The many roles of DOCK2 and DOCK8 in immunology
Keywords: IL-31, immunological synapse, leukocyte migration, Rho family of GTPases, type I interferons
Abstract
Dedicator of cytokinesis (DOCK) proteins constitute a family of evolutionarily conserved guanine nucleotide exchange factors (GEFs) for the Rho family of GTPases. Although DOCK family proteins do not contain the Dbl homology domain typically found in other GEFs, they mediate the GTP–GDP exchange reaction through the DOCK homology region-2 (DHR-2) domain. In mammals, this family consists of 11 members, each of which has unique functions depending on the expression pattern and the substrate specificity. For example, DOCK2 is a Rac activator critical for migration and activation of leukocytes, whereas DOCK8 is a Cdc42-specific GEF that regulates interstitial migration of dendritic cells. Identification of DOCK2 and DOCK8 as causative genes for severe combined immunodeficiency syndromes in humans has highlighted their roles in immune surveillance. In addition, the recent discovery of a naturally occurring DOCK2-inhibitory metabolite has uncovered an unexpected mechanism of tissue-specific immune evasion. On the other hand, GEF-independent functions have been shown for DOCK8 in antigen-induced IL-31 production in helper T cells. This review summarizes multifaced functions of DOCK family proteins in the immune system.
Introduction
The immune system has evolved to recognize and interact with microorganisms to protect our body from invading pathogens. For this purpose, leucocytes continuously patrol the body to identify invading pathogens and elicit immune responses. Advances in molecular biology have enabled us to identify many receptors and their endogenous or exogenous ligands, downstream signaling cascades of which have been extensively analyzed. However, the immune response is not a simple ‘all or none’ type response, but its consequences are complicated and varied. For example, T cells undergo differentiation, proliferation or death through the interaction of the T-cell antigen-receptor (TCR) with antigenic peptide bound to major histocompatibility complex (MHC). This process is critically regulated by the formation of immunological synapses, which are large-scale molecular movements that are accompanied by remodeling of the actin cytoskeleton (1). Membrane polarization and cytoskeletal dynamics are also necessary for leukocytes to migrate efficiently toward a chemoattractant source and both processes are regulated by Rho, Rac and Cdc42, which are members of the Rho family of small GTPases (2, 3).
Rho-family small GTPases act as molecular switches by cycling between GDP-bound inactive and GTP-bound active states, and transmit the signals through interactions with arrays of effector proteins (4). Stimulus-induced generation of the active form of small GTPases is catalyzed by guanine nucleotide exchange factors (GEFs) (5, 6). There are two distinct families of GEFs: Dbl homology (DH)-domain-containing proteins and dedicator of cytokinesis (DOCK) proteins (6). The first GEF to be discovered was a member of the Dbl family, which now includes more than 70 members (5, 6). Until recently, DH-domain-containing proteins have been considered to be the universal GEFs in eukaryotes. However, since our discovery of DOCK2 in lymphocyte migration (7), accumulating evidence indicates that the DOCK proteins act as major GEFs in varied biological settings (8, 9).
The DOCK family consists of 11 members and is classified into four subfamilies—DOCK-A (DOCK1, 2 and 5), DOCK-B (DOCK3 and 4), DOCK-C (DOCK6, 7 and 8) and DOCK-D (DOCK9, 10 and 11)—based on their sequence and substrate specificity. The DOCK family proteins are characterized by two evolutionarily conserved domains: DOCK homology region-1 (DHR-1) and DHR-2 (8, 9). While the DHR-2 domain catalyzes the GDP–GTP exchange reaction for Rac and Cdc42 (10, 11), the DHR-1 domain is involved in recruitment and localization of GEFs to the membrane compartments via binding to phospholipids (12, 13). Here, we review immune regulatory functions of DOCK family proteins in health and diseases, especially focusing on DOCK2 and DOCK8, because these molecules play central roles in immune surveillance in humans and mice.
DOCK2
Expression, structure and localization of DOCK2
DOCK2 was the second DOCK protein to be identified in mammals and belongs to the DOCK-A subfamily [KIAA0209 in (14)]. Whereas other DOCK-A subfamily members, DOCK1 and DOCK5, are expressed in various tissues, DOCK2 expression is restricted to hematopoietic cells (7). In response to various stimuli, DOCK2 binds to phosphatidylinositol 3,4,5 triphosphate (PIP3), a lipid product of phosphatidylinositol 3-kinases (PI3Ks), through the DHR-1 domain and translocates to the plasma membrane (15). In addition, DOCK2 associates with phosphatidic acid (PA) via its C-terminal polybasic amino acid region (PBR) (16).
Although DOCK2 does not contain a DH domain, DOCK2 mediates the GDP–GTP exchange reaction for Rac by means of its DHR-2 domain. The crystal structure of the DOCK2 DHR-2 domain and Rac revealed that this domain consists of three lobes (lobes A–C), but Rac binding is exclusively mediated by lobes B and C (8, 17). Among amino acid residues of lobes B and C, the valine residue located in the catalytic center of the DHR-2 domain (Val1538 in murine DHR-2 and Val1539 in human DHR-2) is important, because it functions as a ‘nucleotide sensor’ that mediates Mg2+ exclusion and GDP release from Rac (17). As Mg2+ is important for coordinating nucleotide binding through neutralizing the negatively charged phosphate groups, its exclusion profoundly reduces nucleotide affinity, leading to GDP release (17). Indeed, the Rac GEF activity of DOCK2 is completely lost when this valine is mutated to alanine (designated the GEF-dead VA mutant) (18).
Additionally, DOCK2 associates with ELMO1 (engulfment and cell motility protein 1) through its N-terminal Src homology 3 (SH3) domain and additional helix bundle formation (19). This interaction is also involved in DOCK2-mediated cellular functions, probably by relieving autoinhibition of ELMO1 or promoting protein stability of DOCK2 (19, 20). On the other hand, unlike DOCK1 and DOCK5, DOCK2 lacks the C-terminal proline-rich motif that mediates the association with the adaptor proteins CrkII and CrkL (Fig. 1) (21).
