Abstract
Cerebral visual impairment (CVI) results from perinatal injury to visual processing structures and pathways and is the most common individual cause of pediatric visual impairment and blindness in developed countries. While there is mounting evidence demonstrating extensive neuroplastic reorganization in early onset, profound ocular blindness, how the brain reorganizes in the setting of congenital damage to cerebral (i.e. retro-geniculate) visual pathways remains comparatively poorly understood. Individuals with CVI exhibit a wide range of visual deficits and, in particular, present with impairments of higher order visual spatial processing (referred to as “dorsal stream dysfunction”) as well as object recognition (associated with processing along the ventral stream). In this review, we discuss the need for ongoing work to develop novel, neuroscience-inspired approaches to investigate functional visual deficits in this population. We also outline the role played by advanced structural and functional neuroimaging in helping to elucidate the underlying neurophysiology of CVI, and highlight key differences with regard to patterns of neural reorganization previously described in ocular blindness.
Keywords: Cerebral visual impairment, ocular blindness, neuroplasticity, functional vision, structural brain imaging, functional brain imaging
1. Introduction
1.1. Blindness and Visual Impairment as a Model to Investigate Developmental Neuroplasticity
Ocular blindness has served as a unique model to investigate developmental neuroplasticity, helping to uncover the neurophysiological mechanisms that characterize the brain’s potential to be molded and adapt to sensory deprivation. Indeed, individuals living with blindness and visual impairment typically develop compensatory behavioral strategies (i.e. relying on non-visual modalities including hearing and touch) to gather relevant sensory information and maintain functional independence in a highly visual-dependent world. Growing experimental evidence obtained from brain imaging studies has shown that these compensatory behaviors in the blind are associated with dramatic neuroplastic reorganization within the brain (see (Bavelier and Neville, 2002; Kupers and Ptito, 2011; Merabet and Pascual-Leone, 2010; Pascual-Leone, et al., 2005; Ricciardi and Pietrini, 2011)). Arguably, the most striking finding has been the observation that the occipital visual cortex (normally associated with visual processing) is functionally recruited for the processing of nonvisual sensory information and cognitive tasks such as memory and language (see also (Bedny, 2017; Fine and Park, 2018; Singh, et al., 2018; Voss, 2019) for more recent discussions on this topic).
While research on neuroplasticity in the setting of ocular blindness has been extensive, comparatively speaking, our understanding of the neurophysiological and clinical repercussions of early developmental damage to cerebral (i.e. retro-geniculate) visual pathways and structures remains very limited. Specifically, what are the neuroplastic changes and developmental consequences resulting from visual impairment and blindness associated with damage to the visual brain as opposed to the eyes? What is the nature of compensatory behaviors in these individuals and what are their neurophysiological correlates? The study of individuals with cerebral visual impairment (CVI) provides an opportunity to explore these important questions.
In this review, we discuss visual processing deficits in CVI, how they differ from those individuals with ocular based visual impairment, and the need to develop novel, neuroscience-inspired approaches to characterize functional visual deficits and compensatory strategies in this population. We will also discuss the role that can be played by advanced structural and functional neuroimaging in elucidating the underlying neurophysiological basis of CVI, and highlight key differences in patterns of neural reorganization that have been previously described in ocular blindness.
1.2. Cerebral Visual Impairment: An Understudied and Underserved Population
In the clinical setting, CVI is defined as significant and verifiable visual dysfunction associated with damage to retrochiasmatic visual pathways and cerebral structures that cannot be attributed to disorders of the anterior visual pathways or any potentially co-occurring ocular impairment (Dutton, 2003; Sakki, et al., 2018). Originally, the term “cortical visual impairment” was coined to describe pediatric visual deficits of non-ocular cause (as opposed to acquired brain injury in adults) and its presumed association with damage to visual cortical processing areas (Whiting, et al., 1985). However, a more contemporary view suggests that neurological damage associated with CVI is likely to be more extensive, implicating subcortical structures, optic radiations and other white matter pathways, as well as higher order visual processing areas. Thus, the term “cerebral visual impairment” is considered a more encompassing and appropriate for this condition (Dutton, 2003; Sakki, et al., 2018).
CVI is now the leading individual cause of pediatric congenital visual impairment in developed countries, including the United States (Good, et al., 2001; Hoyt, 2007; Kong, et al., 2012; Solebo, et al., 2017) and the United Kingdom (Rahi, 2007), with prevalence estimates ranging between 20 to 40% of reported visual impairments (Bosch, et al., 2014; Durnian, et al., 2010; Kozeis, 2010), (see also (Pehere, et al., 2018) for data in developing countries). Furthermore, the incidence of CVI is on the rise worldwide, due in part to dramatically improved infant survival rates from perinatal neurological damage contributing to a steady increase in the number of biologically at-risk infants and children (Dutton, et al., 2004a; Good, et al., 2001; Kozeis, 2010; Taylor, et al., 2009). In parallel, the etiological profile of children attending schools for the blind is also changing. While ocular based visual impairments (i.e. various retinal diseases and other ocular pathologies) were amongst the most common etiologies, CVI is now the leading diagnosis of children in schools for the blind in the United States (Kong, et al., 2012).
Despite this clearly alarming public health concern, individuals with CVI remain greatly underserved and many continue to live with their visual impairments undetected or worse, undiagnosed (Dutton, et al., 2017). A major reason for this disparity is related to our poor understanding of the underlying neurophysiology of this condition and in particular, how brain development in the case of CVI differs from that of individuals with ocular based visual impairment and blindness.
As with ocular visual impairment, the causes of the many variants of CVI are also multifactorial and include perinatal hypoxia/ischemia, head injury/trauma, infection (e.g. encephalitis, meningitis), seizure disorder, genetic, and metabolic disorders (Hoyt, 2003). However, perinatal hypoxic ischemic injury remains the most common culprit and early neurological injury affects the development of white matter tracts coursing between sensory and motor areas of the brain as well as cortical gray matter and subcortical structures (including the thalamus) (Volpe, 2009). Associated developmental impairments are believed to be related to the timing, duration, and severity of the neurological injury (Anderson, 2011). In the case of premature birth (i.e. infants born between 24-32 weeks gestational age), neurological injury is often associated with periventricular white matter damage (referred to as periventricular leukomalacia, or PVL) and is a high risk factor for developing CVI (Pavlova and Krageloh-Mann, 2013). In infants born at term, perinatal hypoxic ischemia results in hypoxic ischemic encephalopathy (HIE) and is the primary cause leading to CVI in term infants (Chong and Dai, 2014; Flodmark, et al., 1990). In HIE, areas that are most commonly damaged are deep gray matter, hippocampus, brainstem, and thalamic regions (Swarte, et al., 2009).
The visual impairments associated with CVI are extensive and include decreased visual acuity ranging from mild to moderate impairment/low vision and even profound blindness (Dutton, 2003; Huo, et al., 1999) (WHO definition of blindness: visual acuity of 20/200 or worse in the better seeing eye with corrective lenses, or visual field restriction to 20 degrees diameter or less in the better seeing eye. WHO definition of visual impairment: visual acuity of 20/60 or worse in the better seeing eye; http://www.who.int/mediacentre/factsheets/fs282/en/). Visual field deficits (typically in the lower hemi-field), reduced contrast sensitivity, and oculomotor abnormalities are also commonly present (Dutton, 2003; Huo, et al., 1999), (see also (Zihl and Dutton, 2015) for a complete description of the phenomenology of visual deficits in CVI). As with the case of ocular based visual impairments, the timing and severity of observed visual deficits are important factors that must be accurately characterized and quantified in order to develop empirically derived and individualized rehabilitative strategies. Further complicating the clinical presentation, individuals with CVI also often show impairments related to higher-order visual processing and other neurodevelopmental delays and co-morbidities (e.g. cerebral palsy) may also be present. Thus, compared to individuals with ocular visual impairment, confirming a diagnosis and developing appropriate rehabilitative strategies for the CVI population represents a significantly greater challenge (Good, 2009).
1.3. Cerebral Visual Impairment: A Model to Investigate Developmental Vulnerability and Neuroplasticity
As mentioned earlier, there is mounting evidence that the brain undergoes extensive neuroplastic reorganization in the case of ocular blindness. A key advancement in our understanding of neuroplasticity in this condition has been the demonstration of how associated changes within the brain correlate with various measures of behavioral performance. For example, occipital cortical thickness has been shown to correlate with performance on auditory discrimination tasks (Voss and Zatorre, 2012). At the functional level, the magnitude of occipital activation (as indexed by functional neuroimaging) appears correlated with performance on sound localization (Gougoux, et al., 2005) and verbal memory (Amedi, et al., 2003) tasks (see (Voss, 2019) for further discussion). In contrast, in the case of CVI, the relationship between clinical behavioral findings and underlying development of neurological structures and function appears much more complex and has yet to be fully understood (Guzzetta, et al., 2013; Hoyt and Fredrick, 1998).
