Abstract
Bone marrow (BM) angiotensin II is a major participant in the regulation of hematopoiesis and immunity. The novel tissue substrate angiotensin-(1–12) and its cleaving enzyme chymase are an essential source of angiotensin II production in cardiac tissue. We hypothesized this non-canonical chymase-mediated angiotensin II producing mechanism exists in the BM tissue. Immunohistostaining and flow cytometry confirmed the presence of angiotensin-(1–12) immunoreaction in the BM of Sprague Dawley rats. Chymase-mediated angiotensin II producing activity in BM was approximately 1,000 fold higher than ACE-mediated angiotensin II producing activity (4,531 ± 137 and 4.2 ± 0.3 fmol/min/mg, respectively, n = 6, P < 0.001) and 280 fold higher than chymase activity in the left ventricle of 16.3 ± 1.7 fmol/min/mg (P < 0.001). Adding a selective chymase inhibitor, TEI-F00806, eliminated almost all 125I-angiotensin II production. Flow cytometry demonstrated that delta median fluorescence intensity of chymase in CD68 positive cells was significantly higher than that in CD68 negative cells (1,546 ± 157 and 222 ± 48 arbitrary unit, respectively. P=0.0021). CD68 positive and side scatter low subsets, considered to be myeloid progenitors, express the highest chymase fluorescence intensity in rat BM. Chymase activity and cellular expression was similar in both male and female rats. In conclusion, myeloid lineage cells, especially myeloid progenitors, have an extraordinary angiotensin II producing activity by chymase in the BM.
Keywords: Chymase; angiotensin converting enzyme; angiotensin-(1–12); bone marrow; CD68, hypertension; inflammation
Summary
Our data highlights an exceedingly high Ang II producing activity by chymase in BM. CD68 positive myeloid lineage cells, especially myeloid progenitors are the main source of chymase in BM. Assessing the relationship between cardiovascular diseases and chymase-expressing BM-derived cells in target organs represents a new window for understanding how tissue-borne Ang II contributes to the early inflammatory processes now linked to the pathogenesis of cardiovascular disease.
Bone marrow (BM) derived cells intervene in the temporal and spatial expression of proteins regulating cardiac and vascular health and repair as a critical component of the low-grade inflammatory mechanisms contributing to cardiovascular and renal disease pathogenesis.1–4 Restorative properties of BM progenitors and mesenchymal stem cells on cardiovascular function are called on by the myocardial remodeling brought about by myocardial ischemia,5 the development of atherosclerotic plaques,6 heart failure progression,7 and the evolution of kidney disease.8,9 The renin angiotensin system (RAS), inextricably linked to the pathogenesis of cardiovascular disease, has a major influence in hematopoiesis10,11 and tissue regeneration by progenitor cells.12 Early reports linking high-dose angiotensin converting enzyme (ACE) inhibitor therapy with the occurrence of anemia and leucopenia13,14 and visualization of immunoreactive angiotensin II (Ang II) in circulating human mononuclear leukocytes15 and macrophages residing in human atherosclerotic vessels,16 led to identifying the expression of RAS’s gene transcripts, enzymes, angiotensin peptides, and Ang II receptors in rat hematopoietic-lineage BM cells and cultured marrow stromal cells by Ferrario’s laboratory.17 In confirming the expression of Ang II-type 1 and 2 receptors (AT1-R and AT2-R, respectively) in monkey and human marrow stromal cells, we further showed an intrinsic paracrine/autocrine function of Ang II in modulation of hematopoietic cell development via activation of arachidonic acid and eicosanoid metabolites.18,19 BM-chimeric experiments revealed the functional importance of local BM Ang II expression in the development of experimentally-induced atherosclerosis.20 Chimeric mice with disruption of AT1-R21,22 or ACE deficiencies23 in BM-derived cells had reduced atherosclerotic lesions. More recently, Kim et al.24 reported that chronic Ang II infusion increased hematopoietic stem cell (HSC) proliferation, myeloid biased differentiation, and impaired efficacy of HSC engraftment, signifying that Ang II is important for mobilization of pro-inflammatory cells from the BM into target organs. The importance of the BM as a “Center Stage” participant in the pathogenesis of chronic cardiovascular disease has been articulated precisely by Swirski and Nahrendorf25 in reviewing Wang’s et al.26 studies showing how reduced lymphocyte adapter protein function in hematopoietic cells impacts the development of atherosclerosis and thrombosis. The interplay between the BM hematopoietic and progenitor cells with the processes engaged in the development of atherosclerosis were previously demonstrated by us in hypercholesterolemia cynomolgus monkeys in which 15 weeks of therapy with the AT1-R antagonist losartan reduced BM cellularity, suppressed peripheral blood and BM monocyte CD11b expression, and normalized CD34 positive cell function.27 Additional support for a critical role of the BM in the pathophysiology of hypertensive vascular disease has been demonstrated by Zubcevic et al.28,29 who showed that hypertension induces a dysfunction in the BM endothelial progenitor and inflammatory cells in spontaneously hypertensive rat.