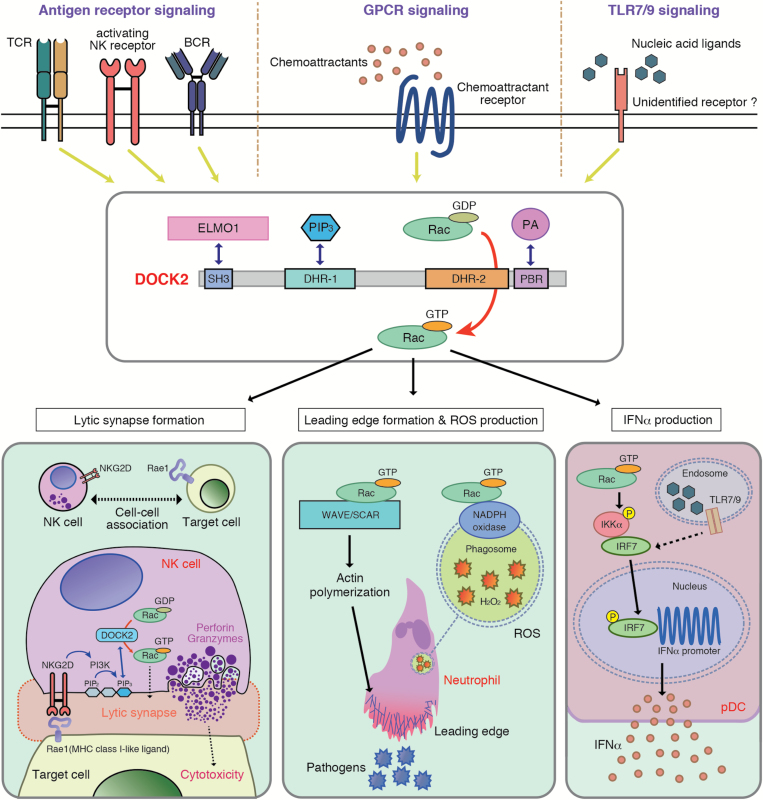
Fig. 1.
Schematic representation highlighting the roles of DOCK2 in immune surveillance. DOCK2 binds to phosphatidylinositol 3,4,5-triphosphate (PIP3) through the DHR-1 region and mediates the GTP–GDP exchange reaction for Rac by means of its DHR-2 domain. DOCK2 is a major Rac GEF that acts downstream of antigen-receptors and chemokine receptors (GPCRs) to control migration, activation and effector functions of leukocytes. On the other hand, although DOCK2 regulates TLR7/9-mediated type I IFN induction in pDCs via Rac activation, how Rac is activated in response to nucleic acid ligands remains unknown. DOCK2 also binds to engulfment and cell motility protein 1 (ELMO1) and phosphatidic acid (PA), and these interactions are functionally important.
Role of DOCK2 in lymphocyte trafficking
Trafficking of lymphocytes is of central importance for induction of adaptive immune responses. T and B cells migrate into the secondary lymphoid organs (SLOs), such as peripheral lymph nodes (PLNs), spleen and Peyer’s patches, via the blood (22). This process is regulated by homeostatic chemokines CXCL13, CCL19 and CCL21 that fulfill two major functions. First, CCL21 presented by high endothelial venules (HEVs) binds to its receptor CCR7 expressed on lymphocytes, which increases adhesiveness of integrins, in particular LFA-1, on blood-borne lymphocytes. Adherent lymphocytes then rearrange their cytoskeleton to squeeze through the HEV cell layer (22). A second function of chemokines is the establishment of specific microenvironments within lymphoid tissue. Interstitial T and B cells segregate into specific T-cell zones and B-cell follicles, which are defined by CXCL13 expression in B-cell follicles and CCL19/CCL21 expression in T-cell areas (22).
When wild-type (WT) lymphocytes were stimulated in vitro with CCL21 and CXCL13, they efficiently migrated in a dose-dependent manner. However, Dock2-deficient (Dock2–/–) lymphocytes did not show any migratory responses to these chemokines, resulting in severe atrophy of the SLOs (7, 23). Using two-photon microscopy, we also revealed that DOCK2 deficiency markedly reduced the migration speed of T and B cells inside lymphoid tissue of live mice (24). In addition, although sphingosine-1-phosphate (S1P) is known to mediate lymphocyte egress from the LNs (25), this process was also impaired in the absence of DOCK2 (24). Thus, DOCK2 plays key roles in all steps of lymphocyte trafficking.
G proteins (guanine nucleotide-binding proteins) are classified into two groups—small GTPases and heterotrimeric G proteins—the latter of which are composed of Gα, Gβ and Gγ subunits and are activated by GPCRs (G-protein-coupled receptors). Chemokine receptors and S1P receptors are coupled with heterotrimeric Gα i-containing proteins (26). Upon binding of chemokines and S1P to their receptors, the G protein is dissociated into the α-subunit and the βγ-subunits, which activates a variety of signaling pathways including Rac (26). In Dock2–/– lymphocytes, chemokine-induced Rac activation and actin polymerization were almost completely abolished, without affecting Akt phosphorylation and Ca2+ mobilization (7). When WT DOCK2 was expressed in Dock2–/– T cells, the migration speed on stromal cells marked increased (18). However, the expression of the VA mutant lacking the Rac GEF activity failed to restore T-cell motility (18). These results indicate that DOCK2 regulates lymphocyte migration by acting as a Rac GEF (Fig. 1).
Role of DOCK2 in immunological synapse formation
Engagement of antigen-receptors induces the formation of immunological synapses at the interface between lymphocytes and antigen-bearing cells or target cells. We found that TCR-mediated Rac activation was almost completely abolished in Dock2−/− T cells (27). The 2B4 TCR recognizes moth cytochrome C (MCC) peptide bound to I-Ek or I-Eb MHC molecules. When WT CD4+ T cells expressing the 2B4 TCR were stimulated in vitro with MCC peptide, both TCR and lipid raft localized to the interface (27). However, such TCR polarization and lipid-raft clustering were impaired in the absence of DOCK2, resulting in a significant reduction of T-cell proliferation (27). Interestingly, the number of double-positive (DP) thymocytes was markedly reduced in Dock2−/− 2B4 TCR transgenic (Tg) mice, suggesting that DOCK2 regulates the threshold for positive selection in the thymus probably through immunological synapse formation (27). Similarly, B-cell antigen-receptor (BCR)-mediated Rac activation and immunological synapse formation were impaired in Dock2−/− B cells, which resulted in defective plasma cell differentiation in vivo (28).
The mechanistic basis for DOCK2-mediated immunological synapse formation was analyzed in natural killer (NK) cells (Fig. 1), which are innate lymphocytes that play an important role in protective immunity against virus infection and tumor progression via contact-dependent cytotoxicity. NK cells express multiple activating-receptors including NKG2D that binds to the MHC class I-like ligand Rae1 expressed on the target cells (29). Ligation of activating-receptors with their ligands induces receptor clustering at the interface and triggers polarized movement of lytic granules to the contact sites. We found that NKG2D-mediated Rac activation and lytic synapse formation were severely impaired in Dock2−/− NK cells (30). This defect was rescued by expressing WT DOCK2, but not the GEF-dead VA mutant, indicating that DOCK2 regulates the lytic synapse formation through Rac activation (30). On the other hand, DOCK2 was recruited to the synapse in a manner dependent on PI3K activation and PIP3 production (30). A similar mechanism has been shown in CD8+ T cells (31).
Collectively, these results indicate that the PI3K–DOCK2–Rac axis plays key roles in antigen-receptor-mediated lymphocyte functions. So far, the DH-domain-containing Vav proteins (Vav1–Vav3) have been considered to be major Rac GEFs acting downstream of antigen-receptors in T cells, B cells and NK cells (32–38). However, considering the result by Miletic et al. showing that immunological defects of Vav-deficient T cells can be rescued by the expression of a Vav mutant lacking the intrinsic GEF activity, it is likely that Vav proteins regulate the development and activation of lymphocytes by functioning as adaptor molecules and assembling signaling complexes (39).