The behavioral characteristics and visual dysfunctions observed in CVI (first described by Jan and colleagues) are markedly different compared to children with ocular causes of blindness and visual impairment. Early studies have described a number of deficits related to high-order visual processing that are particular to CVI, such as impaired attention and highly variable visual functioning in response to increasing visual task demands and environmental complexity (Jan, et al., 1993; Jan, et al., 1987). Published observational reports have documented that children with CVI have difficulties locating objects (e.g. a favorite toy) or familiar people (e.g. a parent) in crowded and visually complex environments (despite being able to easily identify them in isolation), as well as following moving traffic and watching television programs with rapidly moving images and scenes (Dutton, et al., 2006; Fazzi, et al., 2009; Good, et al., 1994; McKillop, 2008). Furthermore, in contrast to their ocular visually impaired peers, individuals with CVI (particularly children) show clear difficulties in developing adaptive and compensatory strategies (Farrenkopf, et al., 1997) when using assistive technology (e.g. sensory substitution devices) or existing visual function abilities (such as using increased magnification in the case of lowered visual acuity). This issue is of even greater concern given the observation that education and (re)habilitative strategies designed for children with ocular visual impairment are largely ineffective (and even possibly detrimental) for children with CVI (Baker-Nobles and Rutherford, 1995; Farrenkopf, et al., 1997; Gordon, 1968; Groenveld, et al., 1990).
It would be reasonable to presume that visual processing impairments observed in CVI are linked to the maldevelopment of key visual processing pathways. In this direction, the classic two-stream (i.e. dorsal-ventral) organization of visual processing (Haxby, et al., 1991; Mishkin, et al., 1983) has served as a useful model to help conceptualize the nature of higher order visual processing deficits in CVI (Goodale, 2013). For example, there is extensive work documenting that individuals with CVI show striking difficulties with visual spatial processing tasks that are putatively associated with damage occurring along the dorsal visual processing stream (i.e. connecting the occipital to parietal cortices and terminating within frontal areas) (Dutton, 2009; Macintyre-Beon, 2010). Indeed, previous reports suggest that CVI can be characterized as a condition of “dorsal stream dysfunction” (or “dorsal stream vulnerability”) and represents the most common type of visual processing impairment observed in children with this condition (Dutton, 2009; Dutton, et al., 2017; Dutton, et al., 2004b; Macintyre-Beon, 2010). Typically, dorsal stream dysfunction presents with visual impairments related to spatial and motion processing, deficits in visual attention, as well as difficulties with visuo-motor integration, and simultanagnosia (Dutton, 2009; Dutton, et al., 2004b; Fazzi, et al., 2009; Macintyre-Beon, 2010; Philip and Dutton, 2014). It has been proposed that dorsal stream dysfunction is associated with the inherent vulnerability of the posterior parietal lobes (specifically, injury to the oligodendrocyte proliferation zone near the trigone area of the lateral ventricles) that are highly susceptible to impaired blood perfusion during early brain development (Dutton, et al., 2017). However, it is also important to realize that individuals with CVI can also present with deficits related to object identification (such as recognizing faces and shapes) (Andersson, et al., 2006; Houliston, et al., 1999). In contrast, these visual deficits would be associated with damage along the ventral visual processing stream (i.e. connecting the occipital to inferior temporal cortex) (Goodale, 2013; Haxby, et al., 1991). While these latter deficits are reportedly less common, when present, they are more often associated with dorsal stream/spatial impairments rather than in isolation (Dutton, 2011; Macintyre-Beon, 2010).
Of note, while the dorsal-ventral dichotomy has been appealing conceptually to help explain the nature of higher order visual processing deficits in CVI, neurophysiological support for this view remains very limited. Could these visual deficits in CVI and behavioral differences compared to individuals with ocular visual impairment be explained in terms of underlying changes in structural and functional neuroplastic reorganization? In the same manner that innovative and carefully designed behavioral and neuroimaging studies have greatly contributed to our knowledge of neuroplasticity and brain-behavioral relationships in the setting of ocular visual impairment and blindness, a similar paradigm shift is needed for advancing our understanding of CVI.
2. Assessing Dorsal Stream Dysfunction: Review of Previous Work and New Approaches
The concept of dorsal stream dysfunction first emerged from behavioral studies investigating visual processing abilities in children with various developmental disorders such as William’s syndrome, autism spectrum disorder, and cerebral palsy (Atkinson, 2017; Atkinson and Braddick, 2011; Braddick, et al., 2003; Dilks, et al., 2008). Using specially designed computerized psychophysical visual tasks, pivotal work by Atkinson, Braddick, and colleagues demonstrated the presence of selective deficits with complex visual processing associated with global motion, but not global form (e.g. “ball in the grass” test) (Braddick and Atkinson, 2007; Braddick, et al., 2005). This provided crucial experimental support for the concept of dorsal stream dysfunction, and further suggested a particular vulnerability for motion processing abilities during early stages of development (Braddick, et al., 2003). In the case of CVI, motion perception deficits have been extensively studied and numerous psychophysical studies have further characterized complex motion perception impairments in this population using a variety of stimuli including biological form from motion, optic flow fields (such as random dot kinematograms or RDK patterns), and spatial integration tasks (Atkinson, 2017; Boot, et al., 2010; Pavlova, et al., 2003; Taylor, et al., 2006; Weinstein, et al., 2012) (see also (Guzzetta, et al., 2009) for a discussion on the effect of prematurity on motion perception deficits).
The presence of these higher-order visual processing deficits (along with behavioral differences between individuals with CVI and ocular based visual impairment) suggests that specific testing for visual spatial deficits (along with eye movement characteristics) may have high diagnostic value (Zihl and Dutton, 2015). However, while characterizing visual spatial deficits in CVI (such as motion processing and visual guidance of movement) has relied on using well controlled psychophysical visual stimuli, they nonetheless remain limited in terms of their ecological validity. In other words, it is difficult to draw inferences from results obtained from these psychophysical studies in relation to what individuals with CVI experience perceptually as they visually interact with the real world. Thus, there remains a need to develop novel and more ecologically valid performance assessments that better characterize visual processing deficits in CVI and how they compare to individuals with ocular based visual impairments.
Virtual reality (VR) is a potentially useful tool that can merge empirical and reliable assessments of functional vision with ecological validity (Tarr and Warren, 2002) as well as with a high degree of control and participant engagement (Loomis, et al., 1999). In this direction, a number of studies have used this approach to investigate higher order visual processing abilities in CVI. For example, Kooiker, Pel and colleagues incorporated a series of desktop computerized tasks combined with capturing eye tracking metrics as a way to assess oculomotor functions in children with CVI. In their task design, participants were instructed to follow a series of moving cartoon images on a screen with their eyes. Using this testing paradigm, this group was able to demonstrate clear deficits related to visual search, fixation and oculomotor pursuit. In particular, the search patterns in CVI appeared more random, and less purposeful (Kooiker, et al., 2016a; Kooiker, et al., 2016b). These observations raise two intriguing questions. First, do impaired search patterns in CVI predict (or even eventually contribute to) their diagnosis, allowing for an estimation of the degree of dorsal stream dysfunction? Secondly, can VR based methods be used to train systematic visual search strategies (e.g. using engaging games to maintain attention and motivation) as a useful (re)habilitative approach?
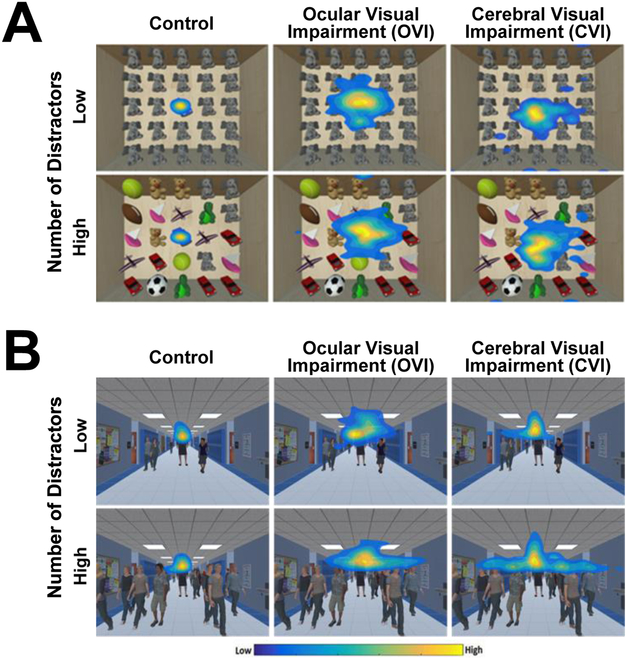
To further explore visual spatial processing abilities in CVI in environments that more closely approximate real world scenarios, our group has developed two VR based visual search simulations. The first assessment, called the “virtual toy box”, has been designed to test static object-based visual search performance. In this task, the participant must search for a target toy presented in a toy box filled with various toys (i.e. other distractor elements). This process is repeated with the target toy appearing at different locations and task difficulty is varied by changing the number of unique surrounding toys (see (Bennett, et al., 2018b) for further details). In a second VR based dynamic visual search task called the “virtual hallway”, the participant views a hallway of a school, and the task is to visually locate and track the movement of a target person walking among other individual humans (i.e. distractors). Task difficulty is varied by continuously changing the number of people present in the hallway (see (Bennett, et al., 2018a) for further details). In both behavioral assessments, visual search performance is quantified by captured eye tracking data aggregated over time to generate heat maps representing the spatial extent of eye gaze patterns (Gibaldi, et al., 2017). Comparing performance between normally sighted controls, individuals with ocular visual impairment, and CVI revealed characteristic visual search patterns across these populations. For both the virtual toy box and hallway tasks, we found that normally sighted controls showed a tight clustering of gaze for both the low and high level of task difficulty (see figure 1 A and B, left panel). In contrast, individuals with ocular visual impairment exhibited a more scattered search pattern with less defined gaze density regions, but again comparable across both the low and high levels of task demand (figure 1 A and B, middle panel). Finally, comparing performance in CVI revealed that these individuals had an intermediate level of gaze spread data for the low distractor task, but a greater increase in scatter in response to the high distractor condition (figure 1 A and B, right panel). This suggests that the CVI group showed the largest change in visual search performance as a function of task difficulty. These latter findings are consistent with previous accounts describing impairments with high-order visual processing associated with dorsal stream dysfunction, and in particular, greater impairments in response to increasing task demands and environmental complexity (Jan, et al., 1993; Jan, et al., 1987). Potential confounding performance deficits related to impaired object recognition (i.e. related to ventral stream dysfunction) could also be further explored by modifying specific features (e.g. color differentiation). Finally, this work also demonstrates the value of VR based and ecologically valid assessments in helping to characterize visual processing impairments using simulations that more closely resemble real world scenarios. Indeed, future behavioral studies will certainly continue to help further characterize visual processing deficits in CVI and how they differ from individuals with ocular visual impairment. Moving forward, a key advancement will be to investigate possible associations between behavioral performance and underlying neurophysiological changes at the level of the brain.
