Abstract
It is often assumed that animals’ temporal activity patterns are highly conserved throughout evolution. While most geckos are nocturnal, the species in the Cnemaspis genus are mostly diurnal (only a few are nocturnal). This raises a question about the evolution of a diel niche in the Cnemaspis genus. Cnemaspis geckos are distributed across Southeast Asia and are often sympatric with Cyrtodactylus, another widespread gecko genus in the same area. Since both genera are mainly rocky habitat specialists, we hypothesize that Cyrtodactylus may influence the temporal activity pattern of Cnemaspis when they are sympatric through competition. By analyzing habitat data, diel activity, and the existence of sympatric Cyrtodactylus species across the phylogeny of the Cnemaspis genus, we found (1) strong phylogenetic signals in the habitat use trait but not in temporal activity, suggesting that the diel niche of this genus is more labile compared with habitat niche, and (2) a significant association with the temporal activity pattern of Cnemaspis and the sympatry between the two genera, with the former tending to be diurnal when they are sympatric. Originated from a diurnal common ancestor, the release from competition with Cyrtodactylus species might open an opportunity for some Cnemaspis species to shift to nocturnal niches.
Subject terms: Evolutionary ecology, Evolutionary theory, Phylogenetics
Introduction
Niche partition among sympatric species is an important mechanism to determine species richness in a community1–3. Although there have been great efforts to study how sympatric species separate their ecological niches along the spatial dimension (e.g.4,5), we still know little about their diel niche segregation6. Many sympatric taxa that share habitats or food resources might be active at different time periods to avoid competition7. However, studies focusing on the diel niche transitions are extremely rare. A good system for such research requires a suitable taxonomic group with a variety of temporal activity patterns, well-resolved phylogenetic relationships, and reliable ecological data.
Based on phylogenetic analyses of diel niche evolution, Anderson and Wiens8 suggested that terrestrial vertebrates may have nocturnal ancestors and show strong phylogenetic signals in this trait. They further supported the idea presented by Vitt et al.9 that the temporal niche partition among current species has been largely conserved over evolution. Among current nocturnal vertebrates, geckos belong to a taxonomic group containing primarily nocturnal species with a nocturnal ancestor10. However, Gamble and colleagues10 found that there were multiple transitions between diurnality and nocturnality in this group during various time periods including several recent ones. Contradicting with the strong conservatism in temporal activity pattern in most vertebrates, the recent shifts between nocturnality and diurnality in geckos provide good chances for testing temporal niche partitioning and ecological community assembly.
Southeast Asia Cnemaspis (rock geckos), composed of 55 species11,12, is a speciose genus in gekkonid lizards. This genus forms a large proportion of species that are endemic to extremely limited distribution ranges12. As rocky habitat specialists, these species tend to maintain strong niche conservatism through a long divergence history13; the high species richness of this genus is thus regarded as a consequence of their strict specialization in fragmented rocky habitats. However, this genus is composed of a majority of diurnal species and a minority of nocturnal species12, implying that they might have experienced multiple diel transitions.
Interestingly, Cnemaspis species are usually sympatric with another nocturnal gecko genus Cyrtodactylus12, which comprises more than 200 species and is the most speciose gekkonid group across Southeast Asia14–16. Similar to Cnemaspis, many Cyrtodactylus geckos are strongly specialized in rocky habitats17–19. The typical habitats of these two genera of geckos, such as rocks, karst topology, or boulder caves, are usually extremely limited in space and food resources. Under this situation, Cnemaspis geckos might face strong competition from Cyrtodactylus geckos when they are sympatric. Since there are many records that diurnal Cnemaspis geckos are co-distributed with nocturnal Cyrtodactylus species12,20,21, we hypothesize that a diel niche partitioning between these two geckos may have evolved to avoid competition.
In this study, we performed phylogenetic comparative analyses on habitat use and temporal activity patterns of the Cnemaspis genus to identify their ancestral states of these traits along the phylogeny. We aim to (1) infer if there is niche conservatism or transition in these traits; (2) examine the correlation between the temporal activity patterns and sympatry of Cnemaspis and Cyrtodactylus species; and (3) determine other potential ecological or environmental factors that impact the temporal activity patterns of Cnemaspis geckos. This will be one of the first studies to examine the interaction between biological competition and diel niche evolution in reptiles.
