Abstract
Oxytocin (OT) subserves various physiological, behavioral, and cognitive processes. This paired with the ability to administer OT with minimal and inconsistent side effects has spurred research to explore its therapeutic potential. Findings from single-dose studies indicate that OT administration may be beneficial, at least under certain circumstances. The state of the field, however, is less clear regarding effects from chronic OT administration, which more closely resembles long-term treatment. To address this gap, this review synthesizes existing findings on the use of chronic OT administration in animal and human work. In addition to detailing the effects of chronic OT administration across different functional domains, this review highlights factors that have contributed to mixed findings. Based on this review, a basic framework of interrelated regulatory functions sensitive to chronic OT administration is offered. The paper also identifies future research directions across different contexts, populations, and outcomes, specifically calling for more systematic and standardized research on chronic OT administration in humans to supplement and expand what is currently known from preclinical work.
Keywords: Oxytocin, Chronic Administration, Intranasal, Pharmacological Research, Peptide, Neuropeptide, Hormone
1. Introduction
Oxytocin (OT) is a complex, multifunctional neuropeptide that is endogenously synthesized by the magnocellular neurosecretory cells of the paraventricular and supraoptic nuclei of the hypothalamus (Insel, 2010). OT release is related to social functioning in mammals along with a range of other evolutionarily adaptive and coordinated functions that span physiological, cognitive, and behavioral domains (Macdonald and Feifel, 2013; Roney, 2016).
The ability to artificially synthesize and intranasally administer OT has catalyzed fast-growing lines of research on clinical and healthy populations. Research has focused on the modulatory effects of OT administration in addition to the mechanisms underlying these effects relative to endogenous functions (Alvares et al., 2017; Du Vigneaud, 1956). The promise of OT administration as an investigative and therapeutic tool has also been supported by the minimal and inconsistent side effects observed in humans, primarily when administered intranasally and in single doses (MacDonald et al., 2011).
Tempering the initial enthusiasm over OT administration as a universal treatment, more recent investigations have indicated that its effects likely depend on contextual and interindividual factors, such as age and sex (Ebner et al., 2016, 2015, 2013; Huffmeijer et al., 2013; Olff et al., 2013; Shamay-Tsoory and Abu-Akel, 2016). Also, much of our current knowledge on the effects of OT administration is restricted to short-term, single-dose trials (MacDonald et al., 2011; Shahrestani, Kemp, & Guastella, 2013). Though short-term and single-dose trials can be useful for determining the acute, modulatory effects of OT on outcomes of interest, significant challenges for clinical translation persist.
Namely, acute, single-dose trials are not representative of long-term treatment. Because certain functional impairments have lifelong impacts on quality of life (e.g., schizophrenia, stress disorders), it is improbable that a single administration will constitute an effective treatment plan (Macdonald and Feifel, 2013). Further, due to the quick degradation of OT after administration (i.e., ~two minutes in the blood and ~20 minutes in the central nervous system) (Mens et al., 1983; Striepens et al., 2013), any effects captured by single-dose trials may not be long-lasting. Inferences made from single-dose OT trials are also limited for clinical application because a single-dose of OT may yield effects that are practically insignificant or even counter to therapeutic intention (Macdonald and Feifel, 2013).
Rather, investigations that use repeated, daily (i.e., chronic) OT administration offer greater ecological validity in assessing the neuropeptide’s efficacy as a treatment. An investigative focus on chronic OT administration can lead to new waves of evidence for treating clinical dysfunction and may even result in support for functional optimization in healthy populations (Bethlehem et al., 2013; Gimpl and Fahrenholz, 2001; Insel, 2010). Thus, larger-scale trials that involve repeated exposure to OT over time in both animals and humans are on the rise.
Exogenous OT can be chronically administered via different routes (e.g., central infusion in preclinical trials, intranasal administration in animals and humans) and dosages (Box 1), which has contributed to a burgeoning literature. Existing studies, however, vary widely in conceptual application depending on the population examined. Given the literature that has proliferated in recent years and the need for safe and effective treatments, there is a demand for consolidating evidence on the effects of chronic OT administration across disciplines.
Box 1: OT Administration Routes.
Non-invasive routes:
Intranasal administration is currently the most practical non-invasive method for investigating central and peripheral OT effects in humans (Quintana et al., 2018). Intranasal administration comprises of OT in an aerosolized form and can be self-applied via a hand-held spray. Common dosages for single administration of OT nasal spray are between 24 and 40 IU but can be as low as 8 IU for humans (Guastella et al., 2013; Quintana et al., 2016). Administration dosage, frequency, and duration vary between studies. Some studies adopt intranasal administration to assess the effects of chronic OT exposure in animals similar to humans (Bales et al., 2014; Parker et al., 2005). Dosages for animal models are often weightadjusted to reflect similar amounts given to humans or other well-established models of OT function like the prairie vole (Bales et al., 2014; Guoynes et al., 2018; Huang et al., 2014). Some researchers argue that intranasal self-administration can lead to interindividual imprecision in dosing (Lawson, 2017). At the same time, however, there is growing evidence for increases in both peripheral and central concentrations of OT after intranasal administration (Dal Monte et al., 2014; Striepens et al., 2013) and several pathways for intranasal OT delivery have been proposed (i.e., olfactory, trigeminal, peripheral) (Quintana et al., 2015a). OT administration via specialized apparatuses (e.g., breath-powered devices, nebulizers) are being explored as they constitute more controlled methods of intranasal delivery to central targets (Chang et al., 2012; Quintana et al., 2016). Structure of the nasal passage and nasal spray formulation also affect OT delivery and absorption (DeMayo et al. 2017). For reviews on intranasal delivery of OT, see Quintana et al. (2015, 2018).
OT administration via a topical gel was developed for the non-invasive study and treatment of wounds and atrophy of the skin and mucous membranes (Al-Saqi et al., 2016). OT gel locally affects the area of application potentially by increasing cell proliferation (Torky et al., 2018) and can increase OT plasma levels via vaginal absorption (Petersson et al., 2005b). Dosages can range from 100 to 600 IU (Al-Saqi et al., 2016; Nielsen et al., 2017).
Invasive routes:
In humans, intravenous administration is largely used in the context of promoting labor and lactation though this method may not be well-tolerated in other circumstances (Guastella et al., 2013). Pre-clinical studies for basic scientific investigation typically employ peripheral injection or central infusion of OT (Quintana et al., 2018). Intraperitoneal (IP) injection involves OT administration into the abdominal cavity; subcutaneous injection involves administration under the skin. Intracerebroventricular (ICV) infusion involves direct delivery via bolus injection or implantation of a catheter into the cerebrospinal fluid or into specific brain regions (DeVos and Miller, 2013; Quintana et al., 2018). Chronic ICV infusion can also be achieved by an osmotic minipump, a device which continuously administers OT over time (DeVos and Miller, 2013). ICV administration is considered to be more stressful than IP administration and can require the use of anesthesia, which can potentially interact with OT (Teng et al., 2013). Dosages for these methods can be bodyweight adjusted.
To address this gap, we conducted a systematic review that synthesizes current findings from animal and human work on chronic OT administration that assess the neuropeptide’s efficacy as a pharmacological treatment. Bringing together cross-disciplinary findings has the potential to advance knowledge on the effects of chronic OT and identify future opportunities for translational research. A synthesis of findings from both animal and human work is especially critical for a comprehensive understanding of chronic OT administration that includes the moderating effects of contextual and interindividual factors (Olff et al., 2013).
We first present the methods for this review, including clarification of exclusion criteria that directed the literature selection (Figure 1). Then a systematic review of the existent literature on the effects of chronic OT administration follows (Figure 2). Next, we highlight conceptual and methodological factors that have contributed to mixed findings across studies (Figure 3). We then introduce a basic framework of the broader, interrelated regulatory functions sensitive to chronic OT administration (Figure 4) and conclude with a reflection of future directions for conducting systematic research in different contexts and populations. As identified through this review, several promising avenues for advancement in this field are apparent, which have great relevance not only for those interested in the OT system but also for pharmacological research more broadly.
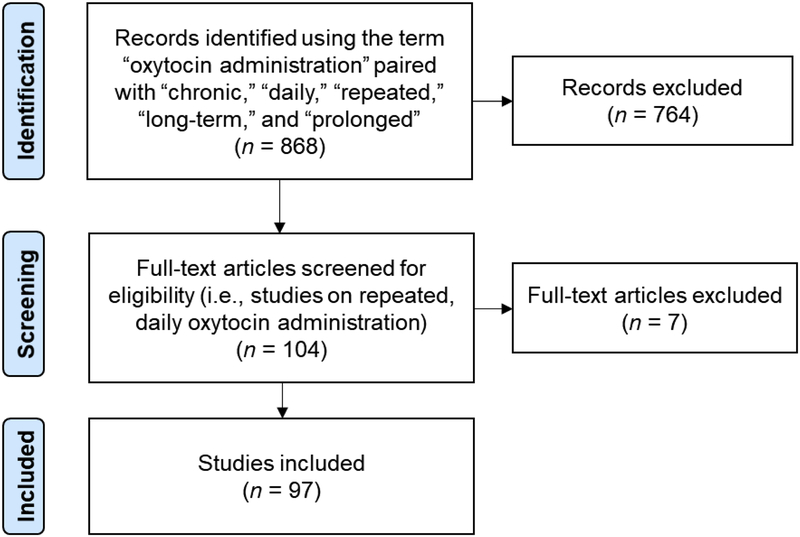
Figure 1.
Diagram of the literature search and selection process. The search and selection process was constrained to studies that administered oxytocin repeatedly, on a daily basis.
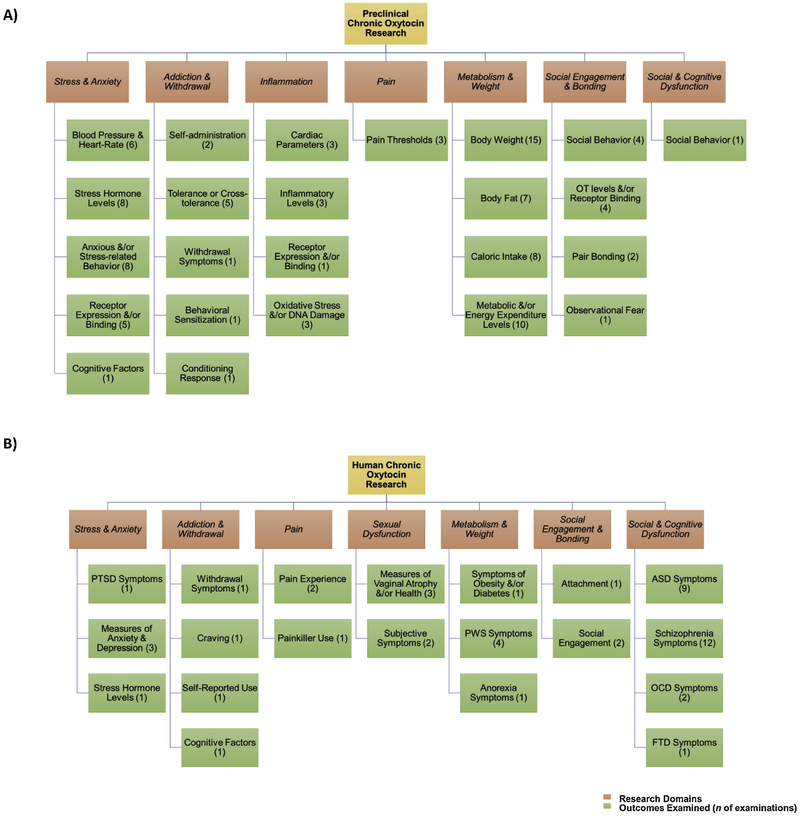
Figure 2.
At-a-glance overview of domains and outcomes of chronic oxytocin research in animals (Panel A) and humans (Panel B). For more details on findings see Tables 1–3. This is not an exhaustive list of all outcomes in each study; also, some studies examined multiple outcomes, which are depicted as individual examinations. ASD = Autism spectrum disorder, FTD = Frontotemporal dementia, OCD = Obsessive-compulsive disorder, PTSD = Post-traumatic stress disorder, PWS = Prader-Willi syndrome.
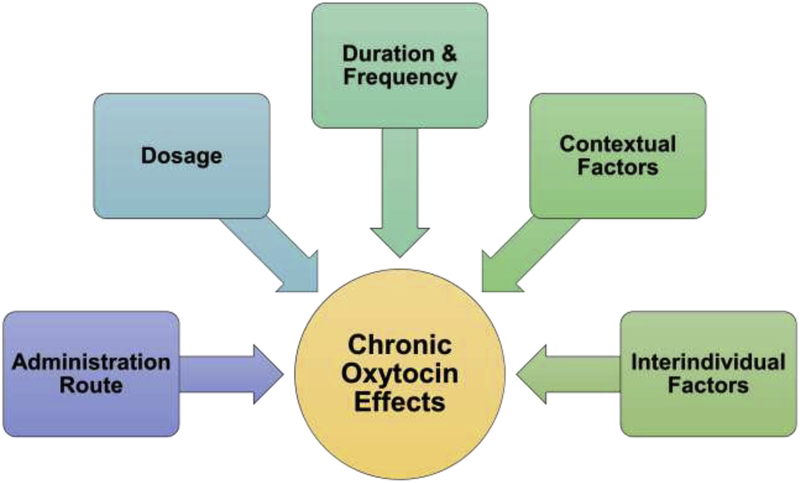
Figure 3.
Conceptual and methodological factors that impact chronic oxytocin administration effects.
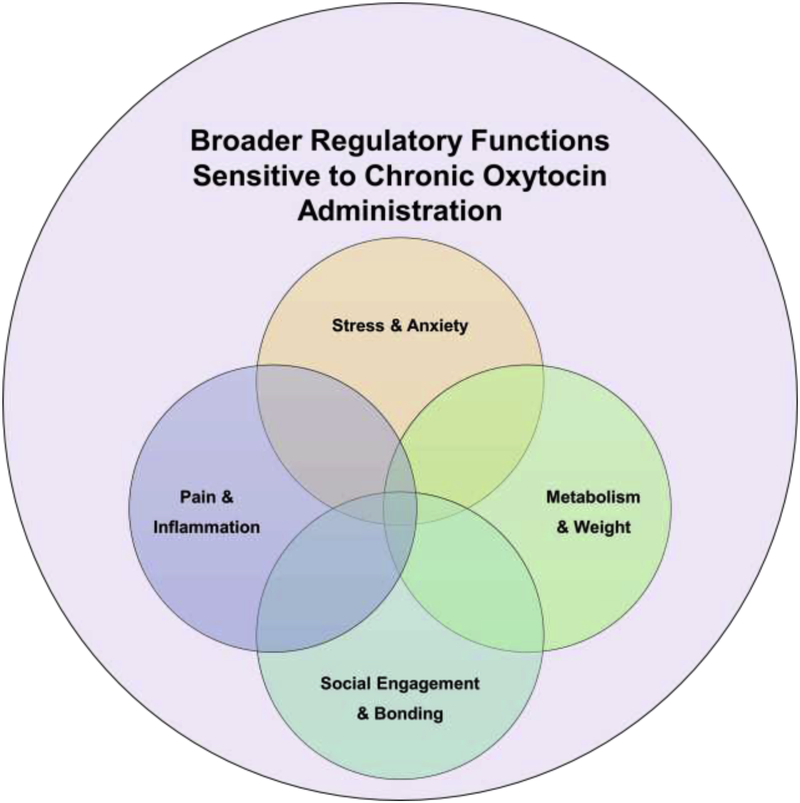
Figure 4.
Basic organizational framework identifying the broader regulatory functions sensitive to chronic oxytocin administration based on this literature review.
2. Methodology and Materials
2.1. Literature search and selection
To keep this review concise and representative of investigations crucial for clinical translation (Macdonald and Feifel, 2013), we defined chronic OT as the repeated, daily administration of OT. The literature search was conducted by the first and second author and a research assistant using online academic repositories, including PubMed, Google Scholar, and OneSearch (Figure 1). Search terms included “oxytocin administration” paired with “chronic,” “daily,” “repeated,” “long-term,” and/or “prolonged.” Bibliographies of resulting publications were also reviewed to identify additional records. Dosage practices and administration routes vary widely in research as summarized in Box 1. No limitations were placed on daily dosage practices or administration routes.
We excluded investigations that involved repeated OT administration in a single session or did not clearly state administration frequency or duration. This review did not include studies involving prolonged OT exposure in the context of labor and lactation (Erickson and Emeis, 2017; Smith and Merrill, 2006). Also, findings that involved repeated exposure to OT agonists or antagonists or utilized OT knockout models were not reviewed. Furthermore, case reports were excluded due to their low potential for generalizability but are summarized elsewhere (Macdonald and Feifel, 2013). Current evidence shows that OT is well-tolerated in different populations (Anagnostou et al., 2014; DeMayo et al., 2017; Finger et al., 2015; Mameli et al., 2014; Miller et al., 2017), therefore a discussion on the side effects of chronic OT is limited.
As summarized in Figure 1, these criteria yielded 97 peer-reviewed, journal articles published between 1985 and early 2018 in English (55 pre-clinical and 41 human records, plus Zhang et al., 2013, a single publication that examined both animal models and human patients). The literature review and selection process began in October 2017 and ended in February 2018.
3. Results
3.1. Domains of chronic OT research
As shown in Figure 2, the literature converged around seven major research domains, including stress and anxiety; addiction and withdrawal; inflammation and pain; sexual dysfunction; metabolism and weight; social engagement and attachment; and social and cognitive dysfunction.
Table 1 (animal work) and Table 2 (human work) summarize the studies included in this review. Studies that involved chronic OT administration in conjunction with other pharmacological and non-pharmacological treatments (i.e., as an adjunctive treatment) are listed separately in Table 3. The Supplementary Material summarizes studies that involved repeated, yet intermittent OT administration (e.g., once per week for 9 weeks) as they did not fit our definition of chronic OT but may still be of interest to readers (Supplementary Tables 1 and 2).
Table 1.
