Abstract
Diploidy is the typical genomic mode in all mammals. Haploid stem cells are artificial cell lines experimentally derived in vitro in the form of different types of stem cells, which combine the characteristics of haploidy with a broad developmental potential and open the possibility to uncover biological mysteries at a genomic scale. To date, a multitude of haploid stem cell types from mouse, rat, monkey and humans have been derived, as more are in development. They have been applied in high-throughput genetic screens and mammalian assisted reproduction. Here, we review the generation, unique properties and broad applications of these remarkable cells.
Keywords: haploidy, parthenogenetic, androgenetic, stem cells, diploidization, functional genomics, imprinting
Introduction
Ploidy refers to the number of sets of chromosomes in a cell or organism, and is considered as a relatively stable cellular characteristic. Changes in ploidy may cause genomic instability, which was proved to promote cancer (Thompson and Compton, 2008; Potapova et al., 2013; Silk et al., 2013). Normally, diploid genomes are typical in most living animals as two homologous sets of chromosomes are existed per nucleus. As the dominant diploid phase is one of the major features of the life cycle in all mammals, haploid cells which contain only one set of chromosomes are generally restricted to gametes.
Evolutionarily speaking, the adaptive significance of diploidy over haploidy lies in two major perspectives. First, diploidy generates more variability or selective possibilities. Second, it is able to mask the deleterious recessive mutations (Paquin and Adams, 1983; Perrot et al., 1991). But when it comes to genetic analysis, these traits endow haploid cells with overwhelming advantages over diploid cells reversely. In haploid cells, loss-of-function mutations can be achieved in a single step, and phenotypes caused by recessive mutations can then be analyzed directly due to the lack of compensation for hemizygous gene mutations (Elling and Penninger, 2014; Wutz, 2014; Horii and Hatada, 2015; Li and Zhou, 2017). However, natural haploidy has not been reported in vertebrates, including mammals.
Thus for long, scientists have been trying to sought yeast-like systems for directly analyzing recessive and disease phenotypes, leading to alternative approaches like using unstable near-haploid cell cultures (Kotecki et al., 1999; Carette et al., 2009) or converting diploidy into haploidy by human-rodent cell fusions (Yan et al., 2000). Since 2009, with the constant optimization of derivation and culture techniques, haploid embryonic stem cell (haESC) lines from medaka fish, mouse, rat, monkey and even humans have been successfully established (Yi et al., 2009; Elling et al., 2011; Leeb and Wutz, 2011; Li et al., 2012; Yang et al., 2012, 2013; Li et al., 2014; Sagi et al., 2016). Most recently, the repertoire of haploid stem cells further expands to include haploid trophoblast stem cells (Cui et al., 2019; Peng et al., 2019), the extraembryonic counterpart of embryonic stem cells. In this review, we present an overview of existing haploid stem cells, as well as their strengths and limitations. We also explore ways in which these unique cells can deepen our understanding of mammalian development and reproductive approaches.
Derivation of haploid mammalian embryos and haploid stem cells
Haploid embryos provide the source of haploid cell lines, which have long been experimentally produced by manipulation of gametes because spontaneous haploid embryos seldom occur naturally in mammals. There are two genetic origins of haploid animals, parthenogenetic (PG) and androgenetic (AG) haploid animals (Li and Zhou, 2017). PG embryos and AG embryos develop from only one of the gametes, the oocyte or the sperm, and therefore contain only the maternal genome or the paternal genome respectively. Parthenogenesis may occur naturally as in many insect species (Pamilo and Crozier, 1981; Beukeboom et al., 2007), or it may be induced experimentally. For parthenogenesis, there are two types. The first is the authentic parthenogenesis, in which chemical or electrical stimuli mimicking fertilization are applied directly to the oocyte and development is therefore initiated without fertilization. In the other circumstance called gynogenesis, the oocyte is stimulated by fertilization but the paternal chromatin is prevented from taking part in embryonic development (EDWARDS, 1957a, b, 1958; Wutz, 2014).
In 1975, Modliński pioneered in obtaining haploid mouse embryos by microsurgical removal of one pronuclei from fertilized eggs (Modliński, 1975), which is the first case in mammals after a success of such experiment on sea urchin eggs by Hiramoto in 1962 (Hiramoto, 1962). However, in the early trials to generate haploid mouse embryos, karyological investigations revealed that the obtained haploid embryos were almost all gynogenetic with an absence of androgenones. That phenomena might be explained by the fact that removal of the female pronucleus was more injurious to the ovum than removal of the male one, as the cell membrane in the region of female pronucleus is more delicate and sensitive to injury than in other parts due to abstraction of the second polar body at this site (Modliński, 1975; Tarkowski and Rossant, 1976). Of note, the parthenogenetic mouse blastocysts were reported to be a mixture of haploid and diploid cells, indicating that during the subsequent developmental process of the activated haploid oocytes, they may become diploid. Haploid cells could be detected up to the egg cylinder stage, though development of haploid embryos became progressively delayed (Tarkowski et al., 1970; Kaufman, 1978; Sagi and Benvenisty, 2017). Overall, these results suggested that it is possible to establish haploid pluripotent cells from the haploid embryos.
Experiments in the 1980s have pioneered the establishment of parthenogenetic haploid pluripotent cells. In 1983, Kaufman et al. attempted to establish pluripotent cell lines from parthenogenetic embryos, but chromosome analysis revealed that all of the four haploid-derived cell lines showed a diploid karyotype. Even though this technique provided a source of homozygous diploid cell lines of parthenogenetic origin (Kaufman et al., 1983), this study revealed a significant diploidization tendency of these haploid cells. Together with other works conducted in Drosophila and human showing that haploid cells were unstable and quickly diploidized (Debec, 1984; Kotecki et al., 1999), they raised the fundamental question about whether haploidy can fully support a stable growth as well as pluripotency in culture.
In 2009, Hong lab generated gynogenetic haploid medaka fish embryos and derived the first haESC lines, demonstrating that haploid genomes can be maintained in proliferating cells cultured in vitro (Yi et al., 2009). And the strong interest in deriving mammalian haESC lines revived. Two years later, two parallel studies reported the successful derivation of mouse parthenogenetic haESCs (phESCs) (Elling et al., 2011; Leeb and Wutz, 2011). Activation of unfertilized oocytes with strontium chloride or 5% ethanol together with subsequent in vitro culture of haploid embryos to the blastocyst stage enabled the establishment of haESC lines (Fig. 1A) (Elling et al., 2011; Leeb and Wutz, 2011). Notably, the application of flow cytometric cell sorting techniques allows for the selection of pure haploid cells with a G1 DNA content, which is a key progress. Meanwhile, advances in culture conditions also benefited the derivation and culture of haESCs (Bryja et al., 2006; Ying et al., 2008).
