ABSTRACT
Meta-analyses have reported higher levels of coffee consumption to be associated with lower mortality. In contrast, some systematic reviews have linked coffee consumption to increased risks for lung cancer and hypertension. Given these inconsistencies, this narrative review critically evaluated the methods and analyses of cohort studies investigating coffee and mortality. A specific focus was adjustment for confounding related to smoking, healthy and unhealthy foods, and alcohol. Assessment of 36 cohort samples showed that many did not adequately adjust for smoking. Consuming 1–5 cups of coffee per day was related to lower mortality among never smokers, in studies that adjusted for pack-years of smoking, and in studies adjusting for healthy and unhealthy foods. Possible reduced health benefits for coffee with added sugar have not been adequately investigated. Research on coffee and health should report separate analyses for never smokers, adjust for consumption of healthy and unhealthy foods, and for sugar added to coffee.
Keywords: coffee, diet, methods, confounding variables, covariates, adjustment, smoking, healthy foods, cohort studies, added sugar
Introduction
Coffee is a popular beverage consumed regularly in many countries. A national survey in the United States reported that ∼75% of adults drink coffee and 49% drink ≥1 cup daily (1). Despite several hundred research studies evaluating the health effects of coffee, including many that associate coffee with beneficial health outcomes, coffee has been largely overlooked as a potential health determinant in dietary research and rarely included as an adjustment variable when assessing the health effects of specific food groups and dietary patterns. A reason for this omission could be inconsistencies in findings from studies examining the health effects of coffee. Although many studies have noted that coffee drinking is associated with better health outcomes, others report no relation and some report adverse health effects. In the face of these conflicting findings, it is important to review in detail the methodological procedures and analyses reported in research on the health effects of coffee. To what extent have decisions made about the research design and analyses affected the findings?
Multiple research designs are used in diet and health research, and these collectively contribute to accumulated evidence regarding healthy and unhealthy foods. Although randomized controlled trials (RCTs) have been regarded by some as the most trustworthy type of research design in dietary research (2), cohort or prospective observational studies are commonly used to ascertain the long-term health effects of specific foods and dietary patterns. Cohort studies have made a substantial contribution to the development of food policy and nutritional guidelines (3). In contrast to RCTs, cohort studies are more suitable for investigating major health events that can take many years to develop, such as cardiovascular disease (CVD), cancer, and mortality. Cohort studies can include exposures that are difficult to randomize or might be considered unethical in experimental studies (e.g., high levels of alcohol consumption).
Some potential limitations of cohort studies are reliance on self-report measures of diet, not adjusting for changes in dietary patterns over time, and unadjusted or “residual” confounding potentially influencing outcomes. An important task for researchers and reviewers of cohort studies is ensuring that cohort study designs are of sufficient quality to contribute to the evidence regarding the health effects of specific foods. Systematic reviews and meta-analyses of cohort studies can use quality assessment checklists to ensure the studies selected for review are of acceptable quality in terms of their research design and analysis.
The Newcastle-Ottawa Scale is commonly used for assessing the quality of nonrandomized studies included in meta-analyses, such as case-control and cohort studies (4). This scale includes a section on comparability. In cohort studies “either exposed and non-exposed individuals must be matched in the design and/or confounders must be adjusted for in the analysis” (4). In the case of coffee consumption, people who do not drink coffee are typically used as the nonexposed reference group and compared with those who drink coffee.
The manual for the Newcastle-Ottawa Scale states that, “If the relative risk for the exposure of interest is adjusted for the confounders listed, then the groups will be considered to be comparable on each variable used in the adjustment” (4). Researchers conducting cohort studies typically adjust a number of variables that might be potentially related to both coffee consumption and health outcomes. These are likely to include variables such as age, gender, risk indicators (such as blood pressure, lipids), noncommunicable diseases, and sometimes dietary measures. Cohort studies typically report the specific covariates for which adjustments were made in relation to either partially or fully adjusted regression models for risk estimates. The Newcastle-Ottawa Scale is not specific as to which potential confounders might need to be adjusted. Each research team makes this decision.
There have been several hundred published research reports on the health effects of coffee, including many cohort studies (5, 6). Systematic reviews and meta-analyses are generally regarded as providing the best-quality evidence, based on summarizing the findings from multiple individual studies. Cohort studies can use mortality, or major diseases such as diabetes, cancer, and CVDs, as end points. These are more robust than the end points typically used in RCTs, which primarily focus on short-term outcomes such as changes in blood lipids, insulin responses, or biological markers of inflammation. In contrast to studies using intermediate end points such as disease states (e.g., diabetes, CVD) or risk factors such as obesity, hypertension, and blood lipids, cohort studies using mortality as an end point do not require underlying assumptions about the link between intermediate end points and mortality.
Research findings investigating the association of coffee consumption and mortality are consistent. Six meta-analyses published between 2013 and 2019 reported an inverse association; people who drank ≥1 cup of coffee per day had significantly lower mortality rates than people who drank little or no coffee (7–12). The maximum protective effect of coffee consumption has been reported as ∼3–4 cups/d. The meta-analysis published in 2016 by Grosso and colleagues (8), which summarized findings from 31 cohort studies, reported an overall RR of 0.84 for people who drink 3–4 cups of coffee per day, indicating a 16% lower mortality risk compared with people who did not drink coffee.
Two umbrella reviews reporting multiple health end points for coffee consumption have been published. Umbrella reviews are syntheses of research for a specific exposure or intervention and examine multiple health outcomes. The umbrella review published in 2017 by Grosso and colleagues (5) included research on 59 specific health outcomes. Types of studies included in their review were meta-analyses from cohort (prospective) studies, case-control studies, and RCTs. The review covered 3 exposures: coffee, decaffeinated coffee, and caffeine. They concluded that, “coffee consumption has beneficial effects for a number of chronic diseases, including cancers, and cardiovascular, metabolic, and neurological conditions.” They also noted that the beneficial health effects were evident for ≤4–5 cups of coffee per day (5).
The second umbrella review published in 2017 included meta-analyses for 67 health outcomes (6). Poole and colleagues (6) concluded that:
Coffee consumption seems generally safe within usual levels of intake, with summary estimates indicating largest risk reduction for various health outcomes at three to four cups a day, and more likely to benefit health than harm.
In contrast to the findings of the beneficial effects of consuming coffee, some systematic reviews of cohort studies have reported associations between higher coffee consumption and negative health outcomes such as increased risk of pancreatic cancer (13), bladder cancer (14), lung cancer (15), and hypertension (16). Other systematic reviews have reported no relation between coffee consumption and health outcomes such as coronary heart disease (17) and hypertension (18).
One of the reasons for the divergent outcomes for the effects of coffee on health could be differences in the selection of covariates for which adjustment was made, and variations in the way covariates have been constructed. For example, some research groups have used binary variables to adjust for smoking (current smoker compared with nonsmoker) whereas others have constructed more complex variables that include both smoking status (never, former, current smoker) and pack-years of smoking. Given the consistent association between smoking and high levels of coffee consumption (19, 20), adequate adjustment for smoking confounding is essential for research on the health effects of coffee.
An important finding from the meta-analysis by Grosso and colleagues (8) was that statistical procedures most commonly used to adjust for smoking did not appear to adequately control for the negative health effects of smoking. An analysis based on 5 subsamples of never smokers showed a linear inverse association between coffee consumption and mortality rates. This contrasted with the overall risk among31 samples adjusted for smoking, which showed a nonlinear association, with the lowest mortality rates among people who drank 3–4 cups of coffee per day (8). These contrasting findings indicate that some of the variables constructed to assess smoking as a covariate can be ineffective in removing confounding. Two articles have reported that variations in constructing smoking variables, when adjusting for smoking in relation to lung cancer risk, lead to significantly different outcomes (21, 22). Use of a binary variable (smoker compared with nonsmoker) was the least effective type of adjustment.
Given both negative and no association outcomes between coffee and health reported from some systematic reviews, the more general question arises: to what extent do specific procedures in the methodologies and analyses in coffee studies lead to divergent and sometimes conflicting outcomes regarding the health impacts of coffee? The aim of this narrative review was to critically analyze methodologies used in human cohort or prospective studies investigating the association between coffee consumption and mortality. A specific focus was on adjustments for smoking and for healthy and unhealthy foods as potential confounding factors. Cohort or prospective studies provide the highest quality evidence currently available about the long-term impacts of foods such as coffee, whereas few long-term RCTs of adequate quality have been published.
Methods
To investigate how typical adjustments made for potential confounding variables, including smoking, diet, and alcohol consumption, might affect the association reported between coffee consumption and mortality, 34 articles were selected for detailed analysis.