A comprehensive overview of selected papers on chronic OT research in animals
| Age | Sex | Model | Route (OT Dosage) | Duration | Design | Main Finding(s) | |
|---|---|---|---|---|---|---|---|
| Stress and Anxiety | |||||||
| Petersson et al., 1996a | NR | M/F | SD rats | SC injection (0.01, 0.1, and 1 mg/kg) or ICV injection (M only, 1 μg/kg) | 5 days | Placebo-controlled | Doses of 0.1 and 1 mg/kg SC OT and ICV OT reduced BP, but not HR, long-term |
| Windle et al., 1997 | NR | F | SD rats | ICV OMP (1, 10, or 100 ng/hr) | 5 days | Placebo-controlled | Doses of 10 and 100 ng/hr reduced corticosterone response and rearing behavior to noise stress |
| OT associated with greater exploratory behaviors in an unfamiliar environment | |||||||
| Petersson et al., 1999a | NR | M | SD rats | SC injection (1 mg/kg) | 5 days | Placebo-controlled | OT reduced plasma levels of corticosterone for 10 days after the last injection |
| Petersson et al., 1999c | NR | F | SD rats | SC injection (1 mg/kg) | 5 days for intact cycling rats or 12 days for OVX rats | Placebo-controlled | OT reduced BP in intact and OVX rats long-term |
| Díaz-Cabiale et al., 2000 | NR | M | SD rats | SC injection (1 mg/kg) | 5 days | Placebo-controlled | OT increased the density of α2-agonist binding sites in the hypothalamus, amygdala, and the paraventricular thalamic nucleus |
| Holst et al., 2002 | 1 day | M/F | SD rats | SC injection (1 mg/kg) | 14 days | Randomized, placebo-controlled | Postnatal OT reduced diastolic BP in M and F adult rats and reduced systolic BP in F adult rats |
| OT also reduced BP in prenatally stressed F rats | |||||||
| Petersson and Uvnäs-Moberg, 2003 | NR | M | SD rats | SC injection (1 mg/kg) | 5 days | Placebo-controlled | OT reduced glucocorticoid receptor mRNA expression and increased mineralocorticoid receptor mRNA expression in the hippocampus |
| Windle et al., 2004 | NR | F | SD rats | ICV OMP (1 or 10 ng/hr) | 7 days | Placebo-controlled, Blinded analyses | 10 ng/hr of OT reduced the release of ACTH and corticosterone levels |
| Rats treated with either dose of OT did not exhibit an increase in stressed-induced corticotropin-releasing factor mRNA expression in the hypothalamic PVN or c-fos mRNA expression in the dorsal hippocampus, ventrolateral septum, and PVN | |||||||
| Petersson et al., 2005a | NR | F | SD rats | SC injection (1 mg/kg) | 10 days | Placebo-controlled | OT increased the density of high-affinity α2-adrenoceptors in hypothalamus, amygdala, and nucleus of the solitary tract |
| OT reduced plasma levels of corticosterone | |||||||
| Parker et al., 2005 | 6–17 years | F | Squirrel monkeys | Intranasal (~23 IU) | 8 days | Randomized, placebo-controlled | OT reduced ACTH levels in monkeys exposed to 90 min of social isolation |
| No OT effects on cortisol levels | |||||||
| Slattery and Neumann, 2010 | NR | M/F | LAB and HAB Wistar rats | ICV OMP (10 ng/hr) | 6 days | Placebo-controlled, Blinded behavioral tests | OT reduced the high level of anxiety-related behavior in F (but not M) HAB rats |
| Rault et al., 2013 | 1 day | M/F | Pigs | Intranasal (24 IU) | 3 days | Placebo-controlled | OT increased aggressive behavior and ACTH concentration |
| OT reduced responsiveness to dexamethasone | |||||||
| Peters et al., 2014 | NR | M | C57BL/6 mice | ICV OMP (1 or 10 ng/hr) | 15 days (both doses) or 19 days (low dose with chronic psychosocial stress exposure) | Randomized, placebo-controlled | High dose of OT increased anxious behavior and weight gain; reduced OXTR binding within the septum, amygdala, and median raphe nucleus |
| The low dose was protective against chronic stress-induced anxious behavior, reduction in OXTR binding, thymus atrophy, and adrenal hypertrophy | |||||||
| Low dose reduced chronic stress-induced corticosterone levels, ACTH sensitivity, and colonic inflammation | |||||||
| Havranek et al., 2015 | NR | M | Wistar Rats | ICV OMP (20 ng/μl) | 7 days | Placebo-controlled | OT-treated rats preferred unknown objects during the object recognition test |
| OT increased gene expression and protein levels of neurotrophins, MAP2, and synapsin I in the hippocampus | |||||||
| No OT effects on anxious behavior | |||||||
| Janezic et al., 2016 | 3–4 months | M | SD rats | SC OMP (0.5 μl/hr) | 7 days or 14 days | Placebo-controlled | OT had a cumulative and long-term anxiolytic effect on behavior in a rodent model of PTSD |
| Addiction and Withdrawal | |||||||
| Kovács et al., 1985a | NR | M | CFY rats | SC injection (0.5 μg/kg) | 8 days | Placebo-controlled | OT slowed acquisition of heroin self-administration in tolerant rats and reduced their rate of self-administration similar to heroin-naive rats |
| Kovács et al., 1985b | NR | M | CFLP mice | SC injection (2, 20, 200 μg/kg) | 4 days | Placebo-controlled | OT pretreatment reduced heroin tolerance with 20 μg/kg being the most effective dose |
| Szabó et al., 1985 | NR | M | CFLP mice | SC injection (0.02, 0.25, 0.5, 1.0, or 2.0 IU) | 3 days | Placebo-controlled | OT pretreatment blocked the development of ethanol tolerance |
| Szabó et al., 1987 | NR | M | CFLP mice | SC injection (0.02, 0.2, 2.0 IU) | 4 days | Compared to alcohol-dependent controls | Increasing doses of OT pretreatment was associated with milder alcohol withdrawal symptoms |
| Sarnyai et al., 1992b | NR | M | CFLP mice | SC injection (0.005, 0.05, 0.5 μg BID) | 5 days | Placebo-controlled | Only highest dose of OT pretreatment increased cocaine-induced behavioral sensitization |
| Sarnyai et al., 1992a | NR | M | Wistar rat | SC injection (0.005 or 0.05 μg) | 4 days | Placebo-controlled | Higher dose of SC OT prevented the development of tolerance to cocaine |
| Intrahippocampal injection (100 pg) | Intrahippocampal OT did not alter the sniffing-inducing effect of cocaine but did prevent the development of tolerance | ||||||
| Kriván et al., 1992 | Adult | M | CFLP mice | SC injection (0.05 μg) | 4 days | Placebo-controlled | OT pretreatment blocked the development of heroin-enkephalin cross-tolerance |
| ICV injection (0.005 μg/2 μl BID) | |||||||
| Tirelli et al., 1992 | 12–15 weeks | M | OF-1 mice | IP injection (0.005 mg/2 ml) | 4 days | Placebo-controlled | OT pretreatment suppressed conditioned compensatory response related to tolerance of hypothermic effects of ethanol |
| Carson et al., 2010 | NR | M | SD rats | IP injection using ascending dose sequence (0.001, 0.01, 0.1, 0.3 and 1 mg/kg) | 5 days | Placebo-controlled | OT dose-dependently reduced methamphetamine self-administration |
| Inflammation | |||||||
| Jankowski et al., 2010 | NR | M | SD rats | SC OMP (25 or 125 ng/kg/hr) | 3 or 7 days | Placebo-controlled | Both doses of OT reduced inflammation and improved echocardiographic parameters in rats with myocardial infarction |
| Ahmed and Elosaily, 2011 | Adult | M | Rats | IP injection (1 mg/kg) | 10 weeks | Randomized, compared to controls with no vehicle or saline administration | OT increased OXTR mRNA; decreased plasma levels of IL-6, MCP-1, and CRP; decreased oxidative stress; improved histological abnormalities in the aorta |
| Szeto et al., 2013 | 2.5 months | M | Watanabe Heritable Hyperlipidemic rabbits | SC OMP (250 ng/kg/hr) | 16 weeks | Placebo-controlled | OT reduced plasma CRP levels and atherosclerosis in the aorta |
| Leffa et al., 2017 | NR | M | Wistar rats | IP injection (0.1, 1.0, or 10 mg/kg) | 21 or 56 days | Randomized, placebo-controlled | 21-day, but not 56-day, OT treatment increased the frequency of DNA-damaged hippocampal cells at all doses |
| DNA damage intensity increased after both durations for most doses | |||||||
| No OT effect on body weight | |||||||
| Pain | |||||||
| Petersson et al., 1996b | NR | M/F | SD rats | SC injection (0.1 and 1.0 mg/kg) or ICV injection (1 μg/kg) | 5 days | Placebo-controlled | SC OT increased nociceptive thresholds in F and M rats long-term at both doses |
| No significant findings for ICV injection | |||||||
| Petersson et al., 2005b | NR | F | SD rats | SC injection (1 mg/kg) or intravaginal gel (100 μg) | 10 days | Placebo-controlled | Both OT administration routes increased spontaneous motor activity and nociceptive thresholds and reduced corticosterone levels |
| Metabolism and Weight | |||||||
| Boer, 1993 | 4 months | M/F | Wistar rats | SC OMP (100 μg) | 2 months | Randomized, placebo-controlled | OT given to pregnant rats from late gestation to delivery and throughout nursing impaired offspring’s growth via weight, brain size, and cerebellar DNA |
| Uvnäs-Moberg et al., 1996 | 10 weeks | F | SGR and RGR SD rats | SC injection (1ml/kg) | 4–5 days | Placebo-controlled | In SGR rats, OT increased weight gain but did not increase food intake |
| In RGR rats, OT reduced food intake | |||||||
| RGR rats had twice as high OT and insulin levels than SGR rats | |||||||
| Uvnäs-Moberg et al., 1998 | 10–14 days | M/F | SD rats | SC injection (1 mg/kg) | 5 days | Placebo-controlled | M and F rats given OT in early development had higher body weight, fat, and nociceptive thresholds than the saline group |
| OT increased CCK for M rats | |||||||
| Petersson et al., 1999b | NR | M | SD rats | SC injection (1 mg/kg) | 5 days | Placebo-controlled | OT reduced plasma levels of insulin, CCK, and gastrin long-term without affecting somatostatin or glucose levels |
| Petersson, 2002 | NR | M | SD rats | SC injection (1 mg/kg) or ICV injection (0.3 μg) | 5 days | Placebo-controlled | ICV OT, but not SC OT, reduced plasma levels of thyroid-stimulating hormone, free thyroxine, and free triiodothyronine |
| Petersson and Uvnäs-Moberg, 2008 | 10–14 days | M | Spontaneously hypertensive rats | SC injection (1 mg/kg) | 5 days | Randomized, placebo-controlled | OT reduced weight by 5–8 weeks of age and reduced systolic BP by adulthood |
| Deblon et al., 2011 | NR | M | Wistar rats | ICV OMP (1.6 or 16 nmol) or SC OMP (50 nmol) | 14 days | Placebo-controlled | ICV and SC OT reduced body weight and increased glucose tolerance, insulin sensitivity, triglyceride uptake, lipolysis, and fatty acid β-oxidation in adipose tissue in DIO rats |
| Eckertova et al., 2011 | Adult | M | Wistar rats | SC OMP (3.6 μg/100g) | 2 weeks | Randomized, placebo-controlled | OT reduced the diameter of adipocytes and increased epididymal adipose tissue protein content with no changes to adipose tissue mass |
| Maejima et al., 2011 | 6 weeks | M | C57BL/6J mice | SC injection (1600 μg/kg) | 17 days (SC injection) | Placebo-controlled | SC OT reduced food intake and weight |
| SC OMP (1600 μg/kg) | 14 days (SC OMP) | SC OMP OT reduced food intake, weight, visceral fat mass, and adipocyte size; improved fatty liver and glucose intolerance | |||||
| Morton et al., 2012 | Adult | M | DIO, HFD-fed rats | Peripheral injection (1000 μg/kg) | 7 days | Placebo-controlled | OT reduced weight, body fat content, and food intake, but maintained levels of energy expenditure, respiratory quotient, and ambulatory activity |
| Zhang et al., 2013 | NR | NR | Streptozotocin-induced diabetic C57BL/6 mice | ICV injection (2 μg) or IP injection (2 mg/kg) | 7 days for ICV injection or 3 days for IP injection | Placebo-controlled | OT improved glucose intolerance and blood insulin levels |
| Blevins et al., 2015 | 10–18 years | M | DIO rhesus monkeys | SC injection (0.2 or 0.4 mg/kg) for 2 weeks at each dose | 4 weeks | Randomized; Monkeys received vehicle during the week prior to OT administration and served as their own control | OT reduced weight and increased lipolysis and energy expenditure |
| Low-dose OT reduced food intake | |||||||
| High-dose OT reduced food and sweetened beverage intake | |||||||
| Iwasaki et al., 2015 | 11 weeks | M | Diabetic db/db mice | SC OMP (1600 μg/kg) | 2 weeks | Placebo-controlled | OT activated vagal afferents, reduced food intake, and ameliorated obesity |
| Plante et al., 2015 | 4 and 22 weeks | M | Obese diabetic-prone db/db mice | SC OMP (125 ng/kg/hr) | 12 weeks | Randomized, placebo-controlled | OT increased circulating OT levels, reduced serum resistin, body fat, fasting blood glucose levels; improved glucose tolerance and insulin sensitivity; had beneficial effects on oxidative stress and inflammation; prevented cardiac dysfunction in young and older mice |
| Additional 6 weeks for a subset of older mice | |||||||
| Balazova et al., 2016 | 10 weeks | M | Obese Zucker rats | SC OMP (3.6 μg/100g) | 2 weeks | Randomized, placebo-controlled | OT reduced food intake in obese and lean rats; and reduced weight and adipocyte size in obese rats |
| Blevins et al., 2016 | Adult | M | SD rats | ICV OMP (16 nmol/day) | 21–26 days | Placebo-controlled | OT limited food intake, weight, and adiposity gain on an HFD, but not chow diet, whether or not rats were obese, which persisted for ~10 days after administration |
| Roberts et al., 2017 | Adult | M | DIO rats (SD and Long Evans) and C57BL/6J mice | ICV OMP (16 nmol/day) | 27–28 days | Randomized, placebo-controlled | Chronic hindbrain (4V) OT resulted in weight loss in DIO HFD-fed rats similar to chronic third ventricular (3V) OT, which reduced caloric intake and prevented weight gain |
| Social Engagement and Bonding | |||||||
| Bowen et al., 2011 | 33 days | M | Wistar Rats | IP injection (1 mg/kg) | 10 days | Placebo-controlled | OT slowed weight gain, reduced anxiety, increased sociability, decreased alcohol consumption, and upregulated OT in plasma and OT mRNA in the hypothalamus that extended into adulthood |
| Bales et al., 2013 | 21 days | M/F | Prairie voles | Intranasal (0.08, 0.8, 8.0 IU/kg) | 3 weeks | Randomized, placebo-controlled | Low and medium dose OT led to deficits in partner preference behavior in M (but not F) |
| No OT effects on anxiety, but OT-treated F had reduced emotionality | |||||||
| Huang et al., 2014 | 12–20 weeks | M | C57BL/6J mice | Intranasal (0.15 and 0.3 IU BID) | 7–21 days | Placebo-controlled; Experimenters blinded during testing and scoring | OT reduced M-F and M-M social interactions |
| Higher dose reduced OXTR binding more than the lower dose in the lateral septum, anterior olfactory nucleus, and amygdala | |||||||
| Both doses reduced OXTR binding in the hippocampus, piriform cortex, and nucleus accumbens | |||||||
| No OT effect on weight, general olfactory, locomotor abilities, nonsocial recognition memory, reactivity to stress, or sensorimotor gating abilities | |||||||
| Calcagnoli et al., 2014 | Adult | M | Wild-type Groningen rats | ICV OMP (0.5 μl/hr) 7 days | 7 days | Placebo-controlled | OT reduced social offensive aggression and increased social explorative behavior |
| Calcagnoli et al., 2015 | Adult | M | Wild-type Groningen rats | Intranasal (1 μg/μl) | 7 days | Randomized, placebo-controlled; blinded researcher | OT exerted anti-aggressive and pro-affiliative effects |
| Pisansky et al., 2017 | 8–12 weeks | M/F | C57/B6 mice | Intranasal (20 μg/kg) | 5 days | Placebo-controlled | OT facilitated long-term observational fear and downregulated transcription of OXTR in the amygdala |
| Guoynes et al., 2018 | 21 days | M/F | Prairie voles | Intranasal (0.08, 0.8, 8.0 IU/kg) | 3 weeks | Randomized, placebo-controlled | Medium dose of OT increased OXTR binding in the nucleus accumbens shell in F, which was correlated with increased pair-bonding, and reduced vasopressin immunoreactive cells in PVN in M, which was correlated with decreased pair bonding |
| Social and Cognitive Dysfunction | |||||||
| ASD | |||||||
| Bales et al., 2014 | 20 days | M/F | BTBR and C57BL/6J mice | Intranasal (0.8 IU/kg) | 30 days | Placebo-controlled | OT increased sniffing of a novel mouse in F BTBR mice only |
| No evidence of major benefits of OT on juvenile and adult sociability, repetitive, or cognitive behaviors | |||||||
Abbreviations:
ACTH: Adrenocorticotropic hormone, ASD: Autism spectrum disorder, BID: Twice-a-day, BP: Blood pressure, CCK: Cholecystokinin, CRP: C-reactive protein, DIO: Diet-induced obesity, F: Female, HAB: High anxiety-related behavior, HPA: Hypothalamic-pituitary-adrenal, ICV: Intracerebroventricular, IP: Intraperitoneal, IU: International Units, LAB: Low anxiety-related behavior, M: Male, MAP2: Microtubule-associated protein 2, NR: Not reported, OMP: Osmotic mini pump, OT: Oxytocin, OVX: Ovariectomized, OXTR: Oxytocin receptor, PVN: Paraventricular nucleus, RGR: Rapidly growing rates, SC: Subcutaneous, SD: Sprague Dawley, SGR: Slowly growing rates
Table 2.