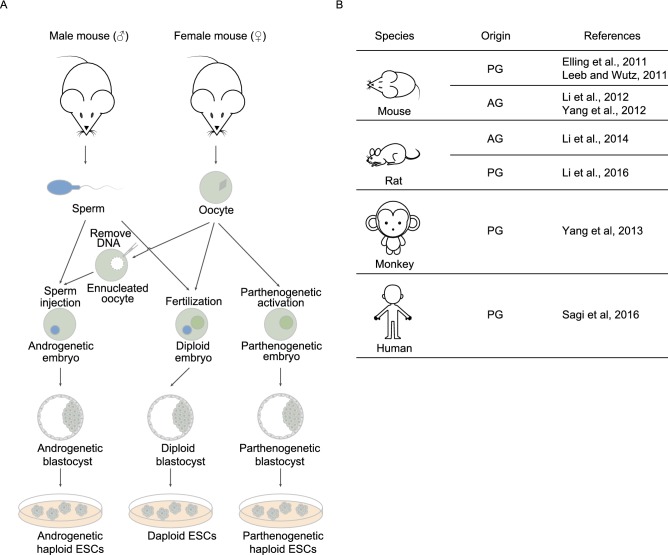
Figure 1.
Derivation of mouse haploid embryonic stem cells (haESCs). (A) Derivation strategies of parthenogenetic haESCs (phESCs) and androgenetic haESCs (ahESCs). Parthenogenetic haploid blastocysts are developed from artificially activated MII oocytes. Androgenetic embryos can be obtained by injecting sperm into the enucleated MII oocytes or removing the female pronucleus from fertilized oocytes. The resulting haploid blastocysts are subsequently cultured to develop haESC lines. (B) The haESC lines of different mammalian species have been generated
The established mouse phESCs exhibited a haploid karyotype, and largely maintain genome integrity. Sharing a similar transcriptional profile with diploid embryonic stem cells (ESCs), these haESCs express all classical pluripotency markers of diploid ESCs. Functionally, these haESCs can differentiate into lineages of all three germ layers in embryoid body (EB) formation assay. Importantly, these haESCs retain the in vivo differentiation potential as apparent coat color chimerism was observed after their being injected into diploid mouse blastocysts (Elling et al., 2011; Leeb and Wutz, 2011).
Hence, whether haESCs can function as haploid gametes to support fertilization and further development remained to be determined. We got the positive answer from androgenetic haESCs (ahESCs). In 2012, mouse ahESCs were established by injecting sperm into the enucleated metaphase II (MII) phase oocyte or removing the female pronucleus from fertilized oocytes (Fig. 1A) (Li et al., 2012; Yang et al., 2012). The ahESCs carry the paternal imprinting, though distinct from the sperm cells. Remarkably, these ahESCs can produce viable and fertile progenies after intracytoplasmic injection into mature oocytes. The production of fertile adult mice bearing haESC-carried genetic traits further shows that the genetic information in haESCs is functionally complete and stable, which significantly enhances the merits of haploid stem cells as a new tool to quickly generate genetic models (Li et al., 2012; Yang et al., 2012; Bai et al., 2016).
Diversified haploid stem cells: from mouse to human
Subsequent trials in gamete manipulation have further yielded haESCs from other mammalian species including the rat and monkey (Fig. 1B) (Yang et al., 2013; Li et al., 2014). These cells with different origins possess a haploid karyotype, and share typical pluripotent stem cell characteristics, such as self-renewal capacity and a pluripotency-specific molecular signature. They are also approved amenable for genetic screening (Yang et al., 2013; Li et al., 2014; Li and Shuai, 2017). Notably, by fusing haESCs of two species, our lab reported the generation of mouse-rat allodiploid ESCs, which possess the pluripotency to differentiate into all three germ layers, and can serve as a powerful tool for identification of X inactivation-escaping genes as well as regulatory mechanisms between species (Li et al., 2016a).
Derivation of human haESCs had been hindered by the limited availability of human oocytes and spontaneous diploidization (Egli et al., 2011; Sagi and Benvenisty, 2017). As artificial activation of unfertilized MII human oocytes resulted in efficient development to the blastocyst stage and subsequent derivation of parthenogenetic ESCs (Kim et al., 2007; Revazova et al., 2007), characterization of these cell lines suggested that they were completely diploid (Paull et al., 2013; Sagi and Benvenisty, 2017). However, it was speculated that rare haploid cells might persist among the majority of diploid cells. The work of Sagi et al. led to the conclusion that human phESCs can be derived within successive rounds of haploid cell enrichment and expansion assisted by fluorescence activated cell sorting (FACS) (Sagi et al., 2016). Like other mammalian haESC lines, after being established, a sorting for the haploid population at every three to four passages is required to maintain the haploid stem cells (Leeb and Wutz, 2011; Li et al., 2012, 2014; Sagi et al., 2016). Notably, the EB generation assay and direct differentiation assays demonstrated that human haESCs can differentiate into various mature somatic cells while retaining a haploid genome. Haploid human neurons, cardiomyocytes and pancreatic cells were generated. In these haploid somatic cells, an X:autosomes dosage imbalance of 1:1 persisted into the differentiated state as haploid cells do not inactivate their single-copy X chromosome like in diploid female cells (Sagi et al., 2016).
However, it seemed more difficult to directly generate haploid somatic cells in other species as diploidization occurs very rapidly after differentiation of mouse, rat and monkey haESCs, which cannot be blocked by FACS-assisted purification (Elling et al., 2011; Leeb et al., 2012; Yang et al., 2013; Li et al., 2014; Sagi and Benvenisty, 2017). Based on a better understanding of the molecular mechanisms underlying diploidization, our lab recently showed that through ROCK inhibition, haploid somatic cellular fates of all three germ layers could be generated when haESCs were grown in defined mediums with different external growth-factor environments (He et al., 2017).
Besides the derivation of haESCs, extensive efforts have been made to devise robust protocols to generate other types of haploid pluripotent cells. Mouse epiblast stem cells (EpiSCs) are primed pluripotent stem cells, which could be derived from post-implantation embryos or via in vitro differentiation of ESCs (Brons et al., 2007; Tesar et al., 2007; Guo et al., 2009). As monkey and human haESCs were generated and shown to maintain haploidy in a putatively primed state (Yang et al., 2013; Sagi et al., 2016), it was also proved possible to generate both androgenetic and parthenogenetic mouse haploid EpiSCs (haEpiSCs) via in vitro differentiation from haESCs, which depends on the Activin/bFGF pathway to maintain self-renewal (Shuai et al., 2015). Subsequent work showed that ahESCs can develop into haploid neural stem cells (haNSCs) given appropriate signals, providing evidence to further prove that haESCs have the potential to undergo patterning events in vitro (Xu et al., 2017).
