Abstract
Ferric chloride-induced distal middle cerebral artery occlusion (MCAO) model of stroke was described in mice several years ago, however it lacked in-depth evaluation of the post-stroke functional outcomes in the animals. In this study, we reproduced the recently developed model and expanded its characterization by thorough evaluation of blood supply, cerebral infarction, and motor function in adult male and female mice up to 14 days after stroke. Our observations indicate near complete interruption of blood flow in the distal MCA shortly after application of 20% ferric chloride over the artery through a cranial window, which remained occluded for at least 4 hours. As expected, infarction of the brain tissue, documented by TTC and hematoxylin stains, was restricted to the cerebral cortex. We also systematically evaluated motor impairment of the animals in this model. For this, a series of studies were carried out in male and female mice up to 14 days after stroke, and motor function was assessed in cylinder and grid-walking tests in blinded manner. Contrary to our expectations, the results of both motor tests indicated minor, transient motor deficit in mice after stroke. Based on these observations, we conclude that the mouse ferric chloride-induced distal MCAO model is likely not suitable for proof-of-concept and preclinical studies where motor function is an important outcome measure.
Keywords: FeCl3-induced distal MCAO model of stroke, motor impairment, grid-walking test, cylinder test, mouse stroke model
1. Introduction
Stroke continues to be a leading cause of death and long-term disability worldwide with an unmet need for new treatments [1]. To aid identification and understanding of pathological mechanisms and development of new therapies, variety of animal stroke models have been established in the last several decades. Among them, the rodent models are most accessible and used by investigators in the stroke research field [2]. Ischemic stroke models in rodents have been developed to induce transient or permanent occlusion of an artery supplying blood to the brain through various techniques spanning from low/no invasive to very invasive procedures [3, 4]. In majority of cases rodent ischemic stroke models focus on occlusion of the middle cerebral artery (MCA), because in humans obstruction of blood flow in the MCA is more common than in other brain arteries. In these models, occlusion of the MCA is done distally or proximally, where the distal occlusion has advantages of low mortality and ischemic lesions occurring primarily in the cerebral cortex (i.e. smaller, more restricted infarction), whereas proximal occlusion being more invasive and leading to larger strokes [4].
It is widely accepted that improvement of impaired neurological function(s) should be a primary endpoint in experimental and proof-of-concept therapeutic preclinical stroke studies, because it is a greatly important but currently unmet therapeutic modality for disabled stroke survivors [5]. Numerous tests have been developed to evaluate the neurobehavioral functional deficit and outcome in rodent stroke models by assessing motor, somatosensory, cognitive or other functions [6–10]. Notably, not all tests are suitable for every rodent stroke model, because different models mimic various clinical forms of stroke and hence result in different neurologic dysfunction(s) [11–14]. In addition, while mice are the primary experimental species in stroke research field and many functional tests have been adapted from the rat, testing methods and principles continue to vary between these two species and make comparison of the test results challenging [8, 13].
Several years ago, Karatas and colleagues introduced a new model of distal MCAO in mice, where ferric chloride was used to permanently occlude the distal MCA [15]. Similar to other distal MCAO models, in this approach opening of a small cranial window was required, followed by brief application of ferric chloride-soaked, small piece of filter paper over dura matter on the artery. The latter offered to be the least invasive approach to achieve occlusion of the artery, because other techniques did so while piercing (ligation method [16]) or compressing the dura matter (compression method [17]), or injuring the brain tissue surrounding the artery (electrocoagulation, [18]). Because of these potential advantages, the purpose of this study was to reproduce the mouse ferric chloride-induced model of stroke and further characterize it in regard to permanency of the distal MCA occlusion, cerebral infarction, and motor functional deficit in adult male and female mice for up to 14 days after stroke. Our observations confirm near complete interruption of blood flow in the distal MCA with application of 20% ferric chloride, which lasts for at least four hours and results in reproducible cerebral cortical infarction. However, contrary to our expectations, systematic evaluation of motor function in cylinder and grid-walking tests indicates minor, transient motor deficit in mice after stroke in this model. Based on these observations, we conclude that the mouse ferric chloride-induced distal MCAO model is likely not suitable for experimental studies where motor function is an important outcome measure.
2. Methods
2.1. Animals and study design
Ten to sixteen-week-old male CD-1 (received from Charles River Laboratories) and male and female B6;129 (received from the Mutant Mouse Regional Resource Center and bred in-house) mice were used in this study which was approved by Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee. Mice were maintained in 12-h light/dark cycle and fed ad libitum in groups of 2 – 4 per cage. CD-1 male mice were used to establish the ferric chloride-induced distal MCAO model, monitor continuity of the artery occlusion for up to 4 hours, and verify cerebral cortical infarction by 2,3,5-triphenyltetrazolium chloride (TTC) staining on days 1 and 3 after stroke. B6;129 mice were used to confirm cerebral cortical infarction by hematoxylin staining at 4 hours and 1, 3, 7 and 14 days post-stroke, and for monitoring of motor function for up to 14 days after stroke. For the latter studies, both male and female mice were used and their assignment to experimental groups was done randomly (https://www.random.org/lists/) before stroke surgeries. The animals were identified by tail-marks and their affiliation with a specific experimental group was blinded from the involved experimenters. Monitoring of motor function was carried out in three independent experimental sets: #1, male mice monitored for up to 7 days post-stroke (n = 8/stroke group and n = 6/sham group); #2, female mice monitored for up to 7 days post-stroke (n = 8/both stroke and sham groups); #3, male mice monitored for up to 14 days post-stroke (n = 8/stroke group and n = 5/sham group).
2.2. Occlusion of the distal middle cerebral artery by ferric chloride
For this we followed the protocol detailed in the original article by Karatas and colleagues [15]. Briefly, under isoflurane anesthesia an incision was made between the left eye and ear of the mouse, the temporalis muscle was cut, and a small cranial window was opened on parietal bone to access the MCA downstream of the lenticulostriate branches, while keeping the overlying dura mater intact. A rectangular piece of Whatman filter paper (~0.3 × 1 mm) was soaked in freshly prepared 20% solution of ferric chloride and placed over the accessed portion of the MCA on dry dura mater. After 4 minutes, the filter paper was removed, the area was gently dried by aspiration and the cranial window was closed by bone wax. Control animals underwent sham operation, which included opening and closure of the cranial window without application of ferric chloride. Note that during pilot experiments, 10% solution of ferric chloride was used as originally described by Karatas and colleagues [15], however, incomplete and reversible occlusion of the artery was observed (data not shown). Because of this, we adopted use of 20% ferric chloride solution, similar to Le Behot and colleagues [19], which resulted in consistent, permanent occlusion of the distal MCA. Throughout the manuscript, the right (contralateral) forelimb of both sham and stroke mice is referred to as ‘affected forelimb’, whereas the left (ipsilateral) forelimb as ‘unaffected forelimb’.