A comprehensive overview of selected papers on chronic OT research in humans
| Age | Sex | Route (OT Dosage) | Duration | Design | Main Finding(s) | |
|---|---|---|---|---|---|---|
| Stress and Anxiety | ||||||
| van Zuiden et al., 2017 | 18–65 years | M/F | Intranasal (40 IU BID) | 8 days | Randomized, double-blind, placebo-controlled trial | OT reduced PTSD scale scores in those with a high baseline score |
| Pain | ||||||
| Ohlsson et al., 2005 | 20–70 years | F | Intranasal (40 IU BID) | 13 weeks | Randomized, double-blind, placebo-controlled trial | OT showed a weak, but positive, effect on abdominal pain, discomfort, and depressed mood in individuals with chronic constipation* |
| No OT effects on gut function | ||||||
| Sexual Dysfunction | ||||||
| Al-Saqi et al., 2015 | 62.0 ± 5.7 years (100 IU) | F | Vaginal gel (100 or 400 IU) | 7 weeks | Randomized, double-blind, placebo-controlled trial | 100 IU reduced vaginal pH and post-menopausal vaginal atrophy* |
| 62.1 ± 5.3 years (400 IU) | 400 IU improved superficial cells in vaginal smears and maturation value* and improved self-reported most bothersome symptoms of post-menopausal vaginal atrophy | |||||
| 62.3 ± 5.8 years (P) | ||||||
| Al-Saqi et al., 2016 | 52–74 years | F | Vaginal gel (600 IU for 2 weeks, then twice-a-week for 10 weeks) | 12 weeks | Randomized, double-blind, placebo-controlled trial | OT improved expressions of post-menopausal vaginal atrophy |
| Torky et al., 2018 | 54.1 ± 4.5 years (OT) | F | Vaginal gel (600 IU) | 1 month | Randomized, placebo-controlled, blinded hospital clinician | OT reduced vaginal atrophy, soreness, and dyspareunia |
| 54.6 ±3.4 years (P) | ||||||
| Metabolism and Weight | ||||||
| Zhang et al., 2013 | 20–60 years | M/F | Intranasal (24 IU, 4 times a day) | 8 weeks | Randomized, placebo-controlled, blinded analyses | OT increased weight loss and reduced waist-to-hip circumference in obese and prediabetic patients |
| Einfeld et al., 2014 | 12–30 years | M | Intranasal (18 IU BID increased to 32 IU BID for 12–15 years old or 24 IU BID increased to 40 IU BID for a subset 16+ years old) | 8 weeks | Randomized, double-blind, placebo-controlled, crossover trial | The higher dose of OT increased temper outbursts |
| No other OT effects on PWS symptoms | ||||||
| Kuppens et al., 2016 | 6–14 years | M/F | Intranasal (12, 16, 20, or 24 IU BID based on body surface) | 4 weeks | Randomized, double-blind, placebo-controlled, crossover trial | OT improved social and food-related behavior in young children (< 11 years) but not older children with PWS (>11 years) |
| Tauber et al., 2017 | < 6 months | M/F | Intranasal (4 IU BID, daily, or once every other day) | 7 days | Proof-of-concept study, no placebo comparison | OT improved social skills, mother infant-interactions, and sucking/swallowing in infants with PWS |
| No adverse events or dose effects | ||||||
| Miller et al., 2017 | 5–11 years | M/F | Intranasal (16 IU) | 5 days | Randomized, double-blind, placebo-controlled, crossover trial | No OT effects on social, anxious, and food-related behaviors in children with PWS |
| Social Engagement and Bonding | ||||||
| Bernaerts et al., 2017 | M = 20.7 years (OT) | M | Intranasal (24 IU) | 2 weeks | Randomized, double-blind, placebo-controlled trial | OT reduced attachment avoidance and improved attachment toward peers |
| M = 21.6 years (P) | ||||||
| Barraza et al., 2013 | 60–95 years | M/F | Intranasal (40 IU) | 10 days | Randomized, double-blind, placebo-controlled trial | OT increased dispositional gratitude and was associated with reduced self-reported physical decline and fatigue |
| No OT effects on social activities, social engagement, and other state and trait affect measures | ||||||
| Social and Cognitive Dysfunction | ||||||
| ASD | ||||||
| Anagnostou et al., 2012 | 33.2 ± 13.3 years | M | Intranasal (24 IU BID) | 6 weeks | Randomized, double-blind, placebo-controlled trial | No OT effects on measures of nonverbal accuracy, Clinical Global Impression, or repetitive behavior |
| OT improved scores on social cognition and quality of life | ||||||
| Tachibana et al., 2013 | 10–14 years | M | Intranasal (8, 16, 24 IU BID) | 2 months per dose | Open-label, trial, 1–2 weeks of placebo per dose of OT but not used for controlled comparison | OT was associated with improved social communication and interaction |
| No OT effects on Child Behavior or Aberrant Behavior Checklist | ||||||
| No side effects or clear dose effects | ||||||
| Anagnostou et al., 2014 | 10–17 years | M/F | Intranasal (0.2, 0.26, 0.33, or 0.4 IU/kg) | 12 weeks | Open-label trial, no placebo comparison | No severe or serious adverse events, metabolic or cardiovascular abnormalities relating to OT at all doses |
| Some improvements on social function, social cognition, anxiety, and repetitive behaviors found but underpowered to compare effects by doses | ||||||
| Dadds et al., 2014 | 7–16 years | M | Intranasal (12 IU for those under 40 kg or 24 IU for those over 40 kg) | 4 days | Randomized, double-blind, placebo-controlled trial | No OT effects on emotion recognition, social interaction skills, or general behavioral adjustment |
| Guastella et al., 2015 | 12–18 years | M | Intranasal (18 IU BID for 12–15 years old or 24 IU BID for 16+ years old) | 8 weeks | Randomized, double-blind, placebo-controlled trial | No OT effects on social deficits or behavior |
| Watanabe et al., 2015 | 18–55 years | M | Intranasal (24 IU BID) | 6 weeks | Randomized, double-blind, placebo-controlled, crossover trial | OT reduced ASD symptoms relating to social reciprocity; increased resting-state functional connectivity between anterior cingulate cortex and dorsomedial prefrontal cortex; and increased brain activity relating to non-verbal information-based judgment |
| Kosaka et al., 2016 | 15–39 years | M/F | Intranasal (16 or 32 IU for double-blind phase and 32 IU for open-label phase) | 12 weeks (double-blind phase) | Randomized, double-blind and open-label, placebo-controlled trial | OT increased Clinical Global Impressions - Improvement scores for M in high-dose (but not low-dose) group in the double-blind phase |
| 12 weeks (open-label phase) | No significant OT improvements in stereotypic or repetitive behaviors and comorbid symptoms in the double-blind phase | |||||
| Munesue et al., 2016 | 15–40 years | M | Intranasal (8 IU BID) | 8 weeks | Randomized, double-blind, placebo-controlled, crossover trial | No OT effects on core social symptoms of ASD but increased reciprocal social interactions were observed |
| Yatawara et al., 2016 | 3–8 years | M/F | Intranasal (12 IU BID) | 5 weeks | Randomized, double-blind, placebo-controlled, crossover trial | OT increased caregiver-rated social responsiveness |
| OCD | ||||||
| den Boer and Westenberg, 1992 | 39.8 ± 7.5 years (OT) | M/F | Intranasal (18 IU for 12 patients and 54 IU for 2 patients) | 6 weeks | Randomized, double-blind, placebo-controlled trial | No OT effects on OCD symptoms at both doses |
| 39.8 ± 8.9 years (Placebo) | ||||||
| Epperson et al., 1996 | 46.3 ± 12.4 years | M/F | Intranasal (160 IU for 5 patients and 320 IU for 2 patients) | 1 week | Randomized, double-blind, placebo-controlled, crossover trial | No OT effects on OCD or anxiety symptoms or memory at both doses |
| FTD | ||||||
| Finger et al., 2015 | 50.3–80.3 years | M/F | Intranasal (24, 48, or 72 IU BID) | 1 week | Randomized, double-blind, placebo-controlled trial | OT is safe for those with behavioral variant FTD or semantic dementia at all examined doses |
| OT is associated with improved apathy, empathy, and patient-caregiver interactions | ||||||
Not significant when compared to placebo
Abbreviations:
ASD: Autism spectrum disorder, BID: Twice-a-day, F: Female, FTD: Frontotemporal Dementia, IU: International Units, M: Male, OCD: Obsessive-compulsive disorder, OT: Oxytocin, PTSD: Post-traumatic stress disorder, PWS: Prader-Willi syndrome
Table 3.
A comprehensive overview of research on chronic OT administered as adjunctive therapy in animal models and humans
| Age | Sex | Model | Route (OT Dosage) | Duration | Design | Main Finding(s) | |
|---|---|---|---|---|---|---|---|
| Stress and Anxiety | |||||||
| Petersson et al., 1999d | NR | M | SD rats | SC injection (1 mg/kg) | 5 days | Placebo-controlled | OT increased the effects of clonidine and reduced BP |
| OT increased the responsiveness of α2-adrenoreceptors involved in regulating BP and spontaneous motor activity | |||||||
| Scantamburlo et al., 2015 | M = 44.8 years (M) | M/F | Human | Intranasal (8 IU BID) | 4 weeks | Open-label trial, no placebo comparison | OT as an adjunct to escitalopram improved scores on measures of depression, anxiety, and severity of illness in those with treatment-resistant depression |
| M = 48.5 years (F) | |||||||
| Addiction and Withdrawal | |||||||
| Pedersen et al., 2013 | 18–65 years | M/F | Human | Intranasal (24 IU BID) | 3 days | Randomized, double-blind, placebo-controlled trial | OT reduced alcohol withdrawal symptoms in individuals with alcohol-dependency demonstrated by less total lorazepam needed for detoxification |
| Stauffer et al., 2016 | M = 49.3 years (OT) | M/F | Human | Intranasal (40 IU BID) | 2 weeks | Randomized, double-blind, placebo-controlled trial | OT was well-tolerated in individuals receiving methadone treatment for opioid use disorder with a cocaine use disorder |
| M = 49.6 years (Placebo) | OT was associated with more honest self-reporting of cocaine use | ||||||
| In the Implicit Association Test, those in the OT group switched from associating cocaine images with the “self” words to “other” words | |||||||
| No OT-related reductions or increases in cocaine or heroin craving | |||||||
| Pain | |||||||
| Mameli et al., 2014 | 18–70 years | F | Human | Intranasal (20 IU BID for Week 1 and 40 IU BID for Week 2 and 3) | 3 weeks | Randomized, double-blind, placebo-controlled, crossover trial | No OT effects on reducing pain severity, symptoms of comorbid disorders, or number of painkillers used by those with fibromyalgia and comorbid disorders |
| Metabolism and Weight | |||||||
| Russell et al., 2018 | 15–67 years | F | Human | Intranasal (18 IU BID) | 4–6 weeks | Randomized, placebo-controlled trial | OT reduced eating concern, cognitive rigidity, and baseline salivary cortisol levels in anorexic patients |
| Social and Cognitive Dysfunction | |||||||
| Schizophrenia | |||||||
| Feifel et al., 2010 | M = 48 years | M/F | Human | Intranasal (20 IU BID for Week 1 and 40 IU BID for Week 2 and 3) | 3 weeks | Randomized, double-blind, placebo-controlled, crossover trial | OT as an adjunct to antipsychotics improved scores on positive symptoms, negative symptoms, and Clinical Global Impressions-Improvement scales |
| Pedersen et al., 2011 | 18–55 years | M | Human | Intranasal (24 IU BID) | 2 weeks | Randomized, double-blind, placebo-controlled trial | OT improved scores on positive and negative symptom scales and social cognition |
| Feifel et al., 2012 | M = 48 years | M/F | Human | Intranasal (20 IU BID for Week 1 and 40 IU BID for Week 2 and 3) | 3 weeks | Randomized, double-blind, placebo-controlled, crossover trial | No OT-related amnesic effect |
| OT as an adjunct to antipsychotics improved verbal memory | |||||||
| Modabbernia et al., 2013 | 18–50 years | M/F | Human | Intranasal (20 IU BID for Week 1 and 40 IU BID for Week 2–8) | 8 weeks | Randomized, double-blind, placebo-controlled trial | OT as an adjunct to risperidone improved scores on positive symptoms, negative symptoms, and general psychopathology scales |
| Lee et al., 2013 | 18–60 years | M/F | Human | Intranasal (20 IU BID) | 3 weeks | Randomized, double-blind, placebo-controlled trial | OT as an adjunct to stable antipsychotic treatment improved odor identification and negative symptoms but not positive symptoms |
| Gibson et al., 2014 | 18–55 years | M/F | Human | Intranasal (24 IU BID) | 6 weeks | Randomized, double-blind, placebo-controlled trial | OT improved scores on fear recognition, perspective-taking, and negative symptoms* |
| Cacciotti-Saija et al., 2015 | 16–35 years | M/F | Human | Intranasal (24 IU BID plus before weekly social cognition training) | 6 weeks | Randomized, double-blind, placebo-controlled trial | No OT effects on Reading the Mind in the Eyes Test, positive and negative symptoms, measures of social functioning and social cognition |
| Dagani et al., 2016 | 18–45 years | M/F | Human | Intranasal (40 IU) | 4 months | Randomized, double-blind, placebo-controlled, crossover trial | No OT effects on positive or negative scores |
| Lee et al., 2016b | 44.7 ± 11.7 years (OT) | M/F | Human | Intranasal (20 IU BID) | 3 weeks | Randomized, double-blind, placebo-controlled trial | No OT effects on endogenous plasma OT levels or symptoms |
| 35.1 ± 8.2 years (Placebo) | |||||||
| Buchanan et al., 2017 | 18–65 years | M/F | Human | Intranasal (24 IU BID) | 6 weeks | Randomized, double-blind, double-dummy, placebo-controlled trial | No OT effects on cognitive impairment, negative symptoms, functional capacity, or ancillary symptoms |
| Jarskog et al., 2017 | 18–65 years | M/F | Human | Intranasal (24 IU BID) | 12 weeks | Randomized, double-blind, placebo-controlled trial | OT improved negative symptoms scores in those with schizophrenia, but not schizoaffective disorder |
| No OT effects on social cognition (i.e., emotion perception, theory-of-mind, and attributional style) or social functioning | |||||||
| Ota et al., 2017 | 40.5 ± 12.8 years | M/F | Human | Intranasal (12 IU BID) | 12 weeks | Open-label trial, no placebo comparison | OT as an adjunct to antipsychotics improved positive and negative symptom scores and verbal fluency |
| Negative correlation between the improvement of negative symptoms by OT and gray matter volumes of the insula and cingulate | |||||||
Not significant when compared to placebo
Abbreviations:
BID: Twice-a-day, BP: Blood pressure, F: Female, IU: International Units, M: Male, NR: Not reported, OT: Oxytocin, SD: Sprague Dawley
3.2. Literature review and synthesis
3.2.1. Stress and anxiety
Stress- and anxiety-inducing stimuli activate the central and peripheral release of OT, supporting stress regulation as one of the major functions of the neuropeptide (Neumann and Slattery, 2016). Similarly, OT release is associated with the suppression of basal and stress-induced hormonal activity across the hypothalamic-pituitary-adrenal (HPA) axis, which is the major neuroendocrine system that coordinates the stress response (Landgraf and Neumann, 2004). OT release has also been discussed as a physiological mechanism that facilitates social support and bonding associated with HPA-axis downregulation (DeVries et al., 2003). The activation of the endogenous OT system in stressful situations has led researchers to consider the extent to which OT administration can reduce stress and anxiety in animals and humans (Landgraf and Neumann, 2004; Neumann and Slattery, 2016).
In animals, chronic OT administration modulated receptor expression and binding in brain regions involved in the stress response (Díaz-Cabiale et al., 2000; Havranek et al., 2015; Petersson et al., 2005a; Petersson and Uvnäs-Moberg, 2003; Windle et al., 2004). For example, peripherally administered chronic OT in rats was associated with higher densities of high-affinity α2-adrenoceptors and α2 agonist binding sites in regions ample in OT receptors (OXTR) and α2-adrenoceptors like the hypothalamus and amygdala (Díaz-Cabiale et al., 2000; Petersson et al., 2005a). Chronic OT has also been shown to prevent reductions in OXTR binding that occurs under chronic stress (Peters et al.,2014).
The stress-regulatory effects of chronic OT were further demonstrated by reductions of stress-related hormones in animals (e.g., corticosterone, adrenocorticotropic hormone (ACTH)) (Parker et al., 2005; Petersson et al., 2005a, 2005b, 1999a; Windle et al., 2004, 1997) (See Rault et al., 2013 for an exception). For example, intranasal chronic OT was associated with attenuated ACTH levels compared to placebo after a socially stressful event in monkeys (Parker et al., 2005). OT-related alterations in receptor expression, binding, and peripheral hormone levels can dampen HPA-axis activity (Parker et al., 2005; Petersson et al., 2005a, 1999a; Petersson and Uvnäs-Moberg, 2003; Windle et al., 2004, 1997) or prevent its upregulation in stressful situations (Peters et al., 2014; Windle et al., 2004).
Chronic OT positively modulated other correlates of stress and anxiety in animals. For example, beneficial behavioral effects have been observed in animal models of high anxiety (Slattery and Neumann, 2010) and post-traumatic stress disorder (PTSD) (Janezic et al., 2016) as well as in animals acutely stressed by white noise (Windle et al., 1997).
Several preclinical studies have also demonstrated that chronic OT has potentially long-lasting effects on cardiovascular responses (Petersson et al., 1999a, 1999c, 1996a). Cardiovascular reactivity to stress (e.g., increased heart rate, blood pressure) can be maladaptive in situations of long-term, persistent stress and may increase the risk of heart disease, hypertension, and/or stroke. OT-related cardiovascular influence is indicated by the presence of OXTR in the heart of animals and humans (Grewen and Light, 2011; Gutkowska and Jankowski, 2012) and the coordinated response of the endogenous OT system (e.g., greater plasma OT) with cardiovascular changes (e.g., greater vasodilation) during stress in humans (Grewen and Light, 2011).
In animals, chronic OT administration was consistently associated with reduced blood pressure (Holst et al., 2002; Petersson et al., 1999c, 1999d, 1996a). One study also demonstrated that chronic OT promoted the effects of clonidine, an α2-adrenoceptor agonist, in reducing blood pressure (Petersson et al., 1999d). This research indicates that chronic OT may be useful as an adjunctive treatment for hypertension.
Other preclinical work, however, has shown that chronic OT increased (Peters et al., 2014; Rault et al., 2013) or had no effect on certain correlates of stress and anxiety (Bales et al., 2013; Havranek et al., 2015; Huang et al., 2014). These mixed findings across preclinical studies may be due to methodological differences, such as the animal model examined (Peters et al., 2014; Rault et al., 2013). Importantly, these mixed effects can also be due to differences in OT dosage. For example, reductions in blood pressure and corticosterone levels were observed in response to higher doses of OT in studies that assessed multiple doses (Petersson et al., 1996a; Windle et al., 1997). In contrast, lower doses appear to be more effective in preventing anxious behavior in response to a stressful experience (Peters et al., 2014).