The initial selection included the 31 articles included in the 2016 meta-analysis by Grosso et al. (8). A further 11 studies were identified from a search of the same databases (PubMed and EMBASE), using the same search terms (“coffee” AND “mortality”) used by Grosso et al. The search was restricted to studies published from January 2016 to May 2019 that were not among studies already reviewed by Grosso et al. The selection criteria were the same as used by Grosso et al.: cohort or prospective studies that reported the association between coffee intake and total or all-cause mortality, and reported HR ratios or RR with 95% CIs, across ≥3 coffee exposure categories. Only studies that had published the full article in English and reported adjusting for smoking as a covariate were selected. It has been clear for several years that studies on coffee and health that do not adjust for smoking are likely to have significant confounding from smoking-related morbidity and mortality (5).
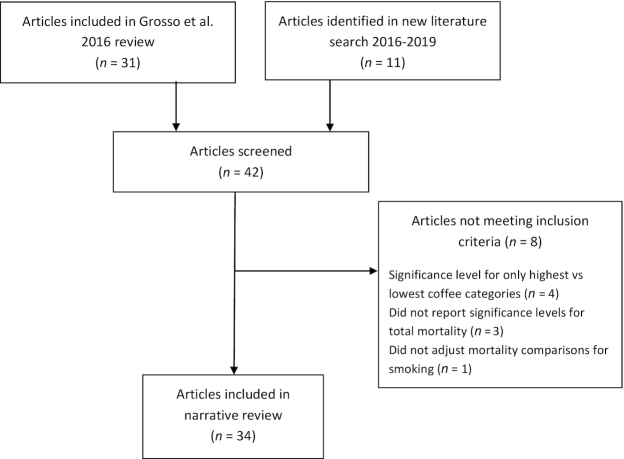
From the 42 articles screened, 8 articles from the Grosso et al. meta-analysis were omitted for the following reasons: they reported only binary comparisons between the highest and lowest coffee consumption groups (n = 4), they did not report HRs or other significance levels for all-cause mortality (n = 3), or did not adjust for smoking for the reported coffee and mortality comparisons (n = 1). The 8 articles omitted are listed in Supplemental Table 1. In total 34 articles, reporting outcomes for 36 samples, were included in the review. The selection process is shown in Figure 1.
FIGURE 1.
Articles selected for detailed analysis.
Analyses
The 34 selected articles, reporting on 36 cohort samples, were read to identify and extract the text describing the variables that were used as covariates or adjustment variables. A specific focus was identifying how smoking and dietary patterns were adjusted. The variables used to adjust for confounding, details of the adjustment variables used for smoking and food consumption, and the reported outcomes (nearly all used HRs, RRs, or ORs) for the association between coffee consumption and mortality were noted.
Four specific ordinal (ranked) variables were constructed for the analyses reported in this review:
the level of significance for the association between coffee consumption and mortality,
the complexity of adjustment for smoking,
the extent of adjustment made for food groups, and
the type of adjustment made for alcohol consumption.
Additional information was also extracted for each cohort, where reported. This included: sample size, the length of the follow-up period, the mean or median age of the sample at baseline measurement, mortality rates for the overall sample and specific subsamples, and whether adjustment was made for total energy consumption. Food groups, such as fruit, vegetables, red meat, and nuts, were used to assess the effectiveness of adjustment for dietary patterns because they are commonly used to construct diet quality assessment tools (23, 24). There has been a trend for food groups to replace macronutrients to measure diet quality in human populations because food groups are more likely to have a direct association with health outcomes than macronutrients (25–27).
Ratings of level of significance
Among the 36 samples, 27 reported HRs, 7 reported RRs, 1 reported ORs, and 1 reported a log-rank test calculated on cumulative survival. The type and significance of the association between coffee consumption and mortality for each sample was assigned to 1 of 3 levels. In all cases, the significance levels extracted from articles were the multivariate or fully adjusted ratios. In 35 of 36 samples the reference group was people who did not drink coffee or had the lowest consumption level. Significance levels were reported for 3 specific groups: 23 samples of women, 22 samples of men, and 10 combined samples comprising both men and women. The following criteria were used to assign the reported findings for each sample to specific significance levels.
Level 1: nonsignificant
Where the specific HRs (or RRs or ORs) for specific categories of coffee consumption, and the linear inverse trend (if reported) for the association with mortality, were nonsignificant (P > 0.05), the finding was categorized as nonsignificant.
Level 2: partially significant
The findings were categorized as partially significant where significance level for the linear trend was either not reported or was not significant (P > 0.05) and if ≥1 of the coffee consumption categories was reported as having significantly lower mortality rates (P < 0.05) than the lowest coffee intake (in 35 of 36 samples the lowest coffee intake was the reference group).
Level 3: significant
Where the association across the categories of coffee consumption and mortality was reported as a significant inverse linear trend (P < 0.05), or where ≥2 coffee consumption categories were significant and the significance level for the linear trend was not reported, the findings were categorized as a significant inverse association.
An additional summary significance score for the 36 samples was constructed to combine separate significance scores for men and women into a single summary score. For the other samples, the single significance level described above was used.
Level 1 The significance levels for both men and women were nonsignificant.
Level 2 The men's and women's samples differed with 1 HR or trend being significant and the other nonsignificant.
Level 3 The levels reported separately for men and women were both significant.
The ratings of the significance levels, for each of the 36 samples, are shown in Supplemental Table 2.
Ratings of adjustment for smoking
A detailed assessment of the smoking adjustments for the 36 samples indicated that categorization into 4 levels of smoking adjustment reflected the relative degree of complexity of the types of adjustments reported. The adjustment ratings were as follows:
Level 1 Binary (current smokers compared with nonsmokers).
Level 2 Smoking status adjustment (binary adjustment based on current, former, and never smokers).
Level 3 Construction of ≥1 smoking variable, typically with both smoking status and ≤4 categories that were based on intensity of smoking (cigarettes per day) and/or years since cessation.
Level 4 Incorporated both intensity of smoking (≥5 categories or continuous variables) using either the number of cigarettes smoked (for both former and current smokers), the number of years of smoking, or the total number of pack-years of smoking at the time of baseline measurement.
These 4 levels were used to measure the relative effectiveness in adjusting for smoking. The levels are consistent with findings from previous articles assessing the effectiveness of statistical adjustment for smoking in relation to lung cancer risk (21, 22, 28). Both level 1 and level 2 binary adjustments are likely to be ineffective, especially given the nonlinear association evident between smoking and coffee consumption in studies that have reported this association. Level 3 adjustment was likely to be more effective than either of the binary adjustments. Level 4 has been shown to be the most effective procedure for statistical adjustment of smoking (21, 22, 28).
The individual scores for ratings of smoking adjustment for the 36 samples are shown in Supplemental Table 2. The text describing the smoking variables used as covariates, extracted from the articles, and the associated rating levels are shown in Supplemental Table 3.
In 17 articles, HRs for subsamples of never smokers were also reported. A summary of these HRs is included in the findings.
Ratings of adjustment for food groups
Adjustments for potential confounding by food groups were rated using 4 levels. The ratings were based on the extent to which the adjusted food groups were likely to be related to mortality and chronic disease based on recent systematic reviews and multisample cohort studies. Adjustment for total energy intake (included in 17 of 36 samples), alcohol consumption, and other beverages (such as green tea and black tea), were not included in the food adjustment ratings.
Level 1 No adjustment for any food variables.
Level 2 Adjustment for macronutrients and/or single foods (e.g., fats, SFAs, leafy vegetables).
Level 3 Adjustment using 2–3 foods selected from the following: healthy foods: fruit, vegetables, wholemeal and wholegrain foods, and nuts; unhealthy foods: red meat, processed meat, refined carbohydrates (including rice and noodles), and sugar-sweetened beverages (SSBs). Where “fruit and vegetables” and “red and processed meat” were reported as single variables they were counted as 1 food group.
Level 4 Adjustment for consumption of ≥4 of the food groups mentioned above or using a food index such as the Alternative Healthy Eating Index (AHEI) or Mediterranean diet.
The text describing the specific food covariates reported in the articles, and their coding, is shown in Supplemental Table 4.
The food ratings were based on specific assumptions about which foods are most likely to be associated with mortality. These assumptions and the supporting evidence were:
Inclusion of macronutrient variables (fats, SFAs, carbohydrates, and protein) as the primary type of food adjustment has little or no effectiveness in reducing confounding given their lack of association with both risk factors (29, 30) and all-cause mortality (31–33). We acknowledge that there are conflicting interpretations from the findings of systematic reviews and meta-analyses on the relation between SFAs and mortality (34). We agree with recent interpretations, supported by a substantial body of recent evidence, that there is no relation between SFAs and total mortality or mortality due to CVDs (35, 36).