Trophoblast stem cells (TSCs) can be viewed as the extraembryonic developmental counterpart of ESCs. They originate from the outer trophectoderm layer, and are committed toward the trophoblast lineage from which ESCs are excluded (Tanaka et al., 1998; Tam and Rossant, 2003; Latos and Hemberger, 2016). As the culture condition of TSCs differs significantly from that of ESCs, it has remained elusive as whether haploid cell lines with a trophoblast lineage could be derived since the establishment of haESCs. A hint that this could be possible came from the early study showing that Gata6-induced extraembryonic cell fate might be compatible with a haploid genome (Leeb et al., 2012). Recently, we have reported the de novo generation of haploid trophoblast stem cells (haTSCs), which exhibit typical expression features of TSCs, possess the multipotency to differentiate into specialized trophoblast cell types and can chimerize developing placentas. Moreover, we showed that haTSCs can facilitate efficient genome-wide screening (Cui et al., 2019). Shuai lab also reported that overexpression of Cdx2 together with deletion of p53 can convert haESCs to haploid-induced TSCs (haiTSCs). By applying haiTSCs for high-throughput genetic screening, they found that Htra1 is a blocker for spongiotrophoblast specification (Peng et al., 2019). The derivation of haTSCs and haiTSCs represents another interesting avenue that might help to explore fundamental biological roadmaps in the extraembryonic trophoblast lineage at a genomic scale.
Being haploid: a fight against diploidization
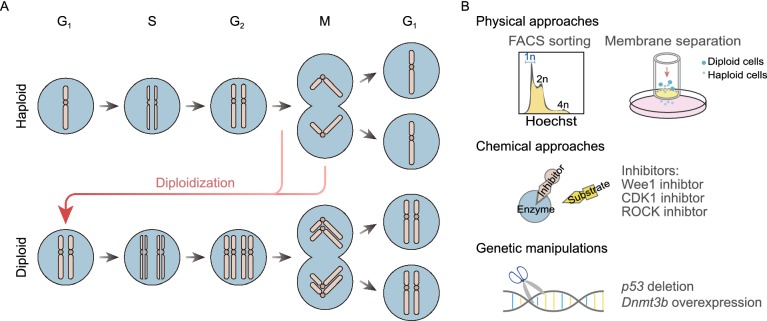
Due to a strong tendency of spontaneous diploidization, it is difficult to maintain the haploid status over time without a frequent cytometric sorting of the G1 phase haploid cells at short intervals (Sagi and Benvenisty, 2017). Gaining more knowledge of principles governing the diploidization process will benefit advancing our experimental approaches to maintain haploidy in culture conditions. Early experiments with mixed cultures of haploid cells expressing different fluorescent proteins indicated that haploid cells do not become diploid via cell fusion, but via failed cytokinesis and/or endoreplication of the genome (Leeb et al., 2012).
Then it was proposed by Takahashi et al. that diploid conversion in haESCs might occur due to abnormal cell cycle regulation, i.e., by G2 arrest and abrupt insertion of an extra G1/S phase. They therefore tried to regulate the haploid cell cycle by adding inhibitors of Wee1 kinase. Experiments conducted in phESCs showed that acceleration of G2/M transition by means of Wee1 kinase inhibitors could prevent the spontaneous diploidization of haESCs and effectively maintain the haploid status for more than 4 weeks without FACS sorting conducted (Takahashi et al., 2014).
A recent study published by our lab showed that mitotic slippage, during which cells directly enter G1/S phase of the next cell cycle without division (Brito and Rieder, 2006), is a major cause of diploidization (Fig. 2A). After screening of a set of inhibitors related to cell cycle regulation, CDK1 and ROCK inhibitors were demonstrated to efficiently suppress diploidization during the culture and differentiation of haESCs (Fig. 2B) (He et al., 2017). Further experiments showed that supplementation with the ROCK inhibitor Y-27632 could facilitate the generation of haploid somatic cells and haTSCs (He et al., 2017; Cui et al., 2019).
Figure 2.
Diploidization of haploid cells. (A) Schematic showing that abnormal cell cycle regulation is the cause for diploidization in haploid cells. (B) Solutions for diploidization include physical approaches using fluorescence-activated cell sorting (FACS) and membranes with micrometer pores, chemical approaches via addition of kinase inhibitors and genetic manipulations in haploid cells
There are also strategies focusing on manipulating the expression of particular genes to reduce diploidization. Olbrich et al. showed that p53 deletion facilitates the maintenance of both human HAP1 cells (Carette et al., 2011b) and mouse haESCs by enabling the survival of genomically unstable cells. They also found that once diploidization occurs, diploid cells will rapidly overtake the culture owing to a better growth property (Olbrich et al., 2017). In another study, overexpression of Dnmt3b, the gene encodes the de novo DNA methyltransferase, was reported to mitigate the self-diploidization in ahESCs as certain G2/M-related genes were downregulated (Fig. 2B) (He et al., 2018).
Notably, based on the findings that haESCs are phenotypically smaller than diploid cells (Sagi et al., 2016), a new method for rapid purification of haploid ESCs from mixed cell populations with high viability was reported, which uses membranes with micrometer pores for force-free separation and allows haploid but not diploid cells to pass through (Fig. 2B). This method does not require the traditional periodic cell sorting and simplifies the culture procedures (Freimann and Wutz, 2017). Yet how efficiently can this approach facilitate the derivation of haploid cell lines remains to be explored.
The haploid cell toolkit for functional genomics
A key goal in genetic analysis is to identify which genes contribute to specific biological phenotypes and diseases. As geneticists have long appreciated, a most effective way to probe genes influencing a phenotype of interest is via genetic screens. The hypothesis-driven, reverse genetic screens can test the effects of pre-defined gene mutations in cells, while forward genetic screens are hypothesis-free approaches that involve metagenesis, selection for the cells with a phenotype of interest, and then characterization of the causative mutation (Grimm, 2004; Shalem et al., 2015).
The genome-wide loss-of-function screen of recessive mutations is challenging in mammalian cells due to the diploid nature of their genomes, because it is time-consuming and rather difficult to generate genome-wide homozygous mutant libraries by standard genetic techniques. In this regard, haploid cells have remarkable advantages in forward genetic screens over diploid cells, as mutations in them are hemizygous and cellular phenotypes can be efficiently revealed (Elling and Penninger, 2014). Yeast cells can grow as haploids and have been used in a wide array of genome-wide phenotypic assays aimed toward an increased understanding of biological functions, response to stress and mechanisms of drug actions over the past decades (Forsburg, 2001; Giaever and Nislow, 2014). The establishment of mammalian haESCs proliferating with an intact haploid chromosome set has opened new and exciting avenues for high-throughput functional interrogation of the genome (Elling et al., 2011; Leeb and Wutz, 2011). Since then, the field of haploid screens has witnessed rapid development.