2.3. Cerebral blood flow monitoring
In each mouse undergoing stroke surgery, blood flow in the MCA was monitored through the skull immediately distally from the cranial window by using a laser Doppler monitor attached to a non-flexible probe (moorLAB, Moor Instruments). The blood flow was continuously recorded 5 minutes before placing of the filter paper, during the period when the filter paper was placed (4 min) and ~15 min after removal of the filter paper. In a cohort of five mice, the blood flow was continuously monitored for 4 hours, under anesthesia, after removal of the filter paper to confirm permanent occlusion of the MCA.
2.4. 2,3,5-Triphenyltetrazolium chloride (TTC) staining
TTC staining was used to document brain infarction in 1 mm coronal forebrain sections (using slicing matrix; Zivic Instruments) obtained from sham and stroke-operated mice 1 and 3 days after stroke. For this we followed a protocol detailed in our earlier publications [20, 21].
2.5. Cryosection and hematoxylin staining
In one set of experiments at 4 hours and 1, 3, 7 and 14 days after stroke, mice were deeply anesthetized with isoflurane and cardially perfused with 4% paraformaldehyde (PFA), followed by overnight incubation of the brain in 4% PFA, cryopreservation in 20 and 30% sucrose solutions and cryosectioning (coronal plane at 50 μm thickness). Hematoxylin staining (Hematoxylin Stain+; Fisher HealthCare) was carried out according the manufacturer’s recommended protocol.
2.6. Measurement of infarct volume
The stained brain sections were digitized using a flat-bed scanner and used to measure the infarct volume using NIH ImageJ software as described earlier [22].
2.7. Behavioral studies
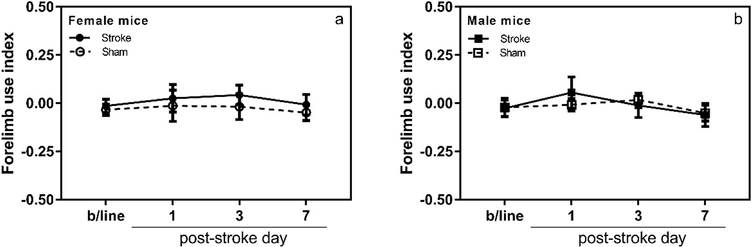
To minimize handling stress during behavioral tests, mice were individually handled for ~2 min once or twice daily for several days before the tests were performed. Grid-walking and cylinder tests were used to assess the motor function by blinded investigators 2 – 3 days before and 1, 3, 7 and 14 days after stroke. The tests were performed during the light cycle (8 to 11 am). Blinded analysis of video recordings for experimental sets #1 and #2 were carried out by one investigator (NS), whereas for experimental set #3 it was done independently by two investigators (NS and FA). The data obtained by two investigators were very similar and the average values from both investigators are presented in Fig. 6.
Figure 6. Grid-walking and cylinder tests (14 day-long study; experimental set #3).
Panel a, grid-walking test, mice showed mild, transient functional deficit in the affected forepaw on day 3 after stroke when compared to its baseline performance (p < 0.05). However, no statistically significant difference was observed in performance of the affected forepaw between sham and stroke-operated mice on day 3 after stroke (p > 0.05). Panel b, grid-walking test, no functional deficit was observed in the un-affected forepaw of sham and stroke-operated animals. Panel c, cylinder test, neither sham nor stroke-operated mice showed functional deficit after ferric chloride-induced distal MCAO on any of the evaluation days (n =5 for sham and n = 12 for stroke groups; *, p < 0.05 in comparison to the stroke group baseline).
2.8. Grid-walking test
In this test, the mice were allowed to freely walk on an elevated wire grid (12 mm square wire mesh with total area of 33 cm x 20 cm) for 5 minutes and their movements were video recorded. The recording was later analyzed for foot faults and total number of steps in slow motion as described in our earlier publication [23].
2.9. Cylinder test
In this test, the mice were placed in a transparent, acrylic cylinder (17 cm height, 10 cm diameter) and their behavior was video recorded for 5 minutes. The recording was later analyzed to evaluate the symmetry of forelimb use upon rearing and exploration of the cylinder wall as described in detail in our earlier publication [23].
2.10. Statistical analysis
Behavioral data were analyzed using two-way repeated measures ANOVA and followed by Dunnett’s or Sidak’s post-hoc tests, as appropriate, for multiple comparisons (Prism 7.02, GraphPad). The statistical significance level was p< 0.05, values shown are mean ± standard error.
3. Results
3.1. Blood flow monitoring after occlusion of the distal MCA by ferric chloride
To confirm permanent occlusion of the MCA after application of ferric chloride, in one set of experiments blood flow in the MCA was continuously monitored in anesthetized mice for 4 hours after removal of the filter paper soaked in 20% ferric chloride solution. The average reduction of the blood flow in the distal MCA reached ~85% of its baseline within ~10 minutes after removal of the filter paper and remained at that level for at least 4 hours (Fig. 1).
Figure 1. Blood flow monitoring in the distal MCA by laser Doppler flowmetry.
Panel a, representative tracing of the blood flow in a mouse distal MCA during occlusion by ferric chloride. Note, ~5 min of tracing is not presented for the period immediately after removal of the filter paper (due to distortion of the recording during removal of the filter paper and drying of the surface by gentle aspiration). Panel b, average tracing of blood flow in the distal MCA for up to 4 hours after stroke (n = 5).