Figure 1. Assessing visual search performance with VR based environments combined with eye tracking.
A) Heat map displays of visual search patterns for the virtual toy box task. The color scheme represents differing levels of gaze data density across spatial regions of the screen space (yellow indicates more time looking in an area and blue indicates less time). Data from a control, ocular visual impaired, and CVI participant are shown for both the low and high number of unique distractor conditions. Note the tight cluster of eye movements in the control subject in both the low and high levels of distractor density, while the ocular visual impaired subject reveals a wide dispersion of eye movements on both levels of distractor density. By comparison, the CVI participant demonstrates an initial intermediate pattern of dispersion at the low level that increases in the high distractor condition. B) Heat map displays of eye search patterns for the virtual hallway task. Similar to performance observed in the toy box task, note how the search pattern in the CVI participant is markedly more diffuse in response to the high compared to the low distractor condition. Adapted from (Bennett, et al., 2018a; Bennett, et al., 2018b).
3. Investigating Brain Structural Connectivity in Ocular Blindness and CVI
Numerous neuroimaging studies have investigated morphological and structural connectivity changes in the case of early onset ocular blindness. Unfortunately, similar initiatives in the case of CVI are lagging far behind. Nonetheless, early comparisons can be drawn giving preliminary insights to help explain observed behavioral differences between these two populations.
While behavioral studies have provided evidence of adaptive and compensatory behaviors in ocular blind individuals (i.e. demonstrating comparable, and in some cases even superior, behavioral skills in tactile and auditory domains as compared to sighted individuals e.g. (Amedi, et al., 2003; Gougoux, et al., 2004; Lessard, et al., 1998; Van Boven, et al., 2000; Wong, et al., 2011)), early neuroanatomical studies suggested that structural brain changes were largely consistent with atrophic losses in response to early visual deprivation (reviewed by (Voss, 2019)). This included atrophy within thalamo-cortical pathways (Breitenseher, et al., 1998), grey matter within calcarine areas (Kitajima, et al., 1997), and white matter connections (e.g. (Bridge, et al., 2009; Ptito, et al., 2008; Shu, et al., 2009a; Shu, et al., 2009b). More recent studies employing diffusion based imaging techniques such as diffusion tensor imaging (DTI) and high angular resolution diffusion imaging (HARDI) have allowed for the characterization of more subtle changes in brain architecture. In general, diffusion based imaging studies have revealed trends of overall decreased connectivity throughout the brain (Lao, et al., 2015; Reislev, et al., 2016; Shu, et al., 2009b; Wang, et al., 2013). In contrast, complementary studies based on resting state functional connectivity MRI (rsfcMRI) have reported mixed results. This includes evidence of enhanced functional connectivity between occipital areas and other regions of the brain such as parietal and frontal areas (Bedny, et al., 2012; Butt, et al., 2013; Heine, et al., 2015; Liu, et al., 2007; Wang, et al., 2014; Watkins, et al., 2012) and patterns of overall decreases in connectivity between occipital areas and somatosensory cortex along with temporal cortical areas implicated with auditory processing (Bedny, et al., 2011; Burton, et al., 2014; Liu, et al., 2007; Striem-Amit, et al., 2015; Yu, et al., 2008) (see also (Bock, et al., 2015) for recent review). As a means to reconcile previous contradictory findings, work from our group has employed a multimodal imaging approach including morphometric analysis combined with structural (i.e. white matter) and functional (i.e. resting state) connectivity analyses to provide complementary information regarding neuroplastic reorganization in early ocular blindness (Bauer, et al., 2017). When comparing the brains of early ocular blind individuals to age matched sighted controls, we found evidence of co-occurring decreases in cortical volume and cortical thickness within visual processing areas of the occipital and temporal cortices while increases in cortical volume in the early blind were evident within regions of parietal cortex. White matter connectivity analysis revealed patterns of increases and decreases (indexed by fiber number), with increases between frontal and temporal areas implicated with language processing, while decreases in structural connectivity were evident involving frontal and somatosensory regions as well as between occipital and cingulate cortices. Finally, rsfcMRI analysis revealed evidence of both increased and decreased functional connectivity in the blind, but moreover, these patterns exhibited a high degree of correlation with observed white matter connectivity networks (Bauer, et al., 2017). In summary, these findings provide complementary evidence, and highlight potential contradictions, regarding the nature of regional and large scale neuroplastic reorganization resulting from early onset blindness. While larger-scale confirmatory studies investigating morphometric and connectivity changes are ongoing, there have been a number of recent reports suggesting that the functional organization (i.e. domain specificity) of the occipital cortex may develop independently of visual experience (e.g. (Dormal, et al., 2016; Matteau, et al., 2010; Reich, et al., 2011; Striem-Amit, et al., 2012b; Striem-Amit, et al., 2015)) and that the overall organization (including the dorsal and ventral processing streams) is maintained by an intrinsic pattern of anatomical and functional connectivity between occipital and other brain regions that process non-visual properties within corresponding cognitive domains (the “connectivity-constraint hypothesis”; see (Bi, et al., 2016; Gomez, et al., 2015; Striem-Amit, et al., 2015)). Thus, it appears that while the exact nature of this neuroplastic reorganization still remains to be fully elucidated, it is these changes that support observed compensatory behaviors in the case of early ocular blindness (see (Bedny, 2017; Fine and Park, 2018; Voss, 2019) for further discussion).
To our knowledge, only a limited number of studies have used advanced imaging modalities in the case of CVI in an attempt to characterize and quantify changes in brain structure and explore potential associations that are clinically and educationally meaningful. In contrast to the case of ocular blindness, characterizing the neurophysiological substrate underlying visual dysfunction in CVI remains considerably more challenging given that damage to cerebral structures is highly heterogeneous across individuals in terms of location, timing, extent, and cause. In fact, a number of studies have attempted to relate functional visual impairments to perinatal brain injury, but with mixed results (Eken, et al., 1994; Flodmark, et al., 1990; Guzzetta, et al., 2001; Uggetti, et al., 1996; Wiklund and Uvebrant, 1991).
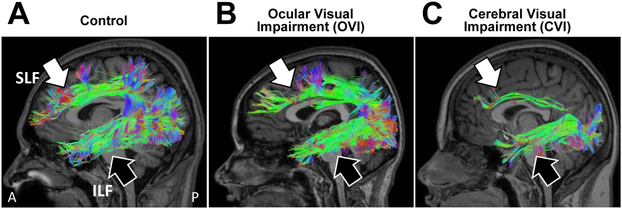
Early work by Cioni and colleagues (2000) attempted to correlate neurodevelopmental outcomes in children with periventricular leukomalacia (PVL) with a variety of indices of visual performance measures (including visual acuity, visual field function, and optokinetic nystagmus). Using a multivariate analysis approach, these investigators found that there was a strong association between the degree of visual impairment and the damage observed to optic radiations (as indexed by structural MRI) (Cioni, et al., 2000). Subsequent work by Serdaroglu and coworkers (2004) showed that the severity of neurological injury associated with PVL was also correlated with neurodevelopmental outcomes. Specifically, children with a low severity of PVL had minor motor problems or mild to normal functional outcomes, whereas the presence of cortical atrophy and thinning of the corpus callosum were associated with more developmental delays (Serdaroglu, et al., 2004). Interestingly, a study by Guzzetta and colleagues (2001) reported that structural MRI defined areas of brain damage were not always predictive of visual field defects in children as expected in adults with the same lesions (Guzzetta, et al., 2001). In a subsequent review, the same investigators reported that many individuals diagnosed with CVI and with early periventricular damage to the optic radiations often presented with normal development of visual field function, suggesting that the preservation of visual field function may be the result of compensatory neuroplastic reorganization (Guzzetta, et al., 2013). More recent studies have employed diffusion based imaging approaches in order to characterize more subtle changes in white matter integrity and architecture in CVI and establish anatomical-functional-behavioral relationships at the individual level. A recent study by Lennartsson and colleagues (2014) employed diffusion weighted MRI in a group of individuals with visual impairment associated with perinatal neurological injury (predominantly in the superior posterior periventricular white matter) and found that early injury to the optic radiations was associated with characteristic patterns of visual field deficits (Lennartsson, et al., 2014). A series of related studies using DTI have demonstrated that impaired visual function (quantified by fixation and tracking abilities) in infants born preterm was associated with lower fractional anisotropy (FA) values (an index of white matter structural integrity) in the optic radiations (Guzzetta, et al., 2010; Guzzetta, et al., 2013). Another study by Ortibus and coworkers (2012) used DTI and found that impaired object processing abilities in children with CVI were correlated with decreases in the structural integrity (indexed by FA values) of the inferior longitudinal fasciculus (ILF; corresponding to the neuroanatomical correlate of the ventral visual processing stream (Ffytche, et al., 2010; Rokem, et al., 2017)) (Ortibus, et al., 2012). While it is important to note that clinical interpretations based on FA outcomes remain an issue of debate (Li, et al., 2013), taken together, these results suggest that associations between losses in white matter integrity and the severity of visual perception deficits in CVI can be revealed using more advanced structural neuroimaging methods. Finally, two more recent studies have explored putative relationships between underlying changes in structural connectivity and visual processing. Braddick and coworkers (2017) used DTI to demonstrate that global motion sensitivity in neurotypical-developed children appears to correlate with the integrity (indexed by FA values) of the superior longitudinal fasciculus (SLF; corresponding to the neuroanatomical correlate of the dorsal visual processing stream (Catani and Thiebaut de Schotten, 2008)) (Braddick, et al., 2017). Building upon these findings, work by our group using HARDI has also demonstrated that individuals with CVI show marked reductions in the volume of the SLF as compared to neurotypical controls and ocular blind individuals. Interestingly, commensurate reductions in the ILF were also observed in the individuals with CVI who also exhibited object recognition visual deficits (Bauer, et al., 2014; Merabet, et al., 2017) (Figure 2). These latter two studies represent early pieces to establishing possible neurophysiological links between dorsal stream dysfunction (as well as potential ventral stream processing impairments) and key extrageniculo-striate visual pathways implicated in visual processing in individuals with CVI (Bauer, et al., 2014).