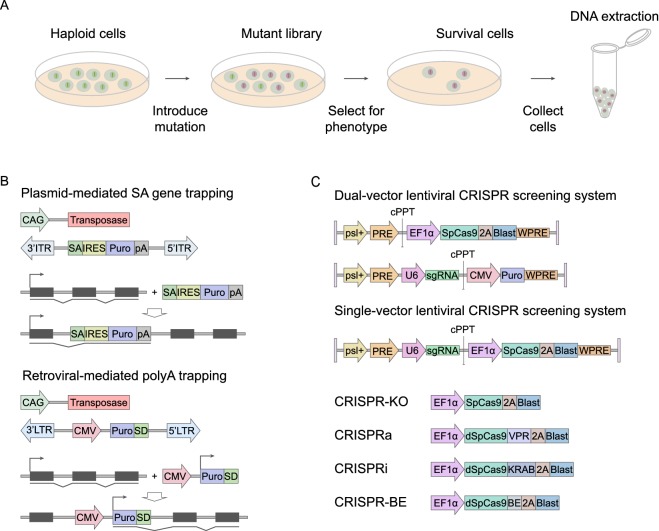
Like all genetic screens, haploid screens start with a mutagenesis step and are followed by the detection of a phenotype. Then the underlying genetic alteration is sought and correlated with a molecular function. In brief, there are three major steps: mutagenesis, selection and mapping of mutations (Fig. 3A). Of note, the validation of the hit target genes identified in secondary screens is also crucial (Elling and Penninger, 2014). Screening applications can be carried out in a wide range of formats using different molecular reagents and delivery vehicles (Fig. 3B and 3C). Usually, mutations in haploid cells are generated by gene trapping and nuclease-mediated gene knockout, including piggyBac and clustered regularly interspaced short palindromic repeats/CRISPR-associated nuclease (CRISPR/Cas) systems (Elling et al., 2011; Leeb and Wutz, 2011; Leeb et al., 2014; Monfort et al., 2015; He et al., 2017; Liu et al., 2017; Wang et al., 2018; Cui et al., 2019; Peng et al., 2019). Meanwhile, it was also reported to generate haESC libraries by chemical mutagens (Forment et al., 2016). Though chemical mutagenesis is easier to induce and results in a wider range of mutant alleles, the causal mutations in the selected clones are initially unknown, making identification of the causal mutations challenging. Different from that, insertional mutagenesis uses defined insertional sequences. Meanwhile, CRISPR sgRNAs can be synthesized to target specific sequences. Therefore, these two strategies have major advantages in the following mapping process as they are amenable to sequencing-based analysis (Elling and Penninger, 2014; Shalem et al., 2015).
Figure 3.
Applying haploid stem cells for functional genomics. (A) Forward genetic screens are powerful tools for the discovery and functional annotation of genetic elements. Three major steps are: mutagenesis to generate high-throughput mutant libraries, selection for the phenotype of interest, and mapping of mutations. (B) Two types of delivery systems used in gene trapping, the plasmid system and the retroviral system. (Top) Schematic diagram of splice acceptor (SA) gene trap, in which transposon elements were integrated in a plasmid vector. Splicing to upstream exons results in gene trap fusion transcript from which puromycin (puro) is transcribed by an endogenous promoter. CAG, a constitutive promoter; IRES, internal ribosome entry site; pA, poly A; ITR, inverted terminal repeat. (Bottom) Schematic diagram of ployA-trap, in which transposon elements were integrated in a retroviral vector. Insertion of a constitutive promoter-driven marker gene into introns results in a gene trap fusion transcript from which puromycin (puro) is terminated by an endogenous polyadenylation site. CMV, a constitutive promoter; SD, splice donor. LTR, long terminal repeat. (C) Schematic diagram of lentiviral expression vector for SpCas9 and sgRNA in a dual-vector form and single-vector form. psl+, Psi packaging singal; PRE, Rev response element; cPPT, central polypurine tract; EF1α, elongation factor 1α promoter; CMV, immediate-early cytomegalovirus enhancer-promoter; U6, RNA polymerase III U6 promoter; 2A, 2A self-cleavage peptide; Blast, blasticidin selection marker; Puro, puromycin selection marker; WPRE, post-transcriptional regulatory element
Due to the vast number of cells and mutations, successful screens largely depend on the strong selection pressure as most haploid screens were based on lethality of cells with toxic agents or viruses and subsequent positive selection by outgrowth of resistant clones (Elling and Penninger, 2014). Cellular reporter systems were also reported (Leeb et al., 2014). To date, reported haploid cell lines have been applied for screens of cellular mechanisms including pathogen mechanisms, cellular pathways, gene essentiality, and targets of drug mechanisms (Table 1) (Wutz, 2014). Notably, the recent creation of Haplobank, a biobank of over 100,000 individual haESC lines targeting 16,970 genes with genetically barcoded, conditional and reversible mutations by genome-saturate mutagenesis, is a major breakthrough, which can be used in reverse and forward genetic screens for high-throughput genetic analysis (Elling et al., 2017).
Table 1.
Genetic screens in haploid cell systems
| Aim of the screen | Cell type | Strain | Genes identified | Reference |
|---|---|---|---|---|
| Mismatch repair pathway | haESCs | Mouse | Msh2 | Leeb and Wutz, 2011 |
| Ricin toxicity | haESCs | Mouse | Gpr107 | Elling et al., 2011 |
| Olaparib resistance | haESCs | Mouse | Parp1 | Pettitt et al., 2013 |
| Promotion of exit from ground state | haESCs | Mouse | Zfp706, Pum1 | Leeb et al., 2014 |
| X inactivation | haESCs | Mouse | Spen | Monfort et al., 2015 |
| Mn2+ induced toxicity | haNSCLCs* | Mouse | Park2 | He et al., 2017 |
| A803467 toxicity | haNPCs# | Rhesus monkey | B4GALT6 | Wang et al., 2018 |
| Block against spongiotrophoblast specification | haiTSCs | Mouse | Htra1 | Peng et al., 2019 |
*haploid neural progenitor cells; #haploid neural stem-cell-like cells
Prior to the recent derivation of human haESCs (Sagi et al., 2016), a near-haploid leukemia cell line KBM-7 had been established from human tumors, which contains one copy of most chromosomes with the exception of Chromosome 8 and a portion of Chromosome 15 being disomic (Kotecki et al., 1999). And the attempt to reprogram KMB-7 into induce pluripotent stem cells (iPSCs) additionally yielded the haploid fibroblast-like cell line HAP1 (Carette et al., 2011b). These cells have been applied to genetic screenings for host genes required for action of toxins or viruses (Kotecki et al., 1999; Carette et al., 2009, 2011a, b; Baggen et al., 2016; Staring et al., 2017), and regulatory genes in biological pathways (Lebensohn et al., 2016). Now with haESCs introducing loss-of-function genetic screenings in human pluripotent cells, they provide new opportunities for functional genomics that would further advance our knowledge of human biology in health and diseases (Yilmaz et al., 2016).
