resistance to 5FC and 5FU in these strains as well, either in pathways unique to Cryptococcus or potentially more broadly conserved.
In addition, UXS1 mutations provide unexpected insight into interaction between nucleotide synthesis and generation of precursors for xylosylation. Surprisingly, accumulation of UDP-glucuronic acid appears to either inhibit the pyrimidine salvage pathway or activate thymidylate synthase (Fig. 5). This suggests that UDP-glucuronic acid may have a role as a source of UDP for the cell, while UDP-xylose does not. While UXS1 orthologs are not found in C. albicans or S. cerevisiae, which lack xylose modifications, there is a UXS1 ortholog in humans. 5FU is commonly used as a chemotherapeutic drug40, and resistance to 5FU is frequently associated with mutations in thymidylate synthase41. Data here suggest that uxs1 mutations may be acting in a similar fashion to either de-repress thymidylate synthase or inhibit Fur1 (Fig. 5). Further exploration of the role of Uxs1 orthologs in humans during 5FU chemotherapy may be of interest.
Methods
Strains and media
The strains and plasmids used in this study are listed in Table S1. The strains were maintained in 25% glycerol stocks at −80 °C and grown on rich YPD media at 30 °C (Yeast extract Peptone Dextrose). Strains with selectable markers were grown on YPD containing 100 µg/mL nourseothricin (NAT) and/or 200 µg/mL G418 (NEO).
Genome sequencing
DNA was isolated for sequencing by expanding individual colonies to 50 mL liquid cultures in YPD at 30 °C. Cultures were then frozen and lyophilized until dry. DNA was extracted using a standard CTAB extraction protocol, as previously described42. Illumina paired-end libraries were prepared and sequenced by the University of North Carolina Next Generation Sequencing Facility using the Kapa Library prep kit and the Hiseq platform. Additional sequencing was performed by the Duke University Sequencing Core using the Kapa Hyper prep kit and performed using a NovaSeq platform. Raw reads are available through the Sequence Read Archive under project accession number PRJNA525019.
Genome assembly and variant calling
Reads were aligned to the V2 R265 reference genome43 using BWA-MEM44. Alignments were further processed with SAMtools45, the Genome Analysis Toolkit (GATK)46, and Picard. SNP and indel calling was performed using the Unified Genotyper Component of the GATK with default settings aside from ploidy = 1. VCFtools47 was utilized for processing of the resulting calls to remove sites common to all strains (errors in the reference assembly) and variants were annotated using SnpEff27. All remaining variant calls were visually examined using the Integrated Genome Viewer (IGV) to remove calls resulting from poor read mapping48. FungiDB was also used to determine putative function and orthology of genes containing called variants in the dataset49.
Strain construction
A ugd1Δ mutant was constructed in the KN99a background as follows. Primers pairs JOHE45233/JOHE45085, JOHE45086/JOHE45087, and JOHE45088/JOHE45234 were used to amplify 1 kb upstream of UGD1, the neomycin resistant marker, and 1 kb downstream of the UGD1 gene, respectively (primer sequences available in Supplementary Table 2). To generate the deletion allele for C. neoformans transformation, all three fragments were cloned into plasmid pRS426 by transforming S. cerevisiae strain FY83450. Recombinant S. cerevisiae transformants were selected on SD-uracil media and verified by spanning PCR with primer pair JOHE45233/JOHE45234. The resulting PCR product was introduced into C. neoformans laboratory strain KN99a by biolistic transformation and transformants were selected on YPD containing neomycin. Putative ugd1Δ deletion mutants were confirmed by PCR.
uxs1Δ single mutants and ugd1Δ uxs1Δ double mutants were generated via a genetic cross51. First, the KN99α uxs1Δ mutant from the Hiten Madhani deletion collection was mated with the wild-type KN99a laboratory strain. Through microdissection, spores were isolated, germinated, and genotyped via PCR for the gene deletion and the mating type locus to isolate a MATa uxs1Δ mutant in the KN99a background. Second, the KN99a uxs1Δ mutant was mated with wild-type H99. Spores were dissected and genotyped via PCR for the gene deletion and the mating type locus to isolate H99 uxs1Δ single mutants. Finally, the H99 uxs1Δ single mutant was crossed with KN99a ugd1Δ to generate ugd1Δ uxs1Δ double mutants, and the H99 ugd1Δ single mutant.