3.2. TTC and hematoxylin staining, and infarct volume measurement
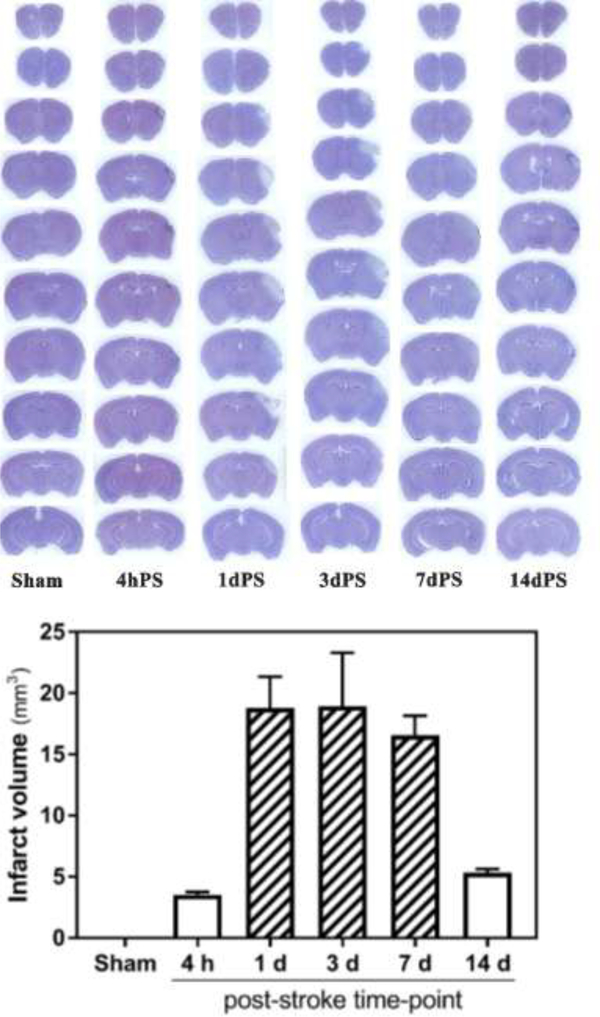
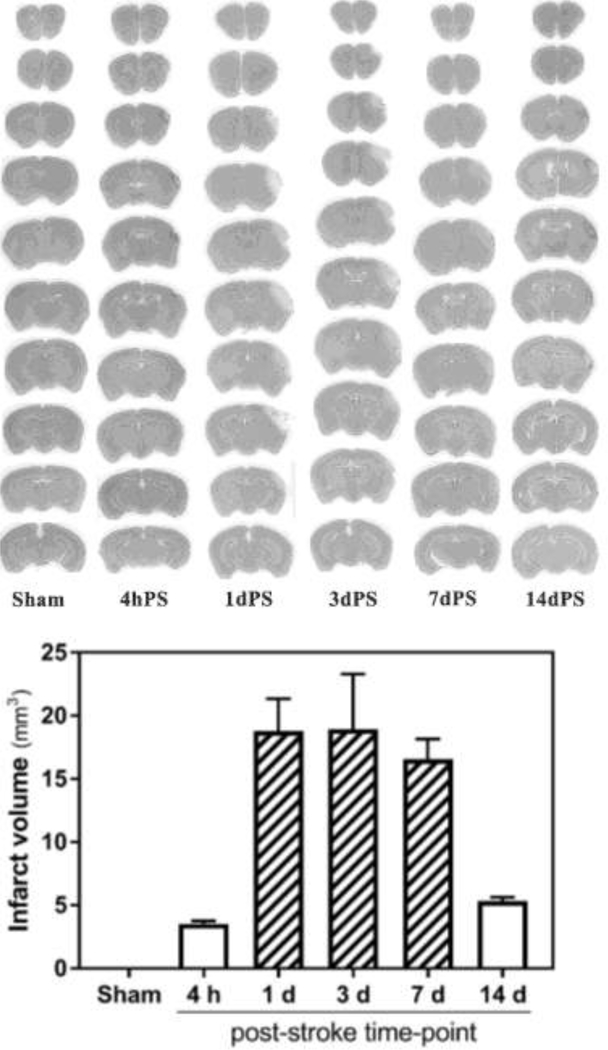
To obtain more complete information about progression of the injury in this stroke model, in a separate set of experiments the brains were collected after stroke followed by staining and measurement of infarction. As expected, both TTC (Fig. 2) and hematoxylin (Fig. 3) staining of the coronal brain sections indicated infarction of the tissue restricted to cerebral cortex without appreciable edema formation. Hematoxylin staining allowed detection of infarction starting from 4 hours after occlusion of the distal MCA which could be observed for at least 14 days after stroke (Fig. 3). The calculated mean infarct volume was 3.52±0.24 mm3 at 4 hours after stroke, 18.78±2.56 mm3 at 1 day after stroke, 18.93±4.37 mm3 at 3 day after stroke, 16.56±1.59 mm3 at 7 days after stroke, and 5.34±0.30 mm3 at 14 days after stroke.
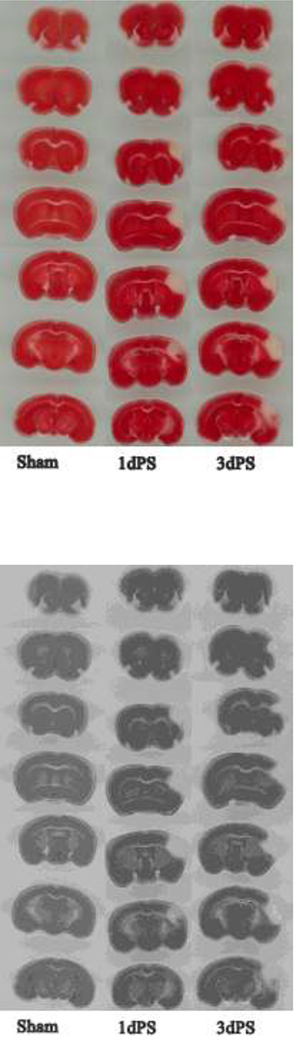
Figure 2. Representative TTC-stained coronal brain sections of sham and distal MCAO-operated CD-1 mice on days 1 and 3 after stroke.
Calculated infarct volume is 23.2 ± 1.68 mm3 on day 1 post-stroke (1d PS) and 23.5 ± 5.3 mm3 on day 3 post-stroke (3d PS).
Figure 3. Hematoxylin-stained coronal brain sections of sham and distal MCAO-operated B6;129 mice.
Top panel, representative brains 4 hours and 1, 3, 7 and 14 days after stroke. Bottom panel, calculated infarct volumes (n = 3 per post-stroke time-point): 3.52 ± 0.24 mm2 (4 h), 18.78 ± 2.56 mm2 (1 d), 18.93 ± 4.37 mm2 (3 d), 16.56 ± 1.59 mm2 (7 d), 5.34 ± 0.30 mm2 (14 d).