Replacing the gametes by haescs
Upon the derivation of haESCs, it was unclear whether haESCs could be used as a substitute for one of the parental gametes. The important conclusion drawn from pioneering studies in mice and rats is that ahESCs have the ability to “fertilize” oocytes by intracytoplasmic injection and produce fertile adult mice with their genetic material being transmitted to the offspring (Fig. 4) (Li et al., 2012, 2014; Yang et al., 2012). Meanwhile, it was proved that phESCs could support embryo development via substituting the maternal genome (Fig. 4) (Wan et al., 2013). Compared with the mature gametes, haESCs are easily engineered and can proliferate indefinitely in vitro. Thus, besides opening a completely new avenue for generating genetically modified animals, they also provide a convenient platform to study effects of genetic and epigenetic issues on animal development, such as genomic imprinting.
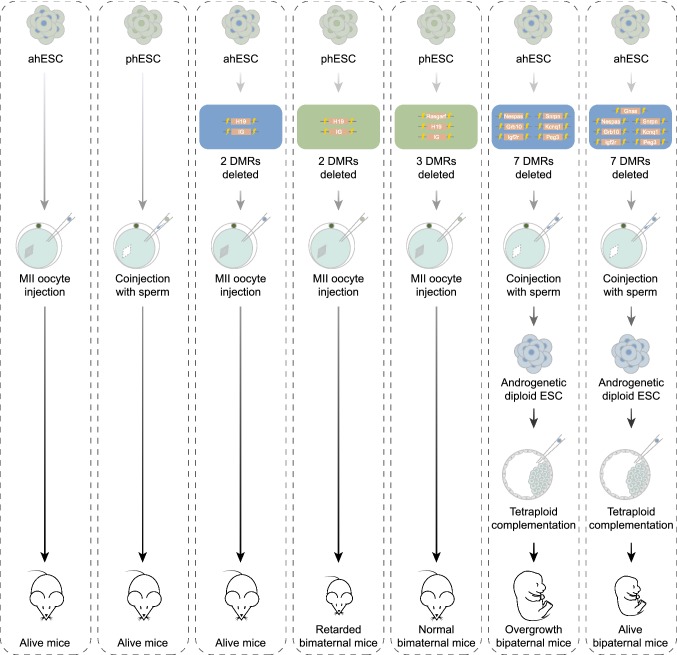
Figure 4.
Haploid embryonic stem cells (haESCs) can be used to replace gametes for the generation of alive mice. Deletions of the specific imprinting regions in haESCs can facilitate to generate normally growing bimaternal mice and live bipaternal mice. DMRs, differentially methylated regions. ahESC, androgenetic haESC. phESC, parthenogenetic haESC
Genomic imprinting is an epigenetic phenomenon that causes genes to be expressed in a parent-of-origin-specific manner. During fertilization, the zygote forms when a sperm carrying paternal imprints enters the oocyte with maternal imprints (Ferguson-Smith, 2011). Previous results showed that neither parthenogenetic nor androgenetic embryos could develop to term (Surani and Barton, 1983; McGrath and Solter, 1984; Surani et al., 1984, 1990). Examination of differentially methylated regions (DMRs) of imprinting genes showed that ahESCs might partially lose sperm-like methylation status during passaging (i.e., H19 and Gtl2). Therefore, though live mice can be obtained by this approach, the developmental efficiency of ahESC-derived embryos is much lower than that of normal fertilized embryos (Li et al., 2012). The challenge was to improve the sperm-like features of ahESCs by optimizing their epigenetic makeup without compromising the genetic integrity and proliferative capacity. Subsequently, it was reported that with H19- and IG-DMRs being knocked out, ahESCs can stably retain the developmental potential and exhibit comparable “fertilizing” capacity as round spermatids (Fig. 4) (Zhong et al., 2015).
Methods to modify imprinting have been further developed. Previous work of our lab showed that H19- and IG-DMR deletions in phESCs enable the generation of vaible bimaternal mice with growth retardation after MII oocyte injection (Li et al., 2016b). In the following studies, we found that phESCs underwent global demethylation during in vitro culture: the demethylated DMRs of highly passaged phESCs mimicked the hypomethylated DMRs of PGCs (Li et al., 2018). After comparing the growth retarded bimaternal mice with the wild type control, we found a loss-of-imprint DMR in the somatic cells of bimaternal mice, Rasgrf1-DMR, which was also demethylated in the phESCs (Li et al., 2018). After deletions of H19-, IG- and Rasgrf1-DMRs in phESCs, we further derived the bimaternal mice with recovered growth curves. On the other hand, the PGC-like genome hypomethylation state was also found in highly passaged ahESCs. After deleting 7 DMRs (Nespas-, Grb10-, Igf2r-, Snrpn-, Kcnq1-, Peg3- and Gnas-DMRs) with CRISPR-Cas9 in ahESCs, they were co-injected into enucleated oocytes with sperm, and the bipaternal diploid blastocysts and androgenetic diploid ESCs (adESCs) were derived from the reconstructed embryos. Impressively, the diploid ESCs were able to produce full-term bipaternal mice after injecting into tetraploid blastocysts (Fig. 4). These results further proved that bipaternal reproduction barriers can also be crossed using haploid cells with specific imprinting regions being deleted (Li et al., 2018).
Future perspectives
The mammalian haploid stem cells introduce new possibilities in a wide range of biological research fields and may offer unprecedented resolutions for genome exploration and reproductive approaches. The ahESCs can functionally take the place of sperms to produce live offspring after injection into the oocytes, which allows for the direct transmission of genomic modifications into the organism level without further time-consuming steps like conventional germline transmission. This is especially valuable for large animals and non-human primates. Therefore, the generation of monkey ahESCs and subsequent analysis of their capacity to take the place of sperms are worth trying for monkey genome engineering. Moreover, although we have gained some knowledge of principles governing diploidization, we are still woefully lacking in the molecular details and parameters that govern this phenomenon in haploid cells. Improving our understanding of this process is thus essential to the development of more stable culture conditions.