Spot dilution assays
Single colonies were inoculated into 5 mL of liquid YPD and grown overnight at 30°C. Cell density was determined using a hemocytometer and the cultures were diluted accordingly such that 100,000 cells were aliquoted on to the most concentrated spot and subsequent spots consisted of 10-fold dilutions per spot. Each strain was spotted onto YPD or YNB alone and onto media also containing 5FC or 5FU at the indicated concentration. Plates were incubated at 30°C until photographed.
Swab assays
To isolate independent drug resistant strains, the original parent strains were subcultured from a frozen glycerol stock. Single colonies were used to inoculate liquid YPD cultures without selection. Those liquid cultures were grown with shaking until saturation. They were then spread onto drug plates (100 µg/mL 5FC on YNB) using sterile cotton swabs to select for resistant colonies. A single drug resistant colony was taken from any given liquid culture to ensure independence. This assay is only semi-quantitative, as the inoculum is not strictly controlled between independent cultures when swabbing.
MIC testing
5-flucytosine was dissolved in water and added to liquid YPD media in a 96-well plate at 400 µg/mL. 2-fold serial dilutions were performed until a concentration of 1.56 µg/mL was achieved. For YNB, 5-flucytosine was dissolved in water and added to liquid YNB media in a 96-well plate at 4 µg/mL. Two-fold serial dilutions were performed until a concentration of 0.016 µg/mL was achieved. Cell density of overnight cultures (liquid YPD, 30°C) was determined using a hemocytometer and cultures were adjusted to 105 cells/mL. 100 µl of cell suspension was added to each well (10,000 cells per well). The 96-well plate was incubated at 30°C and OD600 readings were taken daily using a Sunrise Tecan instrument and Magellan software.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This study was supported by NIH/NIAID R37 MERIT award AI39115-21, NIH/NIAID R01 AI50113-15, NIH/NIAID R01 AI112595-04, and NIH/NIAID P01 AI104533-05 to J.H.; NIH/NIAID R21 AI109623 to T.L.D.; and NIH/NIAID F30 AI120339 to L.X.L. This study utilized a Cryptococcus gene deletion collection deposited at the Fungal Genetics Stock Center and made freely available ahead of publication by the Madhani laboratory and funded by NIH R01 AI100272.
Source Data
Author contributions
R.B.B. and J.H. conceived the study. R.B.B., S.A.C., L.X.L., T.L.D. and J.H. designed and analyzed experiments. R.B.B., S.A.C. and L.X.L. performed experiments. R.B.B. wrote the manuscript with contributions and editing from all other authors. T.L.D. and J.H. provided funding and supervision.
Data availability
Raw reads are available through the Sequence Read Archive under project accession number PRJNA525019. Strains generated in this study are available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Marie Desnos-Ollivier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: R. Blake Billmyre, Shelly Applen Clancey
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13890-z.
References
- 1.Brown GD, et al. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Rajasingham R, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saag MS, et al. Practice guidelines for the management of Cryptococcal disease. Clin. Infect. Dis. 2000;30:710–718. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 4.Bennett JE, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N. Engl. J. Med. 1979;301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 5.Molloy SF, et al. Antifungal combinations for treatment of Cryptococcal meningitis in Africa. N. Engl. J. Med. 2018;378:1004–1017. doi: 10.1056/NEJMoa1710922. [DOI] [PubMed] [Google Scholar]
- 6.Loyse A, Dromer F, Day J, Lortholary O, Harrison TS. Flucytosine and cryptococcosis: time to urgently address the worldwide accessibility of a 50-year-old antifungal. J. Antimicrob. Chemother. 2013;68:2435–2444. doi: 10.1093/jac/dkt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hope W, Tabernero L, Denning D, Anderson M. Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob. Agents Chemother. 2004;48:4377–4386. doi: 10.1128/AAC.48.11.4377-4386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papon N, et al. Molecular mechanism of flucytosine resistance in Candida lusitaniae: contribution of the FCY2, FCY1, and FUR1 genes to 5-fluorouracil and fluconazole cross-resistance. Antimicrob. Agents Chemother. 2007;51:369–371. doi: 10.1128/AAC.00824-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McManus BA, Moran GP, Higgins JA, Sullivan DJ, Coleman DC. A Ser29Leu substitution in the cytosine deaminase Fca1p is responsible for clade-specific flucytosine resistance in Candida dubliniensis. Antimicrob. Agents Chemother. 2009;53:4678–4685. doi: 10.1128/AAC.00607-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelan WL. The genetic basis of resistance to 5-fluorocytosine in Candida species and Cryptococcus neoformans. CRC Crit. Rev. Microbiol. 1987;15:45–56. doi: 10.3109/10408418709104447. [DOI] [PubMed] [Google Scholar]