The efficacy of chronic OT in improving measures of stress and anxiety also depends on contextual factors, such as the developmental period during which chronic OT was administered (Rault et al., 2013) and the type of stress experienced (e.g., social vs. non-social stress; exposure to familiar vs. unfamiliar others) (Parker et al., 2005; Windle et al., 2004, 1997)). Within studies, interindividual factors can also moderate effects, including the sex (Bales et al., 2013) and genotype of the subject (Bales et al., 2014; Slattery and Neumann, 2010).
In humans, repeated and long-term exposure to stressors may dysregulate the HPA-axis and lead to pathological disturbances characterized by persistent anxiety (Charmandari et al., 2005; Engelmann et al., 2004). Thus, OT may be useful as a treatment for stress- and anxiety-related disorders, especially due to its minimal side effects and low potential for dependence compared to anti-anxiety medications (Engelmann et al., 2004; MacDonald and Feifel, 2014; Neumann and Slattery, 2016). For reviews of OT effects on stress and anxiety, see Neumann and Slattery (2016) and MacDonald and Feifel (2014).
To date, however, very few studies have examined the effects of daily, repeated OT administration on stress and anxiety in humans. In patients who scored high on a clinician-administered PTSD symptom severity scale following a traumatic event, chronic OT (40 IU, twice-daily for eight days) compared to placebo was associated with lower symptom severity at follow-up. Thus, chronic OT treatment may be protective for those at increased risk of developing PTSD (van Zuiden et al., 2017). Another study on individuals with treatment-resistant depression found that a low dose of OT (8 IU, twice-daily for four weeks) used in conjunction with the antidepressant escitalopram improved depressive and anxiety severity scores and evaluations on quality of life. However, interpretation of these findings is limited by the absence of placebo comparison (Scantamburlo et al., 2015).
The efficacy of chronic OT in improving measures of stress and anxiety is contextually variable (Parker et al., 2005; Rault et al., 2013; Windle et al., 2004, 1997) and depends on interindividual and methodological factors. More work is needed to distinguish the moderating effects of these factors. Additionally, determination of dose-dependent chronic OT effects on physiological and behavioral outcomes relevant to stress and anxiety reduction will contribute to a mechanistic understanding of OT’s therapeutic effects.
While there is much preclinical evidence on chronic OT effects on physiological and behavioral correlates of stress and anxiety, human research is critically needed. More research on chronic OT effects in individuals with PTSD and other disorders characterized by maladaptive stress and anxiety is especially warranted. Both animal and human work would also benefit from longer-term, randomized, placebo-controlled trials utilizing non-invasive administration to foster translational research.
3.2.2. Addiction and withdrawal
Repeated drug exposure can result in addiction, which is facilitated by dysfunctional learning and memory processes (Lee et al., 2016a; Sarnyai and Kovács, 2014). Repeated drug exposure also impacts the natural production of OT, though the shared mechanism underlying this effect across different substances is unclear (Lee et al., 2016a). A broad body of, mostly preclinical, research has indicated that OT administration inhibits the maladaptive underlying processes of addiction to substances, such as opiates, psychostimulants, and alcohol. In addition to altering learning and memory, OT may counteract and break down addiction and assist with recovering from withdrawal by modulating neural pathways related to reward, stress, and social behavior (Sarnyai and Kovács, 2014). This evidence is largely drawn from acute OT administration studies (Lee et al., 2016a; Sarnyai and Kovács, 2014). Comparatively, there are relatively few studies on the effects of chronic OT on addiction and withdrawal. See Sarnyai and Kovács (2014) and Lee et al. (2016a) for reviews on OT, drug addiction, and withdrawal.
The efficacy of OT administration in altering the neuroadaptations relating to substance abuse can vary by addictive substance and context surrounding OT administration (e.g., whether or not tolerance has been established prior to OT administration). For example, one study demonstrated that heroin self-administration was acquired more quickly in heroin-tolerant over heroin-naïve rats. Although chronic OT had no effect on acquisition of this behavior in naïve rats, it slowed acquisition in tolerant rats. Similarly, chronic OT helped to drop the self-administration rate in tolerant rats to the rate of naïve rats (Kovács et al., 1985a). Peripheral OT pretreatment, furthermore, reduced tolerance in heroin-tolerant mice (Kovács et al., 1985b) and prevented the development of heroin-enkephalin cross-tolerance (Kriván et al., 1992).
Regarding psychostimulants, chronic peripheral OT pretreatment (0.05 μg) and intrahippocampal infusion (100 pg) prevented the development of tolerance in rats (Sarnyai et al., 1992a). Similarly, subcutaneous OT pretreatment at a dose of 0.5 μg, compared to lower doses, increased behavioral sensitization to cocaine in mice (Sarnyai et al., 1992b). Efficacy of chronic OT pretreatment was found to be dose-dependent for methamphetamine self-administration, with higher OT doses associated with reduced drug responding (Carson et al., 2010).
Regarding alcohol, peripheral OT administered daily resulted in reduced consumption in rats (Bowen et al., 2011), mild withdrawal symptoms in mice (Szabó et al., 1987), and prevented tolerance in mice (Szabó et al., 1985). Peripheral OT pretreatment may specifically disrupt conditioning related to alcohol tolerance (Tirelli et al., 1992).
Though much more limited than preclinical research, current evidence in humans supports the notion that chronic OT attenuates addiction and withdrawal (Pedersen et al., 2013; Stauffer et al., 2016). A two-week study demonstrated that intranasal OT (40 IU, twice-daily) along with methadone treatment lowered cocaine craving (while it maintained heroin craving) for individuals with heroin and co-occurring cocaine use disorder, showing promise as a potential adjunctive treatment. OT was also associated with positive changes in drug-related implicit cognition and more accurate self-reporting of cocaine use (Stauffer et al., 2016). Intranasal OT, compared to placebo, administered for three days among alcohol-dependent individuals was also associated with decreased alcohol withdrawal and lorazepam needed for detoxification (Pedersen et al., 2013).
Overall, chronic OT may attenuate symptoms and behaviors related to addiction and withdrawal for different substances. However, these effects depend on the specific substance and context of OT administration. OT mechanisms of action for altering addiction and withdrawal are currently still underspecified. For example, though chronic OT can block tolerance for a variety of drugs this is likely via different mechanisms that are not well understood yet. Human research across distinct phases of addiction and withdrawal is also crucially needed (Lee et al., 2016a). It is possible that, in addition to breaking down the learning and memory processes that facilitate drug addiction and withdrawal, the anxiolytic and anti-stress properties of OT play a role. Also, OT-enhanced social salience may augment therapeutic effects (Lee et al., 2016a; Shamay-Tsoory and Abu-Akel, 2016). For example, OT may be more effective for stress relief among individuals with strong social connections (Heinrichs et al., 2003). It is possible that the relationship between stress-related and social regulatory processes sensitive to OT can be leveraged to prevent stress-induced relapse (Lee et al., 2016a). However, this link has not been systematically examined in a chronic administration context among individuals recovering from addiction.
3.2.3. Inflammation and pain
Inflammatory cytokines released from the spinal cord, dorsal ganglion, and nerve injury sites are associated with pathological pain (Zhang and An, 2007). Little to no pharmacological interventions are effective for long-term pathological pain. This represents a growing public health concern given that many available pain treatments are associated with severe side effects and dependence after prolonged use (Goodin et al., 2015). OT, in contrast, is not associated with these risks.
OT’s involvement in pain signaling is indicated by the presence of oxytocinergic projections from the paraventricular nucleus to the spinal cord and OXTR in nociceptive fibers (Goodin et al., 2015; Rash et al., 2014). OT secretion has also been associated with reduced inflammatory biomarker expression (Ahmed and Elosaily, 2011; Clodi et al., 2008; Jankowski et al., 2010; Szeto et al., 2013) and increased pain tolerance (Rash et al., 2014). OT administration may modulate endogenous OT and inflammatory biomarker levels relevant for pain reduction/tolerance and wound healing (Clodi et al., 2008; Detillion et al., 2004; Gouin et al., 2010; Rash et al., 2014). For a review on OT and analgesia, see Rash et al. (2014).
Although inflammation and pain are interrelated, they have been independently investigated in the context of chronic OT. Associations between inflammation and chronic OT are mixed. On one hand, chronic OT has decreased pro-inflammatory response in animals (Ahmed and Elosaily, 2011). Specifically, continuous OT administration reduced (Jankowski et al., 2010), slowed (Ahmed and Elosaily, 2011), and prevented inflammation and oxidative stress relating to heart disease (Plante et al., 2015; Szeto et al., 2013). Colonic inflammation relating to stress has also been reduced by chronic OT (Peters et al., 2014). On the other hand, chronic OT contributed to DNA damage in the rat hippocampus by increasing oxidative stress over extended periods of treatment (e.g., 21 days). These mixed findings warrant future research on the long-term safety of chronic OT (Leffa et al., 2017). Mechanistic research on chronic OT’s anti-inflammatory properties is furthermore needed, especially in terms of the indirect pathways by which OT modulates inflammation (e.g., via suppression of the HPA-axis) (Peters et al., 2014).
Regarding pain, animal research has reliably demonstrated that central and peripheral chronic OT relieves acute pain and increases long-term pain tolerance (Petersson et al., 2005b, 1996b; Rash et al., 2014; Uvnäs-Moberg et al., 1998). Human research suggests that OT may decrease pain experience and that higher endogenous levels of OT are associated with lower pain sensitivity (Rash et al., 2014). However, the involvement of OT in analgesia in humans is far less researched than in animals and results are less reliable, especially in the context of chronic administration. For example, one study indicated that 13 weeks of OT (40 IU, twice-daily) improved abdominal pain, discomfort, and depressed mood in individuals with chronic constipation (Ohlsson et al., 2005). However, this study was not sufficiently powered to detect significant effects when compared to the placebo group. Additionally, a crossover study among fibromyalgia patients found no therapeutic effects of daily intranasal OT administration (80 IU) for three weeks when used in conjunction with nonsteroidal painkillers (Mameli et al., 2014). This investigation was limited by a small sample. The lack of beneficial effects from OT across these two human studies, however, could have been due to other methodological factors, such as the dosage and duration of administration.
Further investigation is needed to better determine the effectiveness of chronic OT in, and the mechanisms underlying, inflammation and pain reduction in humans. In particular, the indirect mechanisms by which OT can affect pain should be explored further. It is possible that OT indirectly attenuates pain via stress and anxiety reduction (Goodin et al., 2015) and/or via interaction with the endogenous opioid system (García-Boll et al., 2018; Goodin et al., 2015; Taati and Tamaddonfard, 2018; Yang, 1994). Systematic investigation of dosage and treatment duration are also needed to determine therapeutic potential across other pain-related disorders.
3.2.4. Sexual dysfunction
While early investigations in humans centered on the effects of prolonged exposure to OT in the context of labor and post-partum (Bell et al., 2014; Gimpl and Fahrenholz, 2001), recent work has more broadly investigated OT modulation of sexual function and behavior (Behnia et al., 2014; Burri et al., 2008; Carter, 1992). Single-dose trials and case reports indicating an association between OT administration and enhanced sexual function have warranted investigations on chronic OT administration as a potential treatment for sexual dysfunction (Anderson-Hunt and Dennerstein, 1994; IsHak et al., 2008; MacDonald and Feifel, 2012).
Physiological changes in adult development can impact sexual functioning (Kingsberg, 2002). In particular, the thinning, dryness, and inflammation of the vaginal walls due to declines in circulating estrogen negatively affects the experience of intercourse and orgasm in post-menopausal women. Chronic OT exposure through gel application combats post-menopausal vaginal atrophy (Goldstein and Alexander, 2005). Several multidose and multiweek studies have demonstrated improved vaginal atrophy measures and the experience of painful intercourse (dyspareunia) in post-menopausal women using OT gel (Al-Saqi et al., 2016, 2015; Torky et al., 2018).
Improved physiology and symptomatology of post-menopausal vaginal atrophy can potentially enhance sexual function. The widely available estrogen-based treatments for this condition, however, have been associated with little to moderate improvements in sexual function and postmenopausal hormone use is on a decline (Constantine et al., 2015). Chronic OT administration via gel thus may be a viable option associated with minimal side effects for those seeking alternatives to estrogen treatment (Al-Saqi et al., 2016, 2015).
Although these findings are promising, post-menopausal vaginal atrophy represents one sex- and developmentally specific contributor to sexual dysfunction that is closely related to the experience of pain. OT’s involvement in analgesia and stress relief may be beneficial in conditions characterized by pain or anxiety that affect sexual function. There are also other potential targets for chronic OT within the domain of sexual dysfunction to be explored, including psychological and physiological factors that impede sexual motivation and performance apparent in sexual desire/arousal disorders (Baskerville and Douglas, 2010). However, OT administration may be efficacious for specific forms of sexual dysfunction and groups of people. Also, sexually dimorphic responses to chronic OT will require careful consideration in future work (Macdonald and Feifel, 2013).
3.2.5. Metabolism and weight
OT is involved in various metabolic functions and behaviors, such as increasing energy expenditure and reducing caloric intake (Lawson, 2017; Roberts et al., 2017). Dysfunction in the OT system, in particular, may disrupt metabolic processes and contribute to health problems including obesity, heart disease, and diabetes (Miller et al., 2017; Quintana et al., 2017). OT administration may be a promising treatment for disorders associated with abnormal weight due to its endogenous functions in regulating appetite and digestive metabolism (Lawson, 2017). OT-related weight loss, in particular, has been associated with maintained or increased energy expenditure, increased fat oxidation, improved satiety, and reduced caloric intake (Blevins et al., 2016). For reviews on OT effects on metabolism and weight, see Lawson et al. (2017) and Altirriba et al. (2015).
In animals, central and peripheral chronic OT have consistently demonstrated reductions in caloric intake (Balazova et al., 2016; Blevins et al., 2016, 2015; Deblon et al., 2011; Iwasaki et al., 2015; Maejima et al., 2011; Morton et al., 2012; Roberts et al., 2017; Uvnäs-Moberg et al., 1996); weight (Balazova et al., 2016; Blevins et al., 2016, 2015; Deblon et al., 2011; Iwasaki et al., 2015; Maejima et al., 2011; Morton et al., 2012; Petersson and Uvnäs-Moberg, 2008; Roberts et al., 2017); adipocyte size (Balazova et al., 2016; Eckertova et al., 2011; Maejima et al., 2011); and body fat (Blevins et al., 2016; Deblon et al., 2011; Maejima et al., 2011; Morton et al., 2012; Plante et al., 2015; Roberts et al., 2017). Chronic OT also altered endogenous peptide levels (Lawson, 2017; Petersson, 2002; Petersson et al., 1999b; Zhang et al., 2013) and enhanced fat oxidation (Blevins et al., 2016; Deblon et al., 2011), triglyceride uptake and lipolysis (Blevins et al., 2015; Deblon et al., 2011), and satiety in animals (Blevins et al., 2016).
In animal models of diet-induced obesity and diabetes, chronic OT improved glucose intolerance and insulin resistance (Deblon et al., 2011; Maejima et al., 2011; Plante et al., 2015; Zhang et al., 2013; see Balazova et al., 2016 for an exception). Chronic OT can induce weight loss in animals through physiological and neural mechanisms that increase the time between feedings and suppress food-related reward (Altirriba et al., 2015; Arletti et al., 1989; Blevins et al., 2015; Morton et al., 2012). In contrast, chronic OT may not be an effective weight-loss intervention for non-obese or slow-to-grow animals (Balazova et al., 2016; Leffa et al., 2017; Uvnäs-Moberg et al., 1996).
In humans, chronic OT may be an effective intervention for leptin-resistant obesity (Altirriba et al., 2015; Blevins et al., 2015; Zhang et al., 2013). OT has also increased weight loss and decreased waist-to-hip circumference in obese and prediabetic individuals (Zhang et al., 2013). The benefits of chronic OT for treating conditions characterized by disordered eating, however, appear to be more indirect. For example, chronic OT (18 IU, twice-daily) given to women with anorexia undergoing nutritional rehabilitation did not alter weight gain but did modify disordered thoughts and stress relating to food (Russell et al., 2018).
Impaired satiety and obesity in humans may also stem from endogenous OT dysfunction, as observed in Prader-Willi syndrome (PWS) (Einfeld et al., 2014; Kuppens et al., 2016; Miller et al., 2017; Tauber et al., 2017). Infants with PWS experience challenges engaging in appropriate feeding behaviors. Chronic OT improved sucking and swallowing in infants with PWS for several weeks in addition to some positive changes to social behavior (Tauber et al., 2017).
Physical deficiencies in OT-related neural circuitry may impact the efficacy of OT administration in later life. Without this scaffolding, chronic OT may not sufficiently upregulate the endogenous system (Einfeld et al., 2014). Therefore, chronic OT may be most beneficial among infants and young children with PWS (Einfeld et al., 2014; Kuppens et al., 2016; Tauber et al., 2017). However, there have also been null findings in early intervention (Einfeld et al., 2014; Miller et al., 2017). In addition, higher doses (32–40 IU, twice-daily) had unintentional behavioral consequences (i.e., increased outbursts) (Einfeld et al., 2014). Evidence from randomized controlled trials is especially lacking for this population in support of OT as an effective treatment (DeMayo et al., 2017).
Overall, evidence points to the benefits of chronic OT in modulating metabolism and weight, especially in the context of obesity and diabetes. However, the beneficial effects of chronic OT may be more indirect and contextually dependent for humans, especially in clinical populations characterized by disordered eating. Mechanistic research utilizing non-invasive administration in populations impacted by metabolic and weight-related impairments is needed. Factors relating to cognition, social behavior, and development will also require specific attention in future work (Deblon et al., 2011; Zhang et al., 2013).
3.2.6. Social engagement and bonding
Investigation of OT’s involvement in social engagement and bonding in healthy subjects has the potential to inform the treatment of social deficits, with consideration of contextual and interindividual variability (Calcagnoli et al., 2014; Crockford et al., 2014).
According to the current literature, chronic OT modulated social behavior with contextual specificity. For example, positive changes to interactions with familiar and unfamiliar others have been observed in male rats exposed to chronic OT across several administration routes (Bowen et al., 2011; Calcagnoli et al., 2015, 2014). Chronic peripheral OT administration in adolescence was furthermore associated with increased positive social contact in later life along with reductions in anxiety (Bowen et al., 2011). Similarly, reduced aggression and increased social exploration in the presence of unfamiliar others were associated with chronic central (Calcagnoli et al., 2014) and intranasal OT administration (Calcagnoli et al., 2015). Chronic intranasal OT also strengthened pair-bonding with familiar female partners (Calcagnoli et al., 2015).