Role of DOCK2 in neutrophil chemotaxis and production of reactive oxygen species
Neutrophils are highly motile leukocytes and play important roles in the innate immune response to invading pathogens. By sensing the gradient of chemoattractants such as N-formyl-Met-Leu-Phe (fMLP) and C5a via GPCRs, neutrophils migrate to the site of infection. As neutrophil chemotaxis requires Rac activation (40), significant efforts have been made to identify a Rac GEF that functions downstream of chemoattractant receptors in neutrophils. P-Rex1 is a PIP3-dependent and Gβγ-regulated GEF that has been purified from neutrophils (41). Since the discovery of P-Rex1 in 2002 (41), it had been considered that P-Rex1 would be a major Rac activator critical for neutrophil chemotaxis (42). However, it was shown that neutrophil chemotaxis is only mildly affected in P-Rex1-deficient neutrophils and P-Rex1 itself directly activates RhoG (another Rho family of small GTPase), but not Rac (43–45).
On the other hand, we found that fMLP-induced activation of Rac was severely impaired in Dock2–/– neutrophils, resulting in marked reduction of motility and polarity of neutrophils (15). As Rac is a cytosolic component of NADPH oxidases (46), fMLP-induced or phorbol 12-myristate 13-acetate-induced production of reactive oxygen species (ROS) was markedly reduced in Dock2–/– neutrophils (15). In addition, formation of neutrophil extracellular traps (NETs), which is dependent on ROS production, was also defective in Dock2–/– neutrophils (47). Thus, DOCK2 is a major Rac GEF that regulates neutrophil chemotaxis, ROS production and NETs formation (Fig. 2).
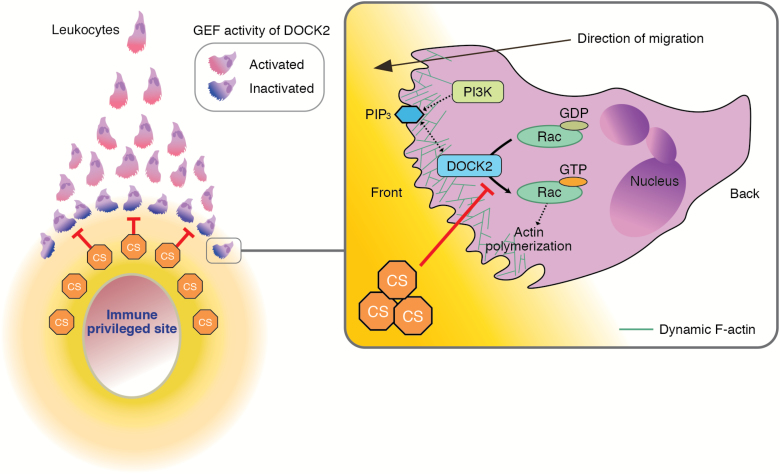
Fig. 2.
A model for CS-mediated chemical barrier formation in immune-privileged sites. CS is a naturally occurring DOCK2 inhibitor and prevents leukocyte infiltration by creating a chemical barrier. It is likely that CS acts locally and contributes to the generation of immune evasive microenvironments in certain tissues and organs known as immune-privileged sites.
To explore the mechanism controlling intracellular DOCK2 dynamics during neutrophil chemotaxis, we developed the ‘knock-in’ mice that express endogenous DOCK2 as a fusion protein with green fluorescent protein (GFP) (15, 16). Upon stimulation, we found that DOCK2 rapidly translocated to the plasma membrane in a PIP3-dependent manner (16). However, subsequent accumulation of DOCK2 at the leading edge required phospholipase-D-mediated synthesis of PA to stabilize DOCK2 localization and increase local actin polymerization (16). When this interaction was blocked, neutrophils failed to form leading edges properly and exhibited defects in chemotaxis (16). These results indicate that intracellular DOCK2 dynamics are sequentially regulated by two distinct phospholipids, PIP3 and PA, to localize Rac activation during neutrophil chemotaxis.
Role of DOCK2 in production of type I interferons by plasmacytoid dendritic cells
Dendritic cells (DCs) are classified into two populations—myeloid DCs (mDCs) and plasmacytoid DCs (pDCs)—according to their morphology, cell surface markers and their functions. We found that DOCK2 deficiency also impaired chemokine-induced Rac activation and migratory responses of pDCs (48). In contrast, Dock2–/– mDCs did not show any defects in Rac activation and migration. Unlike pDCs, mDCs express DOCK1, DOCK2 and DOCK5 (48). Therefore, in mDCs, the effect of DOCK2 deficiency may be functionally compensated by DOCK1 and DOCK5, both of which are known as Rac-specific GEFs.
Upon recognition of Toll-like receptor 7 (TLR7) and TLR9, which are intracellular receptors that recognize nucleic acid ligands, pDCs produce not only inflammatory cytokines, but also large amounts of type I interferons (type I IFNs, i.e. IFN-α and IFN-β) (49). In pDCs, type I IFN induction critically depends on interferon regulatory factor 7 (IRF-7) (50). Although it is known that IKK-α directly binds to and activates IRF-7 (51), the signaling cascades leading to type I IFN induction are not completely defined. We found that the exposure of pDCs to nucleic acid ligands induced Rac activation through a TLR-independent and DOCK2-dependent mechanism (52). This Rac activation was dispensable for production of inflammatory cytokines such as IL-6 and IL-12p40. However, phosphorylation of IKK-α and nuclear translocation of IRF-7 were impaired in Dock2–/– pDCs, resulting in selective loss of type I IFN induction (52). Although the precise mechanism of how Rac is activated in response to nucleic acid ligands remains unknown, these results indicate that the DOCK2–Rac signaling pathway acts in parallel with TLR engagement to control IKK-α activation for type I IFN induction (Fig. 1).
DOCK2 deficiency in humans
It was recently reported that bi-allelic DOCK2 mutations in humans cause severe combined immunodeficiency with early-onset, invasive bacterial and viral infections (53, 54). These mutations include frameshift mutations, missense mutations and splice region mutations, which resulted in absent or markedly reduced levels of DOCK2 protein expression or in expression of a truncated protein lacking the DHR-2 domain (53, 54). Leukocytes from DOCK2-deficient patients exhibit multiple defects, including those in chemotactic responses of T and B cells, degranulation of NK cells, ROS production by neutrophils and type I IFN production by peripheral blood mononuclear cells (53, 54). These abnormalities are quite similar to the phenotypes of Dock2–/– mice as described above, highlighting the central role of DOCK2 in immune surveillance in both humans and mice.