- 11.Vu K, et al. Flucytosine resistance in Cryptococcus gattii is indirectly mediated by the FCY2-FCY1-FUR1 pathway. Med. Mycol. 2018;56:857–867. doi: 10.1093/mmy/myx135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanal Lamichhane A, et al. A novel role of fungal type I myosin in regulating membrane properties and its association with D-amino acid utilization in Cryptococcus gattii. MBio. 2019;10:e01867–19. doi: 10.1128/mBio.01867-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billmyre RB, Clancey SA, Heitman J. Natural mismatch repair mutations mediate phenotypic diversity and drug resistance in Cryptococcus deuterogattii. Elife. 2017;6:e28802. doi: 10.7554/eLife.28802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce, K. J. et al. Mismatch repair of DNA replication errors contributes to microevolution in the pathogenic fungus Cryptococcus neoformans. mBio8, e00595–17 (2017). [DOI] [PMC free article] [PubMed]
- 15.Billmyre RB, et al. Highly recombinant VGII Cryptococcus gattii population develops clonal outbreak clusters through both sexual macroevolution and asexual microevolution. MBio. 2014;5:e01494–14. doi: 10.1128/mBio.01494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes J, et al. A population genomics approach to assessing the genetic basis of within-host microevolution underlying recurrent cryptococcal meningitis infection. G3. 2017;7:1165–1176. doi: 10.1534/g3.116.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healey KR, et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat. Commun. 2016;7:11128. doi: 10.1038/ncomms11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dellière S, et al. Fluconazole and echinocandin resistance of Candida glabrata correlates better with antifungal drug exposure rather than with MSH2 mutator genotype in a French cohort of patients harboring low rates of resistance. Front. Microbiol. 2016;7:2038. doi: 10.3389/fmicb.2016.02038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healey KR, Jimenez Ortigosa C, Shor E, Perlin DS. Genetic drivers of multidrug resistance in Candida glabrata. Front. Microbiol. 2016;7:1995. doi: 10.3389/fmicb.2016.01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, et al. Absence of azole or echinocandin resistance in Candida glabrata isolates in India despite background prevalence of strains with defects in the DNA mismatch repair pathway. Antimicrob. Agents Chemother. 2018;62:e00195–18. doi: 10.1128/AAC.00195-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steenwyk JL, et al. Extensive loss of cell-cycle and DNA repair genes in an ancient lineage of bipolar budding yeasts. PLOS Biol. 2019;17:e3000255. doi: 10.1371/journal.pbio.3000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagen F, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015;78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes J, et al. Tracing genetic exchange and biogeography of Cryptococcus neoformans var. grubii at the global population level. Genetics. 2017;207:327–346. doi: 10.1534/genetics.117.203836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon-Chung, K. J. et al. The case for adopting the “Species Complex” nomenclature for the etiologic agents of Cryptococcosis. mSphere2, e00357-16 (2017). [DOI] [PMC free article] [PubMed]
- 25.Gsaller F, et al. Mechanistic basis of pH-dependent 5-flucytosine resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2018;62:e02593–17. doi: 10.1128/AAC.02593-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav V, et al. RNAi is a critical determinant of centromere evolution in closely related fungi. Proc. Natl Acad. Sci. USA. 2018;115:3108–3113. doi: 10.1073/pnas.1713725115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. (Austin). 