In contexts where unfamiliar others were observed to be under distress, mice that underwent chronic intranasal OT administration exhibited increased sensitivity to this distress. Researchers have argued that this OT-related facilitation of observational fear is associated with emotional state-matching and empathic behavior. Along with this behavioral effect, chronic OT was associated with OXTR downregulation in the amygdala. These findings indicate that rather than universally attenuating stress and anxiety, it is possible that chronic OT selectively enhances certain stressful experiences to motivate prosocial behavior (Pisansky et al., 2017).
In contrast to these positive outcomes, one study in male mice demonstrated that up to 21 days of chronic intranasal OT reduced social interactions regardless of familiarity in addition to reducing OXTR in the brain (Huang et al., 2014). This finding suggests that the endogenous OT system may be overstimulated in healthy subjects.
Other preclinical studies have indicated that chronic OT affects social behavior in a sex- and dose-dependent manner. For example, in prairie voles, chronic intranasal OT at varying doses impacted the development of social behavior, with potentially negative consequences for males. Specifically, low (0.08 IU/kg) and medium doses (0.8 IU/kg) over three weeks, but not a high dose (8 IU/kg), of chronic OT were associated with long-term deficits in pair-bonding. This contrasts with increases in partner preference behavior observed after acute OT administration. Acute and long-term OT effects on partner preference were not significant in female voles (Bales et al., 2013).
Another study in female voles showed that the medium dose facilitated pair-bonding and was associated with increased OXTR binding in the nucleus accumbens. In contrast, the medium dose decreased endogenous vasopressin concentrations and was associated with decreased pair-bonding in males (Guoynes et al., 2018). Thus, according to this preclinical work chronic OT may not enhance social behavior particularly for healthy, developing males. Because OT is under consideration as a potential treatment for disorders that emerge in early life (e.g., autism spectrum disorder, PWS), the extent to which there may be social impairment related to chronic OT in developing subjects needs to be further explored (Young, 2013).
Although investigations on the prosocial effects of single-dose OT in humans are abundant, few chronic OT investigations relating to social engagement or bonding in healthy humans have been conducted. In young men, two weeks of chronic intranasal OT was associated with positive changes to attachment, especially for those with avoidant and insecure attachment styles (Bernaerts et al., 2017). In another study, null effects of chronic OT on social engagement in healthy older adults were observed (Barraza et al., 2013).
Overall, findings on chronic OT effects across different outcomes relating to social engagement and bonding in healthy subjects are mixed with some variation by dosage and sex. Human research is especially scarce in this domain.
3.2.7. Social and cognitive dysfunction
Findings from single-dose and receptor studies on OT’s involvement in social cognition and behavior have led to investigations on chronic OT for the treatment of conditions characterized by social and cognitive dysfunction (Guastella and MacLeod, 2012; Kumsta and Heinrichs, 2013; MacDonald and Macdonald, 2010). To date, findings are still scarce and mixed in this domain.
3.2.7.1. ASD
Autism spectrum disorder (ASD) comprises various neurodevelopmental conditions with core symptoms involving challenges in social communication, obsessive interests, and repetitive behaviors. Effective pharmacological treatments for the social deficits associated with ASD are lacking (Anagnostou et al., 2012). Although behavioral interventions can have positive impacts on certain social skills, access and cost are significant barriers to treatment (DeMayo et al., 2017). OT has emerged as a potential candidate for pharmacological treatment due to its endogenous involvement in facilitating social cognition and behavior and through support from some preclinical and human work using OT administration (Anagnostou et al., 2014; DeMayo et al., 2017; Meyer-Lindenberg et al., 2011). For reviews on OT and ASD, see Anagnostou et al. (2014), Lefevre and Sirigu (2016), and DeMayo et al. (2017).
Various multidose and multiweek investigations have been conducted to assess the efficacy of OT in treating ASD. In male and female genotypic mice models of ASD, peripheral OT once every two days for eight to nine days, but not acute administration, improved social behavior in both adolescence (Teng et al., 2013) and adulthood (Teng et al., 2016). Similar social benefits were observed in other mice models (e.g., male BALB/cByJ) (Teng et al., 2013) and Grin1 knockdown strains (Teng et al., 2016). In female BTBR mice, intranasal OT administered daily for 30 days increased social interactions for unfamiliar others (Bales et al., 2014).
In humans, Anagnostou and colleagues (2012) found that six weeks of intranasal OT (24 IU, twice-daily) in adult males improved scores on the Reading-the-Mind-in-the-Eyes test and quality of life. However, no treatment effects on primary outcomes relating to the core symptoms of ASD, such as repetitive behaviors, were observed (Anagnostou et al., 2012).
A later, open-label pilot study from this research group found that up to 0.4 IU/kg (equivalent to 24 IU) over 3 months was well-tolerated in a younger population (10–17 years old) (Anagnostou et al., 2014). Some improvements to social functioning, social cognition, anxiety, and repetitive behaviors were also observed. However, comparisons across doses were underpowered and findings were not compared to a placebo or control group. Similarly, another open-label study found that 8, 16, and 24 IU of intranasal OT administered twice-daily for two months per dose was well-tolerated in children and associated with improvements in social communication and interactions (Tachibana et al., 2013).
Existing randomized, placebo-controlled, crossover trials have found that chronic OT at either 8 or 24 IU twice-daily over several weeks increased social interactions (Munesue et al., 2016) and improved social reciprocity in children and adults (Watanabe et al., 2015). Chronic OT also enhanced functional activation in and connectivity of the medial prefrontal cortex and improved performance during a social cognition task (Watanabe et al., 2015). Similarly, 12 IU over five weeks in children improved evaluations of social responsiveness (Yatawara et al., 2016). Another multiweek, multidose clinical trial in adolescents and young adults found that higher (32 IU) compared to lower (16 IU) doses were associated with greater global functioning after 12 weeks (Kosaka et al., 2016). In contrast, null findings on social outcomes in children have been observed (Dadds et al., 2014; Guastella et al., 2015).
Although there seem to be some benefits of chronic OT administration, it is important to note that some studies reported null findings on the improvement of core symptoms relating to ASD in adults (Anagnostou et al., 2012; Munesue et al., 2016) and youth (Dadds et al., 2014; Guastella et al., 2015; Munesue et al., 2016; Tachibana et al., 2013). Lack of significant effects could have been due to interindividual factors including variation in the diagnostic subtypes or participant age (Dadds et al., 2014). Also, the outcome measures or dosages used may have been insensitive to behavioral effects (DeMayo et al., 2017; Tachibana et al., 2013).
Mixed evidence across open-label and randomized controlled trials challenge the notion that chronic OT enhances ASD-related social impairment (DeMayo et al., 2017). However, there is promise for expanding future randomized and controlled research on treating ASD symptoms, particularly relating to social deficits, with chronic OT (Anagnostou et al., 2014; Tachibana et al., 2013).
3.2.7.2. Schizophrenia and related disorders
Schizophrenia is associated with social and cognitive dysfunction characterized by positive (i.e., disordered thoughts and behaviors, delusions) and negative symptoms (i.e., blunted affect, apathy, asociality). Although antipsychotics are effective in treating positive symptoms, there are significant risks in using these medications and treatments for negative symptoms and social cognitive dysfunction are still needed (Shilling and Feifel, 2016). To date, the majority of chronic OT research in humans has been conducted as randomized controlled trials to assess the efficacy of chronic OT in treating the symptomatology of schizophrenia and related disorders. For reviews on the role of OT in schizophrenia, see Bradley and Woolley (2017), Shilling and Feifel (2016), and Feifel et al. (2016).
Chronic OT used in conjunction with other therapies and medication over a few weeks to several months has shown potential for improving positive and negative symptoms (Feifel et al., 2010; Gibson et al., 2014; Jarskog et al., 2017; Lee et al., 2013; Modabbernia et al., 2013; Ota et al., 2017; Pedersen et al., 2011). However, some findings point to no significant changes in symptoms and related deficits (Bradley and Woolley, 2017; Buchanan et al., 2017; Cacciotti-Saija et al., 2015; Dagani et al., 2016; Jarskog et al., 2017; Lee et al., 2016b, 2013).
The shortest duration of daily repeated OT administration (i.e., two weeks) was associated with attenuation of scores in from the Positive and Negative Symptom Scale, reductions in anxiety relating to suspiciousness, persecution, and paranoia, and some improvement in a theory-of-mind task. However, this study was limited by small sample size and a lack of data on cognition more broadly (Pedersen et al., 2011).
Three weeks of chronic intranasal OT, in contrast, was associated with mixed evidence across studies. For example, whereas 20 IU administered twice-daily was associated with improved odor identification and negative (but not positive) symptoms (Lee et al., 2013), this dosage yielded no significant change to plasma OT levels or positive and negative symptoms when compared to placebo (Lee et al., 2016b). This lack of findings was attributed to insufficient power and potential lower adherence to nasal spray application protocol among outpatients over inpatients.
In contrast, clinical trials with a crossover design and higher cumulative dosage (20 – 40 IU, twice-daily) were associated with improved positive and negative symptoms (Feifel et al., 2010) and cognition (i.e., improved verbal memory and no amnestic effect) (Feifel et al., 2012). The effects on symptomatology relating to schizophrenia emerged during the last week of OT administration, suggesting a cumulative action of OT (Feifel et al., 2010). In contrast, the cognitive effects of OT were only assessed in the third week of administration and comparison across various time points during treatment was not possible (Feifel et al., 2012).
Six weeks of OT (24 IU, twice-daily) was associated with improved fear recognition, perspective-taking, and negative symptoms. Importantly, these findings were limited by a small sample and were not compared to a placebo group (Gibson et al., 2014). In contrast, randomized controlled trials using the same dosage and duration of OT treatment did not result in improvement in symptomology and functioning relating to schizophrenia or early psychosis when compared to placebo (Buchanan et al., 2017; Cacciotti-Saija et al., 2015). Furthermore, null findings were observed for cognitive impairment (Buchanan et al., 2017) and social cognition (Cacciotti-Saija et al., 2015). This lack of effects could have been due to factors including power, diagnostic heterogeneity within schizophrenia and related disorders (e.g., schizoaffective disorder, early psychosis), insufficient dosage levels for these specific outcomes, and difficulties with nasal spray self-administration among unmonitored outpatients (Buchanan et al., 2017; Cacciotti-Saija et al., 2015).
Longer treatment durations also yielded mixed findings across core symptoms of schizophrenia and related functional outcomes. For example, two months of OT administration (20 – 40 IU, twice daily) as an adjunct to the antipsychotic risperidone improved positive and negative symptoms and general psychopathology (Modabbernia et al., 2013). A three-month open-label trial that involved chronic OT (12 IU, twice-daily) also demonstrated improvements in schizophrenia symptoms, though no comparison to a placebo group was available (Ota et al., 2017). In contrast, a randomized controlled trial of the same duration found that chronic OT (24 IU, twice-daily) had no impact on social cognition when compared to placebo and was only associated with improved negative symptoms in the schizophrenia, but not the schizoaffective, subgroup (Jarskog et al., 2017). Finally, a randomized crossover trial found that four months of OT (40 IU per day) was not associated with any changes to symptoms of schizophrenia or psychosocial functioning (Dagani et al., 2016).
Schizophrenia and related disorders are the most well-researched areas concerning chronic OT effects on social and cognitive dysfunction. Overall, findings across studies vary in their conclusions on chronic OT as a beneficial treatment. All studies involved OT administration in conjunction with established treatment plans that mainly comprised of antipsychotics. Treatment plans vary by individual and it is still unclear how OT interacts with antipsychotics (Lee et al., 2016b). Future research will benefit from systematic assessment of the adjunctive benefit of chronic OT with individual medications when possible, similar to the study conducted by Modabbernia and colleagues (2013), or studies that use OT as a singular treatment (Lee et al., 2016b). Systematic studies on dosage, duration, and administration can help determine effective treatment plans across different contexts that are functionally impaired in schizophrenia and related disorders (Buchanan et al., 2017; Lee et al., 2013). Future studies with samples selected according to specific diagnostic criteria may also uncover whether certain subtypes are associated with more optimal responding to chronic OT (Cacciotti-Saija et al., 2015).
3.2.7.3. OCD
Obsessive-compulsive disorder (OCD) is characterized by cognitive dysfunctions that manifest as obsessions (i.e., pathological, recurrent, uncontrollable thoughts) and compulsions (i.e., avoidant rituals or repetitive behaviors). Some evidence has linked OCD to over-activation of the endogenous OT system whereas others have theorized that OT administration may help treat OCD symptoms, based on findings that low doses of OT attenuate memory and anxiety (Leckman et al., 1994).
However, six weeks of OT (18 IU and 54 IU per day) in individuals with OCD resulted in no reduction in compulsive behaviors or anxious and depressive symptoms. Participants also reported no side effects relating to psychosis and memory (den Boer and Westenberg, 1992). This lack of effects may be due to the low dosage administered (18 IU and 54 IU per day). However, a one-week treatment with much higher doses (160 IU and 320 IU per day) still showed no improvement in OCD symptoms (Epperson et al., 1996). Though investigations on chronic OT for the treatment of OCD are sparse, together these findings suggest that chronic OT, regardless of dosage, may not be an appropriate treatment for this condition.
3.2.7.4. Dementia
To date, only one study has examined chronic OT effects on symptoms of dementia. Specifically, this study was conducted on individuals with frontotemporal dementia, which compromises executive and social functioning. Individuals with the behavioral variant of frontotemporal dementia (bvFTD) experience dysfunctions in social cognition, including empathy and emotion identification (Finger et al., 2015; Jesso et al., 2011; Possin et al., 2013). Chronic OT administered to individuals with bvFTD for one week (24, 48, or 72 IU, twice-daily) was well-tolerated and associated with positive effects on symptoms relating to apathy, empathy, and social interactions (Finger et al., 2015). Further investigation is needed, however, to examine the potential benefits of chronic OT for those with different subtypes of dementia.
4. Discussion
4.1. Challenges and recommendations for future research
The manifold applications of chronic OT are promising and findings from several studies point to potential therapeutic use in different clinical contexts. However, as summarized in Figure 3, conceptual (e.g., contextual and interindividual differences) and methodological factors (e.g., administration route, dosage, frequency, and duration) impact the effects of chronic OT treatment, and challenge the synthesis of this line of research. There is also limited knowledge of the mechanisms of action by which chronic OT exerts its effects (Box 2).
Box 2: Brief Overview of Mechanisms of Action for Chronic OT Administration.
Modulation of receptor expression and binding:
Current evidence suggests that chronic OT administration modulates receptor expression and binding to suppress the activity of the HPA-axis during stress and anxiety. This modulation may be specific to certain stress- and anxiety-related brain regions. For example, OT may reduce HPA-axis activity via modulation of glucocorticoid and mineralocorticoid receptor expression in the hippocampus. The hippocampus is one of the main regions that receive feedback from HPA-axis activation. Glucocorticoid and mineralocorticoid receptors play roles in stress-response and -recovery and dysregulation of these receptors can increase the risk of stress-related disorders (Groeneweg et al., 2012; Petersson and Uvnäs-Moberg, 2003). OT administration may also enhance α2-adrenoceptor and cardiovascular function to promote stress regulation (Díaz-Cabiale et al., 2000; Holst et al., 2002; Petersson et al., 2005a, 1999d, 1996a). Furthermore, OT interaction with dopaminergic receptors is associated with social bonding, cognition, and reward yet systematic work using chronic OT administration is still needed (DeMayo et al., 2017).
Chronic OT also affects OXTR expression and binding, which are subject to individual differences in density, distribution, and polymorphisms (Gimpl and Fahrenholz, 2001; Havranek et al., 2015). OT can have differential effects according to individual endocrine profiles and sensitivity to OT (Bowen et al., 2011; Uvnäs-Moberg et al., 1996). Upregulation of OXTR may be one mechanism by which OT exerts its anti-inflammatory effects (Ahmed and Elosaily, 2011). Repeated exposure to OT, however, can result in lowered OXTR expression, consistent with desensitization and downregulation of the endogenous OT system (Gimpl and Fahrenholz, 2001). For example, at high doses, reductions in OXTR binding and cross-interactions with vasopressin receptors may contribute to anxious behavior (Peters et al., 2014). Further, reductions in OXTR binding may result in impaired social interactions (Huang et al., 2014; Peters et al., 2014). More mechanistic research is needed to determine the extent to which chronic OT either downregulates or upregulates OXTR and whether these effects are dose-dependent.
Modulation of physiological regulators:
Chronic OT acts by modulating the endogenous release of physiological regulators. For example, OT can inhibit or reduce the release of stress-induced HPA-axis regulators, such as corticotropin-releasing factor, ACTH, and corticosterone (Parker et al., 2005; Peters et al., 2014; Petersson et al., 2005b, 1999a; Windle et al., 2004, 1997), likely in a dose-dependent manner (Peters et al., 2014; Windle et al., 2004). OT can increase insulin secretion and sensitivity as well as glucose tolerance in animal models of diabetes and obesity. OT may have indirect benefits on metabolism through downregulation of the HPA-axis and inhibition of cortisol release (Lawson, 2017) and may indirectly exert analgesic effects through facilitating generation and release of endogenous opioids, particularly in the spinal cord (Petersson et al., 1996b; Rash et al., 2014).
Modulation of specific brain regions and networks:
Chronic OT may play a role in regulating hippocampal plasticity and memory formation (Havranek et al., 2015). It has also shown to increase functional activity and connectivity in frontal regions associated with impairment in ASD (Watanabe et al., 2015). Larger insula and anterior cingulate volumes were associated with improved schizophrenia symptoms after chronic OT administration (Ota et al., 2017). OT may also have inhibitory effects on specific brain regions relating to stress, such as the amygdala, though this has not been well-documented in chronic OT work yet (Olff et al., 2013). Very little work to date has incorporated neuroimaging methods to elucidate chronic OT effects on the brain and its neural mechanisms of action.
Modulation of psychological and social factors:
Chronic OT has also shown to modulate psychological and social factors in a context-dependent manner. For example, chronic OT selectively enhanced the experience of stressful situations in a social context in order to elicit prosocial or approach-oriented behavior (Pisansky et al., 2017). OT may also alleviate pain and stress indirectly through improving mood and lowering anxiety (Ohlsson et al., 2005; Rash et al., 2014; Russell et al., 2018). Facilitation of positive social interactions and support, furthermore, constitutes a crucial indirect mechanism by which chronic OT may exert therapeutic effects, which is relevant for cases relating to the alleviation of pain, stress, and addiction (Rash et al., 2014).