Identification of cholesterol sulfate as a naturally occurring DOCK2 inhibitor
Although immune responses are crucial to protect our body from invading pathogens, they generally carry a risk of damaging vital tissues as well. Therefore, certain tissues and organs, such as the eye, the brain and the pregnant uterus, constitute specialized microenvironments that locally inhibit immune reactivity. This phenomenon is classically known as immune privilege (55). However, the underlying mechanisms remain to be determined.
By screening a library of pharmacologically active compounds, we found that cholesterol sulfate (CS) is a potent inhibitor of DOCK2 (56). Indeed, CS directly bound to the DOCK2 DHR-2 domain and inhibited its Rac GEF activity with a half-maximal inhibitory concentration of 2.0 µM (56). Functionally, CS effectively inhibited the migratory response of T cells to CCL21 in a concentration-dependent manner (56). This inhibitory effect was specific to CS, because other cholesterol derivatives did not affect the migration of lymphocytes (56). Consistent with this, treatment of lymphocytes with CS markedly suppressed CCL21-mediated Rac activation (56). Interestingly, we found that in the TAXIScan assay, neutrophils undergoing chemotaxis stopped migration at a defined distance when CS was added to the fMLP source (56). Thus, CS prevents leukocyte infiltration by creating a ‘chemical barrier’ (Fig. 2).
CS has been implicated in many biological processes (57–59), yet its physiological functions remain elusive. We found that CS was most abundantly produced in mice in the Harderian gland (equivalent to Meibomian gland in humans) (56), which provides lipids to form the oily layer of the tear film. Mass spectrometry imaging also revealed that CS was present in the anterior chamber. Sulfation of cholesterol is mediated by the sulfotransferase SULT2B1b and, to a lesser extent, SULT2B1a, which are produced from the same gene Sult2b1 through alternative splicing. By genetically inactivating Sult2b1, we found that the lack of CS augmented ultraviolet-induced and antigen-induced ocular surface inflammation (56). These results indicate that CS contributes to the generation of the immune evasive microenvironments in the eye (Fig. 2).
DOCK8
Expression, structure and localization of DOCK8
DOCK8 is expressed not only in hematopoietic cells, but also in non-immune tissues such as lung, pancreas, kidney and placenta (60). DOCK8 belongs to the DOCK-C subfamily. Although DOCK8 reportedly activates both Rac and Cdc42, the bacterially expressed DOCK8 DHR-2 domain mediated GTP loading on Cdc42, but not Rac. In order to understand the mechanism underlying the Cdc42 specificity of DOCK8, we determined the crystal structure of DOCK8 DHR-2 domain complexed with Cdc42 (61). Lobes B and C of DOCK8 DHR-2 generate a cooperative interface with Cdc42, in a manner similar to DOCK9, a well-known Cdc42-specific GEF (11, 61). A structural comparison between the DOCK8 DHR-2–Cdc42 and DOCK2 DHR-2–Rac1 complexes revealed that lobes B and C of the DHR-2 domain are arranged in different orientations for Cdc42 or Rac1 (8), supporting the idea that DOCK8 acts as a Cdc42-specific GEF (Fig. 3). On the other hand, the amino acid sequence of the DOCK8 DHR-1 domain is totally different from that of DOCK2 DHR-1. Therefore, it is conceivable that phospholipid other than PIP3 binds to the DOCK8 DHR-1 domain and controls its localization. In addition, recent studies have identified DOCK8-binding partners such as mammalian ste-20 like kinase 1 (MST1; see below), leucine repeat adaptor protein 35a (LRAP35a; see below), WASP-interacting protein (WIP), leucine-rich repeat and calponin homology domain-containing protein 1 (LRCH1) and septin 7 (62–66).
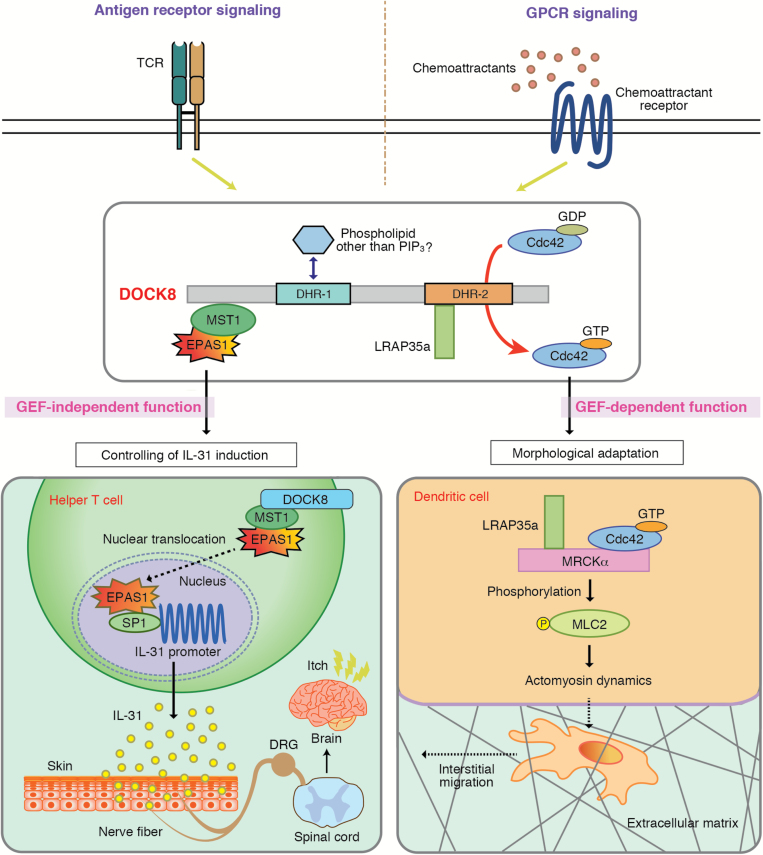
Fig. 3.
GEF-independent or GEF-dependent immune regulatory functions of DOCK8. DOCK8 is a Cdc42-specific GEF that links Cdc42 activation to actomyosin dynamics through the association with LRAP35a. This signaling cascade is required for mDCs to pass through the narrow gaps of three-dimensional (3D) fibrillar networks and transmigrate through the subcapsular sinus floor of the LNs. On the other hand, IL-31 is a major pruritogen associated with AD. DOCK8 negatively regulates antigen-induced IL-31 production by helper T cells. This function does not require the Cdc42 GEF activity, but is mediated by formation of trimolecular complex composed of mammalian ste-20 like kinase 1 (MST1) and endothelial PAS domain 1 (EPAS1). EPAS1 is a master regulator for IL-31 induction, thereby serving as a therapeutic target for controlling AD-associated itch.
DOCK8 deficiency in humans and mice
The bi-allelic DOCK8 mutations in humans cause combined immunodeficiency characterized by recurrent viral infections, early-onset malignancy and atopic dermatitis (AD) (67). Although DOCK8 deficiency is rare, more than 200 cases have been reported worldwide to date and it is becoming a well-recognized primary immunodeficiency (67–69). DOCK8 deficiency syndrome is an autosomal recessive disease caused by loss-of-function mutations in the DOCK8 gene (67). Most cases are associated with large deletions, which results in absent or trace amounts of expressed DOCK8 protein (67–69).