Figure 2. White matter structural connectivity of the dorsal and ventral visual processing streams revealed by HARDI.
Sagittal view of white matter tractography reconstructions of the superior longitudinal fasciculus (SLF; white arrow) and inferior longitudinal fasciculus (ILF; black arrow) corresponding to the dorsal and ventral visual processing streams, respectively. Note the robust appearance of both pathways in a (A) sighted control and (B) ocular blind individual. In contrast, the CVI individual (C) shows a marked reduction in the structural integrity of the SLF while the ILF appears comparatively intact. Adapted from (Bauer, et al., 2014; Merabet, et al., 2017).
In summary, investigating potential associations between measurements of structural integrity of targeted white matter pathways and behavioral measures related to various visual tasks (such as complex visual motion processing in the case of dorsal stream dysfunction, and object recognition deficits in the case of ventral stream involvement) could prove to be a highly informative paradigm to investigate brain-behavioral relationships and help explain the underlying neurophysiological basis of visual impairments in CVI. These aforementioned studies suggest that in contrast to the situation of early ocular blindness, CVI may be associated with a more generalized vulnerability implicating numerous key pathways supporting the developing visual system. Furthermore, neuroplastic changes within the developing brain (such as the “rewiring” of key geniculo-cortical or cortico-cortical connections) may support the sparing of visual function in certain individuals with CVI. While further confirmation of these results are ongoing, they do suggest that a global impairment in overall brain connectivity may be associated with observed cognitive visual dysfunctions as well as other associated sensorimotor and cognitive deficits in CVI. Further analyses at the local and whole brain network level may also provide hints as to why education and (re)habilitative strategies designed for individuals with ocular blindness may not translate to the case of CVI. Indeed, it has been suggested that approaches should be developed that match the skills and adaptive strategies that the child has developed (Dutton and Lueck, 2015).
4. Investigating Functional Neural Correlates Associated with Neuroplasticity in Ocular Blindness and in CVI
Functional neuroimaging approaches (such as fMRI, and PET), and electrophysiology (VEP and EEG) are particularly well-suited in helping to identify the neural correlates associated with behavioral task performance. As mentioned in the introduction, neuroimaging studies have been instrumental in characterizing functional neuroplastic changes in ocular blindness and in particular, revealing how the occipital visual cortex is recruited for the processing of nonvisual sensory information and cognitive tasks such as memory and language (e.g. Braille reading (Sadato, et al., 1996), sound localization (Collignon, et al., 2011; Gougoux, et al., 2005; Voss, et al., 2008), and odor perception (Kupers and Ptito, 2014), along with higher order cognitive tasks including language processing (Bedny, et al., 2011; Burton, et al., 2002; Striem-Amit, et al., 2012a) and verbal memory recall (Amedi, et al., 2003; Raz, et al., 2005). However, studies employing functional neuroimaging to characterize neural correlates with behavioral tasks in CVI have been scant. Given the putative causal relationship between early developmental damage and CVI, it is also crucial that future work incorporate functional neuroimaging modalities as a way to investigate brain processes associated with observed visual impairments and to gain insight into potential neuroplastic compensatory mechanisms (Bauer, et al., 2017; Edmond and Foroozan, 2006; Good, et al., 2001; Murakami, et al., 2008; Pineda, et al., 2014).
To our knowledge, only a handful of studies have employed fMRI to investigate visual cortical function in CVI. An early study by Sie et al. (2001), and later by Yu et al. (2011), both attempted to evaluate primary visual cortex activation in a cohort of anesthetized infants with CVI associated with PVL. Both studies reported that in general, CVI was associated with significant decreases in visual cortex activation in response to visual photic stimulation (with eyes closed) (Sie, et al., 2001; Yu, et al., 2011). While both groups proposed a similar conclusion that early developmental brain injury likely contributed to the observed impairment of visual cortex activation, their results unfortunately do not speak of task elated activity and potential compensatory reorganization that may occur with further development.
Returning to the issue of motion processing/dorsal stream dysfunction, Morrone and colleagues (2008) investigated complex motion perception processing deficits in two young patients with higher-order visual processing impairments associated with PVL. Using random-dot (RDK) displays, this group found that while these patients could perceive rotation and expansion motion stimuli correctly, they would also paradoxically perceive translational motion in the opposite (i.e. inverted) direction of presentation (Morrone, et al., 2008). Furthermore, fMRI testing was carried out in one patient, and revealed that viewing the translational motion stimulus did not elicit activation within the motion sensitive area hMT+ (a key extrastriate visual area implicated in complex visual motion processing and part of the dorsal visual processing stream (Braddick, et al., 2001; Grossman, et al., 2000; Newsome and Pare, 1988)). By comparison, viewing radial and rotational motion stimuli produced normal patterns of activation in this area (Morrone, et al., 2008). The results of the case report highlight how different types of complex motion processing may be selectively vulnerable in the setting of prenatal neurological damage.
Given the technical challenges associated with working with young patients in the MRI environment (e.g. requiring participants to lie sufficiently still), there remains the need to develop novel methods to further characterize visual perceptual deficits and their associated functional neural correlates. EEG affords high temporal resolution (in the order of milliseconds) and thus is very useful in characterizing the temporal profile of task related brain activity. Early work using EEG based paradigms with the CVI population has provided useful insight on visual function and diagnosis, but has not focused on aspects related to higher order visual processing (e.g. (Good, et al., 2001; Granet, et al., 1993; Skoczenski and Norcia, 1999; Watson, et al., 2010)). More recent work by Weinstein and colleagues (2012) explored visual motion processing in CVI using steady state visual evoked potentials (ssVEP) and compared occipital activation in response to viewing local and global motion stimuli as compared to ocular visually impaired (diagnosed with amblyopia and/or strabismus) and neurotypical control individuals. These investigators found that CVI subjects showed selective global (but not local) motion deficits, especially for slower stimulus velocities, with corresponding greater decreases in occipital signal amplitudes (Weinstein, et al., 2012).
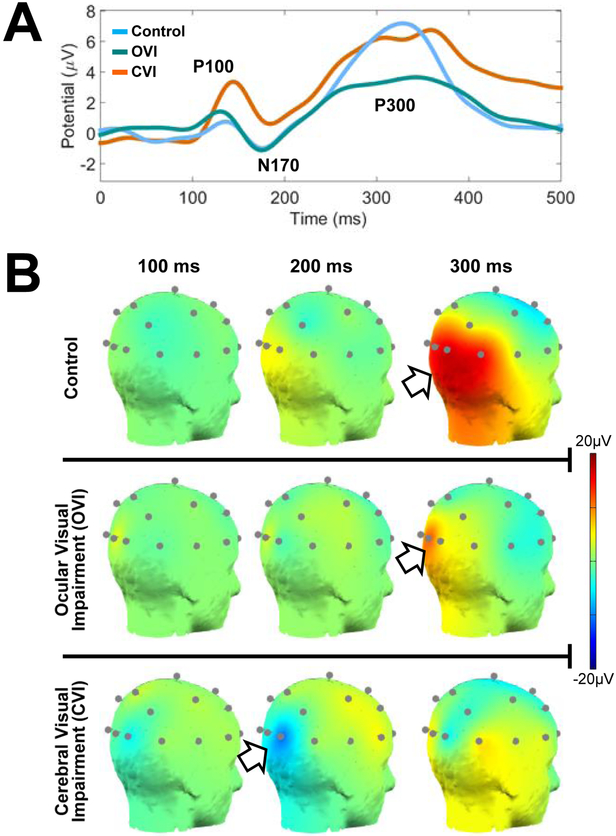
Preliminary work by our group has been investigating the neural correlates associated with visual search using the aforementioned VR based virtual toy box task combined with EEG recordings (Bennett, et al., 2019). Figure 3 A highlights the cortical activation patterns that were observed using this EEG paradigm for key event related potential (ERP) responses namely, the P100, N170, P300. These events are associated with early stages of visual processing and reflect the brain’s ability to parse visual information and execute a response to a given visual task (Lopez-Calderon and Luck, 2014). Early data suggests that individuals with CVI appear to exhibit significant latencies and differences in magnitude for the ERP events compared to controls and individuals with ocular visual impairment. Comparing scalp map projections across representative individuals from all three groups (figure 3 B), we see how the control subject reveals well-timed and robust signals over the occipito-temporal-parietal areas corresponding to higher order visual processing regions. The ocular visually impaired individual demonstrates occipital activation that is more synchronized in time with the control subject, but highly localized within early occipital visual processing areas. Finally, the CVI subject shows reduced cortical activity within occipital areas with highly desynchronized activity along occipito-parietal regions corresponding to the dorsal visual processing stream. Thus, the combined use of VR based visual search tasks and cortical activity as characterized by EEG may prove to be a useful paradigm. Further studies are need to help characterize the neural correlates associated with higher order visual processing deficits in CVI and how they compare with ocular based visual impairment.