Results
There are 51 Cnemaspis species with available ND2 sequences from GenBank, representing 93% among the 55 currently recognized species (Table S1). Among them, 36 were diurnal and 15 were nocturnal. About two-thirds of them were specialists in rocky habitats (35 species) while others (16 species) were terrestrial or habitat generalists. Among them, 20 species were sympatric with Cyrtodactylus species (Table S2). The original phylogeny of Cnemaspis with outgroup species is presented in Fig. S1. Phylogenies with ecological character states are demonstrated in Figs. 1 and 2.
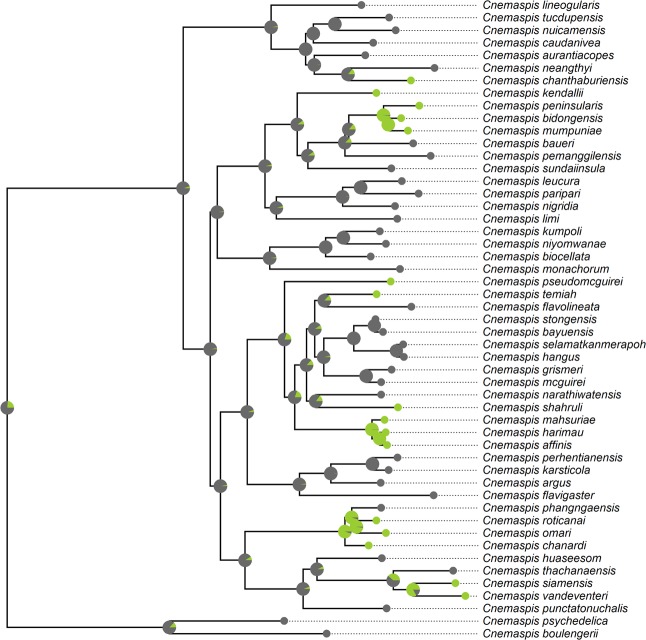
Figure 1.
The evolution of habitat use in Cnemaspis genus. Bayesian ancestral state reconstructions of habitat use were mapped onto the mitochondrial ND2 phylogeny of Cnemaspis using the asymmetric multi-rate model. Circles at the tips of branches indicate the habitat use type for each included species. Pie charts on internal nodes indicate the posterior probability of that ancestor having a particular habitat use type. Species are categorized as rocky habitat specialists (grey) and habitat generalist/other habitat specialists (green).
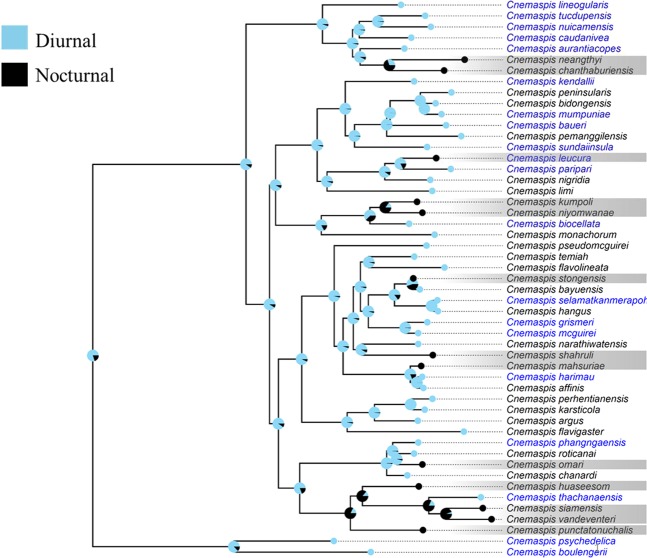
Figure 2.
The evolution of temporal niche in Cnemaspis genus. Bayesian ancestral state reconstructions of temporal activity were mapped onto the mitochondrial ND2 phylogeny of Cnemaspis using the asymmetric multi-rate model. Circles at the tips of branches indicate the temporal niche for each included species. Pie charts on internal nodes indicate the posterior probability of that ancestor having a particular temporal niche. Species are categorized as nocturnal (black) and diurnal (blue). The species names in blue color indicate the existence of sympatric Cyrtodactylus species.
Phylogenetic conservation
The habitat and diel activity niches of the Cnemaspis species showed different levels of phylogenetic signals (Table 1). The estimated D ≈ 0.299 of habitat use suggested a high level of phylogenetic signal in this trait. The simulation test indicated that the level of phylogenetic signal differed significantly from that of a random phylogenetic structure but was consistent with the expectation of Brownian motion. In contrast, the estimated D ≈ 0.789 of the temporal activity pattern indicated a low level of phylogenetic signal. The simulation test on temporal activity showed that the phylogenetic signal level was consistent with the expectation of a random phylogenetic structure.
Table 1.