In the remainder of this paper, we discuss challenges and recommendations for future research, including efforts to improve understanding of mechanistic action of chronic OT, addressing understudied populations and functional outcomes, and determining and standardizing optimal treatment plans.
4.1.1. Advancing knowledge about mechanistic action of chronic OT administration
Chronic OT administration is associated with improvement for various functional outcomes across research domains. However, the effects of chronic OT are not unilateral (i.e., affecting only specific outcomes of interest) or universal (i.e., affecting all individuals in the same way). The current literature discusses several mechanisms of action for chronic OT, briefly summarized in Box 2. OT actions via modulation of receptor expression, receptor binding, and endogenous release of regulatory hormones are the most well-documented mechanisms across, mostly preclinical, studies. However, much is still left to be understood about the endogenous OT system and how it is impacted by chronic administration.
Though it is known that central substrates facilitate OT effects (Quintana et al., 2015a), their response to chronic administration remains unclear. For example, limited work shows that chronic OT administration modulates the structure and function of specific brain regions (Havranek et al., 2015; Ota et al., 2017; Watanabe et al., 2015). However, physiological evidence indicates that the neuropeptide disperses without hard-lined spatial or temporal constraints (Bethlehem et al., 2013). Thus chronic OT administration may have broader impacts on the brain that are not well-documented yet, especially in terms of structural and functional connectivity as well as potential network-level modulation. More investigation on the neural correlates of the OT system and their response to chronic administration is needed to determine this.
Development of models and conceptual frameworks of OT action will help explain findings and drive future research. Existing theoretical approaches of OT action have often served as parsimonious explanations for observed effects of OT administration. These conceptualizations are insufficient as they attempt to distill OT action into a generalized, regulatory role, while not considering the multiple interrelated and coordinated effects OT has on the body and brain (Roney, 2016).
Informed by our comprehensive systematic review, we present a basic organizational framework that outlines the broader, interrelated regulatory functions sensitive to chronic OT (Figure 4). Utilizing this framework, we argue that the intended modulation of one function by chronic OT may lead to, or be facilitated by, cascading effects on others. Some preclinical OT research includes outcome measures across multiple functional domains (Bales et al., 2014; Bowen et al., 2011; Huang et al., 2014; Petersson et al., 2005b; Plante et al., 2015). Following this example, future systematic research in humans may benefit from a similar attempt at an integrated understanding of chronic OT effects on multiple regulatory functions. This approach has the promise to identify generalizable effects as well as context- and/or population-specific moderations and will bring us closer to evaluating chronic OT’s therapeutic efficacy and developing precise analogs to OT for use in treatment.
4.1.2. Addressing understudied populations and functional outcomes
While some findings across different phenotypes and genotypes indicate that chronic OT may have common effects (Teng et al., 2016), certain subpopulations or individuals may be more sensitive to chronic OT than others (DeMayo et al., 2017; Feifel et al., 2010). Interindividual or subgroup-level differences, such as OXTR distribution or specific diagnoses, may contribute to such variations (Feifel et al., 2010). For example, broader conditions, like ASD, schizophrenia, and dementia, can be subdivided into distinct diagnoses with varying symptomology. Future work should seek to distinguish the unique benefits of chronic OT across such diagnostic subtypes to develop individualized and precise treatment plans (Guastella and Hickie, 2016; Saria and Goldenberg, 2015).
However, several challenges exist in subtyping the efficacy of chronic OT. Current research in clinical contexts is often characterized by small and sometimes diagnostically heterogeneous samples (e.g., schizophrenia and schizoaffective disorder) (Jarskog et al., 2017; Lee et al., 2013). Also, comorbid disorders, like co-occurring substance disorders (Stauffer et al., 2016) or ancillary disorders that stem from major pathology (e.g., mood, anxiety, and sleep disorders in those with fibromyalgia; Mameli et al., 2014), are understudied. Larger and more diagnostically homogenous samples will allow for sufficiently powered comparisons across subtypes (Guastella and Hickie, 2016; Saria and Goldenberg, 2015).
Findings from healthy groups also constitute an important comparison of chronic OT effects in clinical populations. To date, only very few studies have investigated chronic OT effects on socioemotional, cognitive, and behavioral functional outcomes in healthy samples. Also, safety and tolerability are not conclusively determined yet across all populations (Anagnostou et al., 2014; DeMayo et al., 2017; Finger et al., 2015; Mameli et al., 2014). As summarized in Box 3, expanded investigations beyond young male subjects to both sexes and different phases of development in healthy and clinical populations are warranted (Ebner et al., 2013; Guastella and Hickie, 2016; Huffmeijer et al., 2013).
Box 3: Chronic OT across development.
Chronic OT in early development:
Preclinical studies have indicated that developmental trajectories can shift as a result of exogenous OT either during prenatal or postnatal development (Bales and Perkeybile, 2012; Carter, 2003; Guoynes et al., 2018). Chronic OT administered over two months in pregnant rats resulted in lower birth weight and postnatal growth in offspring. Reductions in postnatal brain size and cerebellar DNA were also found (Boer, 1993). Though effects were small and indirect, chronic OT may alter prenatal physiology and may not be beneficial for early development (Carter, 2003). OT is a major regulator of physiological and social functions at the end of pregnancy (e.g., labor, lactation, maternal behavior). OT administration prior to delivery may alter plasma OT levels long term (Prevost et al., 2014), cautioning prenatal use for reasons other than assisting in delivery.
In contrast, postnatal chronic OT administered peripherally to rat pups over five days resulted in long-term positive physical and metabolic changes that were similar to the benefits observed in kangaroo care for premature infants (Uvnäs-Moberg et al., 1998). Repeated postnatal OT exposure may also be neuroprotective (e.g., prevention of hypertension; Vargas-Martínez et al., 2014). Preclinical work also reports mixed and sex- and dose-dependent findings regarding the development of social behavior (Bales et al., 2013; Bowen et al., 2011; Guoynes et al., 2018; Rault et al., 2013).
Chronic OT in aging:
Aging is characterized by a variety of physiological, cognitive, social, and emotional changes that may potentially benefit from chronic OT yet research is lacking (Ebner et al., 2014, 2013). To date, only one study has addressed the effects of chronic OT in healthy older adults (Barraza et al., 2013) and found that individuals in the OT, compared to the placebo, group reported less decline in physical functioning and fatigue. Other functional outcomes impacted by aging have yet to be examined.
Safety and tolerability in early and later life:
Evaluation for safety and long-term consequences of chronic OT across development is needed in light of its potential therapeutic use in early-life developmental disorders (e.g., ASD, PWS) (Carter, 2003; Young, 2013) as well as in healthy and pathological aging (Ebner et al., 2013; Finger et al., 2015). In particular, the current data on OT safety and tolerability are insufficient to recommend either short- or long-term OT use in younger populations (Anagnostou et al., 2014; DeMayo et al., 2017; MacDonald et al., 2011; Tachibana et al., 2013). There is also only one systematic investigation on OT safety and tolerability in pathological aging (Finger et al., 2015), which suggested that chronic intranasal OT for one week was generally well-tolerated in frontotemporal dementia at varying doses (24–72 IU) (Finger et al., 2015). Side effects for longer durations and among healthy older adults will still have to be determined. (See DeMayo et al., 2017 for recommendations regarding adequate assessment of OT safety and tolerability).
4.1.2. Determination and standardization of optimal treatment plans
Investigations on OT’s modulatory effects have also proliferated because of the diverse administration routes and dosage practices available (Box 1). However, this methodological breadth impacts generalizability across studies (Lee et al., 2016a). Systematic research to determine optimal administration routes and treatment plans for specific populations and conditions are needed (Lee et al., 2015) with consideration for OT pharmacodynamics (Calcagnoli et al., 2015). This is especially relevant given the short half-life of OT and the central pathways by which OT binds (Quintana et al., 2019, 2015a).
4.1.2.1. Evaluation of OT administration routes
Currently, there is mixed evidence regarding the effectiveness of different routes. For example, central chronic OT administration in rats elicits stronger effects and may be more effective in influencing the endogenous OT system than intranasal administration (Calcagnoli et al., 2015, 2014). However, central OT administration constitutes a stressful experience for animals (Teng et al., 2013), which can perturb the endogenous OT system or interact with OT administration. Well-controlled studies can help disentangle potential confounds.
Also, central OT administration, though important for preclinical investigation, is not pragmatic for use in human research due to its invasive nature. Peripheral OT injections are comparatively less invasive and stress-inducing than central infusion. However, there are concerns that peripheral OT may not efficiently cross or circumvent the blood-brain barrier to bind to central targets and thus degrade in the blood over time. Tolerability of repeated injections is also a limiting factor (Lawson, 2017). Non-invasive administration routes should be prioritized in research that assesses therapeutic efficacy.
Intranasal administration is primarily used in human work though still largely constrained to single-dose application. One advantage of intranasal administration in humans is the ability to self-administer the neuropeptide, allowing for feasibly longer, unmonitored duration of treatment. Adhering to self-administration protocol, however, is a challenge for individuals, particularly in clinical contexts, and monitoring may still be needed (Buchanan et al., 2017; Cacciotti-Saija et al., 2015). Inappropriate self-administration, which can lead to imprecise dosing, may be difficult to identify and control for (Lawson, 2017). Thus the use of specialized devices that consistently deliver the intended dose of OT has been evaluated to reduce user error and ensure delivery to nasal-neural pathways (Quintana et al., 2016, 2015b). Administration through a nebulizer may be more effective than a nasal spray (Calcagnoli et al., 2015; Modi et al., 2014). However, as most studies currently do not compare different administration routes, it is difficult to conclude if one method of nasal administration is superior to another.
One systematic comparison of intravenous and intranasal OT administration found that both routes produce similar OT plasma levels compared to placebo. However, only a lower dose of intranasal OT (8 IU), using a specialized breath-powered device, was associated with the modulation of social cognition compared to placebo. Specifically, the low-dose reduced the perception of anger from ambiguous faces (Quintana et al., 2015b) and reduced amygdala response to angry faces (Quintana et al., 2016). These findings provide mechanistic evidence of nasal-neural OT delivery relevant to social cognition in humans.
4.1.2.2. Evaluation of OT dosages
The majority of current research used dosage levels informed by previous practices and recommendations largely based on single-dose trials (Guastella et al., 2013; MacDonald et al., 2011). However, a clear rationale for the dosages used was not always provided, which may be related to uncertainty surrounding optimal treatment plans and experimental schemes necessary to elicit predicted effects.
Systematically comparing multiple dosages within a study is advantageous for determining the optimal levels to yield effects on outcomes of interest, also in light of dose-dependent, non-linear responses (Guoynes et al., 2018; Yamasue, 2016). For example, higher doses of chronic OT may be less effective than lower doses for particular contexts (e.g., psychosocial stress, drug tolerance) (Kovács et al., 1985a, 1985b; Peters et al., 2014).
Another methodological inconsistency that impacts dosage is the frequency of OT administration per day. Given the fast degradation of OT (Striepens et al., 2013), intranasal administration at least twice-daily, a practice which is already employed in several human studies (Table 2 and 3), may help ensure continuous exposure to OT and lead to lasting effects on the endogenous OT system (Bethlehem et al., 2013). Also, the time of OT administration relative to experimentation is a crucial design factor as acute effects may be inadvertently measured if administration occurs directly prior to the measurement of relevant outcomes.
4.1.2.3. Evaluation of OT administration durations
Whereas some studies investigated chronic OT effects for an extended period of time, other investigations spanned just a few days. Short durations of administration may not be enough to observe lasting effects (Macdonald and Feifel, 2013). Also, many clinical conditions are characterized by long-term dysfunction. Thus, clinical studies should strive for experimental designs that resemble long-term treatment plans and acquire data at various time points.
However, longer treatment does not necessarily lead to optimal outcomes (Yamasue, 2016). Chronic OT may have unintended negative consequences on social behavior in certain populations (Bales et al., 2013; Calcagnoli et al., 2015; Neumann and Slattery, 2016; Young, 2013). Shorter treatment plans may be more adequate for some contexts.
5. Conclusions
There has been a notable surge in research in the last few decades on the functions and therapeutic potential of OT. This review synthesized findings on chronic OT as an investigative tool and clinical intervention. We conclude that growing evidence supports OT as a treatment for certain clinical conditions but the potential for therapeutic application outside of research is still limited. This limited translatability is characterized in part by methodological inconsistencies and divergent findings across studies. We have identified promising avenues for advancement, including mechanistic OT research in humans, investigation of multiple, coordinated functions sensitive to chronic OT as well as systematic dosage comparison, and assessment of safety and tolerability, especially in currently understudied, populations. We are confident that strong theoretical conceptualization and application of rigorous and transparent methodology will propel this field forward.
Supplementary Material
Highlights.
Oxytocin (OT) subserves adaptive physiological, behavioral, and cognitive functions
Literature synthesis shows chronic OT improves outcomes in different circumstances
Multiple, interrelated regulatory functions are sensitive to chronic OT
More systematic and mechanistic research needed to determine therapeutic efficacy
Attention to interindividual and contextual factors will advance the field
Acknowledgments and Disclosures
This work was supported by the National Institute on Aging Pre-Doctoral Fellowship on Physical, Cognitive, and Mental Health in Social Context [T32 AG020499 to MH], a University of Florida Clinical and Translational Science pilot award [NIH/NCATS; UL1 TR000064 to NCE], a University of Florida Claude D. Pepper Older Americans Independence Center pilot award [P30 AG028740 to NCE], a National Institute on Aging grant [R01 AG059809 to NCE] as well as by the Department of Psychology, the Institute on Aging, the Center for Cognitive Aging and Memory, and the McKnight Brain Institute at the University of Florida.
The authors would like to thank Adam Woods, Andreas Keil, Lisa Scott, and Desiree Lussier for their feedback on this project and Gene Liu for his assistance with the literature search.