Figure 3. Visual search combined with EEG data collection.
A) Event related potentials (ERP) for control, ocular visual impaired, and CVI groups in response to the virtual toy box visual search task. Data represents averaging over occipital pole nodes for the first 500 ms after stimulus onset. Note how controls demonstrate well-formed P100, N170, and P300 events. Ocular visually impaired subjects display similar latencies as controls, but with a reduced magnitude of the P300 event. Note that in the CVI group, there are latency delays at all three events (greatest at P300) and noticeable variations in peak amplitudes. B) Scalp map plots of 20 channel EEG data obtained from representative individuals from all three groups in response to the virtual toy box task (posterior view, right side). Scalp maps are displayed at the 100, 200, and 300 ms intervals. The occipital-parietal signal observed in the control (top row) appears robust and peaks just past 300 ms after stimulus onset. The ocular visually impaired individual (middle row) shows less robust and a weaker peak within the occipital pole at 300 ms. The individual with CVI (bottom row) reveals a delayed N170 that peaks close to 200 ms and a delayed P300 that does not peak until close to 375 ms. Note further how the occipital signal does not appear as robustly sustained along the occipital-parietal areas as it is in the control participant. Adapted from (Bennett, et al., 2019).
5. Concluding Thoughts, Limitations, and Future Directions
Investigating neuroplasticity associated with early and profound visual deprivation due to ocular blindness has contributed greatly to our knowledge regarding the developmental constraints and adaptive potential of the brain. In contrast, our understanding of how the brain reorganizes in the setting of congenital damage to cerebral visual processing areas lags far behind despite a clear and pressing public health need. A similar paradigm shift incorporating well controlled behavioral and advanced structural and functional neuroimaging studies is needed to advance our understanding of CVI. In this direction, there are also a number of technical considerations that need to be specifically addressed in order to advance research in this field.
Given the heterogeneity of underlying causes and broad clinical profile of visual impairments, CVI cannot be treated as a monolithic condition in the same way we have approached early onset, profound ocular blindness. Historically, research investigating neuroplasticity associated with visual deprivation has largely focused on individuals characterized as congenitally and profoundly blind as a means to control for the effects of early visual experience on development. In CVI, the majority of individuals have some degree of visual function, and those with more profoundly impaired visual acuity typically present with impaired cognitive abilities in association with more severe neurological injury (Dutton and Lueck, 2015). In fact, the heterogeneous profile of CVI has posed challenges in achieving a common consensus within the medical/scientific community toward a formally accepted definition and diagnosis criteria for this population (see (Colenbrander, 2010; Good, et al., 1994)). Thus, unlike in the case of congenital ocular blindness, it is very difficult to disentangle the effect of early visual experience and changes associated with developmental neuroplasticity. Furthermore, drawing comparisons across studies has been a challenge as characterized visual impairments may be driven not only by the etiology of the neurological injury, its locus, and extent, but also by other developmental factors and co-morbidities. As neurological injury associated with CVI is often extensive, it is likely that other sensory impairments (e.g. auditory, tactile) and cognitive deficits (related to attention, memory, and language) are also present and these need to be carefully characterized and their effect accounted for. Therefore, as with studies in ocular blindness, investigating crossmodal sensory processing and higher order cognitive functions (and their associated neural correlates) in CVI also represents an important, and as of yet, unexplored avenue of research. As the clinical profile and diagnostic criteria for CVI become more defined, it will be crucial that future investigations carefully account for the broad extent of sensory and cognitive deficits, as well as disentangle the effect of etiology and nature of neurological injury.
In the context of dorsal stream dysfunction, further studies are needed to assess broader aspects of visual processing deficits that are more in line with their purported clinical frequency. In evaluating visual impairments in CVI, there are limited validated and reliable diagnostic tools available that can be rapidly administered and at the same time, even handedly assess both dorsal and ventral related visual processing functions. This would be crucial in order to establish more definitively whether there is indeed a strong predilection for spatial processing impairments in CVI. Our current understanding may reflect an inherent bias related to the assessment tools employed or mask deficits that are more associated with cognitive impairments rather than visual processing abilities per se (see (Fazzi, et al., 2009) for further discussion). In parallel, while the dorsal-ventral visual processing stream model serves as a useful framework in conceptualizing higher order visual processing deficits in CVI, it is important to recognize that on a physiological basis, they operate in tandem rather than in isolation. Growing evidence demonstrates that the dorsal-ventral steams interact extensively in the analysis of complex moving visual scenes (Gilaie-Dotan, 2016) and through a network of direct and indirect connections including the vertical occipital fasciculus (VOF) along with hippocampal and parahippocampal regions (de Haan and Cowey, 2011; Kravitz, et al., 2013). As with studies in ocular blindness, the role of these aforementioned areas need to be thoroughly investigated, and may also show evidence of neuroplastic changes related to underlying perceptual deficits (and potentially even compensatory behaviors) in CVI.
In conclusion, future work in CVI would benefit from carrying out well-designed behavioral and advanced multimodal neuroimaging studies similar to those that have greatly contributed to advancing our knowledge in uncovering brain-behavioral relationships and neuroplastic changes associated with ocular blindness. As we continue to characterize the underlying neurophysiology of CVI (including its many variants), this condition may also prove to be a highly intriguing model to investigate visual deprivation and developmental neuroplasticity in the setting of early neurological damage. More importantly, we have the opportunity to help a population that has not only been greatly understudied, but also underserved despite its important public health relevance.
Highlights.
Growing experimental evidence suggests that compensatory behaviors in ocular blind individuals are associated with dramatic neuroplastic reorganization. However, our understanding of the neurophysiological repercussions of visual impairment due to early developmental damage to cerebral visual pathways and structures remains comparatively limited.
Cerebral visual impairment (CVI) is the most common individual cause of pediatric visual impairment and blindness in developed countries. However, large gaps remain in our understanding regarding the underlying neurophysiology of this condition and how the brain reorganizes compared to the case of ocular based blindness.
Along with impaired visual acuity and visual field deficits, individuals with CVI often exhibit impairments in higher order visual spatial processing (referred to as dorsal stream dysfunction). Yet, the neural correlates of these deficits remain poorly understood.
New behavioral testing paradigms, such as using virtual reality (VR) environments, are helpful in further characterizing higher order visual processing deficits in a manner that is ecologically valid and clinically meaningful.
Advanced structural neuroimaging approaches, such as diffusion based imaging, highlight key differences in white matter connectivity in CVI compared to ocular blindness.
Future studies employing functional neuroimaging techniques (such as fMRI and EEG) are needed to identify the neural correlates associated with visual processing deficits in CVI.