Estimated phylogenetic signal strength (D) for the habitat type and temporal activity mode for Cnemaspis genus.
| Habitat type | Temporal activity | |
|---|---|---|
| Estimated D | 0.2989471 | 0.7895734 |
| Random Phylogenetic Structure (p value) | <0.01 | 0.228 |
| Brownian Phylogenetic Structure (p value) | 0.221 | 0.018 |
Ancestral state reconstruction
For both temporal activity and habitat use traits, the transition rates between character states based on single- and two-rate models in BayesTraits did not show a significant difference (temporal activity: single rate = −33.82 and asymmetric rates model = −31.33, logBF = 4.977; habitat use: single rate = −29.25 and asymmetric rates model = −28.29, logBF = 1.933). Both transition rate models favored a diurnal and rocky-specialist common ancestor for the Cnemaspis genus (Table 2).
Table 2.
Estimated probability of ancestral state at the most recent common ancestor of Cnemaspis genus using BayesTraits v2.0.
| Model | Ancestral state | Mean | 95% HPD Interval |
|---|---|---|---|
| (a) Temporal activity: D — diurnal, N — nocturnal | |||
| Asymmetric rate | Root P(D) | 0.503 | [0.4984, 0.5139] |
| Root P(N) | 0.497 | [0.4862, 0.5016] | |
| Equal rate | Root P(D) | 0.625 | [0.5000, 0.8631] |
| Root P(N) | 0.375 | [0.1369, 0.5000] | |
| (b) Habitat use: O — other habitat/generalist, R — rock habitat | |||
| Asymmetric rate | Root P(O) | 0.4184 | [0.2324, 0.5000] |
| Root P(R) | 0.5816 | [0.5000, 0.7676] | |
| Equal rate | Root P(O) | 0.2088 | [7.4 × 10−5, 0.4571] |
| Root P(R) | 0.7912 | [0.5429, 0.9999] | |
The stochastic mapping analyses showed that the asymmetric multi-rate model was preferred (Table S3). The results of ancestral state reconstruction based on the stochastic mapping approach were congruent with those of the Bayestraits analyses (Figs. 1 and 2), suggesting the robustness of these results.
Regression between temporal activity modes and ecological factors
The best model included the existence of sympatric Cyrtodactylus species, along with the mean diurnal range and precipitation of the driest quarter (Tables 3 and 4), together explained 48.1% of the temporal activity patterns in the Cnemaspis genus. Although the mean diurnal range parameter (bio2) was included in the best model, this parameter was weakest, not significant factor, and the performance of the model was slightly decreased without it (Table 3).
Table 3.
Akaike information criterion (AIC) for model selection.
| Model | Df | AIC |
|---|---|---|
| Active_time ~ Cyrto_Sym + bio2 + bio17* | 47 | 45.75073 |
| Active_time ~ Cyrto_Sym + bio17 | 48 | 46.69000 |
| Active_time ~ Cyrto_Sym + dist_range + bio2 + bio17 | 46 | 46.86641 |
| Active_time ~ Cyrto_Sym + dist_range + bio2 + bio17 + bio18 | 45 | 48.3432 |
| Active_time ~ Cyrto_Sym + max_SVL + dist_range + bio2 + bio17 + bio18 | 44 | 49.41486 |
| Active_time ~ bio2 + bio17 | 48 | 50.30900 |
| Active_time ~ Cyrto_Sym + max_SVL + dist_range + Avg_night_temp + bio2 + bio17 + bio18 | 43 | 51.08088 |
| Active_time ~ Cyrto_Sym + bio2 | 48 | 51.91400 |
| Active_time ~ Cyrto_Sym + max_SVL + dist_range + Avg_night_temp + bio2 + bio4 + bio17 + bio18 | 42 | 53.05279 |
| Active_time ~ Cyrto_Sym | 49 | 53.32100 |
| Active_time ~ Cyrto_Sym + max_SVL + Habitat.type + dist_range + Avg_night_temp + bio2 + bio4 + bio17 + bio18 | 41 | 55.04756 |
| Active_time ~ bio2 | 49 | 56.18600 |
| Active_time ~ bio17 | 49 | 56.45700 |
| Active_time ~ Cyrto_Sym + Habitat.type + max_SVL + dist_range + Avg_night_temp + bio2 + bio4 + bio12 + bio17 + bio18 | 40 | 57.04461 |
*The best model.
Table 4.