3.3. Grid-walking test (experimental sets 1 and 2)
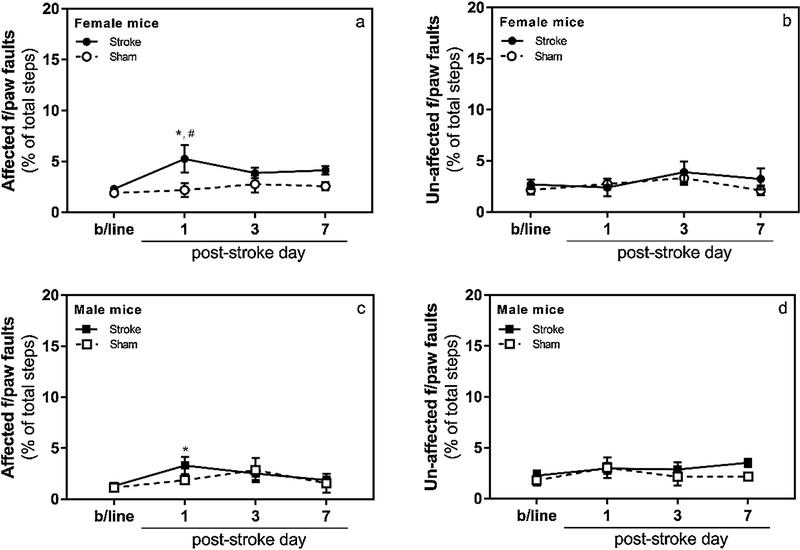
Contrary to our expectation, the focal cerebral stroke in this model did not cause a significant deficit in the function of the contralateral, i.e. stroke-affected, forelimb in the grid-walking test in female (Fig. 4; n =8 sham and 8 stroke; group x day interaction F (3, 42) = 2.158, p = 0.11) and male (Fig. 4; n = 6 sham and 8 stroke; group x day interaction F (3,36) = 0.9028, p = 0.45) mice. Within the stroke group, post-hoc analyses with Dunnett’s correction revealed statistically significant difference in contralateral forelimb function between baseline and post-stroke day 1 for both females and males (p = 0.001 and 0.026, respectively), but not post-stroke days 3 and 7 (p > 0.05). As expected, within the sham group no statistically significant difference was observed in the function of either forelimb between baseline and any of the post-stroke days (p > 0.05; both females and males). Within day comparisons of female sham and stroke animals (post hoc analyses with Sidak’s correction) showed statistically significant difference of the contralateral forelimb function between the two groups only on post-stroke day 1 (p < 0.01). Within day comparisons of male sham and stroke animals did not reveal statistically significant difference between the two groups on any of the evaluation days (p > 0.05). Similar comparisons carried out for the ipsilateral, i.e. unaffected, forelimb did not reveal statistically significant difference between any of the groups on any of the evaluation days (p > 0.05; both females and males).
Figure 4. Grid-walking test.
Following ferric chloride-induced distal MCAO mice exhibited mild to moderate but transient functional deficit in the affected forepaw (i.e., increased number of footfaults; panels a and c) on day 1 after stroke (n =8 for sham and stroke female mice, n = 6 for sham and n = 8 for stroke male mice; *, p < 0.05 in comparison to the stroke group baseline; #, p < 0.05 in comparison to the same day of the sham group). As expected, no functional deficit was observed in the un-affected (i.e., ipsilateral, panels b and d) forepaw of stroke and either forepaw of sham-operated animals.
3.4. Cylinder test (experimental sets 1 and 2)
Similarly, the cylinder test did not reveal a significant deficit in the function of the contralateral, i.e. stroke-affected, forelimb in female (Fig. 5; n = 8 sham and 8 stroke; group x day interaction F (3, 42) = 0.04544, p = 0.98) and male (Fig. 5; n = 6 sham and 8 stroke; group x day interaction F (3, 36) = 0.2311, p = 0.87) mice. Post-hoc analyses with Dunnett’s correction confirmed lack of significant differences between forelimb function at baseline and any of the post-stroke evaluation days within each stroke group (p > 0.05; both females and males). Likewise, within day comparisons of male sham and stroke, or female sham and stroke groups (post-hoc analyses with Sidak’s correction) did not reveal statistically significant difference for any of the evaluation days (p > 0.05).
Figure 5. Cylinder test.
Mice did not show functional deficit in the affected forelimb (i.e., decreased use of the affected forelimb upon rears) after ferric chloride-induced distal MCAO. As expected, sham-operated animals also lacked functional deficit (n =8 for sham and stroke female mice, n = 6 for sham and n = 8 for stroke male mice; p > 0.05 for all within group and within day comparisons).
Because of this unexpected outcome, we carried out a new set of experiments in male mice (n = 12 for stroke group and n = 5 for the sham group) and evaluated the motor function in both tests for up to 14 days after stroke.
3.5. Grid-walking and cylinder tests (experimental set 3)
In this new group of male mice, the grid walking test did not reveal a significant deficit in the function of the stroke-affected forelimb (Fig. 6; n = 5 sham and 12 stroke; group x day interaction F (3, 45) = 0.4714, p = 0.70). Within the stroke group, post-hoc analysis with Dunnett’s correction revealed statistically significant difference in affected forelimb function between baseline and post-stroke day 3 (p = 0.018) but not post-stroke days 7 and 14 (p > 0.05). Within day comparisons of sham and stroke animals (post hoc analyses with Sidak’s correction) did not show statistically significant difference of the affected forelimb function on any of the evaluation days (p > 0.05). Similarly, these mice did not show significant functional deficit in cylinder test (Fig. 6; n = 5 sham and 12 stroke; group x day interaction F (3, 45) = 0.4728, p = 0.70). Both within group (baseline vs post-stroke days) and within day (sham vs stroke on any of the evaluation days) comparisons did not reveal statistically significant differences (p > 0.05 for all comparisons).
4. Discussion
In this study we successfully reproduced ferric chloride-induced distal MCAO model of stroke in mice. Similar to the original report by Karatas and colleagues [15] application of a filter paper soaked in 10% ferric chloride solution did not result in complete occlusion of the distal MCA in our hands. Because of this, we adopted the use of 20% ferric chloride solution as reported by Le Behot and colleagues [19], which consistently resulted in near complete occlusion of the distal MCA lasting for at least 4 hours (Fig. 1). As expected, brain infarction in this model was restricted to cerebral cortex (Figs. 2 and 3). It is important to note that application of ferric chloride over the cerebral cortex away from the MCA, did not result in injury of the brain tissue as determined by TTC staining 24 hours later. This observation does not exclude the possibility that ferric chloride could be harmful to brain cells at microscopic/molecular level, however, it confirms that the documented infarction of the brain by a brief exposure of ferric chloride (4 min in our experiments) is primarily due to occlusion of the distal MCA. Arguably, among various techniques that are utilized to achieve occlusion of the distal MCA in mice (such as electrocoagulation [18], ligation [16], microinjection of thrombin [24], compression with needle [11]), the use of ferric chloride is methodologically the easiest and least invasive. In addition, formation of platelet-rich thrombus and subsequent average size cortical lesion closely mimics the human condition. Therefore, it is not surprising that a number of laboratories have adopted the ferric chloride-induced distal MCAO model of stroke to address important questions related to pathophysiology of ischemic stroke as well as its pharmacological therapy [25–30]. However, it is noteworthy that neither the original study [15], nor most of the experimental studies using this stroke model included robust functional tests to thoroughly evaluate the functional deficit in mice after stroke. In our opinion, this is an important gap, because the improvement of impaired neurological function(s) is considered to be a primary endpoint in experimental and proof-of-concept therapeutic preclinical stroke studies [5, 13]. To address this, we designed a series of experiments to systematically evaluate motor function of adult male and female mice in acute and post-acute phases of stroke (for up to 14 days after stroke) in this model. For this we chose two motor tests (grid-walking, also referred to as foot-fault test, and cylinder test, also referred to as spontaneous forelimb use task), which have been used by multiple laboratories for over a decade and are very sensitive in tracking functional impairment weeks to months following the stroke injury [22, 23, 31–35]. To our surprise, only a marginal impairment of the motor function (in both male and female mice) was observed in grid walking test on day 1 after stroke, which returned to near basal levels on days 3 and 7 (Figs. 4 and 5). However, the transient motor deficit was not observed in the cylinder test in either of the sexes (Figs. 4 and 5). In order to confirm this observation, we carried out a new set of surgeries in a separate cohort of male mice and extended the study up to 14 days after stroke. Similar to the first two cohorts, in this new experiment also we failed to observe substantial motor deficit in mice after stroke (Fig. 6).