Healthier diets, measured using composite food indices such as the AHEI, Dietary Approaches to Stop Hypertension Trial (DASH), and the Mediterranean diet, have been consistently associated with lower mortality (37, 38).
Adjustment for multiple food groups, for which there is extensive evidence of unhealthy or healthy effects, is one of the most effective types of adjustment (27, 39). For the food ratings, healthy foods included vegetables, fruits, nuts, legumes, and wholegrains (40, 41). Unhealthy foods included SSBs (42, 43), refined carbohydrates (44), processed meat, and red meat (25). Many of these foods, but not all, are included in food indices such as the AHEI, DASH, and the multiple versions of the Mediterranean diet.
In summary, the ratings of the quality of food adjustment are based on specific interpretations of recent research findings for which there are conflicting views regarding the relative importance of macronutrients compared with food groups in relation to health outcomes such as CVD and mortality (34, 39). In our view, there is an emerging consensus that use of macronutrients as covariates is not effective compared with using food indices or food groups. A recent commentary on dietary fats noted that, “researchers and public health authorities now agree that to consider the effect of total fat intake alone on health is meaningless….” (34).
The level of adjustment for alcohol consumption as a covariate was rated using 3 levels: 1) no adjustment for alcohol; 2) 2–4 categories of alcohol consumption were used; or 3) ≥5 categories were used or alcohol entered as a continuous variable.
Statistical analyses were carried out using the IBM SPSS Statistics Package (v25). Sample size was set as a continuous variable, and the rating levels set as ordinal variables. Spearman ρ partial correlations (using constructed SPSS syntax files), controlling for sample size, were used to assess the associations between the significance level ratings and rating for the other adjustment variables. It was predicted that higher (more complex) levels of adjustment would be associated with a greater likelihood of a significant inverse association between coffee consumption and mortality. One-tailed significance levels were used for the analyses.
Results
Key characteristics for the 36 cohort samples are shown in Table 1. The samples were from 3 general regions; 15 samples were from the United States, 13 were from Europe (including 2 from the United Kingdom), and 8 from Asia (7 from Japan and 1 from Singapore). Sample sizes ranged from 817 to 451,743. For most samples, the mean age of participants at baseline was between 40 and 59 y (19 samples). In 6 samples the mean age was <50 y, and in 11 samples the mean age was ≥60 y. Most samples had ≥4 categories for coffee intake.
TABLE 1.
Characteristics of 36 samples in selected cohort studies
| First author and date of publication | Country or region | Sample number | Mean age,1 y | Years of follow-up2 | Mortality rate,3 % | Coffee consumption categories4 | Coffee ≥1 cup/d, % | Analyses by sex5 |
|---|---|---|---|---|---|---|---|---|
| Kahn 1984 (45) | USA | 20,969 | NR6 | 21 | 27 | 3 | 22 | Combined |
| Lindsted 1992 (46) | USA | 9484 | 53 | 26 | NR | 3 | 25 | Men |
| Klatsky 1993 (47) | USA | 127,520 | 43 | 8 | 3.5 | 5 | 59 | Combined |
| Woodward 1999 (48) | UK | 11,445 | 50 | 7.7 | 4.9 | 4 | 72 | Separate |
| Kleemola 2000 (49) | Finland | 20,179 | 44 | 10 | 8 | 4 | 95 | Separate |
| Iwai 2002 (50) | Japan | 2855 | 58 | 10 | 12.6 | 3 | 58 | Separate |
| Jazbec 2003 (51) | Croatia | 3364 | 48 | 27 | 28.2 | 4 | 51 | Separate |
| Andersen 2006 (52) | USA | 27,312 | 61 | 15 | 15.6 | 5 | 79 | Women |
| Paganini-Hill 2007 (53) | USA | 13,624 | 74 | 23 | 84.6 | 5 | 49 | Combined |
| Happonen 2008 (54) | Finland | 817 | 76 | 23 | 76 | 5 | 94 | Combined |
| Ahmed 2009 (55) | Sweden | 37,315 | 60 | 9 | NR | 5 | 89 | Men |
| de Koning Gans 2010 (56) | Netherlands | 37,514 | 50 | 11 | 3.7 | 6 | 80 | Combined |
| Sugiyama 2010 (57) | Japan | 37,742 | 51 | 11 | 6.5 | 4 | 46 | Separate |
| Tamakoshi 2011 (58) | Japan | 97,753 | 57 | 16 | 20 | 4 | 28 | Separate |
| Freedman 2012 (59) | USA | 402,260 | 62 | 14 | 13 | 5 | 90 | Separate |
| Liu 2013 (60) | USA | 43,727 | 43 | 16 | 5.7 | 6 | 59 | Separate |
| Gardener 2013 (61) | USA | 2461 | 68 | 11 | 35 | 5 | 69 | Combined |
| Saito 2015 (62) | Japan | 90,914 | 50 | 18.7 | 14.2 | 5 | 41 | Separate |
| Loftfield 2015 (63) | USA | 90,317 | 65 | 11 | 9.7 | 6 | 68 | Separate |
| Lof 2015 (64) | Sweden | 45,140 | 40 | 18 | 3.5 | 3 | 85 | Women |
| Odegaard 2015 (65) | Singapore | 52,584 | 56 | 16 | 19 | 4 | 71 | Combined |
| Ding 2015 NHSII7 (66) | USA | 93,054 | 36 | 23 | 2.2 | 5 | 44 | Women |
| Ding 2015 HPFS7 (66) | USA | 40,557 | 53 | 26 | 31 | 5 | 55 | Men |
| Ding 2015 NHS7 (66) | USA | 74,890 | 51 | 36 | 24 | 5 | 68 | Women |
| Nordestgaard 2016 (67) | Denmark | 95,366 | 58 | 10 | 5.7 | 7 | 78 | Combined |
| Grosso 2017 (68) | Europe | 28,561 | 58 | 6 | 7.4 | 4 | 62 | Separate |
| Gunter 2017 (69) | Europe | 451,743 | 51 | 16 | 9.2 | 5 | NR | Separate |
| Park 2017 (70) | USA | 185,000 | 60 | 16 | 31 | 6 | 64 | Separate |
| Neves 2018 (71) | USA | 3948 | 58 | 5 | 19 | 4 | 46 | Separate |
| Hu 2018 (72) | USA | 2461 | 68 | 8 | 50 | 4 | 47 | Separate |
| van den Brandt 2018 (73) | Netherlands | 90,914 | 61 | 10 | 15 | 6 | 93 | Separate |
| Loftfield 2018 (74) | UK | 90,317 | 58 | 7 | 2.9 | 7 | 77 | Separate |
| Navarro 2018 (75) | Spain | 45,140 | 38 | 10 | 1.7 | 4 | 64 | Combined |
| Sado 2019 (76) | Japan | 52,584 | 53 | 15 | 16 | 5 | 55 | Separate |
| Nohara-Shitama 2019 (77) | Japan | 1117 | 63 | 15 | 18 | 4 | 38 | Combined |
| Yamakawa 2019 (78) | Japan | 15,724 | 53 | 16 | 18 | 5 | NR | Separate |
Sample age at baseline data collection was reported as mean or median.
Based either on time from baseline to end date for total sample or for mean or median follow-up years.
Mortality rate for % deaths in total sample; some samples included both men and women, some men or women only.
Number of coffee consumption categories reported.
HRs reported either on a combined sample, separately for men and women, or only men or women.
NR, not reported.
HPFS, Health Professionals Followup Study; NHS, Nurses' Health Study; NHSII, Nurses' Health Study II.
The category for the highest intake of coffee, for which the HRs were reported, varied among the 34 articles. One article used ≥8 cups/d, 2 used ≥7 cups, 7 used ≥6 cups, 5 used ≥5 cups, 7 used ≥4 cups, 4 used ≥3 cups, and 4 used ≥2 cups/d.. One article reported only quartiles of coffee intake. There were only 10 articles reporting risks for drinking ≥6 cups/d. Because the proportion of each sample in the highest coffee intake categories was usually low, the data are too sparse to justify extrapolation of a continuing direct inverse relation between coffee and mortality for >5 cups of coffee per day.
Significance scores for HRs
The frequencies for type and significance of the association between coffee consumption and mortality are shown in Table 2 for the samples of women, men, and combined samples, which included both men and women. The right-hand column shows the ratings of the summary significance levels, combining both HRs where those for men and women were reported separately. The significance ratings for each of the 36 samples are listed in Supplemental Table 2.
TABLE 2.