Acknowledgements
This work was supported by grants from the NIH/NEI (RO1 EY019924), the Low Vision Research Award from Research to Prevent Blindness (RPB) and Lions Clubs International Foundation (LCIF), and the Massachusetts Lions Eye Research Fund to LBM. The authors would also like to thank Dr. Gordon N. Dutton for helpful comments in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amedi A, Raz N, Pianka P, Malach R, Zohary E (2003) Early 'visual' cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci, 6:758–66. [DOI] [PubMed] [Google Scholar]
- Anderson BJ (2011) Plasticity of gray matter volume: the cellular and synaptic plasticity that underlies volumetric change. Dev Psychobiol, 53:456–65. [DOI] [PubMed] [Google Scholar]
- Andersson S, Persson EK, Aring E, Lindquist B, Dutton GN, Hellstrom A (2006) Vision in children with hydrocephalus. Dev Med Child Neurol, 48:836–41. [DOI] [PubMed] [Google Scholar]
- Atkinson J (2017) The Davida Teller Award Lecture, 2016: Visual Brain Development: A review of "Dorsal Stream Vulnerability"-motion, mathematics, amblyopia, actions, and attention. J Vis, 17:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Braddick O (2011) From genes to brain development to phenotypic behavior: "dorsal-stream vulnerability" in relation to spatial cognition, attention, and planning of actions in Williams syndrome (WS) and other developmental disorders. Prog Brain Res, 189:261–83. [DOI] [PubMed] [Google Scholar]
- Baker-Nobles L, Rutherford A (1995) Understanding cortical visual impairment in children. Am J Occup Ther, 49:899–903. [DOI] [PubMed] [Google Scholar]
- Bauer CM, Heidary G, Koo BB, Killiany RJ, Bex P, Merabet LB (2014) Abnormal white matter tractography of visual pathways detected by high-angular-resolution diffusion imaging (HARDI) corresponds to visual dysfunction in cortical/cerebral visual impairment. J AAPOS, 18:398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CM, Hirsch GV, Zajac L, Koo BB, Collignon O, Merabet LB (2017) Multimodal MR-imaging reveals large-scale structural and functional connectivity changes in profound early blindness. PLoS One, 12:e0173064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ (2002) Cross-modal plasticity: where and how? Nat Rev Neurosci, 3:443–52. [DOI] [PubMed] [Google Scholar]
- Bedny M (2017) Evidence from Blindness for a Cognitively Pluripotent Cortex. Trends Cogn Sci, 21:637–648. [DOI] [PubMed] [Google Scholar]
- Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R (2011) Language processing in the occipital cortex of congenitally blind adults. Proceedings of the National Academy of Sciences of the United States of America, 108:4429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Pascual-Leone A, Dravida S, Saxe R (2012) A sensitive period for language in the visual cortex: distinct patterns of plasticity in congenitally versus late blind adults. Brain and language, 122:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CR, Bailin E, Bauer C, Dubreuil Vall L, Bex P, Merabet LB (2019) Object-based visual search in cerebral visual impairment using a virtual reality and EEG paradigm. Organization for Human Brain Mapping (OHBM). [Google Scholar]
- Bennett CR, Bailin E, Gottlieb T, Bauer C, Bex P, Merabet LB (2018a) Assessing Visual Search Performance in Ocular Compared to Cerebral Visual Impairment Using a Virtual Reality Simulation of Human Dynamic Movement. APA Science, 4:1–6. [Google Scholar]
- Bennett CR, Bailin ES, Gottlieb TK, Bauer CM, Bex PJ, Merabet LB (2018b) Virtual Reality Based Assessment of Static Object Visual Search in Ocular Compared to Cerebral Visual Impairment. HCI, 8:28–38. [Google Scholar]
- Bi Y, Wang X, Caramazza A (2016) Object Domain and Modality in the Ventral Visual Pathway. Trends in cognitive sciences, 20:282–90. [DOI] [PubMed] [Google Scholar]
- Bock AS, Binda P, Benson NC, Bridge H, Watkins KE, Fine I (2015) Resting-State Retinotopic Organization in the Absence of Retinal Input and Visual Experience. The Journal of neuroscience : the official journal of the Society for Neuroscience, 35:12366–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot FH, Pel JJ, van der Steen J, Evenhuis HM (2010) Cerebral Visual Impairment: which perceptive visual dysfunctions can be expected in children with brain damage? A systematic review. Research in developmental disabilities, 31:1149–59. [DOI] [PubMed] [Google Scholar]
- Bosch DG, Boonstra FN, Willemsen MA, Cremers FP, de Vries BB (2014) Low vision due to cerebral visual impairment: differentiating between acquired and genetic causes. BMC ophthalmology, 14:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick O, Atkinson J (2007) Development of brain mechanisms for visual global processing and object segmentation. Prog Brain Res, 164:151–68. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Akshoomoff N, Newman E, Curley LB, Gonzalez MR, Brown T, Dale A, Jernigan T (2017) Individual differences in children's global motion sensitivity correlate with TBSS-based measures of the superior longitudinal fasciculus. Vision Res, 141:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J (2003) Normal and anomalous development of visual motion processing: motion coherence and 'dorsal-stream vulnerability'. Neuropsychologia, 41:1769–84. [DOI] [PubMed] [Google Scholar]
- Braddick O, Birtles D, Wattam-Bell J, Atkinson J (2005) Motion- and orientation-specific cortical responses in infancy. Vision Res, 45:3169–79. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O'Brien JM, Wattam-Bell J, Atkinson J, Hartley T, Turner R (2001) Brain areas sensitive to coherent visual motion. Perception, 30:61–72. [DOI] [PubMed] [Google Scholar]
- Breitenseher M, Uhl F, Prayer Wimberger D, Deecke L, Trattnig S, Kramer J (1998) Morphological dissociation between visual pathways and cortex: MRI of visually-deprived patients with congenital peripheral blindness. Neuroradiology, 40:424–7. [DOI] [PubMed] [Google Scholar]
- Bridge H, Cowey A, Ragge N, Watkins K (2009) Imaging studies in congenital anophthalmia reveal preservation of brain architecture in 'visual' cortex. Brain : a journal of neurology, 132:3467–80. [DOI] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME (2002) Adaptive changes in early and late blind: a fMRI study of Braille reading. Journal of neurophysiology, 87:589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Raichle ME (2014) Resting state functional connectivity in early blind humans. Frontiers in systems neuroscience, 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt OH, Benson NC, Datta R, Aguirre GK (2013) The fine-scale functional correlation of striate cortex in sighted and blind people. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33:16209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a journal devoted to the study of the nervous system and behavior, 44:1105–32. [DOI] [PubMed] [Google Scholar]
- Chong C, Dai S (2014) Cross-sectional study on childhood cerebral visual impairment in New Zealand. J AAPOS, 18:71–4. [DOI] [PubMed] [Google Scholar]
- Cioni G, Bertuccelli B, Boldrini A, Canapicchi R, Fazzi B, Guzzetta A, Mercuri E (2000) Correlation between visual function, neurodevelopmental outcome, and magnetic resonance imaging findings in infants with periventricular leucomalacia. Arch Dis Child Fetal Neonatal Ed, 82:F134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colenbrander A (2010) What's in a name? Appropriate terminology for CVI. Journal of Visual Impairment and Blindness, 104:583–585. [Google Scholar]
- Collignon O, Vandewalle G, Voss P, Albouy G, Charbonneau G, Lassonde M, Lepore F (2011) Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proceedings of the National Academy of Sciences of the United States of America, 108:4435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan EH, Cowey A (2011) On the usefulness of 'what' and 'where' pathways in vision. Trends Cogn Sci, 15:460–6. [DOI] [PubMed] [Google Scholar]
- Dilks DD, Hoffman JE, Landau B (2008) Vision for perception and vision for action: normal and unusual development. Dev Sci, 11:474–86. [DOI] [PubMed] [Google Scholar]
- Dormal G, Rezk M, Yakobov E, Lepore F, Collignon O (2016) Auditory motion in the sighted and blind: Early visual deprivation triggers a large-scale imbalance between auditory and "visual" brain regions. NeuroImage, 134:630–44. [DOI] [PubMed] [Google Scholar]
- Durnian JM, Cheeseman R, Kumar A, Raja V, Newman W, Chandna A (2010) Childhood sight impairment: a 10-year picture. Eye, 24:112–7. [DOI] [PubMed] [Google Scholar]
- Dutton GN (2003) Cognitive vision, its disorders and differential diagnosis in adults and children: knowing where and what things are. Eye (Lond), 17:289–304. [DOI] [PubMed] [Google Scholar]
- Dutton GN (2009) 'Dorsal stream dysfunction' and 'dorsal stream dysfunction plus': a potential classification for perceptual visual impairment in the context of cerebral visual impairment? Developmental medicine and child neurology, 51:170–2. [DOI] [PubMed] [Google Scholar]
- Dutton GN (2011) Structured history taking to characterize visual dysfunction and plan optimal habilitation for children with cerebral visual impairment. Developmental medicine and child neurology, 53:390. [DOI] [PubMed] [Google Scholar]
- Dutton GN, Chokron S, Little S, McDowell N (2017) Posterior parietal visual dysfunction: An exploratory review. Vision Development and Rehabilitation 3:10–22. [Google Scholar]
- Dutton GN, Lueck AH (2015) Impairment of Vision Due to Damage to the Brain In: Dutton A.H.L.a.G.N., editor. Vision and the Brain: Understanding Cerebral Visual Impairment in Children: AFB Press New York. p 3–20. [Google Scholar]
- Dutton GN, McKillop EC, Saidkasimova S (2006) Visual problems as a result of brain damage in children. The British journal of ophthalmology, 90:932–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton GN, Saaed A, Fahad B, Fraser R, McDaid G, McDade J, Mackintosh A, Rane T, Spowart K (2004a) Association of binocular lower visual field impairment, impaired simultaneous perception, disordered visually guided motion and inaccurate saccades in children with cerebral visual dysfunction-a retrospective observational study. Eye, 18:27–34. [DOI] [PubMed] [Google Scholar]
- Dutton GN, Saaed A, Fahad B, Fraser R, McDaid G, McDade J, Mackintosh A, Rane T, Spowart K (2004b) Association of binocular lower visual field impairment, impaired simultaneous perception, disordered visually guided motion and inaccurate saccades in children with cerebral visual dysfunction-a retrospective observational study. Eye (Lond), 18:27–34. [DOI] [PubMed] [Google Scholar]