The authors have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed MA, Elosaily GM, 2011. Role of oxytocin in deceleration of early atherosclerotic inflammatory processes in adult male rats. Int. J. Clin. Exp. Med 4, 169–178. [PMC free article] [PubMed] [Google Scholar]
- Al-Saqi SH, Jonasson AF, Naessén T, Uvnäs-Moberg K, 2016. Oxytocin improves cytological and histological profiles of vaginal atrophy in postmenopausal women. Post Reprod. Heal 22, 25–33. 10.1177/2053369116629042 [DOI] [PubMed] [Google Scholar]
- Al-Saqi SH, Uvnäs-Moberg K, Jonasson AF, 2015. Intravaginally applied oxytocin improves post-menopausal vaginal atrophy. Post Reprod. Heal 21, 88–97. 10.1177/2053369115577328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altirriba J, Poher A-L, Rohner-Jeanrenaud F, 2015. Chronic oxytocin administration as a treatment against impaired leptin signaling or leptin resistance in obesity. Front. Endocrinol. (Lausanne) 6, 1–7. 10.3389/fendo.2015.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvares GA, Quintana DS, Whitehouse AJO, 2017. Beyond the hype and hope: Critical considerations for intranasal oxytocin research in autism spectrum disorder. Autism Res. 10, 25–41. 10.1002/aur.1692 [DOI] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, Jacob S, 2014. Intranasal oxytocin in the treatment of autism spectrum disorders: A review of literature and early safety and efficacy data in youth. Brain Res. 1580, 188–198. 10.1016/j.brainres.2014.01.049 [DOI] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, Wang AT, Pepa L, Tanel N, Kushki A, Hollander E, 2012. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Mol. Autism 3, 16 10.1186/2040-2392-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Hunt M, Dennerstein L, 1994. Increased female sexual response after oxytocin. BMJ 309, 929 10.1136/BMJ.309.6959.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A, 1989. Influence of oxytocin on feeding behavior in the rat. Peptides 10, 89–93. 10.1016/0196-9781(89)90082-X [DOI] [PubMed] [Google Scholar]
- Balazova L, Krskova K, Suski M, Sisovsky V, Hlavacova N, Olszanecki R, Jezova D, Zorad S, 2016. Metabolic effects of subchronic peripheral oxytocin administration in lean and obese zucker rats. J. Physiol. Pharmacol 67, 531–541. [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, 2012. Developmental experiences and the oxytocin receptor system. Horm. Behav 61, 313–319. 10.1016/j.yhbeh.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP, 2013. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol. Psychiatry 74, 180–8. 10.1016/j.biopsych.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, Sahagun E, Puhger KR, Pride MC, Mendoza SP, 2014. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl. Psychiatry 4, e480–e480. 10.1038/tp.2014.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza JA, Grewal NS, Ropacki S, Perez P, Gonzalez A, Zak PJ, 2013. Effects of a 10-day oxytocin trial in older adults on health and well-being. Exp. Clin. Psychopharmacol 21, 85–92. 10.1037/a0031581 [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ, 2010. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci. Ther 16, e92–123. 10.1111/j.1755-5949.2010.00154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia B, Heinrichs M, Bergmann W, Jung S, Germann J, Schedlowski M, Hartmann U, Kruger THC, 2014. Differential effects of intranasal oxytocin on sexual experiences and partner interactions in couples. Horm. Behav 65, 308–318. 10.1016/J.YHBEH.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Bell AF, Erickson EN, Carter CS, 2014. Beyond labor: the role of natural and synthetic oxytocin in the transition to motherhood. J. Midwifery Womens. Health 59, 35–42: quiz 108. 10.1111/jmwh.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaerts S, Prinsen J, Berra E, Bosmans G, Steyaert J, Alaerts K, 2017. Long-term oxytocin administration enhances the experience of attachment. Psychoneuroendocrinology 78, 1–9. 10.1016/j.psyneuen.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Bethlehem RAI, van Honk J, Auyeung B, Baron-Cohen S, 2013. Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 38, 962–974. 10.1016/j.psyneuen.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG, Havel PJ, 2015. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am. J. Physiol. - Regul. Integr. Comp. Physiol 308, R431–R438. 10.1152/ajpregu.00441.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Thompson BW, Anekonda VT, Ho JM, Graham JL, Roberts ZS, Hwang BH, Ogimoto K, Wolden-Hanson T, Nelson J, Kaiyala KJ, Havel PJ, Bales KL, Morton GJ, Schwartz MW, Baskin DG, 2016. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am. J. Physiol. Integr. Comp. Physiol 310, R640–R658. 10.1152/ajpregu.00220.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer GJ, 1993. Chronic oxytocin treatment during late gestation and lactation impairs development of rat offspring. Neurotoxicol. Teratol 15, 383–389. 10.1016/0892-0362(93)90055-S [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS, 2011. Adolescent Oxytocin Exposure Causes Persistent Reductions in Anxiety and Alcohol Consumption and Enhances Sociability in Rats. PLoS One 6, e27237 10.1371/journal.pone.0027237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley ER, Woolley JD, 2017. Oxytocin effects in schizophrenia: Reconciling mixed findings and moving forward. Neurosci. Biobehav. Rev 80, 36–56. 10.1016/j.neubiorev.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Kelly DL, Weiner E, Gold JM, Strauss GP, Koola MM, McMahon RP, Carpenter WT, 2017. A Randomized Clinical Trial of Oxytocin or Galantamine for the Treatment of Negative Symptoms and Cognitive Impairments in People With Schizophrenia. J. Clin. Psychopharmacol 37, 394–400. 10.1097/JCP.0000000000000720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri A, Heinrichs M, Schedlowski M, Kruger THC, 2008. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology 33, 591–600. 10.1016/J.PSYNEUEN.2008.01.014 [DOI] [PubMed] [Google Scholar]
- Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, Moore L, Alvares GA, Redoblado Hodge MA, Guastella AJ, 2015. A Double-Blind Randomized Controlled Trial of Oxytocin Nasal Spray and Social Cognition Training for Young People with Early Psychosis. Schizophr. Bull 41, 483–493. 10.1093/schbul/sbu094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnoli F, Kreutzmann JC, de Boer SF, Althaus M, Koolhaas JM, 2015. Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology 51, 112–121. 10.1016/j.psyneuen.2014.09.019 [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM, 2014. Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm. Behav 65, 427–433. 10.1016/j.yhbeh.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS, 2010. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamineseeking behaviour in rats. Neuropharmacology 58, 38–43. 10.1016/J.NEUROPHARM.2009.06.018 [DOI] [PubMed] [Google Scholar]
- Carter CS, 2003. Developmental consequences of oxytocin. Physiol. Behav 79, 383–397. 10.1016/S0031-9384(03)00151-3 [DOI] [PubMed] [Google Scholar]
- Carter CS, 1992. Oxytocin and sexual behavior. Neurosci. Biobehav. Rev 16, 131–144. 10.1016/S0149-7634(05)80176-9 [DOI] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML, 2012. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl. Acad. Sci. U. S. A 109, 959–964. 10.1073/pnas.1114621109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G, 2005. ENDOCRINOLOGY OF THE STRESS RESPONSE. Annu. Rev. Physiol 67, 259–284. 10.1146/annurev.physiol.67.040403.120816 [DOI] [PubMed] [Google Scholar]
- Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A, 2008. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am. J. Physiol. - Endocrinol. Metab 295, E686–E691. 10.1152/ajpendo.90263.2008 [DOI] [PubMed] [Google Scholar]
- Constantine G, Graham S, Portman DJ, Rosen RC, Kingsberg SA, 2015. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric 18, 226–232. 10.3109/13697137.2014.954996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C, Deschner T, Ziegler TE, Wittig RM, 2014. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front. Behav. Neurosci 8, 68 10.3389/fnbeh.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J, 2014. Nasal oxytocin for social deficits in childhood autism: A randomized controlled trial. J. Autism Dev. Disord 44, 521–531. 10.1007/s10803-013-1899-3 [DOI] [PubMed] [Google Scholar]
- Dagani J, Sisti D, Abelli M, Di Paolo L, Pini S, Raimondi S, Rocchi MB, Saviotti FM, Scocco P, Totaro S, Balestrieri M, de Girolamo G, 2016. Do we need oxytocin to treat schizophrenia? A randomized clinical trial. Schizophr. Res 172, 158–164. 10.1016/j.schres.2016.02.011 [DOI] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB, 2014. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One 9, e103677 10.1371/journal.pone.0103677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier A-L, Petrosino S, Piscitelli F, Legros J-J, Geenen V, Foti M, Wahli W, Di Marzo V, Rohner-Jeanrenaud F, 2011. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One 6, e25565 10.1371/journal.pone.0025565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMayo MM, Song YJC, Hickie IB, Guastella AJ, 2017. A Review of the Safety, Efficacy and Mechanisms of Delivery of Nasal Oxytocin in Children: Therapeutic Potential for Autism and Prader-Willi Syndrome, and Recommendations for Future Research. Pediatr. Drugs 19, 391–410. 10.1007/s40272-017-0248-y [DOI] [PubMed] [Google Scholar]
- den Boer JA, Westenberg HG, 1992. Oxytocin in obsessive compulsive disorder. Peptides 13, 1083–1085. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries AC, 2004. Social facilitation of wound healing. Psychoneuroendocrinology 29, 1004–1011. 10.1016/j.psyneuen.2003.10.003 [DOI] [PubMed] [Google Scholar]
- DeVos SL, Miller TM, 2013. Direct intraventricular delivery of drugs to the rodent central nervous system. J. Vis. Exp e50326 10.3791/50326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE, 2003. Social modulation of stress responses. Physiol. Behav 79, 399–407. 10.1016/S0031-9384(03)00152-5 [DOI] [PubMed] [Google Scholar]
- Díaz-Cabiale Z, Petersson M, Narváez JA, Uvnäs-Moberg K, Fuxe K, 2000. Systemic oxytocin treatment modulates α2-adrenoceptors in telencephalic and diencephalic regions of the rat. Brain Res. 887, 421–425. 10.1016/S0006-8993(00)03017-1 [DOI] [PubMed] [Google Scholar]
- Du Vigneaud V, 1956. Trail of sulfur research: from insulin to oxytocin. Science (80-.). 123, 967–974. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, Cohen RA, 2016. Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology 69, 50–59. 10.1016/j.psyneuen.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Horta M, Lin T, Feifel D, Fischer H, Cohen RA, 2015. Oxytocin modulates meta-mood as a function of age and sex. Front. Aging Neurosci. 7, 175 10.3389/fnagi.2015.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Kamin H, Diaz V, Cohen RA, MacDonald K, 2014. Hormones as “difference makers” in cognitive and socioemotional aging processes. Front. Psychol 5, 1595 10.3389/fpsyg.2014.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Maura GM, Macdonald K, Westberg L, Fischer H, 2013. Oxytocin and socioemotional aging: Current knowledge and future trends. Front. Hum. Neurosci 7, 487 10.3389/fnhum.2013.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckertova M, Ondrejcakova M, Krskova K, Zorad S, Jezova D, 2011. Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis. Br. J. Pharmacol 162, 452–463. 10.1111/j.1476-5381.2010.01037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld SL, Smith E, Mcgregor IS, Steinbeck K, Taffe J, Rice LJ, Horstead SK, Rogers N, Hodge MA, Guastella AJ, 2014. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am. J. Med. Genet. Part A 164, 2232–2239. 10.1002/ajmg.a.36653 [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT, 2004. The hypothalamic–neurohypophysial system regulates the hypothalamic–pituitary–adrenal axis under stress: An old concept revisited. Front. Neuroendocrinol 25, 132–149. 10.1016/J.YFRNE.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Epperson CN, McDougle CJ, Price LH, 1996. Intranasal oxytocin in obsessive-compulsive disorder. Biol Psychiatry 40, 547–549. 10.1016/0006-3223(96)00120-5 [DOI] [PubMed] [Google Scholar]
- Erickson EN, Emeis CL, 2017. Breastfeeding Outcomes After Oxytocin Use During Childbirth: An Integrative Review. J. Midwifery Womens. Health 62, 397–417. 10.1111/jmwh.12601 [DOI] [PubMed] [Google Scholar]
- Feifel D, MacDonald K, Cobb P, Minassian A, 2012. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr. Res 139, 207–210. 10.1016/j.schres.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Feifel D, MacDonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A, 2010. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol. Psychiatry 68, 678–680. 10.1016/j.biopsych.2010.04.039 [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, MacDonald K, 2016. A Review of Oxytocin’s Effects on the Positive, Negative, and Cognitive Domains of Schizophrenia. Biol. Psychiatry 79, 222–233. 10.1016/j.biopsych.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, MacKinley J, Blair M, Oliver LD, Jesso S, Tartaglia MC, Borrie M, Wells J, Dziobek I, Pasternak S, Mitchell DGV, Rankin K, Kertesz A, Boxer A, 2015. Oxytocin for frontotemporal dementia: A randomized dose-finding study of safety and tolerability. Neurology 84, 174–81. 10.1212/WNL.0000000000001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Boll E, Martínez-Lorenzana G, Condés-Lara M, González-Hernández A, 2018. Oxytocin inhibits the rat medullary dorsal horn Sp5c/C1 nociceptive transmission through OT but not V 1A receptors. Neuropharmacology 129, 109–117. 10.1016/j.neuropharm.2017.11.031 [DOI] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA, 2014. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr. Res 156, 261–265. 10.1016/j.schres.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F, 2001. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev 81, 629–683. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Alexander JL, 2005. Practical Aspects in the Management of Vaginal Atrophy and Sexual Dysfunction in Perimenopausal and Postmenopausal Women. J. Sex. Med 2, 154–165. 10.1111/j.1743-6109.2005.00131.x [DOI] [PubMed] [Google Scholar]
- Goodin BR, Ness TJ, Robbins MT, 2015. Oxytocin - a multifunctional analgesic for chronic deep tissue pain. Curr. Pharm. Des 21, 906–913. 10.1016/j.biotechadv.2011.08.021.Secreted [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK, 2010. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology 35, 1082–1090. 10.1016/j.psyneuen.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen KM, Light KC, 2011. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol. Psychol 87, 340–349. 10.1016/j.biopsycho.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joëls M, 2012. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol. Cell. Endocrinol 350, 299–309. 10.1016/j.mce.2011.06.020 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, Keating CM, Cacciotti-Saija C, Einfeld SL, 2015. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: A randomized controlled trial. J. Child Psychol. Psychiatry 56, 444–452. 10.1111/jcpp.12305 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, 2016. Oxytocin Treatment, Circuitry, and Autism: A Critical Review of the Literature Placing Oxytocin Into the Autism Context. Biol. Psychiatry 79, 234–242. 10.1016/j.biopsych.2015.06.028 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan H, Chen TF, Banati RB, 2013. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38, 612–625. 10.1016/j.psyneuen.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C, 2012. A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Horm. Behav 61, 410–418. 10.1016/j.yhbeh.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Guoynes CD, Simmons TC, Downing GM, Jacob S, Solomon M, Bales KL, 2018. Chronic Intranasal Oxytocin has Dose-dependent Effects on Central Oxytocin and Vasopressin Systems in Prairie Voles (Microtus ochrogaster). Neuroscience 369, 292–302. 10.1016/j.neuroscience.2017.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M, 2012. Oxytocin Revisited: Its Role in Cardiovascular Regulation. J. Neuroendocrinol 24, 599–608. 10.1111/j.1365-2826.2011.02235.x [DOI] [PubMed] [Google Scholar]
- Havranek T, Zatkova M, Lestanova Z, Bacova Z, Mravec B, Hodosy J, Strbak V, Bakos J, 2015. Intracerebroventricular oxytocin administration in rats enhances object recognition and increases expression of neurotrophins, microtubule-associated protein 2, and synapsin I. J. Neurosci. Res 93, 893–901. 10.1002/jnr.23559 [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U, 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 54, 1389–1398. 10.1016/S0006-3223(03)00465-7 [DOI] [PubMed] [Google Scholar]
- Holst S, Uvnäs-Moberg K, Petersson M, 2002. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Auton. Neurosci 99, 85–90. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni ML, Papaleo F, 2014. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 39, 1102–14. 10.1038/npp.2013.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffmeijer R, van Ijzendoorn MH, Bakermans-Kranenburg MJ, 2013. Ageing and oxytocin: A call for extending human oxytocin research to ageing populations--a mini-review. Gerontology 59, 32–39. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2010. The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron 65, 768–779. 10.1016/j.neuron.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IsHak WW, Berman DS, Peters A, 2008. Male Anorgasmia Treated with Oxytocin. J. Sex. Med 5, 1022–1024. 10.1111/J.1743-6109.2007.00691.X [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Maejima Y, Suyama S, Yoshida M, Arai T, Katsurada K, Kumari P, Nakabayashi H, Kakei M, Yada T, 2015. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am. J. Physiol. Integr. Comp. Physiol 308, R360–R369. 10.1152/ajpregu.00344.2014 [DOI] [PubMed] [Google Scholar]
- Janezic EM, Uppalapati S, Nagl S, Contreras M, French ED, Fellous J-M, 2016. Beneficial effects of chronic oxytocin administration and social co-housing in a rodent model of post-traumatic stress disorder. Behav. Pharmacol 27, 704–717. 10.1097/FBP.0000000000000270 [DOI] [PubMed] [Google Scholar]
- Jankowski M, Bissonauth V, Gao L, Gangal M, Wang D, Danalache B, Wang Y, Stoyanova E, Cloutier G, Blaise G, Gutkowska J, 2010. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res. Cardiol 105, 205–218. 10.1007/s00395-0090076-5 [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Pedersen CA, Johnson JL, Hamer RM, Rau SW, Elliott T, Penn DL, 2017. A 12-week randomized controlled trial of twice-daily intranasal oxytocin for social cognitive deficits in people with schizophrenia. Schizophr. Res 185, 88–95. 10.1016/j.schres.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesso S, Morlog D, Ross S, Pell MD, Pasternak SH, Mitchell DGV, Kertesz A, Finger EC, 2011. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain 134, 2493–2501. 10.1093/brain/awr171 [DOI] [PubMed] [Google Scholar]
- Kingsberg SA, 2002. The Impact of Aging on Sexual Function in Women and Their Partners. Arch. Sex. Behav 31, 431–437. 10.1023/A:1019844209233 [DOI] [PubMed] [Google Scholar]
- Kosaka H, Okamoto Y, Munesue T, Yamasue H, Inohara K, Fujioka T, Anme T, Orisaka M, Ishitobi M, Jung M, Fujisawa TX, Tanaka S, Arai S, Asano M, Saito DN, Sadato N, Tomoda A, Omori M, Sato M, Okazawa H, Higashida H, Wada Y, 2016. Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: a 24-week randomized clinical trial. Transl. Psychiatry 6, e872 10.1038/tp.2016.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács GL, Borthaiser Z, Telegdy G, 1985a. Oxytocin reduces intravenous heroin self-administration in heroin-tolerant rats. Life Sci. 37, 17–26. 10.1016/0024-3205(85)90620-4 [DOI] [PubMed] [Google Scholar]
- Kovács GL, Faludi M, Telegdy G, 1985b. Oxytocin diminishes heroin tolerance in mice. Psychopharmacology (Berl). 86, 377–379. 10.1007/BF00432233 [DOI] [PubMed] [Google Scholar]
- Kriván M, Szabó G, Sarnyai Z, Kovács GL, Telegdy G, 1992. Oxytocin blocks the development of heroin-enkephalin cross-tolerance in mice. Pharmacol. Biochem. Behav 43, 187–192. 10.1016/0091-3057(92)90656-Z [DOI] [PubMed] [Google Scholar]
- Kumsta R, Heinrichs M, 2013. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Curr. Opin. Neurobiol 23, 11–16. 10.1016/J.CONB.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Kuppens RJ, Donze SH, Hokken-Koelega ACS, 2016. Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial. Clin. Endocrinol. (Oxf) 85, 979–987. 10.1111/cen.13169 [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID, 2004. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol 25, 150–176. 10.1016/j.yfrne.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Lawson EA, 2017. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol 1–10. 10.1038/nrendo.2017.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, Anderson GM, Riddle MA, McDougle CJ, Barr LC, Cohen DJ, 1994. The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology 19, 723–49. 10.1016/0306-4530(94)90021-3 [DOI] [PubMed] [Google Scholar]