Coffee and mortality association: levels of significance of HRs
| HRs | Women | Men | Combined samples1 | Summary significance2 |
|---|---|---|---|---|
| Level 1: not significant | 5 | 7 | 4 | 7 |
| Level 2: partially significant | 2 | 4 | 2 | 14 |
| Level 3: significant inverse linear trend | 16 | 11 | 4 | 15 |
| Sample n | 23 | 22 | 10 | 36 |
Samples that reported HRs for men and women combined, not separately.
Summary significance rating including combined HRs for men and women where both samples were reported.
Several trends were evident in the significance levels across the 36 samples. Studies with shorter follow-up times were less likely to report significant inverse associations between coffee and mortality. Of the 6 samples of men that had follow-up times <10 y, 5 reported findings that were either nonsignificant (48, 71, 72) or only partially significant (55, 68). Samples of women were more likely than samples of men to show a significant inverse association between coffee intake and mortality (16 of 23 samples of women; 11 of 22 samples of men). This difference is likely related to lower rates of smoking in women. Of 10 samples with younger ages (mean age <52 y) and mortality rates <10%, 7 reported a nonsignificant association between coffee and mortality (47–49, 56, 57, 60, 64).
Adjustment for smoking
Table 3 shows the frequencies for 4 levels of adjustment for smoking. The text coded into the smoking adjustment ratings, for each of the 36 samples, is shown in Supplemental Table 3. The association between the ratings of smoking adjustment and the significance of the coffee and mortality outcomes was calculated using Spearman ρ partial correlations, controlling for sample size. The correlation between the significance levels and smoking adjustment rating across all 36 samples was 0.66 (P < 0.001, 1-tailed). For the 22 samples of men, the correlation was 0.29 (NS), among the 23 samples of women the correlation was 0.22 (NS), and for the 10 combined samples the correlation was 0.89 (P = 0.001). The variation in the size of the correlations in the sex-specific, combined, and overall samples could be due to differences in smoking rates. However, this requires further investigation. There was a significant correlation of 0.53 (P < 0.001, 1-tailed) between the year of publication and the smoking adjustment rating, indicating a significant trend for smoking adjustments to become more complex over the period 1984 to 2019.
TABLE 3.
Adjustment for smoking: ratings and frequencies
| Type of smoking adjustment and effectiveness score | Number of samples (n = 36) | Assessment |
|---|---|---|
| Level 1. Binary variable Current smokers vs. nonsmokers | 9 | Least effective type of adjustment with former smokers included with never smokers |
| Level 2. Smoking status Use of 3 categories: current, former, never smokers | 7 | Commonly coded as 2 binary variables. Ineffective adjustment where there are nonlinear associations between smoking and coffee consumption |
| Level 3. Smoking status and intensity One or more smoking variables with ≤4 categories based on smoking status and intensity | 7 | More effective adjustment than smoking status but limited number of intensity categories does not effectively adjust for nonlinear associations |
| Level 4. Complex adjustment Multiple smoking variables including smoking status, intensity and cessation time, or pack-years of smoking | 13 | Most effective type of statistical adjustment, using pack-years of smoking, time since cessation, or similar measures |
The correlation between smoking levels and significance of the coffee and mortality associations indicates that studies that used more complex smoking adjustments were significantly more likely to report a significant inverse association.
The most robust procedure for assessing effectiveness of adjustment for smoking is comparison of significance levels separately in samples of never smokers, former smokers, and current smokers, adjusted for smoking in the 2 latter groups. Of the 36 samples, 17 reported the significance level of the association between coffee consumption and mortality among never smokers. In 14 of these 17 samples there was a significant inverse linear association between coffee consumption and mortality among never smokers. This linear association is consistent with the view that ≤5 cups of coffee per day can be health protective for most people, except for women who are pregnant or people who are sensitive to caffeine. Of the 14 samples reporting an inverse association for never smokers, 5 samples also reported significant linear inverse associations for former and current smokers. One of the articles (66), using pooled results across 3 samples, noted the contrasting results for analyses of never smokers:
Overall, the association of total coffee, caffeinated coffee, and decaffeinated coffee consumption with risk of all-cause mortality changed from a nonlinear association in the overall population to a linear inverse association when restricting to never smokers (total coffee: P value for nonlinearity = 0.32, P value for linear trend < 0.001… [p. 2309]
Of the 17 samples of never smokers, the 3 samples for which the association between coffee intake and mortality was nonsignificant had very low rates of mortality compared with mortality rates in the other cohort studies. Mortality rates were 2.2% for the total sample in the Nurses’ Health Study II sample (over a 23-y follow-up time), compared with 24% in the Nurses’ Health Study (66). In the UK Biobank sample, which had a median 7-y follow-up, the reported mortality rates were 1.5% in never smokers, 3.5% in former smokers, and 5.5% in current smokers (74). The third study, which reported a nonsignificant trend in Japanese men who were never smokers, did not report mortality rates for this subsample (76). The number of mortality cases in male never smokers was low. When mortality rates are very low, such as in samples that recruit younger people, a significant inverse association between coffee consumption and mortality can be less likely. Because of the lower mortality rates in never smokers, a protective effect resulting from coffee consumption appeared more likely to become significant in people aged 55–70 y, in studies that reported HRs for different age groups (63, 66, 74, 75).
These analyses confirm that when findings for never smokers are analyzed separately, higher levels of coffee consumption are consistently associated with lower levels of mortality. Both binary and sometimes more complex adjustments for smoking do not adequately adjust for confounding between smoking and coffee consumption, especially in men. Two possible reasons for the commonly used statistical adjustments for smoking not being effective are: 1) long-term health impacts in former smokers have been underestimated; and 2) smoking and coffee consumption frequently have a nonlinear association, with very heavy smokers likely to be very high coffee consumers. Statistical adjustments for smoking, which can work when there is a linear relation between coffee consumption and smoking, are likely to have residual confounding from the negative health effects of heavy smoking. Supplemental Figure 1 shows the nonlinear relation between smoking and coffee drinking for current smokers. These data were taken from 6 samples included in the current review that reported data for current smokers, using comparable coffee consumption categories.
Adjustment for healthy and unhealthy food
Each of the 34 articles was rated for the extent to which adjustment was made for higher consumption of unhealthy foods, or lower consumption of healthy foods, as covariates of coffee consumption. Adjustments made in the papers for alcohol, total energy intake and other hot beverages (black tea, green tea) were not included in the food adjustment ratings. The ratings are shown in Table 4. The text from each article coded into the specific levels of food adjustment, is shown in Supplemental Table 4. The frequencies indicate 4 distinct patterns in relation to adjusting for food covariates. There was a trend toward a bimodal distribution between the covariates used in the level 2 rating (macronutrient variables) and level 4 rating (specific food groups and dietary indices) indicating contrasting assumptions in relation to adjustment for dietary variables.
TABLE 4.
Adjustment for food: ratings and frequencies
| Type of food adjustment and effectiveness score1 | Number of samples (n = 36) | Examples of text coded into food category2 |
|---|---|---|
| Level 1. No adjustment for food | 12 | None |
| Level 2. Minimal adjustment Adjustment for single food or nutrient | 10 | Fat intake; SFAs and fiber; total carbohydrates; SSBs; leafy vegetables |
| Level 3. Moderate adjustment Adjustment using 2–3 foods | 5 | Nuts, fruit, vegetables; red and processed meat, fruits, vegetables |
| Level 4. Multiple foods adjustment Adjustment using a food index or ≥4 healthy and unhealthy foods |
9 | AHEI and SSBs Mediterranean diet and snacking Rice, bread, meat, fish, eggs, vegetables, fruit Red and processed meat, white meat, SFAs, fruits, vegetables Wholegrains, refined grains, red meat, fish, fruit and vegetables |
Adjustment for alcohol, total energy intake, and other hot beverages (black tea, green tea) was not included in the food adjustment ratings.
AHEI, Alternative Healthy Eating Index; SSB, sugar-sweetened beverage.
The partial correlation, controlling for sample size, between ratings of the effectiveness of food adjustments and the summary significance level for the association between coffee and mortality was significant (ρ = 0.46; P = 0.003, 1-tailed) across 36 samples. The use of more complex food adjustments increased the likelihood of finding a significant inverse association between coffee and mortality. The partial correlation between the food adjustment ratings and significance of the association between coffee and mortality was significant for women (ρ = 0.38; P = 0.043; n = 23) but not for men (ρ = 0.06; NS; n = 22).
There was a significant correlation between the food adjustment and smoking adjustment ratings (ρ = 0.58; P < 0.001; n = 33), when controlling for sample size. This indicated that studies that used more effective smoking adjustment tended also to use more effective food adjustments. When the smoking and food adjustment ratings were combined into a single variable, the partial correlation of the combined variable with the summary significance rating was ρ = 0.64 (P < 0.0001; n = 33). This partial correlation between the combined adjustment rating and significance ratings indicates ∼40% covariance.
