Abstract
Purpose:
To design and build a dual-tuned 17O/1H coil for direct brain oximetry at 3 T
Methods:
A dual tuned 17O/1H coil comprising two degenerate mode birdcage coils was constructed to facilitate high sensitivity 17O and 1H imaging. In vivo 17O brain images were acquired in a healthy volunteer using a FLORET sequence, together with high-resolution structural brain 1H anatomic imaging.
Results:
Natural abundance 17O images with a nominal resolution of 8 mm3 were acquired in under 20 minutes exhibiting clear delineation of the physiologic 17O distribution. 1 mm isotropic 1H structural brain images demonstrated excellent quality and anatomic detail using routine clinical imaging sequence parameters and parallel acceleration.
Conclusion:
A dual tuned 17O/1H array was constructed to enable high sensitivity 17O and 1H imaging under standard clinical 3 T scanning conditions.
Keywords: magnetic resonance imaging, oxygen-17, oximetry, brain
Introduction
Brain homeostasis depends critically upon the steady nutritive flow of oxygenated blood, hinging on the immense energetic advantage of oxidative phosphorylation to sustain the neurovascular unit at rest and during explicit activation. Seminal paradigms of sequential hemodynamic compromise have been expounded using 15O PET, emphasizing a tenuous state of so-called misery perfusion, marked by impaired oxygen metabolism and conferring elevated near-term stroke risk1,2,3. The development of 15O translational and clinical imaging programs, however, proves challenging due to the reliance upon short lived radiotracers (half-life ~90 s) and the dependence upon in situ cyclotron access for in vivo experimentation. Indirect approaches to assessing oxygen metabolism using 1H MRI have therefore been proposed to circumvent the technical obstacles associated with 15O 4–8, but require complex biophysical modeling and suffer generally poor anatomic localization. Direct MR oximetry, exploiting the stable and naturally occurring 17O isotope has been explored in human and non-human species; however, its low natural abundance (0.037%) has demanded ultrahigh field MR systems (> 3 T) coupled to fractionally enriched 17O gas inhalation, limiting widespread translational or clinical applicability 9–15.
While sporadic reports of 17O oximetry at 3 T exist16, the widespread adoption of 17O MRI beyond purely translational applications requires specialized dual-tuned RF coils to enable concurrent 1H imaging for comprehensive, real-time multi-parametric structural, functional, metabolic, and hemodynamic interrogation of the living brain. In this work we developed a dual-tuned 17O/1H coil array to explore the feasibility of natural abundance brain oximetry on a clinical 3 Tesla system, emphasizing high 17O-MRI sensitivity, 1H-MRI performance, and engineering features which could potentially support future simultaneous co-modal, multi-parametric brain PET-MR imaging.
Methods
Design Approach
Given the low natural abundance of 17O, our top design objective was to engineer a coil array with optimal 17O performance while still maintaining clinically acceptable 1H sensitivity, ensuring the full battery of neurovascular proton MRI techniques.
Recent work has shown that dual-nucleus degenerate mode birdcage arrays17,18 can be arranged to achieve improved X-nucleus and 1H performance over dual-tuned birdcage coils. Following this strategy, we built two radially interleaved degenerate mode birdcages that integrated four 17O (16.7 MHz) and eight 1H (123.2 MHz) channels (Fig. 1). The 17O unit was required to operate in transmit/receive (Tx/Rx) mode due to the lack of a commercially available coil. The 1H unit was designed as a Rx-only phased array to enable the system body coil for 1H excitation, facilitating applications such as spin labelling that require cervical RF tagging beyond the coverage of the local coil.
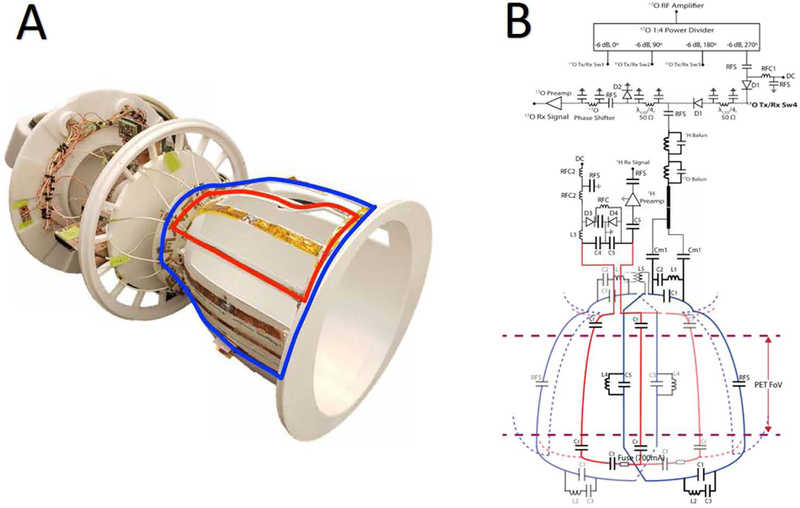
Figure 1.