Although it is a limitation that we used only two motor behavioral tests, these unexpected findings made us take a closer look at published studies which utilized the ferric chloride-induced distal MCAO model of stroke in mice. In their original study Karatas and colleagues used neurological scoring (0 to 5 scale, 0 corresponding to no observable deficit, and 5 to lack of spontaneous motion) to evaluate the neurological status of mice for up to 3 days after stroke and documented mild deficit (score of 2 ± 0.3). Interestingly, another group reported use of the ferric chloride-induced distal MCAO model of stroke in mice nearly at the same time as Karatas and colleagues [36]. The authors of this study did not observe neurobehavioral deficit using the modified neurological severity score (mNSS) test for up to 4 days after stroke. It is noteworthy, that among all published studies which referred to Karatas et al. 2011 paper and used the ferric chloride-induced distal MCAO model of stroke in mice, only four studies included behavioral/functional evaluation of animals after stroke. The most extensive study is by Cisbani and colleagues [37], who reported functional assessment of mice using cylinder and pole tests, and neurological scoring for up to 7 days after stroke. The authors did not observe any appreciable deficit in mice using these tests, however they documented statistically significant differences in nesting behaviors of mice before and after stroke. In a later study, Karatas and colleagues reproduced their earlier observation documenting mild neurological deficit in mice 1 day after stroke [38]. In this study, they used neurological severity scoring (NSS, 0 to 10 sale, 0 corresponding to no observable deficit, and 10 to most severe deficit) and documented NSS of 3 – 4 in control mice subjected to ferric chloride-induced distal MCAO on day 1 after stroke. Two more studies, one by Martinez de Lizarrondo et al. [27] and another by Sanchez-Rojas et al [39], used the ferric chloride-induced distal MCAO model in mice, but were unable to document substantial neurological deficit, using neurological scoring, in the animals.
It is well-documented that in humans suffering stroke obstruction of blood flow in the MCA occurs more often than in other brain arteries [4], and therefore it is not surprising that majority of rodent ischemic stroke models focus on occlusion of the MCA to cause focal brain ischemia. However, an important distinction exists in the functional-anatomical regions of human and rodent brains that receive blood through the MCA. In humans, the cortical branches of the MCA supply blood to the primary motor and somatosensory cortical areas of the upper limbs, face and trunk [40]. Because of this, nearly 85% of stroke survivors experience some degree of paresis in the upper limb acutely after stroke, which persists chronically in about 50% of patients [41]. On the contrary, the MCA and its branches irrigate most of the lateral surface of cerebral cortex in mice [42, 43] which minimally involve the primary motor cortex but rather cover the barrel sensory, auditory and visual sensory areas [44]. Thus, because the MCA minimally supplies blood to the primary motor cortex in mice, it is not surprising that we and others observed only a negligible and/or transient motor deficit in the ferric chloride-induced distal MCAO model of stroke.
If the latter explanation is correct, then it is reasonable to speculate that marginal motor deficit would be observed not just in ferric chloride-induced distal MCAO but in other mouse models which achieve occlusion of the distal MCAO by different techniques. Among such techniques, occlusion of the distal MCA is achieved by a) ligation method, which is done by tying a nylon loop around the distal MCA to block blood flow [16], b) compression method, where a blunted metal tip or needle is used to press the distal MCA and block the blood flow [17], c) thromboembolism by micro-injection of alpha-thrombin [24], and d) electrocoagulation [18], which arguably is the most invasive approach, because the generated heat usually damages the brain tissue surrounding the artery. To verify our assumption related to motor deficit, we reviewed the recent literature reporting use of the distal MCAO model in mice. In a preclinical, randomized, multicenter trial electrocoagulation-induced distal MCAO model was used to study the effect of anti-CD49d treatment in male C57BL/6J mice [45]. Rotarod and adhesive removal tests (performed 1, 3, and 7 days after stroke) were used to evaluate functional deficit, however, no significant functional impairment was documented after stroke in this study. In another multi-laboratory study, Rosell and colleagues evaluated suitability of several behavioral tests (beam walk, rotarod, corner, grip strength, and latency to move) to evaluate functional deficit in male C57BL/6, Balb/C, Swiss, and FVB mice for up to 21 days after distal MCAO induced by electrocoagulation, transient compression or alpha-thrombin-triggered thromboembolism [11]. Contrary to what was expected, none of the tests in this study detected statistically significant differences between sham and stroke-operated mice throughout all evaluation days in all mouse strains. However, a few of the tests (e.g., forelimb force and time to move) detected impairment during the acute phase of stroke in some strains. Notably, forelimb force test detected statistically significant differences between baseline and chronic post-stoke time points (7 and 14 days), however this was true only for Balb/C mice and the deficit was no more than 20% of the baseline value (indicative of marginal impairment). Two more studies had similar findings, in one of which Lubjhun and colleagues used a battery of tests (gait analysis using Catwalk and the DigiGait systems, latency to move, corner and handedness tests) to evaluate functional deficit in male C57BL/6 mice after electrocoagulation-induced distal MCAO for up to 22 days after stroke [46]. When comparing stroke and sham operated cohorts, the authors documented some degree of deficit in all four tests, which however was observed only on days 1 and 2 after stroke but not on day 7 and later time points [46]. In the second study, Freret and colleagues used a large set of sensorimotor and cognitive tests (corner, cylinder, pole, accelerated rotarod, chimney, staircase, adhesive removal, Morris water maze and passive avoidance tests) to monitor functional impairment of male Swiss mice after electrocoagulation-induced distal MCAO for up to 3 weeks after stroke. None of the tests detected functional deficit when comparing sham and stroke-operated groups, with exception of the adhesive removal test which showed sustained deficit starting from week 1 to 3 after stroke. Notably, in their interpretation of the data, the authors recognized that the observed deficit in the adhesive removal test is most likely a result of alterations in face-related somatosensory perception, not motor dysfunction per se. This is because performance of mice in adhesive removal test depends on sensing of the adhesive with whiskers and tongue in order to facilitate the removal [47], and experimental evidence indicating that innervation of these functions involves the barrel sensory and adjacent sensory regions of the cerebral cortex – regions which are primarily affected by occlusion of the distal MCA in mice [42–44]