The correlations reported indicate that adjustments for smoking and healthy and unhealthy foods each make an independent contribution to the overall effectiveness of the adjustments, and their combined contribution to adjustment accounts for considerable variation in the levels of significance.
The level of alcohol adjustment (using the 3-level rating mentioned earlier) was significantly correlated with the smoking adjustment rating (ρ = 0.58; P < 0.001; n = 33) when sample size was controlled. The partial correlation of the alcohol adjustment rating with the significance level rating (ρ = 0.38; P = 0.012) was significant when controlling for sample size, but was nonsignificant (ρ = −0.09) when both sample size and smoking adjustment were controlled. These correlations indicated a trend for studies that used complex adjustment for smoking also to use more complex adjustment for food and alcohol.
The importance of adjusting for specific healthy and unhealthy food groups is consistent with data reported in several of the cohort studies. Of studies that reported the association between specific food groups and coffee consumption, higher coffee consumption was frequently associated with eating less fruit and vegetables (52, 59, 62, 63, 69), higher consumption of red and processed meat and refined grains (52, 59, 62, 63, 69), and drinking more alcohol. One article used an empirically derived “healthy” food pattern, constructed using factor analysis, as a covariate. The vegetable-fruit-soy-rich pattern comprised high intakes of vegetables, fruits, and soy foods, and low intakes of rice, noodles, and red and processed meats. This pattern was inversely associated with coffee consumption in both never smokers and ever smokers in a Singaporean sample (65).
These findings emphasize the importance of adjusting for healthy and unhealthy foods in research on coffee and health outcomes. Based on the food ratings, ∼60% of the studies (22/36) did not adjust for any food groups or used only macronutrients and/or a single food in the adjustment.
Higher coffee consumption had a linear association with higher alcohol consumption in 25 of 31 samples that reported the association for alcohol consumption. However, a different pattern of coffee and alcohol consumption was evident in 5 of the 7 Japanese samples that reported data separately for men and women. In Japanese men, higher levels of coffee drinking (4+ cups/d) tended to be associated with lower rates of alcohol consumption, in samples where 50–70% of men reported drinking alcohol regularly. In contrast, in Japanese women there tended to be a linear relation, with higher coffee associated with higher alcohol consumption in female samples where 5–25% of women reported that they drank alcohol (50, 57, 58, 62, 76). The high levels of coffee drinking (4+ cups/d) associated with lower levels of alcohol consumption in Japanese men could indicate a pattern of drinking coffee as an alternative to alcohol in social or work-related groups. This pattern was not evident in men in other countries.
Sugar added to coffee has been generally ignored in research on coffee, despite sugar being shown to have negative health effects. Of the 34 articles included in the analysis, 29 made no mention of added sugar to coffee. In 5 articles, 1 mentioned not measuring added sugar as a limitation of the study (57) and 4 mentioned that they checked whether sugar added to coffee altered the findings. All of the latter 4 reported no difference to the HRs from added sugar (35, 36, 38, 49). The 4 articles appear to have coded added sugar as a binary variable (yes/no), and none reported adjusting for the amount of added sugar (e.g., teaspoon equivalents) in coffee drinkers.
Discussion
Cohort studies provide a crucial source of evidence for investigating the long-term effects of specific foods and dietary patterns on health. Critical analyses can help improve the quality of cohort research designs. The focus of this narrative review was to assess the quality of adjustment for potential confounding in research on coffee. Although this report is based on a small number of published articles, as far as we are aware, it is the first to systematically examine the relation between adequacy of adjustment for smoking and food as covariates, and the significance of these findings for research on coffee and health outcomes such as mortality. Evidence from 34 published studies supported the view that inadequate adjustment for confounding for both smoking and unhealthy foods reduced the likelihood of finding a significant health protective effect for coffee. The review also noted that the potentially negative health effects of sugar added to coffee have not been adequately investigated.
Inadequate adjustment for confounding between coffee consumption and smoking has led to misleading findings in both cohort studies and meta-analyses, particularly for the association between coffee and lung cancer and pancreatic cancer. Two meta-analyses reported a significant association between higher coffee consumption and increased risk of lung cancer (15, 79). Both these meta-analyses did not assess the effectiveness of adjustment for smoking in the individual studies included in the meta-analyses. An association has been reported between coffee consumption and pancreatic cancer, but this association becomes nonsignificant in nonsmokers and in studies that have adjusted for smoking (13). As noted earlier, the Grosso et al. (8) meta-analysis reported a significant linear inverse trend between coffee intake and mortality rates among never smokers. The differences between never and ever smokers were most evident in cancer deaths. Never smokers showed significantly lower cancer death rates with increasing coffee consumption. In contrast, in former and current smokers, increasing coffee consumption did not reduce risks for cancer mortality.
Given the inadequacy of adjustments commonly used for smoking, studies reporting on the health effects of coffee, and other exposures that could be linked to smoking, should report RRs separately for never smokers (5, 80). Because some groups show large differences in smoking rates between men and women, separate analyses by sex should also be reported. Future studies might also need to include the use of e-cigarettes (vaping).
These findings have implications for other dietary studies making adjustments for smoking status, where smoking might be associated with other food variables or lifestyle patterns. For example, an extensive review on risk thresholds for alcohol consumption based on 83 prospective studies used a binary variable (current smoker compared with nonsmoker) to adjust for smoking (81). Adjustment using binary variables is more likely to be linked to misleading assessments of RRs, especially where an exposure variable (alcohol) and the potential confounder (smoking) have a nonlinear association. Many published reports that have used binary covariates to adjust for smoking could have residual confounding resulting from smoking-associated health risks.
When smoking adjustments are used, authors should report explicitly how the variable or variables were constructed for smoking adjustment and how these variables were entered into regression analyses. Only 1 article included in the current review reported this detail (52). Another concern is avoiding use of the term “nonsmokers.” This term is ambiguous, and has been used to refer to both “never smokers” and “noncurrent smokers.” It is better to use the terms for which the meaning is clear, such as never, former (past, previous), and current smokers.
Higher levels of coffee intake were commonly associated with consumption of unhealthy foods in the studies reviewed. Additional evidence for this association is evident in studies on dietary patterns using factor analysis. Six systematic reviews focused on food patterns were found where coffee was reported among the specific foods related to healthy and unhealthy eating patterns (82–87). From the 6 reviews, 101 individual studies using factor analysis were examined. Of the individual studies, 14 reported the association of coffee with the primary factors. Eleven of 14 studies reported coffee as loading on factors commonly labeled as “Western” or unhealthy in samples from 9 countries. This “unhealthy” pattern consisted of red meat, processed meat, refined grains, alcohol, sweet foods, and coffee (82, 84, 86, 87). These findings are consistent with the importance of adjusting for food groups. Where potential covariation between coffee and unhealthy foods has not been adjusted, it is less likely that higher coffee consumption will be associated with reduced mortality and morbidity.
Current research indicates that added sugar is a risk factor for health problems such as obesity, CVD, and diabetes (42, 88, 89). Taking coffee with added sugar, or flavored coffees with sugar as a sweetener, is likely to reduce the health benefits of coffee (90). A literature search for studies investigating the association between coffee and health outcomes found few that reported the proportion of coffee drinkers who added sugar, and none that reported the amount of sugar added.
The omission of sugar as a potential confounder in research on coffee could be based on the assumption that sugar intake has negligible health effects. The continued omission of added sugar is likely to be a legacy from dietary questionnaires constructed prior to 2000, which are unlikely to have included questions to measure sugar added to coffee. The influential NHANES question set used for repeated national surveys in the United States illustrates this problem. In NHANES, sugar added to coffee was measured by the following questions:
123 How many cups of coffee, caffeinated or decaffeinated, did you drink? (over the last 12 months)
Ten response categories were provided from “None” to “6 or more cups per day.”
126 How often did you add sugar or honey to your coffee or tea?
Ten response categories were provided from “Never” to “6 or more times per day.”
The question on added sugar or honey does not provide a quantity estimate for added sugar (e.g., teaspoons). Reports on tea and coffee consumption based on the NHANES surveys have ignored added sugar in the profiles of groups consuming tea or coffee. For example, a 2016 article reported ∼75% of adults in the United States drinking coffee in the past 12 mo, and ∼49% drinking coffee daily (1). No mention was made of the proportion of coffee drinkers who added sugar.
In contrast, the more recently constructed UK Biobank question set, used in a cohort for which recruitment started in 2003, does allow for calculation of added sugar (91). This survey included the following question:
How much sugar did you add to your coffee (per drink)?