Parameter estimates for the regression models of temporal activity on the ecological factors.
| Slope | Std. Error | z value | Pr(>|z|) | |
|---|---|---|---|---|
| Sympatric Cyrtodactylus present | 2.53E + 00 | 1.19E + 00 | 2.127 | 0.0334* |
| Mean diurnal range | 8.93E − 01 | 5.64E − 01 | 1.584 | 0.1132 |
| Precipitation of Driest Quarter | −8.34E − 04 | 3.33E − 03 | −2.506 | 0.0122* |
R2 model = 0.481. *p < 0.05.
Discussion
Temporal niche responses to competition and environmental factors
With the existence of sympatric Cyrtodactylus species, the mean diurnal range and precipitation of the driest quarter are the factors in the best-supported model that explain the temporal activity patterns of Cnemaspis genus. Our study might present the first evidence supporting that the evolution of a diel niche of a vertebrate species can be influenced by a sympatric, ecologically similar species. The absence of Cyrtodactylus geckos in the distribution range increases the chance that Cnemaspis species are active at night. Except for Cnemaspis leucura, almost all nocturnal Cnemaspis species are not sympatric with Cyrtodactylus species. Considering that the most recent common ancestor of Cnemaspis species was diurnal, the recent shift to nocturnality of these species may be the result of ecological release22 from the competition of Cyrtodactylus species. Nonetheless, we also notice that some Cnemaspis species without sympatric Cyrtodactylus are diurnal (Fig. 2). This pattern might be caused by incomplete records. Firstly, even though several new species of the Cyrtodactylus genus have been described in south Thailand and Malaysia23–26, there is still insufficient information about the fine-scaled distribution of some Cyrtodactylus species, especially for those potentially new ones. Secondly, competition from other geckos, including other sympatric Cnemaspis congeners, might also led to the temporal niche partition, leading to the somewhat inconsistent relationship between diel niche and sympatry between Cnemaspis and Cyrtodactylus species. For example, it has been reported that Cnemaspis congeners could have sympatric distribution and temporal niche partition (C. kendallii versus C. nigridia, C. leucura versus C. kendallii and C. monachorum versus C. roticanai12,27,28). Furthermore, competition pressure might also come from nocturnal geckos other than Cyrtodactylus species, as they are abundant in Southeast Asia29.
Our results are consistent with those of Vidan et al.29 in supporting the precipitation of the driest quarter as a strong predictor for the distribution of nocturnal lizards. The negative relationship between the rainfall in dry quarters and the nocturnal activity levels of Cnemaspis species indicates that the geckos tend to be more active at night when the dry season is drier. This might be related to the water retention of the geckos; they have higher rates of water loss than other lizards30 and the water loss rate increases with ambient temperature31. Therefore, Cnemaspis species tend to be nocturnal in arid habitats to avoid high water loss. Moreover, in accordance with the results of Cunningham et al.32 and Vidan et al.29, the mean diurnal range is also determined to be one factor in our best model. However, this parameter does not seem to contribute much to the model since the support of our model is slightly decreased without it. Furthermore, despite the positive relationship with nocturnal activity levels, the Cnemaspis species with the highest diurnal range are still active at day time (Table S2).
Conservatism in temporal and spatial niches
In contrast to Anderson and Wiens8, who suggest that diel activity patterns have long-term conservatism, our results show little phylogenetic signal or conservatism in the temporal activity pattern of Cnemaspis geckos. However, we find strong niche conservatism in the habitat use of Cnemaspis geckos. These results suggest that Cnemaspis might change their active times more frequently than their habitat use. Theoretically, the pupil of geckos could be categorized into vertical narrow pupils for nocturnal taxa and circular ones for diurnal taxa33. However, the real relationship between pupil shapes and temporal active modes is controversial34. Although all species of Cnemaspis have circular pupils, multiple species in this genus are nocturnal. The labile diel niche Cnemaspis geckos might reflect the history that they could have evolved from a nocturnal ancestor that predated the diurnal one (i.e., their most recent common ancestor) inferred in this study, and such shift might have occurred repeatedly over their evolution. In addition, many diurnal geckos (e.g., Sphaerodactylus macrolepis) can be active in dim light conditions35. Some diurnal lizards can even opportunely switch to a nocturnal mode to avoid predators or competition, or to utilize novel resources36. Therefore, diurnal Cnemaspis species, which are likely used to low light conditions since they mostly restrict their movements to the shadow under rocks, should be able to easily change to a night active mode. Such adaptive flexibility may explain their labile temporal active patterns.