Acknowlegments
This study was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA16030400), the National Basic Research Program of China (Grant No. 2014CB964800), the National Natural Science Foundation of China (Grant Nos. 31621004, 31422038), the National Key Research and Development Program (Grant No. 2017YFA0103803), CAS Key Projects (Grant No. QYZDY-SSW-SMC022, QYZDB-SSW- SMC002).
Compliance with ethics guidelines
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Abbreviations
- ahESC
androgenetic haESC
- AG
androgenetic
- CRISPR/Cas
clustered regularly interspaced short palindromic repeats/CRISPR-associated nuclease
- DMR
differentially methylated region
- EB
embryoid body
- EpiSC
epiblast stem cell
- ESC
embryonic stem cell
- FACS
fluorescence activated cell sorting
- haEpiSC
haploid EpiSC
- haESC
haploid embryonic stem cell
- haiTSC
haploid-induced trophoblast stem cell
- haNSC
haploid neural stem cell
- haTSC
haploid trophoblast stem cell
- iPSC
induce pluripotent stem cell
- MII
metaphase II
- PG
parthenogenetic
- phESC
parthenogenetic haESC
References
- Baggen J, Thibaut HJ, Staring J, Jae LT, Liu Y, Guo H, Slager JJ, de Bruin JW, van Vliet AL, Blomen VA, et al. Enterovirus D68 receptor requirements unveiled by haploid genetics. Proc Natl Acad Sci USA. 2016;113:1399–1404. doi: 10.1073/pnas.1524498113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Wu Y, Li J. Generation and application of mammalian haploid embryonic stem cells. J Intern Med. 2016;280:236–245. doi: 10.1111/joim.12503. [DOI] [PubMed] [Google Scholar]
- Beukeboom LW, Kamping A, Louter M, Pijnacker LP, Katju V, Ferree PM, Werren JH. Haploid females in the parasitic wasp Nasonia vitripennis. Science. 2007;315:206. doi: 10.1126/science.1133388. [DOI] [PubMed] [Google Scholar]
- Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, de Sousa Chuva, Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Bryja V, Bonilla S, Arenas E. Derivation of mouse embryonic stem cells. Nat Protoc. 2006;1:2082–2087. doi: 10.1038/nprot.2006.355. [DOI] [PubMed] [Google Scholar]
- Carette JE, Guimaraes CP, Varadarajan M, Park AS, Wuethrich I, Godarova A, Kotecki M, Cochran BH, Spooner E, Ploegh HL, et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science. 2009;326:1231–1235. doi: 10.1126/science.1178955. [DOI] [PubMed] [Google Scholar]
- Carette JE, Guimaraes CP, Wuethrich I, Blomen VA, Varadarajan M, Sun C, Bell G, Yuan B, Muellner MK, Nijman SM, et al. Global gene disruption in human cells to assign genes to phenotypes by deep sequencing. Nat Biotechnol. 2011;29:542–546. doi: 10.1038/nbt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui T, Jiang L, Li T, Teng F, Feng G, Wang X, He Z, Guo L, Xu K, Mao Y, et al. Derivation of mouse haploid trophoblast stem cells. Cell Rep. 2019;26(407–414):e405. doi: 10.1016/j.celrep.2018.12.067. [DOI] [PubMed] [Google Scholar]
- Debec A. Evolution of karyotype in haploid cell lines of Drosophila melanogaster. Exp Cell Res. 1984;151:236–246. doi: 10.1016/0014-4827(84)90371-9. [DOI] [PubMed] [Google Scholar]
- Edwards RG. The experimental induction of gynogenesis in the mouse. I. Irradiation of the sperm by x-rays. Proc R Soc Lond Ser B Biol Sci. 1957;146:469–487. doi: 10.1098/rspb.1957.0025. [DOI] [PubMed] [Google Scholar]
- Edwards RG. The experimental induction of gynogenesis in the mouse. II. Ultra-violet irradiation of the sperm. Proc R Soc Lond Ser B Biol Sci. 1957;146:488–504. doi: 10.1098/rspb.1957.0026. [DOI] [PubMed] [Google Scholar]
- Edwards RG. The experimental induction of gynogenesis in the mouse. III. Treatment of sperm with trypaflavine, toluidine blue, or nitrogen mustard. Proc R Soc Lond Ser B Biol Sci. 1958;149:117–129. doi: 10.1098/rspb.1958.0056. [DOI] [PubMed] [Google Scholar]
- Egli D, Chen Alice E, Saphier G, Powers D, Alper M, Katz K, Berger B, Goland R, Leibel Rudolph L, Melton Douglas A, et al. Impracticality of egg donor recruitment in the absence of compensation. Cell Stem Cell. 2011;9:293–294. doi: 10.1016/j.stem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Elling U, Penninger JM. Genome wide functional genetics in haploid cells. FEBS Lett. 2014;588:2415–2421. doi: 10.1016/j.febslet.2014.06.032. [DOI] [PubMed] [Google Scholar]
- Elling U, Taubenschmid J, Wirnsberger G, O’Malley R, Demers SP, Vanhaelen Q, Shukalyuk AI, Schmauss G, Schramek D, Schnuetgen F, et al. Forward and reverse genetics through derivation of haploid mouse embryonic stem cells. Cell Stem Cell. 2011;9:563–574. doi: 10.1016/j.stem.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elling U, Wimmer RA, Leibbrandt A, Burkard T, Michlits G, Leopoldi A, Micheler T, Abdeen D, Zhuk S, Aspalter IM, et al. A reversible haploid mouse embryonic stem cell biobank resource for functional genomics. Nature. 2017;550:114–118. doi: 10.1038/nature24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith AC. Genomic imprinting: the emergence of an epigenetic paradigm. Nat Rev Genet. 2011;12:565–575. doi: 10.1038/nrg3032. [DOI] [PubMed] [Google Scholar]
- Forment JV, Herzog M, Coates J, Konopka T, Gapp BV, Nijman SM, Adams DJ, Keane TM, Jackson SP. Genome-wide genetic screening with chemically mutagenized haploid embryonic stem cells. Nat Chem Biol. 2016;13:12–14. doi: 10.1038/nchembio.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL. The art and design of genetic screens: yeast. Nat Rev Genet. 2001;2:659–668. doi: 10.1038/35088500. [DOI] [PubMed] [Google Scholar]