Accumulating evidence indicates that human patients with DOCK8 mutations have morphological and functional abnormalities of leukocytes (70–72). In addition, the important roles of DOCK8 in leukocytes have been demonstrated using animal models. For example, N-ethyl-N-nitrosourea (ENU)-mediated mutagenesis in mice has shown that DOCK8 regulates immunological synapse formation in B cells and is required for development or survival of memory CD8+ T cells, NKT cells and group 3 innate lymphoid cells (72–77). On the other hand, by generating DOCK8-deficient (Dock8–/–) mice, we and others have shown that DOCK8 is essential for interstitial migration of mDCs (61, 78, 79).
Role of DOCK8 in migration of interstitial mDCs
The mDCs are the most potent antigen-presenting cells that reside in peripheral tissues such as skin. Upon antigen exposure, mDCs phagocytose antigens and migrate via the afferent lymphatic vessels into the draining PLNs to stimulate T cells (22). During this process, mDCs switch their sessile sampling behavior to a highly migratory behavior, which is characterized by the acquisition of a polarized morphology and increased expression of the chemokine receptor CCR7. Although CCR7 signals guide mDCs to the LN parenchyma (80), mDCs must pass through three-dimensional interstitial space composed of fibrillar extracellular matrix (ECM) before reaching their destination. To perform this task efficiently, mDCs constantly adapt their shape to the given structure of the interstitial ECM and follow the path of least resistance (81). This amoeboid migration of mDCs occurs independently of adhesion to specific substrates and ECM degradation (82, 83), yet its regulatory mechanisms are poorly understood.
We found that, in the absence of DOCK8, mDCs failed to accumulate in the LN parenchyma for T-cell priming (61). Although DOCK8-deficient mDCs migrated normally on two-dimensional surfaces, DOCK8 was required for mDCs to pass through the narrow gaps of three-dimensional (3D) fibrillar networks and transmigrate through the subcapsular sinus floor of the LNs (61). In this process, the GEF activity of DOCK8 was required. We found that DOCK8 associated with LRAP35a, an adaptor molecule that binds to the Cdc42 effector MRCKα (myotonic dystrophy kinase-related Cdc42-binding kinase α), and facilitated its activity to phosphorylate myosin II regulatory light chain (MLC2) (63). When this interaction was disrupted in WT mDCs, they showed a migration defect, as seen in Dock8–/– mDCs (63). Thus, during mDC migration, DOCK8 links Cdc42 activation to actomyosin dynamics through an interaction with LRAP35a (Fig. 3).
DOCK8 acts as negative regulator for IL-31 induction in helper T cells
IL-31 is a cytokine that is related to the IL-6 cytokine family in terms of its structure and receptor complex (84). Recently, much attention has been paid to IL-31 as an AD-associated itch mediator since the discovery of the pruritogenic action of IL-31 in mice (85). IL-31 is mainly produced by CD4+ T cells and transmits signals via a heterodimeric receptor composed of IL-31 receptor A (IL-31RA) and oncostatin M receptor (OSMR), both of which are expressed in various cell types including dorsal root ganglion (DRG) neurons (86). A recent clinical study has demonstrated that blockade of IL-31 signals by a specific antibody for IL-31RA alleviates pruritus in patients with AD (87). However, the mechanisms controlling IL-31 induction in helper T cells are poorly understood.
To examine the role of DOCK8 in antigen-specific T-cell responses, we developed Dock8–/– mice expressing the OTII TCR that recognizes OVA peptide bound to I-Ab MHC molecules. OVA-specific T cell proliferation occurred normally even in the absence of DOCK8. However, these CD4+ T cells produced large amounts of IL-31, as compared with those of Dock8+/– OTII Tg CD4+ T cells. To examine whether this effect could be extended to other CD4+ T cells with different antigen specificity, we developed Dock8–/– mice expressing the AND TCR on the genetic background of C57BL/6 mice. The AND TCR is a product of artificial TCRαβ-chain combination, which recognizes the MCC peptide in the context of I-Ek MHC molecules; yet, it is known that CD4+CD8+ thymocytes expressing AND are also selected to mature in the presence of I-Ab MHC molecules (88). As seen in Dock8–/– OTII Tg mice, CD4+ T cells from Dock8–/– AND Tg mice produced large amounts of IL-31 upon stimulation with MCC peptide. Thus, DOCK8 generally acts as a negative regulator for IL-31 induction in CD4+ T cells (62).
Recent evidence indicates that the AND TCR shows high self-reactivity to selecting I-Ab MHC molecules (89). Surprisingly, we found that Dock8–/– AND Tg mice spontaneously developed severe skin inflammation with scratching behavior and increased serum IL-31 levels (62). However, this skin inflammation was completely lost in Dock8–/– AND Tg mice when OSMR expression was deleted. These results indicate that Dock8–/– AND Tg mice spontaneously develop atopic skin inflammation through the mechanism dependent on IL-31 signaling (62).
By functionally analyzing the transcription factors up-regulated in Dock8–/– CD4+ T cells in microarrays, we identified endothelial PAS domain 1 (EPAS1) as a master regulator for IL-31 induction in CD4+ T cells (62). Indeed, induction of Il31 in Dock8–/– AND CD4+ T cells was markedly suppressed by knocking down Epas1 gene expression using small interfering RNA (siRNA). Similar results were obtained when Il31 gene expression was analyzed in CD4+ T cells from Dock8–/– AND Tg mice lacking EPAS1 expression in a T-cell-specific manner (CD4-Cre+Epas1lox/loxDock8–/– AND Tg mice). More importantly, scratching behavior, skin disease development and increased serum IL-31 level were cancelled in all CD4-Cre+Epas1lox/loxDock8–/– AND Tg mice tested. Although EPAS1 is known to form a complex with aryl hydrocarbon receptor nuclear translocator (ARNT) and control hypoxic responses (90), EPAS1-mediated Il31 promoter activation was independent of ARNT, but acted in collaboration with SP1 (62).
To understand the mechanism by which DOCK8 negatively regulates IL-31 induction, we examined the effect of DOCK8 deficiency on subcellular localization of EPAS1 in mouse embryonic fibroblasts (MEFs). We found that nuclear localization of EPAS1 was markedly augmented in Dock8–/– MEFs, as compared with that in WT MEFs. This effect of DOCK8 deficiency was cancelled when either WT DOCK8 or a DOCK8 mutant lacking the DHR-2 domain was stably expressed in Dock8–/– MEFs. However, the expression of the DOCK8 mutant lacking the N-terminal 527 amino acid residues failed to suppress nuclear accumulation of EPAS1 in Dock8–/– MEFs, indicating that the N-terminal region of DOCK8 is important for controlling subcellular localization of EPAS1.