The diurnal ancestral state of the Cnemaspis genus, which belongs to a lizard groups with mostly nocturnal lineages10, suggests that the ancestor of this genus might have used a day time niche in order to avoid competition from other sympatric nocturnal geckos29. Moreover, their ancestral state of habitat use was the rocky habitat that often has limited space and resources, and thus might drive the diel niche partition among sympatric geckos to avoid direct competition. Our analyses of temporal activity pattern and habitat use across the Cnemaspis phylogeny suggest that the Cnemaspis species are more likely to change their active time than their habitat use to avoid competition over evolution. Given that nocturnal Cnemaspis species mostly occur exclusively where there is no existence of sympatric Cyrtodactylus species, the former might be inferior competitors than the latter. Therefore, the empirical evidence found in this study supports that the recent shifts to nocturnality in Cnemaspis species might be the result of ecological release from the competition of Cyrtodactylus species.
Methods
Phylogenetic relationship
The mitochondrial ND2 sequences of 51 Cnemaspis species (Table S1) were retrieved from GenBank and aligned using CLUSTAL37 implemented in MEGA 638. We estimated the phylogenetic relationships among Cnemaspis species in a Bayesian framework using MrBayes v3.2.639. The optimal model of sequence evolution (GTR + I + G) was determined using Bayesian information criterion (BIC) in jModelTest 2.1.840. We ran two independent Metropolis-coupled MCMC analyses; each one was run for 2 million generations, with sampling every 1000 generations. Thirty-one species from Alsophylax, Gehyra, Hemiphyllodactylus, Microgecko, and Perochirus genera were used as outgroups (Table S1).
Ecology data collection
The life history information of each Cnemaspis species, including temporal activity pattern, maximum body size (SVL), habitat use, and the existence of sympatric Cyrtodactylus species, was retrieved from the published literature (i.e.12,41,42) (Table S2). For the temporal activity pattern, we defined the study species as “diurnal” or “nocturnal” based on the description of their active time in the literature. The existence of sympatric Cyrtodactylus species was indicated by two states (yes/no). For the main habitat type, species that specialized in rocky habitat were recorded as “rocks”, and those used all other habitats (terrestrial or habitat generalists) were recorded as “others”. For the dispersal potential, the distances between all localities of each species were calculated using the distm function of the R package “geosphere”43, and the maximum distance was selected as the representative dispersal potential of each species.
Following the results of Cunningham et al.32 and Vidan et al.29, we chose the following seven climate variables that strongly effect the distribution of reptiles and diurnal lizards from WorldClim version 244: annual mean temperature (bio1); mean diurnal range (bio2); temperature seasonality (bio4); mean temperature of coldest quarter (bio6); precipitation seasonality (bio12); precipitation of driest quarter (bio17); and precipitation of warmest quarter (bio18). The variables were extracted from 2.5-minute resolution layers of WorldClim version 244. Furthermore, we included night temperature from the Moderate Resolution Imaging Spectroradiometer (MODIS) Land Surface Temperature and Emissivity dataset (MOD11A2 Terra)45; the values were extracted from high-resolution (~1 km) remote-sensed land surface temperature (LST) data by using the R package “MODISTools”46.
Phylogenetic conservation
We used the method developed by Fritz and Purvis47, which is specific for measuring phylogenetic signals in binary traits, to estimate the character dispersion on a phylogeny (D) using the R package “caper”48. Here, a D < 0 suggests a highly clustered trait, D ~ 0 indicates that the trait is phylogenetically conserved as expected under a Brownian threshold model, D = 1 suggests that trait values are random at the tips of the phylogeny, and D > 1 suggests phylogenetic over-dispersion47. In order to assess significance for each trait of temporal activity and habitat use, we performed 1000 simulated permutations based on random or Brownian motion patterns of evolution and compared the observed patterns to these two distributions.
Ancestral state reconstruction
The ancestral states of temporal activity patterns and habitat use were reconstructed using two methods: Bayesian ancestral state reconstruction and stochastic mapping. Bayesian ancestral state reconstruction was performed using BayesTraits v2.049. A set of 2000 trees, drawn from the posterior distribution of trees inferred by MrBayes analyses, was used to incorporate phylogenetic uncertainty. Analyses were conducted for 1 million generations, sampled every 1000 generations, and the first 10,000 generations were discarded as burn-in. We conducted three chains of each analysis to assess convergence of the results by checking their MCMC trend lines. Models with different transition rates were built and compared based on Bayes factors.
For stochastic mapping, we mapped the temporal activity and habitat use states onto the maximum credibility tree from MrBayes analysis using the simmap function in the R package “phytools”50. The transition models that best fitted the data were estimated based on maximum likelihood using the ace function in the R package “APE”51.