Photograph of the 17O/1H array without protective cover (A). The blue and red overlays indicate the contour of individual 17O and 1H coils, respectively. The interface components, including preamplifiers, power dividers, and transmit/receive switches, are located superior to the coils to reduce PET attenuation in future MR/PET studies.
Schematic diagram of the 17O/1H coil array and interface (B). 17O coils are represented in blue and 1H coils in red. For simplicity one 17O and 1H coil are highlighted. For both the 17O and 1H arrays the status of the DC bias is forward in transmit mode and reverse in receive mode. In receive mode the diodes D1 provide isolation between the RF amplifier and preamplifiers. The diode D2 provides isolation between the RF amplifier and the preamplifier in transmit mode. During proton body coil excitation the diode D3 along with L3 and C4 forms the detuning circuit, the diode D4 provides protection for the preamplifier. Typical component values are C1=47 pF, C2=200pF, C3=300pF, C4 =15pF, C5=27pF, Cm1=180pF, Cr=18pF, Ct = 2–15 pF, L1= 38nH, L2 = 80nH, L3= 105 nH and L4 = 60 nH, L5 = 230 nH, RFC1 = 10μH, RFC2 =2.2μH, RFS =1000pF.
To reduce PET attenuation, we consolidated the arrays into a two “layer” structure (layer 1 is the 17O Tx/Rx array and layer 2 is the 1H Rx array), moved interface components such as the preamplifiers, Tx/Rx switches, and power dividers, and majority of tuning capacitors outside the FOV that extends radially outward from the brain, and enclosed the device in a 3D printed (Fortus 360, Stratasys, Minneapolis) stealth polycarbonate shell. A detailed description of the two arrays is provided in the following sections.
17O Array
Given the low operating frequency of 17O (16.7 MHz) the coil array design had to be chosen to achieve a balance between the high peripheral SNR of a high channel array and high central SNR of a low channel array. To select the number of coils in the 17O array, we built test coils with 45° and 90° apertures to emulate individual coils in eight and four channel arrays, respectively. The coils were built on a head-sized domed substrate that was 27 cm long. We measured the unloaded and loaded quality factor (Q) in three cases: 1) 90° aperture coil with two distributed capacitors, 2) 90° aperture coil with four distributed capacitors, and 3) 45° aperture coil with two distributed capacitors. The loaded Q measurements were made on a tissue equivalent head phantom19. Of the candidates tested, we found that the 90° aperture coil with two distributed capacitors had the highest unloaded Q and unloaded-to-loaded Q ratio (Table 1). Additionally, previous work17 suggests that a low-frequency, eight-channel array that encircles the head can be expected to exhibit significant coupling between third and fourth neighbors, which can be complicated to decouple.
Table 1.
Q measurements on candidate 17O coils with various apertures and tuning components. The coil length was fixed at 27 cm, with apertures to emulate a single coil in a 4 (90°) or 8 channel (45°) array. The coil with 90° aperture and 2 distributed capacitors represents the baseline (row 1), while the others show compromised performance primarily due to increased capacitor loss (row 2) or reduced coil-to-sample coupling (row 3). This information motivated the implemented design (row 4), in which the aperture provided sufficient coil-to-sample coupling, while loss due to 1H blocking circuits was considered a necessary penalty to enable 1H body coil excitation.