During the course of screening for DOCK8-binding proteins, we found that DOCK8 bound to MST1 through the N-terminal region. When Mst1 gene expression was knocked down in WT MEFs, nuclear translocation of EPAS1 was significantly augmented. More importantly, knockdown of Mst1 markedly induced Il31 gene expression in CD4+ T cells from Dock8+/– AND Tg mice. These results indicate that the DOCK8–MST1 axis negatively regulates IL-31 induction by inhibiting nuclear translocation of EPAS1 (62) (Fig. 3).
A cautionary tale about immune phenotypes of gene-targeted mice
Gene targeting has become a powerful approach to dissect the role of genes in vivo. To assess the phenotypes of gene-targeted mice, it is a common practice to backcross into the C57BL/6 background. Recently, however, a homozygous copy-number variant that disrupts the function of DOCK2 was found in a commercially available C57BL/6 strain (C57BL/6NHsd) that is widely used for backcrossing (91). The originally reported immune defective phenotypes of mice deficient in interferon regulatory factor 5 (Irf5), sialic acid acetyl esterase (Siae) and cytidine monophosphate N-acetylneuraminic acid hydroxylase (Cmah) on this background turned out to be due to the DOCK2 deficiency (91–93). Fortunately, a range of other commercially available C57BL/6J and C57BL/6N mice have only WT Dock2 (91).
Apparently unrelated, a role in the adaptive immune response was wrongly assigned to the inflammasome adaptor ASC (apoptosis-associated speck-like protein containing a CARD) because of the loss of DOCK2 expression in some backgrounds (94). Similarly, coincidental loss of DOCK8 in NLRP10 (NOD-like receptor family pyrin domain-containing 10)-deficient mice led to an mDC migration defect, which is unrelated to NLRP10 function (78). At least one strain of C3H/HeJ mice harbors a Dock8 mutation that partially impairs mDC migration (78). The sheer size of Dock2 (52 exons in >550 kb) and Dock8 (48 exons in >200 kb) may potentially give rise to the problems. Nonetheless, these observations independently confirmed the crucial roles of DOCK2 and DOCK8 in immune surveillance. Thus, it should be recommended as a cautionary measure to check the status and functions of DOCK2 and/or DOCK8 when interpreting any immune related phenotypes of gene-targeted mice.
Summary and perspectives
Studies over the last two decades have established that DOCK2 is a major Rac activator critical for migration and activation of leukocytes. The DOCK2 DHR-2 domain exhibits 100- to 200-fold stronger Rac GEF activity in vitro, as compared with those of the DH–PH (pleckstrin homology) domains of the classical Rac GEFs such as Trio and Tiam (56). This is likely to make DOCK2 a ‘special’ Rac GEF that mediates non-redundant functions during immune responses.
Recent advances in biochemical and structural studies have provided a novel insight into how DOCK2 activates Rac and induces cytoskeletal reorganization. Additionally, identification of DOCK2-deficient patients revealed that DOCK2 plays key roles in immune surveillance in both humans and mice. As such, it was surprising to find that CS acts as a naturally occurring DOCK2 inhibitor and mediates immune evasion in the eye by creating a chemical barrier. Besides the classically known immune-privileged sites, various tissues, including tumors, also create microenvironments that help them evade immune surveillance. Therefore, further studies are needed to unravel the potential roles of CS in other tissues.
On the other hand, DOCK8 acts as a Cdc42-specific GEF and its mutations in humans cause combined immunodeficiency characterized by recurrent viral infections, early-onset malignancy, and AD. We found that Dock8–/– AND Tg mice spontaneously developed severe skin inflammation with increased serum IL-31 levels. By analyzing these mice, we identified EPAS1 as a master regulator for IL-31 induction in helper T cells. Recently, the use of Dock8–/– AND Tg mice also led us to identify neurokinin B as a key molecule that transmits an IL-31-induced itch sensation in the spinal cord (95). Thus, Dock8–/– AND Tg mice could be a useful animal model to understand the pathogenesis of AD. Interestingly, although DOCK8-deficient patients also suffer from severe food allergies (96), this manifestation does not improve after bone marrow transplantation (96, 97). Therefore, elucidation of the underlying mechanism for food allergies would be a challenging clinical problem in this field.
Funding
This work was supported by Japan Agency for Medical Research and Development (grant JP19gm0010001, JP19gm1310005 and JP19ek0410064 to Y.F.) and Japan Society for the Promotion of Science (grant 19H00983 to Y.F.).
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Dustin M. L. and Cooper J. A. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23. [DOI] [PubMed] [Google Scholar]
- 2. Bokoch G. M. 2005. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 15:163. [DOI] [PubMed] [Google Scholar]
- 3. Tybulewicz V. L. and Henderson R. B. 2009. Rho family GTPases and their regulators in lymphocytes. Nat. Rev. Immunol. 9:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jaffe A. B. and Hall A. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt A. and Hall A. 2002. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16:1587. [DOI] [PubMed] [Google Scholar]
- 6. Rossman K. L., Der C. J. and Sondek J. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6:167. [DOI] [PubMed] [Google Scholar]
- 7. Fukui Y., Hashimoto O., Sanui T. et al. 2001. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 412:826. [DOI] [PubMed] [Google Scholar]
- 8. Nishikimi A., Kukimoto-Niino M., Yokoyama S. and Fukui Y. 2013. Immune regulatory functions of DOCK family proteins in health and disease. Exp. Cell Res. 319:2343. [DOI] [PubMed] [Google Scholar]
- 9. Côté J. F. and Laurin M. 2014. Insights into the biological functions of Dock family guanine nucleotide exchange factors. Genes Dev. 15:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Côté J. F. and Vuori K. 2002. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 115(Pt 24):4901. [DOI] [PubMed] [Google Scholar]
- 11. Yang J., Zhang Z., Roe S. M., Marshall C. J. and Barford D. 2009. Activation of Rho GTPases by DOCK exchange factors is mediated by a nucleotide sensor. Science 325:1398. [DOI] [PubMed] [Google Scholar]
- 12. Côté J. F., Motoyama A. B., Bush J. A. and Vuori K. 2005. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat. Cell Biol. 7:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Premkumar L., Bobkov A. A., Patel M. et al. 2010. Structural basis of membrane targeting by the Dock180 family of Rho family guanine exchange factors (Rho-GEFs). J. Biol. Chem. 285:13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu Y. C. and Horvitz H. R. 1998. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 392:501. [DOI] [PubMed] [Google Scholar]