Regression between temporal active patterns and ecological factors
We used logistic regression analysis to test the relationship between temporal activity modes and the ecological factors (existence of sympatric Cyrtodactylus species, habitat types, dispersal potential, and climate variables). The variables were tested for collinearity examining the variance inflation factor with the vif function in the R package “car”52. The mean annual temperature (bio1) and mean temperature of coldest quarter (bio6) were excluded due to a high correlation between them and other climate variables. We used a backwards stepwise model selection process, and selected the best model according to Akaike Information Criterion (AIC) scores by using the stepAIC function in the R package “MASS”53.
Supplementary information
Acknowledgements
We appreciate Miss Tsui-Wen Li, Dr. Hui-Yun Tseng, Dr. Larry Lee Grismer, and Dr. Nikolay A. Poyarkov Jr. for their assistance and advice during data analyses. This research was partially supported by the Ministry of Science and Technology, Taiwan (MOST 108-2311-B-003-001-MY3 and MOST 108-2621-B-003-003-MY3).
Author contributions
H.N.N. and S.M.L. conceived the ideas and designed the methodology; H.N.N. and M.Y.Y. analyzed the data; and H.N.N., C.M.H. and S.M.L. led the writing. All authors contributed critically to the drafts and gave final approval for publication.
Data availability
Data relevant to the study, including GenBank Accession numbers and tree files, are provided in Supporting Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56549-x.
References
- 1.Ricklefs RE. Species richness and morphological diversity of passerine birds. Proc. Natl. Acad. Sci. 2012;109(36):14482–14487. doi: 10.1073/pnas.1212079109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nippert JB, Knapp AK. Soil water partitioning contributes to species coexistence in tallgrass prairie. Oikos. 2007;116(6):1017–1029. doi: 10.1111/j.0030-1299.2007.15630.x. [DOI] [Google Scholar]
- 3.Paoli GD, Curran LM, Zak DR. Soil nutrients and beta diversity in the Bornean Dipterocarpaceae: Evidence for niche partitioning by tropical rain forest trees. J. Ecol. 2006;94(1):157–170. doi: 10.1111/j.1365-2745.2005.01077.x. [DOI] [Google Scholar]
- 4.Rouag R, Djilali H, Gueraiche H, Luiselli L. Resource partitioning patterns between two sympatric lizard species from Algeria. J. Arid. Environ. 2007;69(1):158–168. doi: 10.1016/j.jaridenv.2006.08.008. [DOI] [Google Scholar]
- 5.Luiselli L. Community ecology of African reptiles: Historical perspective and a meta-analysis using null models. Afr. J. Ecol. 2008;46(3):384–394. doi: 10.1111/j.1365-2028.2007.00870.x. [DOI] [Google Scholar]
- 6.Magurran AE. Species abundance distributions over time. Ecol. Lett. 2007;10(5):347–354. doi: 10.1111/j.1461-0248.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 7.Goodyear SE, Pianka ER, Desert GV. Spatial and Temporal Variation in Diets of Sympatric Lizards (Genus Ctenotus) in the Great Victoria Desert, Western Australia Published By: The Society for the Study of Amphibians and Reptiles Spatial and Temporal Variation in Diets of Sympatric. J. Herpetol. 2011;45(3):265–271. doi: 10.1670/10-190.1. [DOI] [Google Scholar]
- 8.Anderson SR, Wiens JJ. Out of the dark: 350 million years of conservatism and evolution in diel activity patterns in vertebrates. Evolution (N Y). 2017;71(8):1944–1959. doi: 10.1111/evo.13284. [DOI] [PubMed] [Google Scholar]
- 9.Vitt LJ, Pianka ER, Cooper WEJ, Schwenk K. History and the Global Ecology of Squamate Reptiles. Am Nat. 2003;162(1):44–60. doi: 10.1086/375172. [DOI] [PubMed] [Google Scholar]
- 10.Gamble T, Greenbaum ELI, Jackman TR, Bauer AM. Into the light: diurnality has evolved multiple times in geckos. Biol. J. Linn. Soc. 2015;115(4):896–910. doi: 10.1111/bij.12536. [DOI] [Google Scholar]
- 11.Wood PJL, et al. Three new karst-dwelling Cnemaspis Strauch, 1887 (Squamata; Gekkoniade) from Peninsular Thailand and the phylogenetic placement of C. punctatonuchalis and C. vandeventeri. Crandall, K. ed. PeerJ. 2017;5:e2884. doi: 10.7717/peerj.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grismer LL, et al. Systematics and natural history of Southeast Asian Rock Geckos (genus Cnemaspis Strauch, 1887) with descriptions of eight new species from Malaysia, Thailand, and Indonesia. Zootaxa. 2014;3880(1):147. doi: 10.11646/zootaxa.3880.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Wiens JJ, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010;13(10):1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 14.Shea, G., Couper, P., Wilmer, J. W. & Amey, A. Revision of the Genus Cyrtodactylus Gray, 1827 (Squamata: Gekkonidae) in Australia. Vol. 63 (2011).