- Edmond JC, Foroozan R (2006) Cortical visual impairment in children. Curr Opin Ophthalmol, 17:509–12. [DOI] [PubMed] [Google Scholar]
- Eken P, van Nieuwenhuizen O, van der Graaf Y, Schalij-Delfos NE, de Vries LS (1994) Relation between neonatal cranial ultrasound abnormalities and cerebral visual impairment in infancy. Dev Med Child Neurol, 36:3–15. [DOI] [PubMed] [Google Scholar]
- Farrenkopf C, McGregor D, Nes SL, Koenig AJ (1997) Increasing a functional skill for an adolescent with cortical visual impairment. Journal of of Visual Impairment and Blindness, 91:484–493. [Google Scholar]
- Fazzi E, Bova S, Giovenzana A, Signorini S, Uggetti C, Bianchi P (2009) Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Developmental medicine and child neurology, 51:974–81. [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Blom JD, Catani M (2010) Disorders of visual perception. J Neurol Neurosurg Psychiatry, 81:1280–7. [DOI] [PubMed] [Google Scholar]
- Fine I, Park JM (2018) Blindness and Human Brain Plasticity. Annu Rev Vis Sci, 4:337–356. [DOI] [PubMed] [Google Scholar]
- Flodmark O, Jan JE, Wong PK (1990) Computed tomography of the brains of children with cortical visual impairment. Developmental medicine and child neurology, 32:611–20. [PubMed] [Google Scholar]
- Gibaldi A, Vanegas M, Bex PJ, Maiello G (2017) Evaluation of the Tobii EyeX Eye tracking controller and Matlab toolkit for research. Behav Res Methods, 49:923–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilaie-Dotan S (2016) Visual motion serves but is not under the purview of the dorsal pathway. Neuropsychologia, 89:378–392. [DOI] [PubMed] [Google Scholar]
- Gomez J, Pestilli F, Witthoft N, Golarai G, Liberman A, Poltoratski S, Yoon J, Grill-Spector K (2015) Functionally defined white matter reveals segregated pathways in human ventral temporal cortex associated with category-specific processing. Neuron, 85:216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good WV (2009) Cortical visual impairment: new directions. Optometry and vision science : official publication of the American Academy of Optometry, 86:663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good WV, Jan JE, Burden SK, Skoczenski A, Candy R (2001) Recent advances in cortical visual impairment. Developmental medicine and child neurology, 43:56–60. [DOI] [PubMed] [Google Scholar]
- Good WV, Jan JE, DeSa L, Barkovich AJ, Groenveld M, Hoyt CS (1994) Cortical visual impairment in children. Survey of ophthalmology, 38:351–64. [DOI] [PubMed] [Google Scholar]
- Goodale MA (2013) Separate visual systems for perception and action: a framework for understanding cortical visual impairment. Developmental medicine and child neurology, 55 Suppl 4:9–12. [DOI] [PubMed] [Google Scholar]
- Gordon N (1968) Visual agnosia in childhood. VI. Preliminary communication. Dev Med Child Neurol, 10:377–9. [DOI] [PubMed] [Google Scholar]
- Gougoux F, Lepore F, Lassonde M, Voss P, Zatorre RJ, Belin P (2004) Neuropsychology: pitch discrimination in the early blind. Nature, 430:309. [DOI] [PubMed] [Google Scholar]
- Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F (2005) A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol, 3:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granet DB, Hertle RW, Quinn GE, Breton ME (1993) The visual-evoked response in infants with central visual impairment. Am J Ophthalmol, 116:437–43. [DOI] [PubMed] [Google Scholar]
- Groenveld M, Jan JE, Leader P (1990) Observations on the habilitation of children with cortical visual impairment. Journal of Visual Impairment and Blindness, 84:11–15. [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R (2000) Brain areas involved in perception of biological motion. Journal of cognitive neuroscience, 12:711–20. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Cioni G, Cowan F, Mercuri E (2001) Visual disorders in children with brain lesions: 1. Maturation of visual function in infants with neonatal brain lesions: correlation with neuroimaging. Eur J Paediatr Neurol, 5:107–14. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, D'Acunto G, Rose S, Tinelli F, Boyd R, Cioni G (2010) Plasticity of the visual system after early brain damage. Developmental medicine and child neurology, 52:891–900. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Fiori S, Scelfo D, Conti E, Bancale A (2013) Reorganization of visual fields after periventricular haemorrhagic infarction: potentials and limitations. Developmental medicine and child neurology, 55 Suppl 4:23–6. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Tinelli F, Del Viva MM, Bancale A, Arrighi R, Pascale RR, Cioni G (2009) Motion perception in preterm children: role of prematurity and brain damage. Neuroreport, 20:1339–43. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI (1991) Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proceedings of the National Academy of Sciences of the United States of America, 88:1621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine L, Bahri MA, Cavaliere C, Soddu A, Laureys S, Ptito M, Kupers R (2015) Prevalence of increases in functional connectivity in visual, somatosensory and language areas in congenital blindness. Front Neuroanat, 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houliston MJ, Taguri AH, Dutton GN, Hajivassiliou C, Young DG (1999) Evidence of cognitive visual problems in children with hydrocephalus: a structured clinical history-taking strategy. Dev Med Child Neurol, 41:298–306. [DOI] [PubMed] [Google Scholar]
- Hoyt CS (2003) Visual function in the brain-damaged child. Eye, 17:369–84. [DOI] [PubMed] [Google Scholar]
- Hoyt CS (2007) Brain injury and the eye. Eye, 21:1285–9. [DOI] [PubMed] [Google Scholar]
- Hoyt CS, Fredrick DR (1998) Cortically visually impaired children: a need for more study. The British journal of ophthalmology, 82:1225–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo R, Burden SK, Hoyt CS, Good WV (1999) Chronic cortical visual impairment in children: aetiology, prognosis, and associated neurological deficits. The British journal of ophthalmology, 83:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan JE, Groenveld M, Anderson DP (1993) Photophobia and cortical visual impairment. Dev Med Child Neurol, 35:473–7. [DOI] [PubMed] [Google Scholar]
- Jan JE, Groenveld M, Sykanda AM, Hoyt CS (1987) Behavioural characteristics of children with permanent cortical visual impairment. Dev Med Child Neurol, 29:571–6. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Korogi Y, Hirai T, Hamatake S, Ikushima I, Sugahara T, Shigematsu Y, Takahashi M, Mukuno K (1997) MR changes in the calcarine area resulting from retinal degeneration. AJNR Am J Neuroradiol, 18:1291–5. [PMC free article] [PubMed] [Google Scholar]
- Kong L, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG (2012) An update on progress and the changing epidemiology of causes of childhood blindness worldwide Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus, 16:501–7. [DOI] [PubMed] [Google Scholar]
- Kooiker MJ, Pel JJ, van der Steen-Kant SP, van der Steen J (2016a) A Method to Quantify Visual Information Processing in Children Using Eye Tracking. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooiker MJ, Pel JJ, Verbunt HJ, de Wit GC, van Genderen MM, van der Steen J (2016b) Quantification of visual function assessment using remote eye tracking in children: validity and applicability. Acta Ophthalmol, 94:599–608. [DOI] [PubMed] [Google Scholar]
- Kozeis N (2010) Brain visual impairment in childhood: mini review. Hippokratia, 14:249–51. [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M (2013) The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci, 17:26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Ptito M (2011) Insights from darkness: what the study of blindness has taught us about brain structure and function. Prog Brain Res, 192:17–31. [DOI] [PubMed] [Google Scholar]
- Kupers R, Ptito M (2014) Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neuroscience and biobehavioral reviews, 41:36–52. [DOI] [PubMed] [Google Scholar]
- Lao Y, Kang Y, Collignon O, Brun C, Kheibai SB, Alary F, Gee J, Nelson MD, Lepore F, Lepore N (2015) A study of brain white matter plasticity in early blinds using tract-based spatial statistics and tract statistical analysis. Neuroreport, 26:1151–4. [DOI] [PubMed] [Google Scholar]
- Lennartsson F, Nilsson M, Flodmark O, Jacobson L (2014) Damage to the immature optic radiation causes severe reduction of the retinal nerve fiber layer, resulting in predictable visual field defects. investigative ophthalmology & visual science, 55:8278–88. [DOI] [PubMed] [Google Scholar]
- Lessard N, Pare M, Lepore F, Lassonde M (1998) Early-blind human subjects localize sound sources better than sighted subjects. Nature, 395:278–80. [DOI] [PubMed] [Google Scholar]
- Li J, Liu Y, Qin W, Jiang J, Qiu Z, Xu J, Yu C, Jiang T (2013) Age of onset of blindness affects brain anatomical networks constructed using diffusion tensor tractography. Cerebral cortex, 23:542–51. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T (2007) Whole brain functional connectivity in the early blind. Brain : a journal of neurology, 130:2085–96. [DOI] [PubMed] [Google Scholar]
- Loomis JM, Blascovich JJ, Beall AC (1999) Immersive virtual environment technology as a basic research tool in psychology. Behav Res Methods Instrum Comput, 31:557–64. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ (2014) ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci, 8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre-Beon C, Ibrahim H, Hay I, Cockburn D, Calvert J, Dutton GN, Bowman R. (2010) Dorsal Stream Dysfunction in Children: A Review and an Approach to Diagnosis and Management Current Pediatric Reviews, 6:00–00. [Google Scholar]
- Matteau I, Kupers R, Ricciardi E, Pietrini P, Ptito M (2010) Beyond visual, aural and haptic movement perception: hMT+ is activated by electrotactile motion stimulation of the tongue in sighted and in congenitally blind individuals. Brain research bulletin, 82:264–70. [DOI] [PubMed] [Google Scholar]
- McKillop E, Dutton GN (2008) Impairment of vision in children due to damage to the brain: a practical approach. Br Ir Orthopt J:8–14. [Google Scholar]