- 15. Kunisaki Y., Nishikimi A., Tanaka Y. et al. 2006. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J. Cell Biol. 174:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishikimi A., Fukuhara H., Su W. et al. 2009. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 324:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulkarni K., Yang J., Zhang Z. and Barford D. 2011. Multiple factors confer specific Cdc42 and Rac protein activation by dedicator of cytokinesis (DOCK) nucleotide exchange factors. J. Biol. Chem. 286:25341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Terasawa M., Uruno T., Mori S. et al. 2012. Dimerization of DOCK2 is essential for DOCK2-mediated Rac activation and lymphocyte migration. PLoS ONE 7:e46277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanawa-Suetsugu K., Kukimoto-Niino M., Mishima-Tsumagari C. et al. 2012. Structural basis for mutual relief of the Rac guanine nucleotide exchange factor DOCK2 and its partner ELMO1 from their autoinhibited forms. Proc. Natl Acad. Sci. USA 109:3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevenson C., de la Rosa G., Anderson C. S. et al. 2014. Essential role of Elmo1 in Dock2-dependent lymphocyte migration. J. Immunol. 192:6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanui T., Inayoshi A., Noda M. et al. 2003. DOCK2 regulates Rac activation and cytoskeletal reorganization through interaction with ELMO1. Blood 102:2948. [DOI] [PubMed] [Google Scholar]
- 22. Stein J. V. and Nombela-Arrieta C. 2005. Chemokine control of lymphocyte trafficking: a general overview. Immunology 116:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nombela-Arrieta C., Lacalle R. A., Montoya M. C. et al. 2004. Differential requirements for DOCK2 and phosphoinositide-3-kinase gamma during T and B lymphocyte homing. Immunity 21:429. [DOI] [PubMed] [Google Scholar]
- 24. Nombela-Arrieta C., Mempel T. R., Soriano S. F. et al. 2007. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J. Exp. Med. 204:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matloubian M., Lo C. G., Cinamon G. et al. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355. [DOI] [PubMed] [Google Scholar]
- 26. Thelen M. and Stein J. V. 2008. How chemokines invite leukocytes to dance. Nat. Immunol. 9:953. [DOI] [PubMed] [Google Scholar]
- 27. Sanui T., Inayoshi A., Noda M. et al. 2003. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity 19:119. [DOI] [PubMed] [Google Scholar]
- 28. Ushijima M., Uruno T., Nishikimi A. et al. 2018. The Rac activator DOCK2 mediates plasma cell differentiation and IgG antibody production. Front. Immunol. 9:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilfillan S., Ho E. L., Cella M., Yokoyama W. M. and Colonna M. 2002. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat. Immunol. 3:1150. [DOI] [PubMed] [Google Scholar]
- 30. Sakai Y., Tanaka Y., Yanagihara T. et al. 2013. The Rac activator DOCK2 regulates natural killer cell-mediated cytotoxicity in mice through the lytic synapse formation. Blood 122:386. [DOI] [PubMed] [Google Scholar]
- 31. Le Floc’h A., Tanaka Y., Bantilan N. S. et al. 2013. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J. Exp. Med. 210:2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tarakhovsky A., Turner M., Schaal S. et al. 1995. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature 374:467. [DOI] [PubMed] [Google Scholar]
- 33. Zhang R., Alt F. W., Davidson L., Orkin S. H. and Swat W. 1995. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature 374:470. [DOI] [PubMed] [Google Scholar]
- 34. Fischer K. D., Zmuldzinas A., Gardner S., Barbacid M., Bernstein A. and Guidos C. 1995. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature 374:474. [DOI] [PubMed] [Google Scholar]
- 35. Doody G. M., Bell S. E., Vigorito E. et al. 2001. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat. Immunol. 2:542. [DOI] [PubMed] [Google Scholar]
- 36. Tedford K., Nitschke L., Girkontaite I. et al. 2001. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2:548. [DOI] [PubMed] [Google Scholar]
- 37. Billadeau D. D., Brumbaugh K. M., Dick C. J., Schoon R. A., Bustelo X. R. and Leibson P. J. 1998. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J. Exp. Med. 188:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galandrini R., Palmieri G., Piccoli M., Frati L. and Santoni A. 1999. Role for the Rac1 exchange factor Vav in the signaling pathways leading to NK cell cytotoxicity. J. Immunol. 162:3148. [PubMed] [Google Scholar]
- 39. Miletic A. V., Graham D. B., Sakata-Sogawa K. et al. 2009. Vav links the T cell antigen receptor to the actin cytoskeleton and T cell activation independently of intrinsic Guanine nucleotide exchange activity. PLoS ONE 4:e6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu Y., Filippi M. D., Cancelas J. A. et al. 2003. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302:445. [DOI] [PubMed] [Google Scholar]
- 41. Welch H. C., Coadwell W. J., Ellson C. D. et al. 2002. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell 108:809. [DOI] [PubMed] [Google Scholar]
- 42. Weiner O. D. 2002. Rac activation: P-Rex1 - a convergence point for PIP(3) and Gbetagamma? Curr. Biol. 12:R429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dong X., Mo Z., Bokoch G., Guo C., Li Z. and Wu D. 2005. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr. Biol. 15:1874. [DOI] [PubMed] [Google Scholar]
- 44. Welch H. C., Condliffe A. M., Milne L. J. et al. 2005. P-Rex1 regulates neutrophil function. Curr. Biol. 15:1867. [DOI] [PubMed] [Google Scholar]
- 45. Damoulakis G., Gambardella L., Rossman K. L. et al. 2014. P-Rex1 directly activates RhoG to regulate GPCR-driven Rac signalling and actin polarity in neutrophils. J. Cell Sci. 127(Pt 11):2589. [DOI] [PubMed] [Google Scholar]
- 46. Panday A., Sahoo M. K., Osorio D. and Batra S. 2015. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watanabe M., Terasawa M., Miyano K. et al. 2014. DOCK2 and DOCK5 act additively in neutrophils to regulate chemotaxis, superoxide production, and extracellular trap formation. J. Immunol. 193:5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gotoh K., Tanaka Y., Nishikimi A. et al. 2008. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood 111:2973. [DOI] [PubMed] [Google Scholar]
- 49. Kawai T. and Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373. [DOI] [PubMed] [Google Scholar]
- 50. Honda K., Yanai H., Negishi H. et al. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772. [DOI] [PubMed] [Google Scholar]
- 51. Hoshino K., Sugiyama T., Matsumoto M. et al. 2006. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature 440:949. [DOI] [PubMed] [Google Scholar]
- 52. Gotoh K., Tanaka Y., Nishikimi A. et al. 2010. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J. Exp. Med. 207:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dobbs K., Domínguez Conde C., Zhang S. Y. et al. 2015. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N. Engl. J. Med. 372:2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moens L., Gouwy M., Bosch B. et al. 2019. Human DOCK2 deficiency: report of a novel mutation and evidence for neutrophil dysfunction. J. Clin. Immunol. 39:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Niederkorn J. Y. 2006. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat. Immunol. 7:354. [DOI] [PubMed] [Google Scholar]
- 56. Sakurai T., Uruno T., Sugiura Y. et al. 2018. Cholesterol sulfate is a DOCK2 inhibitor that mediates tissue-specific immune evasion in the eye. Sci. Signal. 11:eaao4874. [DOI] [PubMed] [Google Scholar]
- 57. Langlais J., Zollinger M., Plante L., Chapdelaine A., Bleau G. and Roberts K. D. 1981. Localization of cholesteryl sulfate in human spermatozoa in support of a hypothesis for the mechanism of capacitation. Proc. Natl Acad. Sci. USA 78:7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Merten M., Dong J. F., Lopez J. A. and Thiagarajan P. 2001. Cholesterol sulfate: a new adhesive molecule for platelets. Circulation 103:2032. [DOI] [PubMed] [Google Scholar]