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bar-Peled M, Griffith CL, Doering TL. Functional cloning and characterization of a UDP- glucuronic acid decarboxylase: the pathogenic fungus Cryptococcus neoformans elucidates UDP-xylose synthesis. Proc. Natl Acad. Sci. USA. 2001;98:12003 LP–12012008. doi: 10.1073/pnas.211229198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gish SR, et al. Computational analysis reveals a key regulator of cryptococcal virulence and determinant of host response. MBio. 2016;7:e00313–e00316. doi: 10.1128/mBio.00313-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LX, Rautengarten C, Heazlewood JL, Doering TL. Xylose donor transport is critical for fungal virulence. PLoS Pathog. 2018;14:e1006765. doi: 10.1371/journal.ppat.1006765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klutts JS, Doering TL. Cryptococcal xylosyltransferase 1 (Cxt1p) from Cryptococcus neoformans plays a direct role in the synthesis of capsule polysaccharides. J. Biol. Chem. 2008;283:14327–14334. doi: 10.1074/jbc.M708927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffith CL, Klutts JS, Zhang L, Levery SB, Doering TL. UDP-glucose dehydrogenase plays multiple roles in the biology of the pathogenic fungus Cryptococcus neoformans. J. Biol. Chem. 2004;279:51669–51676. doi: 10.1074/jbc.M408889200. [DOI] [PubMed] [Google Scholar]
- 33.Harris BE, Manning BW, Federle TW, Diasio RB. Conversion of 5-fluorocytosine to 5-fluorouracil by human intestinal microflora. Antimicrob. Agents Chemother. 1986;29:44 LP–44 48. doi: 10.1128/AAC.29.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenaillon O, Le Nagard H, Godelle B, Taddei F. Mutators and sex in bacteria: conflict between adaptive strategies. Proc. Natl Acad. Sci. USA. 2000;97:10465–10470. doi: 10.1073/pnas.180063397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyrand F, Klaproth B, Himmelreich U, Dromer F, Janbon G. Isolation and characterization of capsule structure mutant strains of Cryptococcus neoformans. Mol. Microbiol. 2002;45:837–849. doi: 10.1046/j.1365-2958.2002.03059.x. [DOI] [PubMed] [Google Scholar]
- 36.Leipheimer J, Bloom ALM, Baumstark T, Panepinto JC. CNBP homologues Gis2 and Znf9 interact with a putative G-quadruplex-forming 3’ untranslated region, altering polysome association and stress tolerance in Cryptococcus neoformans. mSphere. 2018;3:e00201–e00218. doi: 10.1128/mSphere.00201-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz P, Janbon G, Dromer F, Lortholary O, Dannaoui E. Combination of amphotericin B with flucytosine is active in vitro against flucytosine-resistant isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 2007;51:383–385. doi: 10.1128/AAC.00446-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapeland-Leclerc F, et al. Inactivation of the FCY2 gene encoding purine-cytosine permease promotes cross-resistance to flucytosine and fluconazole in Candida lusitaniae. Antimicrob. Agents Chemother. 2005;49:3101–3108. doi: 10.1128/AAC.49.8.3101-3108.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Florent M, et al. Nonsense and missense mutations in FCY2 and FCY1 genes are responsible for flucytosine resistance and flucytosine-fluconazole cross-resistance in clinical isolates of Candida lusitaniae. Antimicrob. Agents Chemother. 2009;53:2982–2990. doi: 10.1128/AAC.00880-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moertel CG. Chemotherapy for colorectal cancer. N. Engl. J. Med. 1994;330:1136–1142. doi: 10.1056/NEJM199404213301608. [DOI] [PubMed] [Google Scholar]
- 41.Pullarkat ST, et al. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 2001;1:65. doi: 10.1038/sj.tpj.6500012. [DOI] [PubMed] [Google Scholar]
- 42.Pitkin JW, Panaccione DG, Walton JD. A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology. 1996;142:1557–1565. doi: 10.1099/13500872-142-6-1557. [DOI] [PubMed] [Google Scholar]
- 43.Farrer RA, et al. Genome evolution and innovation across the four major lineages of Cryptococcus gattii. MBio. 2015;6:e00868–15. doi: 10.1128/mBio.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenna A, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stajich JE, et al. FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res. 2012;40:D675–D681. doi: 10.1093/nar/gkr918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ianiri, G., Averette, A. F., Kingsbury, J. M., Heitman, J. & Idnurm, A. Gene function analysis in the ubiquitous human commensal and pathogen Malassezia genus. mBio7, e01853–16 (2016). [DOI] [PMC free article] [PubMed]
- 51.Sun S, Priest SJ, Heitman J. Cryptococcus neoformans mating and genetic crosses. Curr. Protoc. Microbiol. 2019;53:e75. doi: 10.1002/cpmc.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Raw reads are available through the Sequence Read Archive under project accession number PRJNA525019. Strains generated in this study are available upon request.