- Merabet LB, Mayer DL, Bauer CM, Wright D, Kran BS (2017) Disentangling How the Brain is "Wired" in Cortical (Cerebral) Visual Impairment. Semin Pediatr Neurol, 24:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A (2010) Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci, 11:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA (1983) Object vision and spatial vision: two cortical pathways. Trends in neurosciences, 6:414–417. [Google Scholar]
- Morrone MC, Guzzetta A, Tinelli F, Tosetti M, Del Viva M, Montanaro D, Burr D, Cioni G (2008) Inversion of perceived direction of motion caused by spatial undersampling in two children with periventricular leukomalacia. J Cogn Neurosci, 20:1094–106. [DOI] [PubMed] [Google Scholar]
- Murakami A, Morimoto M, Yamada K, Kizu O, Nishimura A, Nishimura T, Sugimoto T (2008) Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics, 122:500–6. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB (1988) A selective impairment of motion perception following lesions of the middle temporal visual area (MT). The Journal of neuroscience : the official journal of the Society for Neuroscience, 8:2201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L (2012) Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: a diffusion tensor imaging study. Developmental medicine and child neurology, 54:38–43. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB (2005) The plastic human brain cortex. Annu Rev Neurosci, 28:377–401. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Staudt M, Sokolov A, Birbaumer N, Krageloh-Mann I (2003) Perception and production of biological movement in patients with early periventricular brain lesions. Brain : a journal of neurology, 126:692–701. [DOI] [PubMed] [Google Scholar]
- Pavlova MA, Krageloh-Mann I (2013) Limitations on the developing preterm brain: impact of periventricular white matter lesions on brain connectivity and cognition. Brain : a journal of neurology, 136:998–1011. [DOI] [PubMed] [Google Scholar]
- Pehere N, Chougule P, Dutton GN (2018) Cerebral visual impairment in children: Causes and associated ophthalmological problems. Indian J Ophthalmol, 66:812–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip SS, Dutton GN (2014) Identifying and characterising cerebral visual impairment in children: a review. Clinical & experimental optometry, 97:196–208. [DOI] [PubMed] [Google Scholar]
- Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, Reynolds LC, Walker S, Rogers C, Mathur AM, Van Essen DC, Inder T (2014) Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. The Journal of pediatrics, 164:52–60 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptito M, Schneider FC, Paulson OB, Kupers R (2008) Alterations of the visual pathways in congenital blindness. Experimental brain research, 187:41–9. [DOI] [PubMed] [Google Scholar]
- Rahi JS (2007) Childhood blindness: a UK epidemiological perspective. Eye (Lond), 21:1249–53. [DOI] [PubMed] [Google Scholar]
- Raz N, Amedi A, Zohary E (2005) V1 activation in congenitally blind humans is associated with episodic retrieval. Cerebral cortex, 15:1459–68. [DOI] [PubMed] [Google Scholar]
- Reich L, Szwed M, Cohen L, Amedi A (2011) A ventral visual stream reading center independent of visual experience. Current biology : CB, 21:363–8. [DOI] [PubMed] [Google Scholar]
- Reislev NL, Dyrby TB, Siebner HR, Kupers R, Ptito M (2016) Simultaneous Assessment of White Matter Changes in Microstructure and Connectedness in the Blind Brain. Neural Plast, 2016:6029241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi E, Pietrini P (2011) New light from the dark: what blindness can teach us about brain function. Curr Opin Neurol, 24:357–63. [DOI] [PubMed] [Google Scholar]
- Rokem A, Takemura H, Bock AS, Scherf KS, Behrmann M, Wandell BA, Fine I, Bridge H, Pestilli F (2017) The visual white matter: The application of diffusion MRI and fiber tractography to vision science. J Vis, 17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M (1996) Activation of the primary visual cortex by Braille reading in blind subjects. Nature, 380:526–8. [DOI] [PubMed] [Google Scholar]
- Sakki HEA, Dale NJ, Sargent J, Perez-Roche T, Bowman R (2018) Is there consensus in defining childhood cerebral visual impairment? A systematic review of terminology and definitions. Br J Ophthalmol, 102:424–432. [DOI] [PubMed] [Google Scholar]
- Serdaroglu G, Tekgul H, Kitis O, Serdaroglu E, Gokben S (2004) Correlative value of magnetic resonance imaging for neurodevelopmental outcome in periventricular leukomalacia. Developmental medicine and child neurology, 46:733–9. [DOI] [PubMed] [Google Scholar]
- Shu N, Li J, Li K, Yu C, Jiang T (2009a) Abnormal diffusion of cerebral white matter in early blindness. Human brain mapping, 30:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu N, Liu Y, Li J, Li Y, Yu C, Jiang T (2009b) Altered anatomical network in early blindness revealed by diffusion tensor tractography. PloS one, 4:e7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie LTL, Rombouts SA, Valk IJ, Hart AA, Scheltens P, van der Knaap MS (2001) Functional MRI of visual cortex in sedated 18 month-old infants with or without periventricular leukomalacia. Dev Med Child Neurol, 43:486–90. [DOI] [PubMed] [Google Scholar]
- Singh AK, Phillips F, Merabet LB, Sinha P (2018) Why Does the Cortex Reorganize after Sensory Loss? Trends Cogn Sci, 22:569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoczenski AM, Norcia AM (1999) Development of VEP Vernier acuity and grating acuity in human infants. Invest Ophthalmol Vis Sci, 40:2411–7. [PubMed] [Google Scholar]
- Solebo AL, Teoh L, Rahi J (2017) Epidemiology of blindness in children. Arch Dis Child, 102:853–857. [DOI] [PubMed] [Google Scholar]
- Striem-Amit E, Cohen L, Dehaene S, Amedi A (2012a) Reading with sounds: sensory substitution selectively activates the visual word form area in the blind. Neuron, 76:640–52. [DOI] [PubMed] [Google Scholar]
- Striem-Amit E, Dakwar O, Reich L, Amedi A (2012b) The large-scale organization of "visual" streams emerges without visual experience. Cerebral cortex, 22:1698–709. [DOI] [PubMed] [Google Scholar]
- Striem-Amit E, Ovadia-Caro S, Caramazza A, Margulies DS, Villringer A, Amedi A (2015) Functional connectivity of visual cortex in the blind follows retinotopic organization principles. Brain : a journal of neurology, 138:1679–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarte R, Lequin M, Cherian P, Zecic A, van Goudoever J, Govaert P (2009) Imaging patterns of brain injury in term-birth asphyxia. Acta Paediatr, 98:586–92. [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Warren WH (2002) Virtual reality in behavioral neuroscience and beyond. Nat Neurosci, 5 Suppl:1089–92. [DOI] [PubMed] [Google Scholar]
- Taylor HR, Pezzullo ML, Keeffe JE (2006) The economic impact and cost of visual impairment in Australia. The British journal of ophthalmology, 90:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NM, Jakobson LS, Maurer D, Lewis TL (2009) Differential vulnerability of global motion, global form, and biological motion processing in full-term and preterm children. Neuropsychologia, 47:2766–78. [DOI] [PubMed] [Google Scholar]
- Uggetti C, Egitto MG, Fazzi E, Bianchi PE, Bergamaschi R, Zappoli F, Sibilla L, Martelli A, Lanzi G (1996) Cerebral visual impairment in periventricular leukomalacia: MR correlation. AJNR Am J Neuroradiol, 17:979–85. [PMC free article] [PubMed] [Google Scholar]
- Van Boven RW, Hamilton RH, Kauffman T, Keenan JP, Pascual-Leone A (2000) Tactile spatial resolution in blind braille readers. Neurology, 54:2230–6. [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet neurology, 8:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P (2019) Brain (re)organization following visual loss. Wiley Interdiscip Rev Cogn Sci, 10:e1468. [DOI] [PubMed] [Google Scholar]
- Voss P, Gougoux F, Zatorre RJ, Lassonde M, Lepore F (2008) Differential occipital responses in early- and late-blind individuals during a sound-source discrimination task. NeuroImage, 40:746–58. [DOI] [PubMed] [Google Scholar]
- Voss P, Zatorre RJ (2012) Occipital cortical thickness predicts performance on pitch and musical tasks in blind individuals. Cereb Cortex, 22:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Qin W, Liu Y, Zhang Y, Jiang T, Yu C (2013) Altered white matter integrity in the congenital and late blind people. Neural Plast, 2013:128236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Qin W, Liu Y, Zhang Y, Jiang T, Yu C (2014) Altered resting-state network connectivity in congenital blind. Human brain mapping, 35:2573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Cowey A, Alexander I, Filippini N, Kennedy JM, Smith SM, Ragge N, Bridge H (2012) Language networks in anophthalmia: maintained hierarchy of processing in 'visual' cortex. Brain : a journal of neurology, 135:1566–77. [DOI] [PubMed] [Google Scholar]
- Watson T, Orel-Bixler D, Haegerstrom-Portnoy G (2010) Early visual-evoked potential acuity and future behavioral acuity in cortical visual impairment. Optom Vis Sci, 87:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JM, Gilmore RO, Shaikh SM, Kunselman AR, Trescher WV, Tashima LM, Boltz ME, McAuliffe MB, Cheung A, Fesi JD (2012) Defective motion processing in children with cerebral visual impairment due to periventricular white matter damage. Developmental medicine and child neurology, 54:e1–8. [DOI] [PubMed] [Google Scholar]
- Whiting S, Jan JE, Wong PK, Flodmark O, Farrell K, McCormick AQ (1985) Permanent cortical visual impairment in children. Dev Med Child Neurol, 27:730–9. [DOI] [PubMed] [Google Scholar]
- Wiklund LM, Uvebrant P (1991) Hemiplegic cerebral palsy: correlation between CT morphology and clinical findings. Dev Med Child Neurol, 33:512–23. [DOI] [PubMed] [Google Scholar]
- Wong M, Gnanakumaran V, Goldreich D (2011) Tactile spatial acuity enhancement in blindness: evidence for experience-dependent mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience, 31:7028–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Guo Q, Fan G, Liu N (2011) Assessment of cortical visual impairment in infants with periventricular leukomalacia: a pilot event-related FMRI study. Korean J Radiol, 12:463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Liu Y, Li J, Zhou Y, Wang K, Tian L, Qin W, Jiang T, Li K (2008) Altered functional connectivity of primary visual cortex in early blindness. Human brain mapping, 29:533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihl J, Dutton GH (2015) Visual Disorders In: Zihl J, G.N.D., editor. Cerebral Visual Impairment in Children: Visuoperceptive and Visuocogntive Disorders: Springer-Verlag Wien; p 61–115. [Google Scholar]