- Lee MR, Rohn MCH, Tanda G, Leggio L, 2016a. Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date. CNS Drugs 30, 109–23. 10.1007/s40263-016-0313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Wehring HJ, McMahon RP, Linthicum J, Cascella N, Liu F, Bellack A, Buchanan RW, Strauss GP, Contoreggi C, Kelly DL, 2013. Effects of adjunctive intranasal oxytocin on olfactory identification and clinical symptoms in schizophrenia: results from a randomized double blind placebo controlled pilot study. Schizophr. Res 145, 110–115. 10.1016/j.schres.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Wehring HJ, Mcmahon RP, Liu F, Linthicum J, Verbalis JG, Buchanan RW, Strauss GP, Rubin LH, Kelly DL, 2016b. Relationship of Plasma Oxytocin Levels to Baseline Symptoms and Symptom Changes during Three Weeks of Daily Oxytocin Administration in People with Schizophrenia. Schizophr. Res 172, 165–168. 10.1016/j.schres.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Lee AR, Hwangbo R, Han J, Hong M, Bahn GH, 2015. Is Oxytocin Application for Autism Spectrum Disorder Evidence-Based? Exp. Neurobiol 24, 312–24. 10.5607/en.2015.24.4.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Sirigu A, 2016. The two fold role of oxytocin in social developmental disorders: A cause and a remedy? Neurosci. Biobehav. Rev 63, 168–176. 10.1016/j.neubiorev.2016.01.011 [DOI] [PubMed] [Google Scholar]
- Leffa DD, Daumann F, Damiani AP, Afonso AC, Santos MA, Pedro TH, Souza RP, Andrade VM, 2017. DNA damage after chronic oxytocin administration in rats: a safety yellow light? Metab. Brain Dis 32, 51–55. 10.1007/s11011-016-9885-z [DOI] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ, 2011. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology 36, 1114–1126. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Feifel D, 2013. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front. Neurosci 7, 35 10.3389/fnins.2013.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, Feifel D, 2014. Oxytocin’s role in anxiety: A critical appraisal. Brain Res. 1580, 22–56. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D, 2012. Dramatic Improvement in Sexual Function Induced by Intranasal Oxytocin. J. Sex. Med 9, 1407–1410. 10.1111/J.1743-6109.2012.02703.X [DOI] [PubMed] [Google Scholar]
- MacDonald K, Macdonald TM, 2010. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv. Rev. Psychiatry 18, 1–21. 10.3109/10673220903523615 [DOI] [PubMed] [Google Scholar]
- Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T, 2011. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany. NY) 3, 1169–77. 10.18632/aging.100408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli S, Pisanu GM, Sardo S, Marchi A, Pili A, Carboni M, Minerba L, Trincas G, Carta MG, Melis MR, Agabio R, 2014. Oxytocin nasal spray in fibromyalgic patients. Rheumatol. Int 34, 1047–1052. 10.1007/s00296-014-2953-y [DOI] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wimersma Greidanus TB, 1983. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 262, 143–9. 10.1016/0006-8993(83)90478-x [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M, 2011. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci 12, 524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Miller JL, Tamura R, Butler MG, Kimonis V, Sulsona C, Gold JA, Driscoll DJ, 2017. Oxytocin treatment in children with Prader–Willi syndrome: A double-blind, placebo-controlled, crossover study. Am. J. Med. Genet. Part A 173, 1243–1250. 10.1002/ajmg.a.38160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SMR, Tajdini M, Ghaleiha A, Akhondzadeh S, 2013. Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia: An 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs 27, 57–65. 10.1007/s40263-012-0022-1 [DOI] [PubMed] [Google Scholar]
- Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA, 2014. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology 45, 49–57. 10.1016/j.psyneuen.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE, 2012. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab 302, E134–44. 10.1152/ajpendo.00296.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munesue T, Nakamura H, Kikuchi M, Miura Y, Takeuchi N, Anme T, Nanba E, Adachi K, Tsubouchi K, Sai Y, Miyamoto KI, Horike SI, Yokoyama S, Nakatani H, Niida Y, Kosaka H, Minabe Y, Higashida H, 2016. Oxytocin for male subjects with autism spectrum disorder and comorbid intellectual disabilities: A randomized pilot study. Front. Psychiatry 7, 1–11. 10.3389/fpsyt.2016.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA, 2016. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiatry 79, 213–221. 10.1016/J.BIOPSYCH.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Nielsen EI, Al-Saqi SH, Jonasson AF, Uvnäs-Moberg K, 2017. Population Pharmacokinetic Analysis of Vaginally and Intravenously Administered Oxytocin in Postmenopausal Women. J. Clin. Pharmacol 57, 1573–1581. 10.1002/jcph.961 [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Truedsson M, Bengtsson M, Torstenson R, Sjölund K, Björnsson ES, Simrèn M, 2005. Effects of long-term treatment with oxytocin in chronic constipation; a double blind, placebo-controlled pilot trial. Neurogastroenterol. Motil 17, 697–704. 10.1111/j.1365-2982.2005.00679.x [DOI] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M, 2013. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 38, 1883–1894. 10.1016/J.PSYNEUEN.2013.06.019 [DOI] [PubMed] [Google Scholar]
- Ota M, Yoshida S, Nakata M, Yada T, Kunugi H, 2017. The effects of adjunctive intranasal oxytocin in patients with schizophrenia. Postgrad. Med 1–7. 10.1080/00325481.2018.1398592 [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM, 2005. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology 30, 924–929. 10.1016/j.psyneuen.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL, 2011. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr. Res 132, 50–53. 10.1016/j.schres.2011.07.027 [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC, 2013. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol. Clin. Exp. Res 37, 484–489. 10.1111/j.1530-0277.2012.01958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID, 2014. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 42, 225–236. 10.1016/j.psyneuen.2014.01.021 [DOI] [PubMed] [Google Scholar]
- Petersson M, 2002. Oxytocin decreases plasma levels of thyroid-stimulating hormone and thyroid hormones in rats. Regul. Pept 108, 83–7. [DOI] [PubMed] [Google Scholar]
- Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K, 1996a. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol. Behav 60, 1311–5. [DOI] [PubMed] [Google Scholar]
- Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K, 1996b. Oxytocin increases nociceptive thresholds in a long-term perspective in female and male rats. Neurosci. Lett 212, 87–90. 10.1016/0304-3940(96)12773-7 [DOI] [PubMed] [Google Scholar]
- Petersson M, Diaz-Cabiale Z, Angel Narváez J, Fuxe K, Uvnäs-Moberg K, 2005a. Oxytocin increases the density of high affinity α2-adrenoceptors within the hypothalamus, the amygdala and the nucleus of the solitary tract in ovariectomized rats. Brain Res. 1049, 234–239. 10.1016/j.brainres.2005.05.034 [DOI] [PubMed] [Google Scholar]
- Petersson M, Eklund M, Uvnäs-Moberg K, 2005b. Oxytocin decreases corticosterone and nociception and increases motor activity in OVX rats. Maturitas 51, 426–433. 10.1016/j.maturitas.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Petersson M, Hulting A-L, Uvnäs-Moberg K, 1999a. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neurosci. Lett 264, 41–44. 10.1016/S0304-3940(99)00159-7 [DOI] [PubMed] [Google Scholar]
- Petersson M, Hulting A, Andersson R, Uvnäs-Moberg K, 1999b. Long-term changes in gastrin, cholecystokinin and insulin in response to oxytocin treatment. Neuroendocrinology 69, 202–8. 10.1159/000054420 [DOI] [PubMed] [Google Scholar]
- Petersson M, Lundeberg T, Uvnäs-Moberg K, 1999c. Short-term increase and long-term decrease of blood pressure in response to oxytocin-potentiating effect of female steroid hormones. J. Cardiovasc. Pharmacol 33, 102–108. [DOI] [PubMed] [Google Scholar]
- Petersson M, Lundeberg T, Uvnäs-Moberg K, 1999d. Oxytocin enhances the effects of clonidine on blood pressure and locomotor activity in rats. J. Auton. Nerv. Syst 78, 49–56. 10.1016/S0165-1838(99)00061-2 [DOI] [PubMed] [Google Scholar]
- Petersson M, Uvnäs-Moberg K, 2008. Postnatal oxytocin treatment of spontaneously hypertensive male rats decreases blood pressure and body weight in adulthood. Neurosci. Lett 440, 166–169. 10.1016/j.neulet.2008.05.091 [DOI] [PubMed] [Google Scholar]
- Petersson M, Uvnäs-Moberg K, 2003. Systemic oxytocin treatment modulates glucocorticoid and mineralocorticoid receptor mRNA in the rat hippocampus. Neurosci. Lett 343, 97–100. 10.1016/S0304-3940(03)00334-3 [DOI] [PubMed] [Google Scholar]
- Pisansky MT, Hanson LR, Gottesman II, Gewirtz JC, 2017. Oxytocin enhances observational fear in mice. Nat. Commun 8, 2102 10.1038/s41467-017-02279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante E, Menaouar A, Danalache BA, Yip D, Broderick TL, Chiasson J-L, Jankowski M, Gutkowska J, 2015. Oxytocin Treatment Prevents the Cardiomyopathy Observed in Obese Diabetic Male db/db Mice. Endocrinology 156, 1416–1428. 10.1210/en.2014-1718 [DOI] [PubMed] [Google Scholar]
- Possin KL, Feigenbaum D, Rankin KP, Smith GE, Boxer AL, Wood K, Hanna SM, Miller BL, Kramer JH, 2013. Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology 80, 2180–2185. 10.1212/WNL.0b013e318296e940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost M, Zelkowitz P, Tulandi T, Hayton B, Feeley N, Carter CS, Joseph L, Pournajafi-Nazarloo H, Yong Ping E, Abenhaim H, Gold I, 2014. Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front. Public Heal. 2, 1 10.3389/fpubh.2014.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Alvares GA, Hickie IB, Guastella AJ, 2015a. Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2014.12.011 [DOI] [PubMed] [Google Scholar]
- Quintana DS, Dieset I, Elvsåshagen T, Westlye LT, Andreassen OA, 2017. Oxytocin system dysfunction as a common mechanism underlying metabolic syndrome and psychiatric symptoms in schizophrenia and bipolar disorders. Front. Neuroendocrinol 45, 1–10. 10.1016/J.YFRNE.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Quintana DS, Rokicki J, van der Meer D, Alnæs D, Kaufmann T, Córdova-Palomera A, Dieset I, Andreassen OA, Westlye LT, 2019. Oxytocin pathway gene networks in the human brain. Nat. Commun 10, 668 10.1038/s41467-019-08503-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Smerud KT, Andreassen OA, Djupesland PG, 2018. Evidence for intranasal oxytocin delivery to the brain: recent advances and future perspectives. Ther. Deliv 9, 515–525. 10.4155/tde-2018-0002 [DOI] [PubMed] [Google Scholar]
- Quintana DS, Westlye LT, Alnæs D, Rustan ØG, Kaufmann T, Smerud KT, Mahmoud RA, Djupesland PG, Andreassen OA, 2016. Low dose intranasal oxytocin delivered with Breath Powered device dampens amygdala response to emotional stimuli: A peripheral effect-controlled within-subjects randomized dose-response fMRI trial. Psychoneuroendocrinology 69, 180–188. 10.1016/j.psyneuen.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Quintana DS, Westlye LT, Rustan ØG, Tesli N, Poppy CL, Smevik H, Tesli M, Røine M, Mahmoud RA, Smerud KT, Djupesland PG, Andreassen OA, 2015b. Low-dose oxytocin delivered intranasally with Breath Powered device affects social-cognitive behavior: a randomized four-way crossover trial with nasal cavity dimension assessment. Transl. Psychiatry 5, e602–e602. 10.1038/tp.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JA, Aguirre-Camacho A, Campbell TS, 2014. Oxytocin and Pain: A Systematic Review and Synthesis of Findings. Clin. J. Pain 00, 1–10. 10.1097/AJP.0b013e31829f57df [DOI] [PubMed] [Google Scholar]
- Rault J-L, Carter CS, Garner JP, Marchant-Forde JN, Richert BT, Lay DC, 2013. Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol. Behav 112–113, 40–48. 10.1016/j.physbeh.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Roberts ZS, Wolden-Hanson T, Matsen ME, Ryu V, Vaughan CH, Graham JL, Havel PJ, Chukri DW, Schwartz MW, Morton GJ, Blevins JE, 2017. Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am. J. Physiol. Integr. Comp. Physiol 313, R357–R371. 10.1152/ajpregu.00169.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roney JR, 2016. Theoretical frameworks for human behavioral endocrinology. Horm. Behav 84, 97–110. 10.1016/J.YHBEH.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Russell J, Maguire S, Hunt GE, Kesby A, Suraev A, Stuart J, Booth J, McGregor IS, 2018. Intranasal oxytocin in the treatment of anorexia nervosa: Randomized controlled trial during re-feeding. Psychoneuroendocrinology 87, 83–92. 10.1016/j.psyneuen.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Saria S, Goldenberg A, 2015. Subtyping: What It is and Its Role in Precision Medicine. IEEE Intell. Syst 30, 70–75. 10.1109/MIS.2015.60 [DOI] [Google Scholar]
- Sarnyai Z, Bíró É, Babarczy E, Vecsernyés M, Laczi F, Szabó G, Kriván M, Kovács GL, Telegdy G, 1992a. Oxytocin modulates behavioural adaptation to repeated treatment with cocaine in rats. Neuropharmacology 31, 593–598. 10.1016/0028-3908(92)90192-R [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovács GL, 2014. Oxytocin in learning and addiction: From early discoveries to the present. Pharmacol. Biochem. Behav 119, 3–9. 10.1016/j.pbb.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Szabó G, Kovács GL, Telegdy G, 1992b. Opposite actions of oxytocin and vasopressin in the development of cocaine-induced behavioral sensitization in mice. Pharmacol. Biochem. Behav 43, 491–494. 10.1016/0091-3057(92)90182-F [DOI] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Geenen V, Legros JJ, Ansseau M, 2015. Additional intranasal oxytocin to escitalopram improves depressive symptoms in resistant depression: An open trial. Eur. Psychiatry 30, 65–68. 10.1016/j.eurpsy.2014.08.007 [DOI] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, Guastella AJ, 2013. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology 38, 1929–1936. 10.1038/npp.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A, 2016. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202. 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Shilling PD, Feifel D, 2016. Potential of Oxytocin in the Treatment of Schizophrenia. CNS Drugs 30, 193–208. 10.1007/s40263-016-0315-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID, 2010. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology 58, 56–61. 10.1016/j.neuropharm.2009.06.038 [DOI] [PubMed] [Google Scholar]
- Smith JG, Merrill DC, 2006. Oxytocin for induction of labor. Clin. Obstet. Gynecol 49, 594–608. [DOI] [PubMed] [Google Scholar]
- Stauffer CS, Musinipally V, Suen A, Lynch KL, Shapiro B, Woolley JD, 2016. A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addict. Res. Theory 24, 490–498. 10.3109/16066359.2016.1173682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R, 2013. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep 3, 3440 10.1038/srep03440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó G, Kovács GL, Székeli S, Telegdy G, 1985. The effects of neurohypophyseal hormones on tolerance to the hypothermic effect of ethanol. Alcohol 2, 567–574. 10.1016/0741-8329(85)90082-5 [DOI] [PubMed] [Google Scholar]
- Szabó G, Kovács GL, Telegdy G, 1987. Effects of neurohypophyseal peptide hormones on alcohol dependence and withdrawal. Alcohol Alcohol 22, 71–4. [PubMed] [Google Scholar]
- Szeto A, Rossetti MA, Mendez AJ, Noller CM, Herderick EE, Gonzales JA, Schneiderman N, McCabe PM, 2013. Oxytocin administration attenuates atherosclerosis and inflammation in Watanabe Heritable Hyperlipidemic rabbits. Psychoneuroendocrinology 38, 685–693. 10.1016/j.psyneuen.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taati M, Tamaddonfard E, 2018. Ventrolateral orbital cortex oxytocin attenuates neuropathic pain through periaqueductal gray opioid receptor. Pharmacol. Reports 70, 577–583. 10.1016/J.PHAREP.2017.12.010 [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, Oishi M, Kimura T, Onaka T, Ozono K, Taniike M, 2013. Long-Term Administration of Intranasal Oxytocin Is a Safe and Promising Therapy for Early Adolescent Boys with Autism Spectrum Disorders. J. Child Adolesc. Psychopharmacol 23, 123–127. 10.1089/cap.2012.0048 [DOI] [PubMed] [Google Scholar]
- Tauber M, Boulanouar K, Diene G, Çabal-Berthoumieu S, Ehlinger V, Fichaux-Bourin P, Molinas C, Faye S, Valette M, Pourrinet J, Cessans C, Viaux-Sauvelon S, Bascoul C, Guedeney A, Delhanty P, Geenen V, Martens H, Muscatelli F, Cohen D, Consoli A, Payoux P, Arnaud C, Salles J-P, 2017. The use of oxytocin to improve feeding and social skills in infants with Prader–Willi Syndrome. Pediatrics 139, e20162976 10.1542/peds.2016-2976 [DOI] [PubMed] [Google Scholar]
- Teng BL, Nikolova VD, Riddick NV, Agster KL, Crowley JJ, Baker LK, Koller BH, Pedersen CA, Jarstfer MB, Moy SS, 2016. Reversal of social deficits by subchronic oxytocin in two autism mouse models. Neuropharmacology 105, 61–71. 10.1016/j.neuropharm.2015.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, Baker LK, Pedersen CA, Jarstfer MB, Moy SS, 2013. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology 72, 187–96. 10.1016/j.neuropharm.2013.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Jodogne C, Legros J-J, 1992. Oxytocin blocks the environmentally conditioned compensatory response present after tolerance to ethanol-induced hypothermia in mice. Pharmacol. Biochem. Behav 43, 1263–1267. 10.1016/0091-3057(92)90512-E [DOI] [PubMed] [Google Scholar]
- Torky HA, Taha A, Marie H, El-Desouky E, Raslan O, Moussa AA, Ahmad AM, Abo-Louz A, Zaki S, Fares T, Eesa A, 2018. Role of topical oxytocin in improving vaginal atrophy in postmenopausal women: a randomized, controlled trial. Climacteric 21, 174–178. 10.1080/13697137.2017.1421924 [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Alster P, Petersson M, 1996. Dissociation of oxytocin effects on body weight in two variants of female Sprague-Dawley rats. Integr. Physiol. Behav. Sci 31, 44–55. 10.1007/BF02691480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Alster P, Petersson M, Sohlström A, Björkstrand E, 1998. Postnatal Oxytocin Injections Cause Sustained Weight Gain and Increased Nociceptive Thresholds in Male and Female Rats. Pediatr. Res 43, 344–348. 10.1203/00006450-199803000-00006 [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Frijling JL, Nawijn L, Koch SBJ, Goslings JC, Luitse JS, Biesheuvel TH, Honig A, Veltman DJ, Olff M, 2017. Intranasal Oxytocin to Prevent Posttraumatic Stress Disorder Symptoms: A Randomized Controlled Trial in Emergency Department Patients. Biol. Psychiatry 81, 1030–1040. 10.1016/j.biopsych.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Vargas-Martínez F, Uvnäs-Moberg K, Petersson M, Olausson HA, Jiménez-Estrada I, 2014. Neuropeptides as neuroprotective agents: Oxytocin a forefront developmental player in the mammalian brain. Prog. Neurobiol 123, 37–78. 10.1016/j.pneurobio.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, Takao H, Nippashi Y, Kawakubo Y, Kunimatsu A, Kasai K, Yamasue H, 2015. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 138, 3400–3412. 10.1093/brain/awv249 [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD, 2004. Oxytocin Attenuates Stress-Induced c-fos mRNA Expression in Specific Forebrain Regions Associated with Modulation of Hypothalamo-Pituitary-Adrenal Activity. J. Neurosci 24, 2974–2982. 10.1523/JNEUROSCI.3432-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD, 1997. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 138, 2829–34. 10.1210/endo.138.7.5255 [DOI] [PubMed] [Google Scholar]
- Yamasue H, 2016. Promising evidence and remaining issues regarding the clinical application of oxytocin in autism spectrum disorders. Psychiatry Clin. Neurosci 70, 89–99. 10.1111/pcn.12364 [DOI] [PubMed] [Google Scholar]
- Yang J, 1994. Intrathecal administration of oxytocin induces analgesia in low back pain involving the endogenous opiate peptide system. Spine (Phila. Pa. 1976). 19, 867–871. [DOI] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ, 2016. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol. Psychiatry 21, 1225–1231. 10.1038/mp.2015.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, 2013. When Too Much of a Good Thing is Bad: Chronic Oxytocin, Development, and Social Impairments. Biol. Psychiatry 74, 160–161. 10.1016/j.biopsych.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, Cai D, 2013. Treatment of Obesity and Diabetes Using Oxytocin or Analogs in Patients and Mouse Models. PLoS One 8, 1–11. 10.1371/journal.pone.0061477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-M, An J, 2007. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin 45, 27–37. 10.1097/AIA.0b013e318034194e [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.