- 15.Wood PL, Heinicke MP, Jackman TR, Bauer AM. Phylogeny of bent-toed geckos (Cyrtodactylus) reveals a west to east pattern of diversification. Mol. Phylogenet Evol. 2012;65(3):992–1003. doi: 10.1016/j.ympev.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Uetz, P. The Reptile Database, http://reptile-database.reptarium.cz. Published 2019. Accessed January 20 (2019).
- 17.Grismer LL, Wood PJL. Ngo T Van, Murdoch ML. The systematics and independent evolution of cave ecomorphology in distantly related clades of Bent-toed Geckos (Genus Cyrtodactylus Gray, 1827) from the Mekong Delta and islands in the Gulf of Thailand. 2015. 2015;3980(1):21. doi: 10.11646/zootaxa.3980.1.6. [DOI] [PubMed] [Google Scholar]
- 18.Phung TM, Schingen MV, Ziegler T, Nguyen TQ. A third new Cyrtodactylus (Squamata: Gekkonidae) from Ba Den Mountain Tay Ninh Province, southern Vietnam. Zootaxa. 2014;3764(3):347–363. doi: 10.11646/zootaxa. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch ML, et al. Six new species of the Cyrtodactylus intermedius complex (Squamata: Gekkonidae) from the Cardamom Mountains and associated highlands of Southeast Asia. Zootaxa. 2019;4554(1):1–62. doi: 10.11646/zootaxa.4554.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Grismer LL, Ngo VT. Four new species of the Gekkonid genus Cnemaspis Strauch 1887 (Reptilia: Squamata) from Southern Vietnam. Herpetologica. 2007;63(4):482–500. doi: 10.1655/0018-0831(2007)63[482:FNSOTG]2.0.CO;2. [DOI] [Google Scholar]
- 21.Ngo HN, Nguyen TQ, Nguyen TV, van Schingen M, Ziegler T. Microhabitat selection and communal nesting in the insular Psychedelic Rock Gecko, Cnemaspis psychedelica, in Southern Vietnam with updated information on trade. Nat. Conserv. 2018;31:1–16. doi: 10.3897/natureconservation.31.28145. [DOI] [Google Scholar]
- 22.Bolnick DI, et al. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B. Biol. Sci. 2010;277(1689):1789–1797. doi: 10.1098/rspb.2010.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grismer, L. L. Lizards of Peninsular Malaysia, Singapore and Their Adjacent Archipelagos. Edition Chimaira (2011).
- 24.Grismer LL, et al. A phylogeny and taxonomy of the Thai-Malay Peninsula Bent-toed Geckos of the Cyrtodactylus pulchellus complex (Squamata: Gekkonidae): combined morphological and molecular analyses with descriptions of seven new species. Zootaxa. 2012;3520:1–55. doi: 10.11646/zootaxa.3520.1.1. [DOI] [Google Scholar]
- 25.Davis HR, et al. Checklist of the herpetofauna of Hutan Lipur Gunung Senyum, Pahang, Peninsular Malaysia. Russ. J. Herpetol. 2018;25(3):207–220. [Google Scholar]
- 26.Grismer LL, et al. A new species of forest-dwelling Cyrtodactylus Gray (Squamata: Gekkonidae) from the Indawagyi Wildlife Sanctuary, Kachin State, Myanmar. Zootaxa. 2019;4623(1):1–25. doi: 10.11646/zootaxa.4623.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Grismer LL, et al. Another new, diminutive Rock Gecko (Cnemaspis Strauch) from Peninsular Malaysia and a discussion of resource partitioning in sympatric species pairs. Zootaxa. 2010;2569(2569):55–66. doi: 10.5281/zenodo.197342. [DOI] [Google Scholar]
- 28.Kurita T, Nishikawa K, Matsui M, Hikida T. A new species of Rock Gecko genus Cnemaspis (Squamata: Gekkonidae) from Western Sarawak, Malaysia. Zootaxa. 2017;4258(6):525–538. doi: 10.11646/zootaxa.4258.6.2. [DOI] [PubMed] [Google Scholar]
- 29.Vidan E, et al. The Eurasian hot nightlife: Environmental forces associated with nocturnality in lizards. Glob. Ecol. Biogeogr. 2017;26(11):1316–1325. doi: 10.1111/geb.12643. [DOI] [Google Scholar]
- 30.Leclair RJ. Water loss and microhabitat in three sympatric species of lizards (Reptilia, Lacertilia) from Martinique, West Indie. J. Herpetol. 1978;12(2):177–182. doi: 10.2307/1563405. [DOI] [Google Scholar]