| Aperture | Number of Distributed capacitors |
Unloaded Q Qun |
Loaded Q Ql |
Ratio Qun/Ql |
|---|---|---|---|---|
| 90° | 2 | 420 | 190 | 2.21 |
| 90° | 4 | 320 | 180 | 1.78 |
| 45° | 2 | 285 | 170 | 1.68 |
| 90° | 1 first-order and 2 second-order blocking circuits | 320 | 180 | 1.78 |
We therefore chose to build a four-channel 17O array (Fig. 1) whose coils were distributed with radial symmetry around a head-shaped polycarbonate substrate (20 cm x 33 cm x 27 cm). The 17O coils were constructed with 10 mm wide, 0.06 mm thick copper tape and matched to 50Ω at 16.7 MHz using two distributed capacitors in the presence of the tissue-equivalent head phantom. The perimeter of the 17O coils was 114 cm, potentially long enough to support standing waves at the 1H frequency (123.2 MHz). To reduce interaction between the 1H and 17O coils, the distributed capacitors in the 17O coils were replaced with lumped element second-order filters that were set up to provide high impedance at the 1H frequency and the appropriate capacitance to resonate the coil at 17O frequency20, along with one first-order filter21 that provided high impedance at the 1H frequency (Fig. 1).
While the coils were set up individually (in other words, without shared rungs), we collapsed the modes to achieve degeneracy by decoupling neighboring elements by geometrical overlap and the next nearest neighbors by linked counter-wound inductors (Fig. 1). Scattering (S) parameters were measured to determine decoupling efficacy. The 17O array was interfaced to the system’s broadband power amplifier through a one-to-four way power splitter. To achieve circularly polarized transmit excitation, home-built lumped element phase shift networks were added to provide 90⁰ phase offsets based on the azimuthal location of the coil elements. Individual power splitter outputs were connected to Tx/Rx switches22 to isolate and protect the preamplifiers during 17O excitation (Fig. 1). The coil ground was separated from the common system ground by two cable traps, one tuned to 17O and the other tuned to 1H frequency. Preamplifier decoupling was achieved by adding a phase shift network in the receive path that transformed the low input impedance of the preamplifier into an inductance that formed a parallel resonant circuit with the match capacitor23.
1H Array
The 1H unit was a degenerate mode birdcage with 8-channels in which neighboring coils have shared rungs. In the past, degenerate mode birdcages have been utilized when both a uniform excitation field and phased array signal reception are desired24,25. In this case, we used the concept in Rx only mode in order to reduce the copper footprint and therefore the PET attenuation compared to an overlapped array. The coils were laid out with radial symmetry around the substrate using 5 mm wide, 0.06 mm thick copper tape to circumscribe loops that were 24 cm long with 45° apertures (12 cm arc length). The coils were tuned to 123.2 MHz using six distributed capacitors and matched to 50Ω in the presence of the head phantom. The ratio between the shared rung and end ring capacitors was chosen to decouple nearest neighbor coils. Next nearest neighbor decoupling was addressed using preamplifier decoupling. The coil Q values were approximately 310 (unloaded) and 85 (loaded).
Spin excitation was produced by system body coil. To isolate the receive array and to ensure patient safety during body coil operation one active detuning circuit and one current fuse rated at 700 mA were integrated per 1H coil.
MRI and PET Measurements
All imaging experiments were performed on Siemens 3 T Prisma scanner (Siemens Healthineers, Erlangen, Germany). Human subjects were scanned after obtaining their informed written consent. 1H and MNO “TIM to 4G” adapter interfaces (Siemens Healthineers, Erlangen, Germany) were used to route the received 1H and 17O signals from the array to the respective receive chains. The 17O transmit power was restricted to enforce a two-fold safety buffer below the 10 W/kg limit set by the International Electrotechnical Commission (IEC document 60601–2-33 2010).
1H Flip angle maps were measured in the head phantom with and without the 17O/1H array to ensure the integrity of the 1H transmit field produced by the system body coil was preserved.
The 17O reference pulse amplitude to generate a 90º flip angle was determined by recording the maximum signal amplitude acquired with a Stimulated Echo Acquisition Mode (STEAM)26 sequence (TE=20ms, TR= 2000ms, TM = 10 ms, BW =1200 Hz, Ave = 16) for a range of pulses (200 to 300 V in steps of 20 V). In vivo 17O-MRI was demonstrated using a three-dimensional non-Cartesian Fermat Looped Orthogonally Encoded Trajectories (FLORET) 27,28 acquisition with the following parameters: TR = 50 ms, TE = 0.2 ms, flip angle = 90°, resolution = 8 mm isotropic, 3 hubs at 45º, 140 interleaves/hub, field of view = 512 mm isotropic, 56 averages, readout time = 10.3 ms, acquisition time = 19:36 min.