It is important to note that there are some studies which documented functional deficit in mice after distal MCAO. For example, Caballero-Garrido and colleagues used CatWalk gait analysis system to characterize long-lasting gait and locomotion deficits in male C57BL/6 mice after electrocoagulation-induced distal MCAO for up to 4 weeks after stroke [48]. The authors documented statistically significant changes in some spatial, temporal and kinetic parameters when comparing sham and stroke-operated groups, and concluded that the CatWalk system is sensitive for monitoring of long-term functional recovery in the mouse distal MCAO model. This study is valuable because it is among few where a comprehensive analysis of most parameters that are tracked by the CatWalk system was carried out. However, it is noteworthy that for most of the parameters that were deemed affected by stroke, changes were documented in all limbs/paws rather than in paws contralateral to the stroke side (lack of expected unilateral alterations). In majority of cases the documented changes were observed transiently on day 7 after stroke, however, the pre-stroke performance of animals (i.e. baseline) was not included in data analyses, making it difficult to evaluate the extent of deficit within the same experimental group [48]. In a similar study, Barios and colleagues used cylinder and grid-walking tests to evaluate motor function of male C57BL/6 mice after distal MCAO, induced by ligation, for up to 8 weeks after stroke [49]. In addition to behavioral tests, somatosensory evoked potentials (SSEPs) were evaluated in the same animals to monitor responses in both hemispheres after contralateral and ipsilateral forepaw stimulation. The authors took great care in the design and execution of their study and observed substantial and sustained deficit in motor function of animals in both tests throughout the study period. Notably, the regions of infarcted cerebral cortex that were documented in this stud were similar to observations made by us and others and included the barrel sensory and adjacent lateral cortical regions but not the primary motor cortex. Therefore, findings of substantial and sustained motor deficit by Barios and colleagues is somewhat surprising and is in contrast with the above discussed studies which documented negligible or small but transient motor deficit in mice after distal MCAO. While Barios and colleagues did not discuss the published studies documenting negligible or small motor deficit in mice after distal MCAO, they acknowledged a possibility that repetitive katamine administration (for measurement of SSEPs under anesthesia) could have contributed to their observations of the motor deficit [49].
It is important to note that size of the infarction produced by occlusion of the distal MCA was shown to be strain dependent in mice. In a recent study comparing C57BL/6J and BALB/cJ mice, Doyle and Buckwalter documented smaller cerebral infarction restricted to the barrel region of the somatosensory cortex in C57BL/6J mice, which had negligible sensorimotor deficit after distal MCAO induced by ligation [16]. On the contrary, the authors observed a larger lesion, incorporating more of the somatosensory cortex and part of the primary motor cortex, in BALB/cJ mice after distal MCAO, which was also accompanied by a clear sensorimotor deficit [50]. To overcome the lack of robust sensorimotor deficit in C57BL/6J mice after distal MCAO, the authors suggested combination of the established distal MCAO model with hypoxia to generate a larger lesion, which is comparable to that of BALB/cJ mice and is accompanied by functional deficit [16]. Notably, the latter modification is similar to other established approaches in which occlusion of the distal MCA is combined with ligation of one or both carotid arteries to produce larger infarctions involving motor areas and clearer sensorimotor deficit in mice [16, 51].
Thus, based on majority of the published studies which were specifically designed to evaluate neurobehavioral function in the mouse distal MCAO model of stroke, it is reasonable to suggest that occlusion of the distal MCA, irrespective of the technique, leads to minimal and/or transient motor deficit in mice. On the contrary, some of these studies appear to indicate more pronounced and lasting sensory deficit in mice after distal MCAO. It is important to note, that strain differences and variability in vascular network branching from the MCA likely contribute to some of the inconsistencies in published literature.
In summary, our observations confirm that topical application of ferric chloride is a methodologically convenient and robust technique leading to reproducible cortical infarcts in mice. This model is likely well-suited for studies focusing on pathophysiology of stroke in regard to cell-death mechanisms and the response of brain tissue to injury. However, our functional behavioral experiments indicate that only marginal and transient motor deficit is observed in mice in this model of stroke. Because of the latter, in our opinion, the ferric chloride-induced model of distal MCAO is not suitable for studies focusing on motor functional recovery as a stroke outcome. However, it is important to recognize that the brain regions that are affected in this model (barrel sensory, auditory and visual sensory [44]) are responsible for other important functions, and hence it is reasonable to expect that behavioral tests focusing on such functions could very well detect significant and sustained deficit in animals after ferric chloride-induced distal MCAO. This assumption warrants future studies which would require validation of robust somatosensory, auditory and/or visual tests to evaluate the anticipated functional impairment of mice in this model of stroke.
Highlights.