- 31.Snyder GK, Weathers WW. Physiological responses to temperature in the tropical lizard, Hemidactylus frenatus (Sauria: Gekkonidae) Herpetologica. 1976;32(3):252–256. [Google Scholar]
- 32.Cunningham Heather R., Rissler Leslie J., Buckley Lauren B., Urban Mark C. Abiotic and biotic constraints across reptile and amphibian ranges. Ecography. 2015;39(1):1–8. doi: 10.1111/ecog.01369. [DOI] [Google Scholar]
- 33.Werner YL. Eye size in geckos of various ecological types (Reptilia: Gekkonidae and Sphaerodactylidae) Isr. J. Zool. 1969;18(2):291–316. [Google Scholar]
- 34.Werner YL, Seifan T. Eye size in geckos: Asymmetry, allometry, sexual dimorphism, and behavioral correlates. J. Morphol. 2006;267(12):1486–1500. doi: 10.1002/jmor.10499. [DOI] [PubMed] [Google Scholar]
- 35.Nava SS, Conway MA, Martins EP. Divergence of visual motion detection in diurnal geckos that inhabit bright and dark habitats. Funct. Ecol. 2009;23(4):794–799. doi: 10.1111/j.1365-2435.2009.01565.x. [DOI] [Google Scholar]
- 36.Gordon CE, Dickman CR, Thompson MB. What factors allow opportunistic nocturnal activity in a primarily diurnal desert lizard (Ctenotus pantherinus)? Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 2010;156(2):255–261. doi: 10.1016/j.cbpa.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic. Acids Res., 22(22):4673–4680, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC308517/ (1994). [DOI] [PMC free article] [PubMed]
- 38.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 40.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grismer LL, Onn CK. A new species of karst dwelling Cnemaspis Strauch 1887 (Squamata: Gekkonidae) from Sarawak, Borneo. Development. 2009;31(October 2009):5326-5326.
- 42.Wood PL, Quah ESH, Anuar S, Muin MA. A new species of lowland karst dwelling Cnemaspis strauch 1887 (Squamata: Gekkonidae) from northwestern Peninsular Malaysia. Zootaxa. 2013;3691(5):538–558. doi: 10.11646/zootaxa.3691.5.2. [DOI] [PubMed] [Google Scholar]
- 43.Hijmans, R. J. Geosphere: Spherical Trigonometry, https://cran.r-project.org/package=geosphere (2017).
- 44.Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37(12):4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 45.Wan, Z., Hook, S. & Hulley, G. MOD11A2 MODIS/Terra Land Surface Temperature/Emissivity 8-Day L3 Global 1km SIN Grid V006 [Data set]. NASA EOSDIS L. Process DAAC, 10.5067/MODIS/MOD11A2.006 (2015).
- 46.Tuck SL, et al. MODISTools - downloading and processing MODIS remotely sensed data in R. Ecol. Evol. 2014;4(24):4658–4668. doi: 10.1002/ece3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fritz SA, Purvis A. Selectivity in mammalian extinction risk and threat types: A new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 2010;24(4):1042–1051. doi: 10.1111/j.1523-1739.2010.01455.x. [DOI] [PubMed] [Google Scholar]
- 48.Orme, C. D. L., Freckleton, R. P., Thomas, G. H., Petzold, T. & Fritz, S. A. caper: comparative analyses of phylogenetics and evolution in R, http://r-forge.r-project.org/projects/caper/ (2011).
- 49.Pagel M, Meade A, Barker D. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol. 2004;53(5):673–684. doi: 10.1080/10635150490522232. [DOI] [PubMed] [Google Scholar]
- 50.Revell LJ. phytools: An R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 51.Paradis E, Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2018;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 52.Fox, J. & Weisberg, S. An {R} Companion to Applied Regression. Second. Thousand Oaks, CA: Sage, http://socserv.socsci.mcmaster.ca/jfox/Books/Companion (2011).
- 53.Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S. Fourth. New York: Springer, http://www.stats.ox.ac.uk/pub/MASS4 (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data relevant to the study, including GenBank Accession numbers and tree files, are provided in Supporting Information.