- Freimann R, Wutz A. A fast and efficient size separation method for haploid embryonic stem cells. Biomicrofluidics. 2017;11:054117. doi: 10.1063/1.5006326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Nislow C. The yeast deletion collection: a decade of functional genomics. Genetics. 2014;197:451–465. doi: 10.1534/genetics.114.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S. The art and design of genetic screens: mammalian culture cells. Nat Rev Genet. 2004;5:179–189. doi: 10.1038/nrg1291. [DOI] [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Zhang X, Zhang Y, Zheng W, Xiong Z, Hu X, Wang M, Zhang L, Zhao K, Qiao Z, et al. Reduced self-diploidization and improved survival of semi-cloned mice produced from androgenetic haploid embryonic stem cells through overexpression of Dnmt3b. Stem Cell Rep. 2018;10:477–493. doi: 10.1016/j.stemcr.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZQ, Xia BL, Wang YK, Li J, Feng GH, Zhang LL, Li YH, Wan HF, Li TD, Xu K, et al. Generation of mouse haploid somatic cells by small molecules for genome-wide genetic screening. Cell Rep. 2017;20:2227–2237. doi: 10.1016/j.celrep.2017.07.081. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. An analysis of the mechanism of fertilization by means of enucleation of sea urchin eggs. Exp Cell Res. 1962;28:323–334. doi: 10.1016/0014-4827(62)90286-0. [DOI] [PubMed] [Google Scholar]
- Horii T, Hatada I. Genome editing using mammalian haploid cells. Int J Mol Sci. 2015;16:23604–23614. doi: 10.3390/ijms161023604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH. Chromosome analysis of early postimplantation presumptive haploid parthenogenetic mouse embryos. J Embryol Exp Morphol. 1978;45:85–91. [PubMed] [Google Scholar]
- Kaufman MH, Robertson EJ, Handyside AH, Evans MJ. Establishment of pluripotential cell lines from haploid mouse embryos. J Embryol Exp Morphol. 1983;73:249–261. [PubMed] [Google Scholar]
- Kim K, Ng K, Rugg-Gunn PJ, Shieh JH, Kirak O, Jaenisch R, Wakayama T, Moore MA, Pedersen RA, Daley GQ. Recombination signatures distinguish embryonic stem cells derived by parthenogenesis and somatic cell nuclear transfer. Cell Stem Cell. 2007;1:346–352. doi: 10.1016/j.stem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Kotecki M, Reddy PS, Cochran BH. Isolation and characterization of a near-haploid human cell line. Exp Cell Res. 1999;252:273–280. doi: 10.1006/excr.1999.4656. [DOI] [PubMed] [Google Scholar]
- Latos PA, Hemberger M. From the stem of the placental tree: trophoblast stem cells and their progeny. Development. 2016;143:3650–3660. doi: 10.1242/dev.133462. [DOI] [PubMed] [Google Scholar]
- Lebensohn AM, Dubey R, Neitzel LR, Tacchelly-Benites O, Yang E, Marceau CD, Davis EM, Patel BB, Bahrami-Nejad Z, Travaglini KJ, et al. Comparative genetic screens in human cells reveal new regulatory mechanisms in WNT signaling. Elife. 2016 doi: 10.7554/eLife.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb M, Dietmann S, Paramor M, Niwa H, Smith A. Genetic exploration of the exit from self-renewal using haploid embryonic stem cells. Cell Stem Cell. 2014;14:385–393. doi: 10.1016/j.stem.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb M, Walker R, Mansfield B, Nichols J, Smith A, Wutz A. Germline potential of parthenogenetic haploid mouse embryonic stem cells. Development. 2012;139:3301–3305. doi: 10.1242/dev.083675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb M, Wutz A. Derivation of haploid embryonic stem cells from mouse embryos. Nature. 2011;479:131–134. doi: 10.1038/nature10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li X, Li T, Jiang MG, Wan H, Luo GZ, Feng C, Cui X, Teng F, Yuan Y, et al. Genetic modification and screening in rat using haploid embryonic stem cells. Cell Stem Cell. 2014;14:404–414. doi: 10.1016/j.stem.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Li W, Shuai L, Wan H, Dong M, Wang M, Sang L, Feng C, Luo GZ, Li T, Li X, et al. Androgenetic haploid embryonic stem cells produce live transgenic mice. Nature. 2012;490:407–411. doi: 10.1038/nature11435. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou Q. New stem cell types for versatile applications. Natl Sci Rev. 2017;4:528–530. [Google Scholar]
- Li X, Cui XL, Wang JQ, Wang YK, Li YF, Wang LY, Wan HF, Li TD, Feng GH, Shuai L, et al. Generation and application of mouse-rat allodiploid embryonic stem cells. Cell. 2016;164:279–292. doi: 10.1016/j.cell.2015.11.035. [DOI] [PubMed] [Google Scholar]
- Li Y, Shuai L. A versatile genetic tool: haploid cells. Stem Cell Res Ther. 2017;8:197. doi: 10.1186/s13287-017-0657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wan H, Feng G, Wang L, He Z, Wang Y, Wang XJ, Li W, Zhou Q, Hu B. Birth of fertile bimaternal offspring following intracytoplasmic injection of parthenogenetic haploid embryonic stem cells. Cell Res. 2016;26:135–138. doi: 10.1038/cr.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZK, Wang LY, Wang LB, Feng GH, Yuan XW, Liu C, Xu K, Li YH, Wan HF, Zhang Y, et al. Generation of bimaternal and bipaternal mice from hypomethylated haploid ESCs with imprinting region deletions. Cell Stem Cell. 2018;23(665–676):e664. doi: 10.1016/j.stem.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Liu G, Wang X, Liu Y, Zhang M, Cai T, Shen Z, Jia Y, Huang Y. Arrayed mutant haploid embryonic stem cell libraries facilitate phenotype-driven genetic screens. Nucleic Acids Res. 2017;45:e180. doi: 10.1093/nar/gkx857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37:179–183. doi: 10.1016/0092-8674(84)90313-1. [DOI] [PubMed] [Google Scholar]
- Modliński JA. Haploid mouse embryos obtained by microsurgical removal of one pronucleus. J Embryol Exp Morphol. 1975;33:897–905. [PubMed] [Google Scholar]
- Monfort A, Di Minin G, Postlmayr A, Freimann R, Arieti F, Thore S, Wutz A. Identification of spen as a crucial factor for xist function through forward genetic screening in haploid embryonic stem cells. Cell Rep. 2015;12:554–561. doi: 10.1016/j.celrep.2015.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich T, Mayor-Ruiz C, Vega-Sendino M, Gomez C, Ortega S, Ruiz S, Fernandez-Capetillo O. A p53-dependent response limits the viability of mammalian haploid cells. Proc Natl Acad Sci. 2017;114:9367–9372. doi: 10.1073/pnas.1705133114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamilo P, Crozier RH. Genic variation in male haploids under deterministic selection. Genetics. 1981;98:199–214. doi: 10.1093/genetics/98.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin C, Adams J. Frequency of fixation of adaptive mutations is higher in evolving diploid than haploid yeast populations. Nature. 1983;302:495–500. doi: 10.1038/302495a0. [DOI] [PubMed] [Google Scholar]