FeCl3 induces complete occlusion of the distal MCA and cortical infarction in mice
Grid-walking and cylinder tests were used to study motor function in this model
Negligible motor deficit was observed in (fe)male mice up to 14 days after stroke
This model is not suitable for studies where motor function is an outcome measure
Acknowledgments
This work was partly supported by research grants from the American Heart Association (14BGIA20380826) and NIH (1R01NS106879). Breeding pairs of B6;129 mouse strain used for this research project were obtained from the Mutant Mouse Regional Resource Center, a NIH funded strain repository, and was donated to the MMRRC by the MMRRC at University of California, Davis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Karamyan VT, Peptidase neurolysin is an endogenous cerebroprotective mechanism in acute neurodegenerative disorders, Med Hypotheses 131 (2019) 109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Durukan A, Tatlisumak T, Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia, Pharmacol Biochem Behav 87(1) (2007) 179–97. [DOI] [PubMed] [Google Scholar]
- [3].Casals JB, Pieri NC, Feitosa ML, Ercolin AC, Roballo KC, Barreto RS, Bressan FF, Martins DS, Miglino MA, Ambrosio CE, The use of animal models for stroke research: a review, Comp Med 61(4) (2011) 305–13. [PMC free article] [PubMed] [Google Scholar]
- [4].Fluri F, Schuhmann MK, Kleinschnitz C, Animal models of ischemic stroke and their application in clinical research, Drug Des Devel Ther 9 (2015) 3445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Corbett D, Carmichael ST, Murphy TH, Jones TA, Schwab ME, Jolkkonen J, Clarkson AN, Dancause N, Weiloch T, Johansen-Berg H, Nilsson M, McCullough LD, Joy MT, Enhancing the Alignment of the Preclinical and Clinical Stroke Recovery Research Pipeline: Consensus-Based Core Recommendations From the Stroke Recovery and Rehabilitation Roundtable Translational Working Group, Neurorehabil Neural Repair 31(8) (2017) 699–707. [DOI] [PubMed] [Google Scholar]
- [6].Liguz-Lecznar M, Zakrzewska R, Daniszewska K, Kossut M, Functional assessment of sensory functions after photothrombotic stroke in the barrel field of mice, Behav Brain Res 261 (2014) 202–9. [DOI] [PubMed] [Google Scholar]
- [7].Linden J, Fassotte L, Tirelli E, Plumier JC, Ferrara A, Assessment of behavioral flexibility after middle cerebral artery occlusion in mice, Behav Brain Res 258 (2014) 127–37. [DOI] [PubMed] [Google Scholar]
- [8].Gerlai R, Thibodeaux H, Palmer JT, van Lookeren Campagne M, Van Bruggen N, Transient focal cerebral ischemia induces sensorimotor deficits in mice, Behav Brain Res 108(1) (2000) 63–71. [DOI] [PubMed] [Google Scholar]
- [9].Schaar KL, Brenneman MM, Savitz SI, Functional assessments in the rodent stroke model, Exp Transl Stroke Med 2(1) (2010) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Caballero-Garrido E, Pena-Philippides JC, Galochkina Z, Erhardt E, Roitbak T, Characterization of long-term gait deficits in mouse dMCAO, using the CatWalk system, Behav Brain Res 331 (2017) 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosell A, Agin V, Rahman M, Morancho A, Ali C, Koistinaho J, Wang X, Vivien D, Schwaninger M, Montaner J, Distal occlusion of the middle cerebral artery in mice: are we ready to assess long-term functional outcome?, Transl Stroke Res 4(3) (2013) 297–307. [DOI] [PubMed] [Google Scholar]
- [12].Frechou M, Margaill I, Marchand-Leroux C, Beray-Berthat V, Behavioral tests that reveal long-term deficits after permanent focal cerebral ischemia in mouse, Behav Brain Res 360 (2019) 69–80. [DOI] [PubMed] [Google Scholar]
- [13].Balkaya MG, Trueman RC, Boltze J, Corbett D, Jolkkonen J, Behavioral outcome measures to improve experimental stroke research, Behav Brain Res 352 (2018) 161–171. [DOI] [PubMed] [Google Scholar]
- [14].Andrews MMM, Peruzzaro S, Raupp S, Wilks J, Rossignol J, Dunbar GL, Using the behavioral flexibility operant task to detect long-term deficits in rats following middle cerebral artery occlusion, Behav Brain Res 356 (2019) 1–7. [DOI] [PubMed] [Google Scholar]
- [15].Karatas H, Erdener SE, Gursoy-Ozdemir Y, Gurer G, Soylemezoglu F, Dunn AK, Dalkara T, Thrombotic distal middle cerebral artery occlusion produced by topical FeCl(3) application: a novel model suitable for intravital microscopy and thrombolysis studies, J Cereb Blood Flow Metab 31(6) (2011) 1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Doyle KP, Buckwalter MS, A mouse model of permanent focal ischemia: distal middle cerebral artery occlusion, Methods Mol Biol 1135 (2014) 103–10. [DOI] [PubMed] [Google Scholar]
- [17].Morancho A, Garcia-Bonilla L, Barcelo V, Giralt D, Campos-Martorell M, Garcia S, Montaner J, Rosell A, A new method for focal transient cerebral ischaemia by distal compression of the middle cerebral artery, Neuropathol Appl Neurobiol 38(6) (2012) 617–27. [DOI] [PubMed] [Google Scholar]
- [18].Llovera G, Roth S, Plesnila N, Veltkamp R, Liesz A, Modeling stroke in mice: permanent coagulation of the distal middle cerebral artery, J Vis Exp (89) (2014) e51729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Le Behot A, Gauberti M, Martinez De Lizarrondo S, Montagne A, Lemarchand E, Repesse Y, Guillou S, Denis CV, Maubert E, Orset C, Vivien D, GpIbalpha-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice, Blood 123(21) (2014) 3354–63. [DOI] [PubMed] [Google Scholar]
- [20].Rashid M, Wangler NJ, Yang L, Shah K, Arumugam TV, Abbruscato TJ, Karamyan VT, Functional up-regulation of endopeptidase neurolysin during post-acute and early recovery phases of experimental stroke in mouse brain, J Neurochem 129(1) (2014) 179–89. [DOI] [PubMed] [Google Scholar]
- [21].Jayaraman S, Al Shoyaib A, Kocot J, Villalba H, Alamri FF, Rashid M, Wangler NJ, Chowdhury EA, German N, Arumugam TV, Abbruscato TJ, Karamyan VT, Peptidase neurolysin functions to preserve the brain after ischemic stroke in male mice, J Neurochem (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vijayan M, Alamri FF, Al Shoyaib A, Karamyan VT, Reddy PH, Novel miRNA PC-5P-12969 in Ischemic Stroke, Mol Neurobiol (2019). [DOI] [PubMed] [Google Scholar]
- [23].Alamri FF, Shoyaib AA, Biggers A, Jayaraman S, Guindon J, Karamyan VT, Applicability of the grip strength and automated von Frey tactile sensitivity tests in the mouse photothrombotic model of stroke, Behav Brain Res 336 (2018) 250–255. [DOI] [PubMed] [Google Scholar]
- [24].Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, Agin V, Vivien D, Mouse model of in situ thromboembolic stroke and reperfusion, Stroke 38(10) (2007) 2771–8. [DOI] [PubMed] [Google Scholar]
- [25].Denorme F, Langhauser F, Desender L, Vandenbulcke A, Rottensteiner H, Plaimauer B, Francois O, Andersson T, Deckmyn H, Scheiflinger F, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF, ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice, Blood 127(19) (2016) 2337–45. [DOI] [PubMed] [Google Scholar]