Six responses categories were provided, from none to 3+ teaspoons.
One of the few studies that mentioned sugar in coffee was a report on the US NIH-AARP (American Association of Retired Persons) Diet and Health case-control study of older people (aged 50–71 y at recruitment) (92). Of 242,171 tea and coffee drinkers in the control group, 49% did not add sugar or honey to tea or coffee, 25% added sugar or honey, and 26% added other sweeteners. Those who did not add sweeteners to tea or coffee had a lower risk for depression than people who added any type of sweetener (92). A Korean study reported that instant coffee mixes with added sugar were associated with an increased risk of metabolic syndrome, compared with other types of coffee (93).
There has been sufficient evidence at least since 2010 to justify the inclusion of added sugar as a potential confounder in studies of the association between coffee and health outcomes. In 2 umbrella reviews on coffee and health, 1 did not mention sugar at all (6) and the other mentioned it as a possible limitation of the existing research (5). Given the practice of ignoring added sugar in studies of coffee and health, nearly all the published findings on health outcomes from drinking coffee could reflect unadjusted confounding, which could reduce the likelihood of finding health benefits from coffee. Confounding is most likely for health outcomes where sugar has been reported as a risk factor, such as weight gain, obesity, metabolic syndrome, diabetes, and blood lipids. Confounding with added sugar could be most likely to occur among people drinking ≥3 more cups of coffee per day and who add >1 teaspoon of sugar per cup. For example, a person drinking 5 cups/d with 2 teaspoons of sugar per cup would have an added sugar intake of around 50 g/d (1 teaspoon ≈ 5 g) just from their coffee consumption. It is possible that part of the reduced protective effect of coffee for consumption of 5+ cups/d, which some studies have reported, could be due to added sugar.
In terms of implications for further research related to the health effects of coffee, there were several topics for which no research was found. These include studies investigating the association of coffee consumption with other dietary and lifestyle patterns. A particular pattern of interest is the use of coffee as a substitute for other beverages such as alcohol or SSBs, especially in social settings. In addition, no studies were found that included self-reporting of reactions to coffee, especially among occasional drinkers of coffee. Some people can be allergic to coffee or have adverse reactions to caffeine. Research is needed on the strategies people use to self-manage coffee consumption at comfortable levels. One RCT, examining the effects of coffee, required participants to drink 1 L of coffee every day for 2 wk. Negative reactions (“palpitations and tremor during the first days of drinking the cafetière coffee”) were reported as being sufficiently severe for 1 participant to drop out of the study. However, as was evident in this report (94), RCT studies are unlikely to gather information about participants’ reactions to exposures, in this case drinking more coffee than usual.
For some people, coffee drinking is associated with social contact and social support (95, 96). Studies linking coffee consumption with, for example, reduced risk of depression, could have confounding due to increased social contact and support being associated with coffee consumption (97). More research is needed on the social contexts associated with coffee consumption and the extent to which these contexts can have beneficial effects on health.
A pattern evident among Japanese men, and that could occur in other societies, is that some people consume coffee instead of alcohol in settings where both types of drinks are available. This pattern of substitution does not appear to have been investigated. As well, changes in coffee consumption over time, and reasons for change, appear not to have been investigated. Only a few studies have reported consistency of coffee consumption over several years (72). A pattern needing further research is the extent to which people take up, or increase, their coffee consumption as a substitute for drinking alcohol or SSBs.
Research using self-report measures of coffee consumption should clearly describe the questions used to measure coffee and should note whether added sugar was measured, including flavorings that include sugar. No information was found about the various types of milks added to coffee. For some consumers, milk can be used instead of sweeteners to reduce the bitterness of coffee and is likely to be a healthier option than sugar.
More reviews are needed to document which research studies on food groups and patterns have included coffee as a food variable, and which included coffee but did not report it because it was not associated with outcomes of interest. Research using cohort samples should assess whether coffee drinking is associated with unhealthy eating patterns and if so, allow for this association when adjusting for potential confounders.
This review has several limitations. It was restricted to cohort or observational studies. Cohort studies can have unadjusted confounding, which is a limitation for attributing a causal relation between exposures and health outcomes. The findings reported here were dependent on the assumptions made when assessing the quality of smoking adjustment and adjustment for healthy and unhealthy foods. The assessment of the quality and levels of significance between coffee consumption and mortality was dependent on the information reported in each of the 34 articles reviewed. If this information was inaccurate or incomplete, it could affect the findings reported.
Conclusions
A detailed analysis of 34 cohort studies, covering 36 samples investigating the association between coffee consumption and mortality, showed that samples with less effective smoking adjustment and ineffective adjustment of healthy and unhealthy foods were less likely to report a significant inverse association between coffee and mortality. Based on the findings reviewed, coffee consumption is likely to be health protective for up to 5 cups of coffee per day for most people. Fourteen of 17 samples of never smokers showed a significant linear inverse relation between coffee consumption and mortality.
The findings of the current review indicate that many reports and reviews linking coffee to adverse health outcomes, or that have reported no association with health outcomes, have failed to adequately adjust for the negative health effects of smoking or the association of coffee drinking with unhealthy dietary patterns. This failure was particularly evident in studies using binary adjustment for smoking such as smokers/nonsmokers or smoking status (current, former, never). Future studies on the health effects of coffee should report RRs separately for “never smokers” or use adjustments that incorporate pack-years of smoking or equivalent. Researchers using cohort samples also need to assess the extent to which coffee drinking is associated with unhealthy eating patterns and if so, allow for this association when adjusting for potential confounders.
There has been sufficient evidence since at least 2010 to justify the inclusion of added sugar as a potential confounder in studies of the links between coffee and health. Because sugar has been almost totally ignored as a confounder, existing research could underestimate the health-protective effects of coffee.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Associate Professor Roger Marshall, School of Population Health, University of Auckland, for his constructive comments on an earlier version of this article.
The authors’ contributions were as follows—DRT: responsible for conceiving the review, planning and carrying out the literature review, analyzing the reports, constructing the rating scales, and writing the first draft of the manuscript; IDH: verified the links between the text in the 34 articles and the ratings made, read drafts of the manuscript, and rewrote sections of the text; and both authors: reviewed and revised the manuscript, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–4 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AHEI, Alternative Healthy Eating Index; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension Trial; RCT, randomized controlled trial; SSB, sugar-sweetened beverage.