- 59. Wang F., Beck-García K., Zorzin C., Schamel W. W. and Davis M. M. 2016. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat. Immunol. 17:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ruusala A. and Aspenström P. 2004. Isolation and characterisation of DOCK8, a member of the DOCK180-related regulators of cell morphology. FEBS Lett. 572:159. [DOI] [PubMed] [Google Scholar]
- 61. Harada Y., Tanaka Y., Terasawa M. et al. 2012. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 119:4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamamura K., Uruno T., Shiraishi A. et al. 2017. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat. Commun. 8:13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shiraishi A., Uruno T., Sanematsu F. et al. 2017. DOCK8 protein regulates macrophage migration through Cdc42 protein activation and LRAP35a protein interaction. J. Biol. Chem. 292:2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Janssen E., Tohme M., Hedayat M. et al. 2016. A DOCK8-WIP-WASp complex links T cell receptors to the actin cytoskeleton. J. Clin. Invest. 126:3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xu X., Han L., Zhao G. et al. 2017. LRCH1 interferes with DOCK8-Cdc42-induced T cell migration and ameliorates experimental autoimmune encephalomyelitis. J. Exp. Med. 214:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schauer M., Kleinwort K. J. H., Degroote R. L. et al. 2018. Interaction of septin 7 and DOCK8 in equine lymphocytes reveals novel insights into signaling pathways associated with autoimmunity. Sci. Rep. 8:12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Q., Davis J. C., Lamborn I. T. et al. 2009. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361:2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Engelhardt K. R., Gertz M. E., Keles S. et al. 2015. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J. Allergy Clin. Immunol. 136:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Biggs C. M., Keles S. and Chatila T. A. 2017. DOCK8 deficiency: insights into pathophysiology, clinical features and management. Clin. Immunol. 181:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jabara H. H., McDonald D. R., Janssen E. et al. 2012. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat. Immunol. 13:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mizesko M. C., Banerjee P. P., Monaco-Shawver L. et al. 2013. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J. Allergy Clin. Immunol. 131:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang Q., Dove C. G., Hor J. L. et al. 2014. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J. Exp. Med. 211:2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Randall K. L., Lambe T., Johnson A. L. et al. 2009. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat. Immunol. 10:1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Randall K. L., Chan S. S., Ma C. S. et al. 2011. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J. Exp. Med. 208:2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lambe T., Crawford G., Johnson A. L. et al. 2011. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur. J. Immunol. 41:3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Crawford G., Enders A., Gileadi U. et al. 2013. DOCK8 is critical for the survival and function of NKT cells. Blood 122:2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Singh A. K., Eken A., Fry M., Bettelli E. and Oukka M. 2014. DOCK8 regulates protective immunity by controlling the function and survival of RORγt+ ILCs. Nat. Commun. 5:4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Krishnaswamy J. K., Singh A., Gowthaman U. et al. 2015. Coincidental loss of DOCK8 function in NLRP10-deficient and C3H/HeJ mice results in defective dendritic cell migration. Proc. Natl Acad. Sci. USA 112:3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Krishnaswamy J. K., Gowthaman U., Zhang B. et al. 2017. Migratory CD11b+ conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci. Immunol. 2:eaam9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Förster R., Schubel A., Breitfeld D. et al. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 102:3262. [DOI] [PubMed] [Google Scholar]
- 81. Friedl P. and Weigelin B. 2008. Interstitial leukocyte migration and immune function. Nat. Immunol. 9:960. [DOI] [PubMed] [Google Scholar]
- 82. Wolf K., Müller R., Borgmann S., Bröcker E. B. and Friedl P. 2003. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102:3262. [DOI] [PubMed] [Google Scholar]
- 83. Lämmermann T., Bader B. L., Monkley S. J. et al. 2008. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453:51. [DOI] [PubMed] [Google Scholar]
- 84. Cornelissen C., Lüscher-Firzlaff J., Baron J. M. and Lüscher B. 2012. Signaling by IL-31 and functional consequences. Eur. J. Cell Biol. 91:552. [DOI] [PubMed] [Google Scholar]
- 85. Dillon S. R., Sprecher C., Hammond A. et al. 2004. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 5:752. [DOI] [PubMed] [Google Scholar]
- 86. Cevikbas F., Wang X., Akiyama T. et al. 2014. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 133:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ruzicka T., Hanifin J. M., Furue M. et al. ; XCIMA Study Group 2017. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N. Engl. J. Med. 376:826. [DOI] [PubMed] [Google Scholar]
- 88. Kaye J., Vasquez N. J. and Hedrick S. M. 1992. Involvement of the same region of the T cell antigen receptor in thymic selection and foreign peptide recognition. J. Immunol. 148:3342. [PubMed] [Google Scholar]
- 89. Mandl J. N., Monteiro J. P., Vrisekoop N. and Germain R. N. 2013. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 38:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu D., Potluri N., Lu J., Kim Y. and Rastinejad F. 2015. Structural integration in hypoxia-inducible factors. Nature 524:303. [DOI] [PubMed] [Google Scholar]
- 91. Mahajan V. S., Demissie E., Mattoo H. et al. 2016. Striking immune phenotypes in gene-targeted mice are driven by a copy-number variant originating from a commercially available C57BL/6 Strain. Cell Rep. 15:1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Naito Y., Takematsu H., Koyama S. et al. 2007. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol. Cell. Biol. 27:3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Purtha W. E., Swiecki M., Colonna M., Diamond M. S. and Bhattacharya D. 2012. Spontaneous mutation of the Dock2 gene in Irf5-/- mice complicates interpretation of type I interferon production and antibody responses. Proc. Natl Acad. Sci. USA 109:E898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ippagunta S. K., Malireddi R. K., Shaw P. J. et al. 2012. Addendum: defective Dock2 expression in a subset of ASC-deficient mouse lines. Nat. Immunol. 13:701. [DOI] [PubMed] [Google Scholar]
- 95. Sakata D., Uruno T., Matsubara K. et al. 2019. Selective role of neurokinin B in IL-31-induced itch response in mice. J. Allergy Clin. Immunol. 144:1130. [DOI] [PubMed] [Google Scholar]
- 96. Su H. C., Jing H., Angelus P. and Freeman A. F. 2019. Insights into immunity from clinical and basic science studies of DOCK8 immunodeficiency syndrome. Immunol. Rev. 287:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Happel C. S., Stone K. D., Freeman A. F. et al. 2016. Food allergies can persist after myeloablative hematopoietic stem cell transplantation in dedicator of cytokinesis 8-deficient patients. J. Allergy Clin. Immunol. 137:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]