- [26].Gauberti M, Montagne A, Marcos-Contreras OA, Le Behot A, Maubert E, Vivien D, Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes, Stroke 44(7) (2013) 1988–96. [DOI] [PubMed] [Google Scholar]
- [27].Martinez de Lizarrondo S, Gakuba C, Herbig BA, Repesse Y, Ali C, Denis CV, Lenting PJ, Touze E, Diamond SL, Vivien D, Gauberti M, Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi, Circulation 136(7) (2017) 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pautus S, Alami M, Adam F, Bernadat G, Lawrence DA, De Carvalho A, Ferry G, Rupin A, Hamze A, Champy P, Bonneau N, Gloanec P, Peglion JL, Brion JD, Bianchini EP, Borgel D, Characterization of the Annonaceous acetogenin, annonacinone, a natural product inhibitor of plasminogen activator inhibitor-1, Sci Rep 6 (2016) 36462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rai G, Joshi N, Jung JE, Liu Y, Schultz L, Yasgar A, Perry S, Diaz G, Zhang Q, Kenyon V, Jadhav A, Simeonov A, Lo EH, van Leyen K, Maloney DJ, Holman TR, Potent and selective inhibitors of human reticulocyte 12/15-lipoxygenase as anti-stroke therapies, J Med Chem 57(10) (2014) 4035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Theriault P, Le Behot A, ElAli A, Rivest S, Sub-acute systemic erythropoietin administration reduces ischemic brain injury in an age-dependent manner, Oncotarget 7(24) (2016) 35552–35561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cook DJ, Nguyen C, Chun HN, I LL, Chiu AS, Machnicki M, Zarembinski TI, Carmichael ST, Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke, J Cereb Blood Flow Metab 37(3) (2017) 1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Doeppner TR, Kaltwasser B, Bahr M, Hermann DM, Effects of neural progenitor cells on post-stroke neurological impairment-a detailed and comprehensive analysis of behavioral tests, Front Cell Neurosci 8 (2014) 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, Bahjat FR, Stenzel-Poore MP, Kawaguchi R, Coppola G, Carmichael ST, GDF10 is a signal for axonal sprouting and functional recovery after stroke, Nat Neurosci 18(12) (2015) 1737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Minnerup J, Kim JB, Schmidt A, Diederich K, Bauer H, Schilling M, Strecker JK, Ringelstein EB, Sommer C, Scholer HR, Schabitz WR, Effects of neural progenitor cells on sensorimotor recovery and endogenous repair mechanisms after photothrombotic stroke, Stroke 42(6) (2011) 1757–63. [DOI] [PubMed] [Google Scholar]
- [35].Zhou LY, Wright TE, Clarkson AN, Prefrontal cortex stroke induces delayed impairment in spatial memory, Behav Brain Res 296 (2016) 373–8. [DOI] [PubMed] [Google Scholar]
- [36].Mora-Lee S, Sirerol-Piquer MS, Gutierrez-Perez M, Lopez T, Casado-Nieto M, Jauquicoam C, Abizanda G, Romaguera-Ros M, Gomez-Pinedo U, Prosper F, Garcia-Verdugo JM, Histological and ultrastructural comparison of cauterization and thrombosis stroke models in immune-deficient mice, J Inflamm (Lond) 8(1) (2011) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cisbani G, Le Behot A, Plante MM, Prefontaine P, Lecordier M, Rivest S, Role of the chemokine receptors CCR2 and CX3CR1 in an experimental model of thrombotic stroke, Brain Behav Immun 70 (2018) 280–292. [DOI] [PubMed] [Google Scholar]
- [38].Karatas H, Eun Jung J, Lo EH, van Leyen K, Inhibiting 12/15-lipoxygenase to treat acute stroke in permanent and tPA induced thrombolysis models, Brain Res 1678 (2018) 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sanchez-Rojas L, Gomez-Pinedo U, Benito-Martin MS, Leon-Espinosa G, Rascon-Ramirez F, Lendinez C, Martinez-Ramos C, Matias-Guiu J, Pradas MM, Barcia JA, Biohybrids of scaffolding hyaluronic acid biomaterials plus adipose stem cells home local neural stem and endothelial cells: Implications for reconstruction of brain lesions after stroke, J Biomed Mater Res B Appl Biomater 107(5) (2019) 1598–1606. [DOI] [PubMed] [Google Scholar]
- [40].Navarro-Orozco D, Sanchez-Manso JC, Neuroanatomy, Middle Cerebral Artery, StatPearls, Treasure Island (FL), 2019. [PubMed] [Google Scholar]
- [41].Santisteban L, Teremetz M, Bleton JP, Baron JC, Maier MA, Lindberg PG, Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review, PLoS One 11(5) (2016) e0154792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xiong B, Li A, Lou Y, Chen S, Long B, Peng J, Yang Z, Xu T, Yang X, Li X, Jiang T, Luo Q, Gong H, Precise Cerebral Vascular Atlas in Stereotaxic Coordinates of Whole Mouse Brain, Front Neuroanat 11 (2017) 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dorr A, Sled JG, Kabani N, Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study, Neuroimage 35(4) (2007) 1409–23. [DOI] [PubMed] [Google Scholar]
- [44].Mohajerani MH, Chan AW, Mohsenvand M, LeDue J, Liu R, McVea DA, Boyd JD, Wang YT, Reimers M, Murphy TH, Spontaneous cortical activity alternates between motifs defined by regional axonal projections, Nat Neurosci 16(10) (2013) 1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Llovera G, Hofmann K, Roth S, Salas-Perdomo A, Ferrer-Ferrer M, Perego C, Zanier ER, Mamrak U, Rex A, Party H, Agin V, Fauchon C, Orset C, Haelewyn B, De Simoni MG, Dirnagl U, Grittner U, Planas AM, Plesnila N, Vivien D, Liesz A, Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia, Sci Transl Med 7(299) (2015) 299ra121. [DOI] [PubMed] [Google Scholar]
- [46].Lubjuhn J, Gastens A, von Wilpert G, Bargiotas P, Herrmann O, Murikinati S, Rabie T, Marti HH, Amende I, Hampton TG, Schwaninger M, Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery, J Neurosci Methods 184(1) (2009) 95–103. [DOI] [PubMed] [Google Scholar]
- [47].Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, Schumann-Bard P, Boulouard M, Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test, Behav Neurosci 123(1) (2009) 224–30. [DOI] [PubMed] [Google Scholar]
- [48].Caballero-Garrido E, Pena-Philippides JC, Galochkina Z, Erhardt E, Roitbak T, Characterization of long-term gait deficits in mouse dMCAO, using the CatWalk system, Behav Brain Res 331 (2017) 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barios JA, Pisarchyk L, Fernandez-Garcia L, Barrio LC, Ramos M, Martinez-Murillo R, Gonzalez-Nieto D, Long-term dynamics of somatosensory activity in a stroke model of distal middle cerebral artery oclussion, J Cereb Blood Flow Metab 36(3) (2016) 606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Doyle KP, Fathali N, Siddiqui MR, Buckwalter MS, Distal hypoxic stroke: a new mouse model of stroke with high throughput, low variability and a quantifiable functional deficit, J Neurosci Methods 207(1) (2012) 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, Shaw C, Fidanboylu M, Orr AW, Ogunshola O, Fertala A, Thomas SA, Bix GJ, Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents, J Clin Invest 121(8) (2011) 3005–23. [DOI] [PMC free article] [PubMed] [Google Scholar]