References
- 1. Loftfield E, Freedman ND, Dodd KW, Vogtmann E, Xiao Q, Sinha R, Graubard BI. Coffee drinking is widespread in the United States, but usual intake varies by key demographic and lifestyle factors. J Nutr. 2016;146(9):1762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ioannidis JA. The challenge of reforming nutritional epidemiologic research. JAMA. 2018;320(10):969–70. [DOI] [PubMed] [Google Scholar]
- 3. Hu FB, Willett WC.. Current and future landscape of nutritional epidemiologic research. JAMA. 2018;320(20):2073–4. [DOI] [PubMed] [Google Scholar]
- 4. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa Hospital Research Institute; 2016. Retrieved Accessed on 20 April 2019 from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 5. Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr. 2017;37:131–56. [DOI] [PubMed] [Google Scholar]
- 6. Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. Br Med J. 2017;359:j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180(8):763–75. [DOI] [PubMed] [Google Scholar]
- 8. Grosso G, Micek A, Godos J, Sciacca S, Pajak A, Martínez-González MA, Giovannucci EL, Galvano F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: a dose-response meta-analysis. Eur J Epidemiol. 2016;31(12):1191–205. [DOI] [PubMed] [Google Scholar]
- 9. Je Y, Giovannucci E.. Coffee consumption and total mortality: a meta-analysis of twenty prospective cohort studies. Br J Nutr. 2014;111(7):1162–73. [DOI] [PubMed] [Google Scholar]
- 10. Li Q, Liu Y, Sun X, Yin Z, Li H, Cheng C, Liu L, Zhang R, Liu F, Zhou Q et al.. Caffeinated and decaffeinated coffee consumption and risk of all-cause mortality: a dose-response meta-analysis of cohort studies. J Hum Nutr Diet. 2019;32(3):279–87. [DOI] [PubMed] [Google Scholar]
- 11. Malerba S, Turati F, Galeone C, Pelucchi C, Verga F, La Vecchia C, Tavani A. A meta-analysis of prospective studies of coffee consumption and mortality for all causes, cancers and cardiovascular diseases. Eur J Epidemiol. 2013;28(7):527–39. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Y, Wu K, Zheng J, Zuo R, Li D. Association of coffee drinking with all-cause mortality: a systematic review and meta-analysis. Public Health Nutr. 2015;18(7):1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li T-D, Yang H-W, Wang P, Song C-H, Wang K-J, Dai L-P, Shi J-X, Zhang J-Y, Ye H. Coffee consumption and risk of pancreatic cancer: a systematic review and dose–response meta-analysis. Int J Food Sci Nutr. 2019;70(5):519–29. [DOI] [PubMed] [Google Scholar]
- 14. Wu W, Tong Y, Zhao Q, Yu G, Wei X, Lu Q. Coffee consumption and bladder cancer: a meta-analysis of observational studies. Sci Rep. 2015;5:9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie Y, Qin J, Nan G, Huang S, Wang Z, Su Y. Coffee consumption and the risk of lung cancer: an updated meta-analysis of epidemiological studies. Eur J Clin Nutr. 2016;70(2):199–206. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Z, Hu G, Caballero B, Appel L, Chen L. Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies. Am J Clin Nutr. 2011;93(6):1212–9. [DOI] [PubMed] [Google Scholar]
- 17. Sofi F, Conti AA, Gori AM, Eliana Luisi ML, Casini A, Abbate R, Gensini GF. Coffee consumption and risk of coronary heart disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2007;17(3):209–23. [DOI] [PubMed] [Google Scholar]
- 18. Steffen M, Kuhle C, Hensrud D, Erwin PJ, Murad MH. The effect of coffee consumption on blood pressure and the development of hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(12):2245–54. [DOI] [PubMed] [Google Scholar]
- 19. Bjørngaard JH, Nordestgaard AT, Taylor AE, Treur JL, Gabrielsen ME, Munafò MR, Nordestgaard BG, Åsvold BO, Romundstad P, Davey Smith G. Heavier smoking increases coffee consumption: findings from a Mendelian randomization analysis. Int J Epidemiol. 2017;46(6):1958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Treur JL, Taylor AE, Ware JJ, McMahon G, Hottenga J-J, Baselmans BML, Willemsen G, Boomsma DI, Munafò MR, Vink JM. Associations between smoking and caffeine consumption in two European cohorts. Addiction. 2016;111(6):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matukala Nkosi T, Parent M-É, Siemiatycki J, Rousseau M-C. Socioeconomic position and lung cancer risk: how important is the modeling of smoking?. Epidemiology. 2012;23(3):377–85. [DOI] [PubMed] [Google Scholar]
- 22. Menvielle G, Boshuizen H, Kunst AE, Dalton SO, Vineis P, Bergmann MM, Hermann S, Ferrari P, Raaschou-Nielsen O, Tjønneland A et al.. The role of smoking and diet in explaining educational inequalities in lung cancer incidence. J Natl Cancer Inst. 2009;101(5):321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fung TT, Isanaka S, Hu FB, Willett WC. International food group–based diet quality and risk of coronary heart disease in men and women. Am J Clin Nutr. 2018;107(1):120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146(8):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng Y, Li Y, Satija A, Pan A, Sotos-Prieto M, Rimm E, Willett WC, Hu FB. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. Br Med J. 2019;365:l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neuenschwander M, Ballon A, Weber KS, Norat T, Aune D, Schwingshackl L, Schlesinger S. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. Br Med J. 2019;366:l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Micha R, Shulkin ML, Peñalvo JL, Khatibzadeh S, Singh GM, Rao M, Fahimi S, Powles J, Mozaffarian D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One. 2017;12(4):e0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leffondré K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. Am J Epidemiol. 2002;156(9):813–23. [DOI] [PubMed] [Google Scholar]
- 29. Mente A, Dehghan M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, Lear S, Li W, Chen H, Yi S et al.. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: a cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol. 2017;5(10):774–87. [DOI] [PubMed] [Google Scholar]
- 30. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG et al.. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398–406. [DOI] [PubMed] [Google Scholar]
- 31. Hooper L, Martin N, Abdelhamid A, Davey SG. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2015;(6):CD011737. doi: 10.1002/14651858.CD011737. [DOI] [PubMed] [Google Scholar]
- 32. Khan SU, Khan MU, Riaz H, Valavoor S, Zhao D, Vaughan L, Okunrintemi V, Riaz IB, Khan MS, Kaluski E et al.. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171(3):190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J et al.. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. Br Med J. 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. Br Med J. 2018;361:k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DuBroff R, de Lorgeril M. Fat or fiction: the diet-heart hypothesis. BMJ Evid Based Med[Internet] 2019. doi: 10.1136/bmjebm-2019-111180. [DOI] [PubMed] [Google Scholar]
- 36. Hamley S. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: a meta-analysis of randomised controlled trials. Nutr J. 2017;16(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, Boushey CJ, Schap TE, Reedy J. The dietary patterns methods project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145(3):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144(6):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mozaffarian D, Rosenberg I, Uauy R. History of modern nutrition science—implications for current research, dietary guidelines, and food policy. Br Med J. 2018;361:k2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, Swaminathan S, Machiweni T, Mapanga R. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390(10107):2037–49. [DOI] [PubMed] [Google Scholar]
- 41. Zhang B, Zhao Q, Guo W, Bao W, Wang X. Association of whole grain intake with all-cause, cardiovascular, and cancer mortality: a systematic review and dose–response meta-analysis from prospective cohort studies. Eur J Clin Nutr. 2018;72:57–65. [DOI] [PubMed] [Google Scholar]
- 42. Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100(1):65–79. [DOI] [PubMed] [Google Scholar]
- 43. Malik VS, Hu FB.. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11(8):1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–45. [DOI] [PubMed] [Google Scholar]
- 45. Kahn HA, Phillips RL, Snowdon DA, Choi W. Association between reported diet and all-cause mortality: twenty-one-year follow-up on 27,530 adult Seventh-Day Adventists. Am J Epidemiol. 1984;119(5):775–87. [DOI] [PubMed] [Google Scholar]
- 46. Lindsted KD, Kuzma JW, Anderson JL. Coffee consumption and cause-specific mortality association with age at death and compression of mortality. J Clin Epidemiol. 1992;45(7):733–42. [DOI] [PubMed] [Google Scholar]
- 47. Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993;3(4):375–81. [DOI] [PubMed] [Google Scholar]
- 48. Woodward M, Tunstall-Pedoe H.. Coffee and tea consumption in the Scottish Heart Health Study follow up: conflicting relations with coronary risk factors, coronary disease, and all cause mortality. J Epidemiol Community Health. 1999;53(8):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kleemola P, Jousilahti P, Pietinen P, Vartiainen E, Tuomilehto J. Coffee consumption and the risk of coronary heart disease and death. Arch Intern Med. 2000;160(22):3393–400. [DOI] [PubMed] [Google Scholar]
- 50. Iwai N, Ohshiro H, Kurozawa Y, Hosoda T, Morita H, Funakawa K, Okamoto M, Nose T. Relationship between coffee and green tea consumption and all-cause mortality in cohort of a rural Japanese population. J Epidemiol. 2002;12(3):191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jazbec A, Šimić D, Čorović N, Duraković Z, Pavlović M. Impact of coffee and other selected factors on general mortality and mortality due to cardiovascular disease in Croatia. J Health Popul Nutr. 2003;21(4):332–40. [PubMed] [Google Scholar]
- 52. Andersen LF, Carlsen MH, Blomhoff R, Jacobs DR Jr.. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women's Health Study. Am J Clin Nutr. 2006;83(5):1039–46. [DOI] [PubMed] [Google Scholar]