1H SNR and flip angle measurements were performed with the developed array and a product commercial phased array (Head/Neck 20, Siemens Healthineers, Erlangen, Germany, and (23cm x 38 cm x 27 cm) available at our center. The commercial array has twenty coils arranged in three rows along the Z direction of 8, 8, and 4 coils. The final row of 4 coils is used for neck imaging and was disabled in our measurements. SNR maps were calculated from separate signal and noise (with the RF pulse amplitude set to zero) measurements acquired with a gradient echo pulse sequence and processed with the optimal array combination method29. The SNR acquisition parameters were as follows: TR = 500 ms, TE = 3.82 ms, flip angle = 10°, voxel size = 0.9×0.9×3 mm3. The 1H flip angle maps were measured using the method described in Ref30. Proton anatomical imaging was performed with a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence: TR = 2200 ms, TE = 3.2 ms, TI = 900 ms, flip angle = 8°, resolution = 1 mm isotropic, parallel acceleration factor = 2, and acquisition time = 4:30 min.
To measure the influence of the MRI coils on 511 keV photons, we recorded the true net counts over 5 minute intervals that were emitted from a 5.4 L water phantom doped with 1.14 miC 18F-FDG in three environments on a 3T PET-MR system (Biograph mMR, Siemens Healthineers, Erlangen Germany): 1) no local coil present (reference), 2) inside the 17O/1H array, and 3) inside the commercial head/neck PET coil (mMR head array, Siemens Healthineers, Erlangen, Germany). The value recorded in the reference environment was used to predict the net counts in subsequent measurements while accounting for FDG decay. The attenuation loss from the coils is reported as the ratio between the actual and predicted true net counts.
Results
17O Array
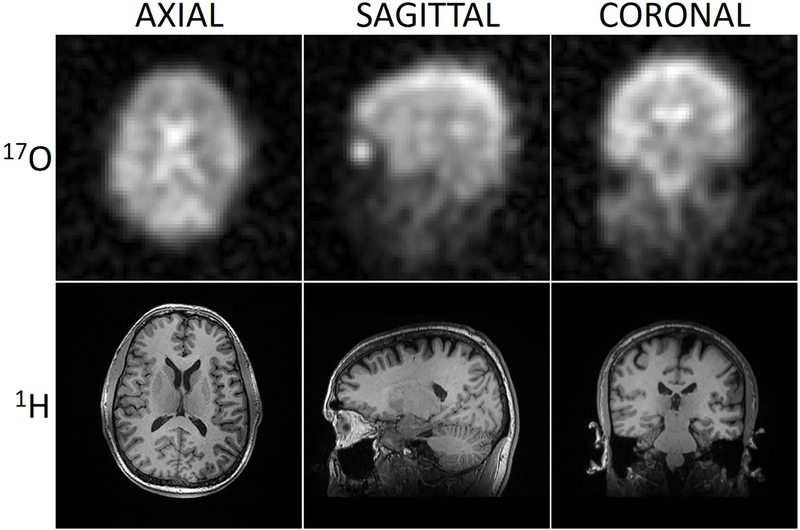
For the 17O coils, the average S-parameter reflection was −15 dB and the average isolation was −14 dB and −18 dB between neighbors and next nearest neighbors, respectively. The worst isolation between neighbor and next nearest neighbors were −13.6 and −17.4 dB respectively. The insertion loss of the 17O power dividers and Tx/Rx switches was - 0.3 ± 0.1 dB with 90º ± 1º phase intervals at each output. A 17O transmit pulse with 240 V amplitude and 500 μs duration produced a 90º flip angle. Orthogonal 17O images acquired with an isotropic 3D FLORET sequence in a healthy adult volunteer (Fig. 2 top row) demonstrate the feasibility of direct oximetry under natural abundance conditions without enrichment. Anatomical delineation of the cerebral hemispheres and substructure is apparent at the current spatial resolution.
Figure 2.
In vivo FLORET 17O images (top row) and co-registered MPRAGE 1H images (bottom row) acquired using the 17O/1H array. 17O FLORET images reflect the underlying water pool with greatest intensity in the ventricular system, cisterns/sulci, grey matter and intraocular vitreous. The 1H MPRAGE images demonstrate excellent T1 contrast in the whole brain.