- Paull D, Emmanuele V, Weiss KA, Treff N, Stewart L, Hua H, Zimmer M, Kahler DJ, Goland RS, Noggle SA, et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature. 2013;493:632–637. doi: 10.1038/nature11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Li X, Wu C, Wang Y, Yu J, Zhang J, Gao Q, Zhang W, Zhang Q, Fan Y, et al. Derivation of haploid trophoblast stem cells via conversion in vitro. iScience. 2019;11:508–518. doi: 10.1016/j.isci.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot V, Richerd S, Valero M. Transition from haploidy to diploidy. Nature. 1991;351:315–317. doi: 10.1038/351315a0. [DOI] [PubMed] [Google Scholar]
- Potapova TA, Zhu J, Li R. Aneuploidy and chromosomal instability: a vicious cycle driving cellular evolution and cancer genome chaos. Cancer Metastasis Rev. 2013;32:377–389. doi: 10.1007/s10555-013-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, Pryzhkova MV. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432–449. doi: 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- Sagi I, Benvenisty N. Haploidy in humans: an evolutionary and developmental perspective. Dev Cell. 2017;41:581–589. doi: 10.1016/j.devcel.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Sagi I, Chia G, Golan-Lev T, Peretz M, Weissbein U, Sui L, Sauer MV, Yanuka O, Egli D, Benvenisty N. Derivation and differentiation of haploid human embryonic stem cells. Nature. 2016;532:107–111. doi: 10.1038/nature17408. [DOI] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR–Cas9. Nat Rev Genet. 2015;16:299–311. doi: 10.1038/nrg3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai L, Wang Y, Dong M, Wang X, Sang L, Wang M, Wan H, Luo G, Gu T, Yuan Y, et al. Durable pluripotency and haploidy in epiblast stem cells derived from haploid embryonic stem cellsin vitro. J Mol Cell Biol. 2015;7:326–337. doi: 10.1093/jmcb/mjv044. [DOI] [PubMed] [Google Scholar]
- Silk AD, Zasadil LM, Holland AJ, Vitre B, Cleveland DW, Weaver BA. Chromosome missegregation rate predicts whether aneuploidy will promote or suppress tumors. Proc Natl Acad Sci USA. 2013;110:E4134–4141. doi: 10.1073/pnas.1317042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staring J, von Castelmur E, Blomen VA, van den Hengel LG, Brockmann M, Baggen J, Thibaut HJ, Nieuwenhuis J, Janssen H, van Kuppeveld FJ, et al. PLA2G16 represents a switch between entry and clearance of picornaviridae. Nature. 2017;541:412–416. doi: 10.1038/nature21032. [DOI] [PubMed] [Google Scholar]
- Surani MA, Barton SC. Development of gynogenetic eggs in the mouse: implications for parthenogenetic embryos. Science. 1983;222:1034–1036. doi: 10.1126/science.6648518. [DOI] [PubMed] [Google Scholar]
- Surani MA, Barton SC, Norris ML. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984;308:548–550. doi: 10.1038/308548a0. [DOI] [PubMed] [Google Scholar]
- Surani MA, Kothary R, Singh PB, Fundele R, Ferguson-Smith AC, Barton SC. Genome imprinting and development in the mouse. Dev Suppl. 1990;1990:89–98. [PubMed] [Google Scholar]
- Takahashi S, Lee J, Kohda T, Matsuzawa A, Kawasumi M, Kanai-Azuma M, Kaneko-Ishino T, Ishino F. Induction of the G2/M transition stabilizes haploid embryonic stem cells. Development. 2014;141:3842–3847. doi: 10.1242/dev.110726. [DOI] [PubMed] [Google Scholar]
- Tam PP, Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155–6163. doi: 10.1242/dev.00893. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK, Rossant J. Haploid mouse blastocysts developed from bisected zygotes. Nature. 1976;259:663–665. doi: 10.1038/259663a0. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK, Witkowska A, Nowicka J. Experimental partheonogenesis in the mouse. Nature. 1970;226:162–165. doi: 10.1038/226162a0. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, He Z, Dong M, Gu T, Luo GZ, Teng F, Xia B, Li W, Feng C, Li X, et al. Parthenogenetic haploid embryonic stem cells produce fertile mice. Cell Res. 2013;23:1330–1333. doi: 10.1038/cr.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang W, Yu J, Wu C, Gao Q, Li X, Li Y, Zhang J, Tian Y, Tan T, et al. Genetic screening and multipotency in rhesus monkey haploid neural progenitor cells. Development. 2018 doi: 10.1242/dev.160531. [DOI] [PubMed] [Google Scholar]
- Wutz A. Haploid mouse embryonic stem cells: rapid genetic screening and germline transmission. Annu Rev Cell Dev Biol. 2014;30:705–722. doi: 10.1146/annurev-cellbio-100913-012920. [DOI] [PubMed] [Google Scholar]
- Xu H, Yue C, Zhang T, Li Y, Guo A, Liao J, Pei G, Li J, Jing N. Derivation of haploid neurons from mouse androgenetic haploid embryonic stem cells. Neurosci Bull. 2017;33:361–364. doi: 10.1007/s12264-017-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Papadopoulos N, Marra G, Perrera C, Jiricny J, Boland CR, Lynch HT, Chadwick RB, de la Chapelle A, Berg K, et al. Conversion of diploidy to haploidy. Nature. 2000;403:723–724. doi: 10.1038/35001659. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu Z, Ma Y, Zhong C, Yin Q, Zhou C, Shi L, Cai Y, Zhao H, Wang H, et al. Generation of haploid embryonic stem cells from Macaca fascicularis monkey parthenotes. Cell Res. 2013;23:1187–1200. doi: 10.1038/cr.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Shi L, Wang BA, Liang D, Zhong C, Liu W, Nie Y, Liu J, Zhao J, Gao X, et al. Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell. 2012;149:605–617. doi: 10.1016/j.cell.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Yi M, Hong N, Hong Y. Generation of medaka fish haploid embryonic stem cells. Science. 2009;326:430–433. doi: 10.1126/science.1175151. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Peretz M, Sagi I, Benvenisty N. Haploid human embryonic stem cells: half the genome, double the value. Cell Stem Cell. 2016;19:569–572. doi: 10.1016/j.stem.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Yin Q, Xie Z, Bai M, Dong R, Tang W, Xing YH, Zhang H, Yang S, Chen LL, et al. CRISPR-Cas9-mediated genetic screening in mice with haploid embryonic stem cells carrying a guide RNA library. Cell Stem Cell. 2015;17:221–232. doi: 10.1016/j.stem.2015.06.005. [DOI] [PubMed] [Google Scholar]