- 53. Paganini-Hill A, Kawas CH, Corrada MM. Non-alcoholic beverage and caffeine consumption and mortality: the Leisure World Cohort Study. Prev Med. 2007;44(4):305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Happonen P, Läärä E, Hiltunen L, Luukinen H. Coffee consumption and mortality in a 14-year follow-up of an elderly northern Finnish population. Br J Nutr. 2008;99(6):1354–61. [DOI] [PubMed] [Google Scholar]
- 55. Ahmed HN, Levitan EB, Wolk A, Mittleman MA. Coffee consumption and risk of heart failure in men: an analysis from the Cohort of Swedish Men. Am Heart J. 2009;158(4):667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Koning Gans JM, Uiterwaal CSPM, van der Schouw YT, Boer JMA, Grobbee DE, Verschuren WMM, Beulens JWJ. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler Thromb Vasc Biol. 2010;30(8):1665–71. [DOI] [PubMed] [Google Scholar]
- 57. Sugiyama K, Kuriyama S, Akhter M, Kakizaki M, Nakaya N, Ohmori-Matsuda K, Shimazu T, Nagai M, Sugawara Y, Hozawa A et al.. Coffee consumption and mortality due to all causes, cardiovascular disease, and cancer in Japanese women. J Nutr. 2010;140(5): 1007–13. [DOI] [PubMed] [Google Scholar]
- 58. Tamakoshi A, Lin Y, Kawado M, Yagyu K, Kikuchi S, Iso H. Effect of coffee consumption on all-cause and total cancer mortality: findings from the JACC study. Eur J Epidemiol. 2011;26(4):285–93. [DOI] [PubMed] [Google Scholar]
- 59. Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366(20):1891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu J, Sui X, Lavie CJ, Hebert JR, Earnest CP, Zhang J, Blair SN. Association of coffee consumption with all-cause and cardiovascular disease mortality. Mayo Clin Proc. 2013;88(10):1066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gardener H, Rundek T, Wright CB, Elkind MS, Sacco RL. Coffee and tea consumption are inversely associated with mortality in a multiethnic urban population. J Nutr. 2013;143(8):1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saito E, Inoue M, Sawada N, Shimazu T, Yamaji T, Iwasaki M, Sasazuki S, Noda M, Iso H, Tsugane S. Association of coffee intake with total and cause-specific mortality in a Japanese population: the Japan Public Health Center–based prospective study. Am J Clin Nutr. 2015;101:1029–37. [DOI] [PubMed] [Google Scholar]
- 63. Loftfield E, Freedman ND, Graubard BI, Guertin KA, Black A, Huang WY, Shebl FM, Mayne ST, Sinha R. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182(12):1010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lof M, Sandin S, Yin L, Adami HO, Weiderpass E. Prospective study of coffee consumption and all-cause, cancer, and cardiovascular mortality in Swedish women. Eur J Epidemiol. 2015;30(9):1027–34. [DOI] [PubMed] [Google Scholar]
- 65. Odegaard AO, Koh W-P, Yuan J-M, Pereira MA. Beverage habits and mortality in Chinese adults. J Nutr. 2015;145(3):595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ding M, Satija A, Bhupathiraju SN, Hu Y, Sun Q, Han J, Lopez-Garcia E, Willett W, van Dam RM, Hu FB. Association of coffee consumption with total and cause-specific mortality in 3 large prospective cohorts. Circulation. 2015;132(24):2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nordestgaard AT, Nordestgaard BG. Coffee intake, cardiovascular disease and all-cause mortality: observational and Mendelian randomization analyses in 95 000–223 000 individuals. Int J Epidemiol. 2016;45(6):1938–52. [DOI] [PubMed] [Google Scholar]
- 68. Grosso G, Stepaniak U, Micek A, Stefler D, Bobak M, Pajak A. Coffee consumption and mortality in three Eastern European countries: results from the HAPIEE (Health, Alcohol and Psychosocial factors In Eastern Europe) study. Public Health Nutr. 2017;20(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gunter MJ, Murphy N, Cross AJ, Dossus L, Dartois L, Fagherazzi G, Kaaks R, Kühn T, Boeing H, Aleksandrova K et al.. Coffee drinking and mortality in 10 European countries: a multinational cohort study. Ann Intern Med. 2017;167(4):236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Park S, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan V. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann Intern Med. 2017;167:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Neves JS, Leitão L, Magriço R, Bigotte Vieira M, Viegas Dias C, Oliveira A, Carvalho D, Claggett B. Caffeine consumption and mortality in diabetes: an analysis of NHANES 1999–2010. Front Endocrinol (Lausanne). 2018;9:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hu Y, Ding M, Yuan C, Wu K, Smith-Warner SA, Hu FB, Chan AT, Meyerhardt JA, Ogino S, Fuchs CS et al.. Association between coffee intake after diagnosis of colorectal cancer and reduced mortality. Gastroenterology. 2018;154(4):916–26. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van den Brandt PA. Coffee or tea? A prospective cohort study on the associations of coffee and tea intake with overall and cause-specific mortality in men versus women. Eur J Epidemiol. 2018;33(5):183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Loftfield E, Cornelis MC, Caporaso N, Yu K, Sinha R, Freedman N. Association of coffee drinking with mortality by genetic variation in caffeine metabolism: findings from the UK Biobank. JAMA Intern Med. 2018;178(8):1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Navarro AM, Martínez-González M, Gea A, Grosso G, Martín-Moreno JM, Lopez-Garcia E, Martín-Calvo N, Toledo E. Coffee consumption and total mortality in a Mediterranean prospective cohort. Am J Clin Nutr. 2018;108(5):1113–20. [DOI] [PubMed] [Google Scholar]
- 76. Sado J, Kitamura T, Kitamura Y, Liu R, Ando E, Sobue T, Sugawara Y, Matsuo K, Nakayama T, Tsuji I et al.. Coffee consumption and all-cause and cardiovascular mortality: three-prefecture cohort in Japan. Circ J. 2019;83(4):757–66. [DOI] [PubMed] [Google Scholar]
- 77. Nohara-Shitama Y, Adachi H, Enomoto M, Fukami A, Nakamura S, Kono S, Morikawa N, Sakaue A, Hamamura H, Toyomasu K et al.. Habitual coffee intake reduces all-cause mortality by decreasing heart rate. Heart Vessels. 2019;34(11):1823–9. [DOI] [PubMed] [Google Scholar]
- 78. Yamakawa M, Wada K, Mizuta F, Koda S, Uji T, Nagata C. Associations between coffee consumption and all-cause and cause-specific mortality in a Japanese city: the Takayama study. Public Health Nutr. 2019;22(14):2561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tang N, Wu Y, Ma J, Wang B, Yu R. Coffee consumption and risk of lung cancer: a meta-analysis. Lung Cancer. 2010;67(1):17–22. [DOI] [PubMed] [Google Scholar]
- 80. Galarraga V, Boffetta P.. Coffee drinking and risk of lung cancer—a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25(6):951–7. [DOI] [PubMed] [Google Scholar]
- 81. Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S et al.. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391(10129):1513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Albuquerque RC, Baltar VT, Marchioni DM. Breast cancer and dietary patterns: a systematic review. Nutr Rev. 2014;72(1):1–17. [DOI] [PubMed] [Google Scholar]
- 83. Fabiani R, Minelli L, Bertarelli G, Bacci S. A Western dietary pattern increases prostate cancer risk: a systematic review and meta-analysis. Nutrients. 2016;8(10):626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maghsoudi Z, Ghiasvand R, Salehi-Abargouei A. Empirically derived dietary patterns and incident type 2 diabetes mellitus: a systematic review and meta-analysis on prospective observational studies. Public Health Nutr. 2016;19(2):230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McEvoy CT, Cardwell CR, Woodside JV, Young IS, Hunter SJ, McKinley MC. A posteriori dietary patterns are related to risk of type 2 diabetes: findings from a systematic review and meta-analysis. J Acad Nutr Diet. 2014;114(11):1759–75. e4. [DOI] [PubMed] [Google Scholar]
- 86. Rodríguez-Monforte M, Sánchez E, Barrio F, Costa B, Flores-Mateo G. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2017;56(3):925–47. [DOI] [PubMed] [Google Scholar]
- 87. Shab-Bidar S, Golzarand M, Hajimohammadi M, Mansouri S. A posteriori dietary patterns and metabolic syndrome in adults: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2018;21(9):1681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang Q, Zhang Z, Gregg EW, Flanders W, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174(4):516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. Br Med J. 2013;346:e7492. [DOI] [PubMed] [Google Scholar]
- 90. O'Connor L, Imamura F, Brage S, Griffin SJ, Wareham NJ, Forouhi NG. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin Nutr. 2018;37(4):1313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M et al.. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guo X, Park Y, Freedman ND, Sinha R, Hollenbeck AR, Blair A, Chen H. Sweetened beverages, coffee, and tea and depression risk among older US adults. PLoS One. 2014;9(4):e94715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kim HJ, Cho S, Jacobs DR Jr, Park K. Instant coffee consumption may be associated with higher risk of metabolic syndrome in Korean adults. Diabetes Res Clin Pract. 2014;106(1):145–53. [DOI] [PubMed] [Google Scholar]
- 94. Grubben MJ, Boers GH, Blom HJ, Broekhuizen R, de Jong R, van Rijt L, de Ruijter E, Swinkels DW, Nagengast FM, Katan MB. Unfiltered coffee increases plasma homocysteine concentrations in healthy volunteers: a randomized trial. Am J Clin Nutr. 2000;71(2):480–4. [DOI] [PubMed] [Google Scholar]
- 95. Samoggia A, Riedel B.. Consumers’ perceptions of coffee health benefits and motives for coffee consumption and purchasing. Nutrients. 2019;11:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Broughton KA, Payne L, Liechty T. An exploration of older men's social lives and well-being in the context of a coffee group. Leis Sci. 2017;39(3):261–76. [Google Scholar]
- 97. Grosso G, Micek A, Castellano S, Pajak A, Galvano F. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60(1):223–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.