1H Array
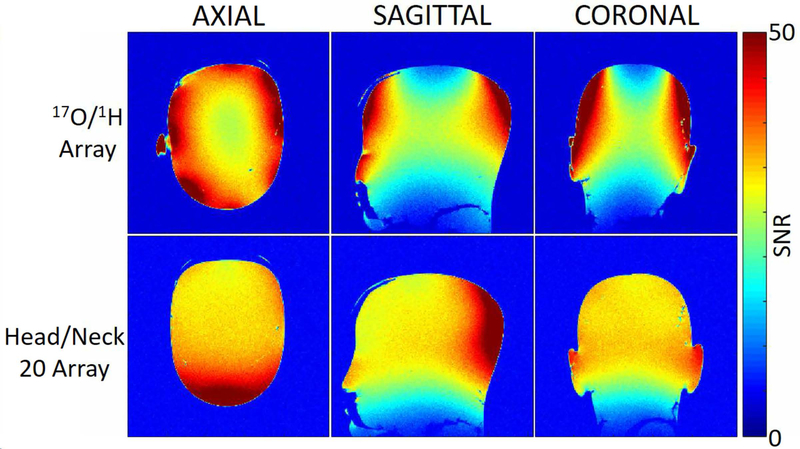
For the 1H coils, the average S-parameter reflection was −15 dB and the average isolation was −9 dB and −11 dB between neighbors and next nearest neighbors, respectively. The worst isolation was −8.4 dB and −10.6 dB between neighbors and next nearest neighbors respectively. The system body coil required a 202 V, 500 μs hard pulse to achieve 90º flip angle when the array was present and 188 V when the array was absent. 1H flip angle maps acquired with and without the 17O/1H array (Fig. 3) show that it caused minimal distortion to the transmit field produced by the system body coil. 1H SNR at the center of the head phantom was 20% lower when compared to the commercial Head/Neck 20 array (Fig. 4). Concurrent multi-planar reconstructions from an MPRAGE sequence obtained in the same imaging session (Fig. 2 Bottom row) confirm the suitable performance of the 1H array, exhibiting high anatomic detail and tissue contrast at isotropic 1 mm resolution.
Figure 3.
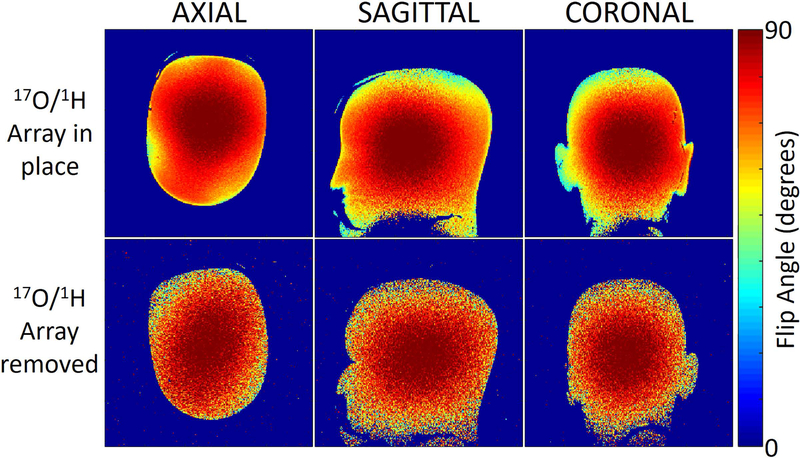
1H flip angle maps measured in a head shaped phantom with and without the 17O/1H array. The flip angle maps show that the 17O/1H array caused minimal distortion to the transmit field produced by the system body coil. The low SNR on the bottom row is attributed to the use of system body coil for signal reception.
Figure 4.
1H SNR maps measured in a head shaped phantom show that the commercial Head/Neck 20 array outperforms the 17O/1H array by 20% at the center of the phantom.
PET
The commercial mMR head array attenuated 15.1% of the net true photon counts from the FDG-doped phantom, while the 17O/1H array attenuated 7.7%, indicating the developed array’s PET compatibility for future co-modal measurements.
Discussion and Conclusions
In this work we developed a dual tuned 17O/1H array comprising radially interleaved degenerate mode birdcage coils. The 17O unit operated in Tx/Rx mode to ensure uniform excitation during transmission and high sensitivity during signal reception. The 17O image quality confirms the feasibility of natural abundance oximetry at 3 T16.
The 1H unit was engineered to support the full battery of neurovascular proton MRI. Hence we chose to build a 1H Rx only array and utilize the system body coil for spin excitation, which represents a departure from most dual-tuned arrays that consist of a pair of local Tx/Rx arrays17,18,25,31–34.
To preserve the integrity of the 1H excitation field produced by the system body coil we incorporated 1H blocking circuits20,21 in 17O elements, which caused a reduction in the unloaded to loaded Q ratio (Table 1). Using the well-known relationship between Q ratio and SNR we can equate this reduction in Q to a SNR penalty of 8%. We chose to implement the blocking circuits to harvest the potential advantages of an Rx only 1H array such as improved excitation coverage and sensitivity over a local 1H transmit array. Eight of the 12 blocking circuits were positioned outside the FOV to improve PET transparency. While a μ-map generated from a high-dose CT scan to enable local coil attenuation correction and simultaneous PET/MR are pending at the time of this report, the PET true photon count measurements showed that the 17O/1H array provided ~50% improved PET transparency over the commercial head coil. This result suggests that the 17O/1H array is congruent with attenuation correction and co-modal operation.
In conclusion we designed and implemented a multi-channel 17O/1H array for direct brain oximetry at 3 T. The array is underpinned with dual degenerate mode birdcages to reduce its electrical component footprint for co-modal PET measurements, a 4-channel 17O receiver to enhance sensitivity, and a receive-only 8-channel 1H array to enable body coil functionality. We anticipate that the head coil will allow a new set of functional, metabolic, and hemodynamic measurements in the brain.
Acknowledgements.
The authors thank Jerzy Walczyk for help with the coil housing and Riccardo Lattanzi for the SNR calculation tool. This work was partially supported by National Institutes of Health grants R01DK106292, R01NS097494, R21CA213169, R01EB026456, R21AG061579, and R01DK114428 and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at the New York University School of Medicine, which is an NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).
References
- 1.Derdeyn CP, Carpenter DA, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125(3):595–607. [DOI] [PubMed] [Google Scholar]
- 2.Grubb RL, Powers WJ, Clarke WR, Videen TO, Adams HP, Derdeyn CP. Surgical results of the Carotid Occlusion Surgery Study. 2013;118(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. 1991;29(3):231–240. [DOI] [PubMed] [Google Scholar]
- 4.Bolar DS, Rosen BR, Sorensen AG, Adalsteinsson E. QUantitative Imaging of extraction of oxygen and TIssue consumption (QUIXOTIC) using venular-targeted velocity-selective spin labeling. 2011;66(6):1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulte DP, Kelly M, Germuska M, et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. NeuroImage. 2012;60(1):582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauthier CJ, Hoge RD. Magnetic resonance imaging of resting OEF and CMRO2 using a generalized calibration model for hypercapnia and hyperoxia. NeuroImage. 2012;60(2):1212–1225. [DOI] [PubMed] [Google Scholar]
- 7.An H, Lin W. Impact of intravascular signal on quantitative measures of cerebral oxygen extraction and blood volume under normo- and hypercapnic conditions using an asymmetric spin echo approach. 2003;50(4):708–716. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Zhou D, Nguyen TD, Spincemaille P, Gupta A, Wang Y. Cerebral metabolic rate of oxygen (CMRO2) mapping with hyperventilation challenge using quantitative susceptibility mapping (QSM). 2017;77(5):1762–1773. [DOI] [PubMed] [Google Scholar]
- 9.Zhu XH, Chen W. In vivo oxygen-17 NMR for imaging brain oxygen metabolism at high field. Progress in Nuclear Magnetic Resonance Spectroscopy. 2011;59:319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurzhunov D, Borowiak R, Hass H, et al. Quantification of oxygen metabolic rates in Human brain with dynamic 17O MRI: Profile likelihood analysis. 2017;78(3):1157–1167. [DOI] [PubMed] [Google Scholar]
- 11.Kurzhunov D, Borowiak R, Reisert M, Krafft AJ, Ozen AC, Bock M. 3D CMRO2 mapping in human brain with direct 17O MRI: Comparison of conventional and proton-constrained reconstructions. NeuroImage. 2017;155:612–624. [DOI] [PubMed] [Google Scholar]
- 12.Lu M, Zhang Y, Ugurbil K, Chen W, Zhu X-H. In vitro and in vivo studies of 17O NMR sensitivity at 9.4 and 16.4 T. 2013;69(6):1523–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann SH, Begovatz P, Nagel AM, et al. A measurement setup for direct 17O MRI at 7 T. 2011;66(4):1109–1115. [DOI] [PubMed] [Google Scholar]
- 14.Niesporek SC, Umathum R, Lommen JM, et al. Reproducibility of CMRO2 determination using dynamic 17O MRI. 2018;79(6):2923–2934. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson IC, Thulborn KR. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. Neuroimage. 2010;51(2):723–733. [DOI] [PubMed] [Google Scholar]
- 16.Borowiak R, Groebner J, Haas M, Hennig J Bock, MJMRMiP, Biology, Medicine. Direct cerebral and cardiac 17O-MRI at 3 Tesla: initial results at natural abundance. 2014;27(1):95–99. [DOI] [PubMed] [Google Scholar]
- 17.Lakshmanan K, Brown R, Madelin G, Qian Y, Boada F, Wiggins GC. An eight-channel sodium/proton coil for brain MRI at 3 T. NMR in Biomedicine. 2018;31(2):e3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown R, Khegai O, Parasoglou P. Magnetic Resonance Imaging of Phosphocreatine and Determination of BOLD Kinetics in Lower Extremity Muscles using a Dual-Frequency Coil Array. Scientific Reports. 2016;6:30568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ianniello C, de Zwart JA, Duan Q, et al. Synthesized tissue-equivalent dielectric phantoms using salt and polyvinylpyrrolidone solutions. 2018;80(1):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerspeer M, Roig ES, Gruetter R, Magill AW. An improved trap design for decoupling multinuclear RF coils. 2014;72(2):584–590. [DOI] [PubMed] [Google Scholar]
- 21.Dabirzadeh A, McDougall MP. Trap Design for Insertable Second-Nuclei Radiofrequency Coils for Magnetic Resonance Imaging and Spectroscopy. Concepts in Magnetic Resonance Part B (Magnetic Resonance Engineering),. 2009;35B(3):121–132. [Google Scholar]
- 22.Shajan G, Hoffmann J, Budde J, Adriany G, Ugurbil K, Pohmann R. Design and evaluation of an RF front-end for 9.4 T human MRI. Magn Reson Med 2011;66(2):596–604. [DOI] [PubMed] [Google Scholar]
- 23.Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med 1990;16(2):192–225. [DOI] [PubMed] [Google Scholar]
- 24.Alagappan V, Nistler J, Adalsteinsson E, et al. Degenerate mode band-pass birdcage coil for accelerated parallel excitation. Magn Reson Med 2007;57(6):1148–1158. [DOI] [PubMed] [Google Scholar]
- 25.Lin F, Kwong K, Huang I, Belliveau J, Wald L. Degenerate mode birdcage volume coil for sensitivity-encoded imaging. Magn Reson Med 2003;50:1107–1111. [DOI] [PubMed] [Google Scholar]
- 26.Frahm J, Merboldt K-D, Hänicke W. Localized proton spectroscopy using stimulated echoes. Journal of Magnetic Resonance (1969). 1987;72(3):502–508. [Google Scholar]
- 27.Pipe JG, Zwart NR, Aboussouan EA, Robison RK, Devaraj A, Johnson KO. A new design and rationale for 3D orthogonally oversampled k-space trajectories. Magn Reson Med 2011;66(5):1303–1311. [DOI] [PubMed] [Google Scholar]
- 28.Madelin G, Kline R, Walvick R, Regatte RR. A method for estimating intracellular sodium concentration and extracellular volume fraction in brain in vivo using sodium magnetic resonance imaging. Sci Rep 2014;4:4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005;54(6):1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fautz HP, Vogel M, Gross P, Kerr A, Zhu Y. B1 mapping of coil arrays for parallel transmission ISMRM; 2008; Toronto, Ontario. [Google Scholar]
- 31.Brown R, Madelin G, Lattanzi R, et al. Design of a nested eight-channel sodium and four-channel proton coil for 7T knee imaging. Magn Reson Med 2013;70(1):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaggie JD, Hadley JR, Badal J, et al. A 3 T sodium and proton composite array breast coil. Magn Reson Med 2014;71(6):2231–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon CH, Kim J-H, Zhao T, Bae KT. Quantitative 23Na MRI of human knee cartilage using dual-tuned 1H/23Na transceiver array radiofrequency coil at 7 tesla. 2013;38(5):1063–1072. [DOI] [PubMed] [Google Scholar]
- 34.Shajan G, Mirkes C, Buckenmaier K, Hoffmann J, Pohmann R, Scheffler K. Three-layered radio frequency coil arrangement for sodium MRI of the human brain at 9.4 Tesla. Magn Reson Med 2015. [DOI] [PubMed] [Google Scholar]