Although the existence of a local system forming Ang II within the BM is beyond question, the primary enzymatic pathway accounting for BM Ang II production remains to be fully characterized. While renin, angiotensinogen (AGT) and ACE are reported in BM cells,10,11,17 their primacy as Ang II forming mechanism has not been resolved. In recent years, evidence of non-canonical pathways for tissue Ang II formation independent of renin and ACE has resurfaced as the potential mechanism explaining intracrine Ang II functions.30–32 Remerging concepts of non-renin Ang II production were brought to the forefront by the identification of an extended form of angiotensin I (Ang I)-the dodecapeptide angiotensin-(1–12) [Ang-(1–12)] in the blood and tissues of Wistar rats.33 Through a series of studies performed in rat and human heart and kidney tissues,33–40 Ang-(1–12) functions as an endogenous alternate tissue-forming system generating Ang II directly from Ang-(1–12) by chymase. However, reported observations that the BM expresses high chymase activity remain to be explained41 while chymase’s role in inflammation, immunity, and cardiovascular remodeling is established.42 In advancing the hypothesis that Ang-(1–12) may be the primary cellular substrate for intracrine actions of Ang II via chymase, the current study interrogated whether a non-canonical mechanism for Ang II production independent of renin exists in the BM. Unraveling the biochemical pathways for Ang II critical modulatory function in hematopoiesis will uncover novel therapeutic strategies for cardiovascular disease treatment.
Methods
Detailed protocols and supporting data are available within the article and in the online-only Data Supplement. In addition, the data that support the findings of this study are available from the corresponding author upon reasonable request.
Animal Protocol
Experiments were conducted on 20 male and 14 female Sprague Dawley (SD) (12 weeks old) obtained from Charles River Inc. (Wilmington, MA). All procedures were performed in accordance with National Institutes of Health guidelines and were approved by the Wake Forest University Animal Care and Use Committees. Details of protocols are described in the online-only Data Supplements.
Flow Cytometry
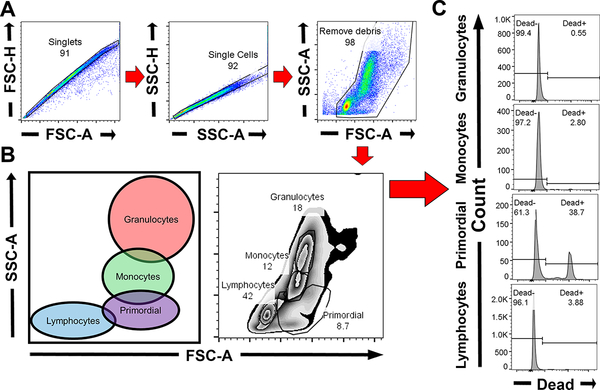
Figure 1 illustrates the gating strategy employed using flow cytometry. Doublets and debris were excluded by appropriate gating of forward (FSC) and side scatter (SSC) as documented in Figure 1A. Subpopulations of BM cells with common characteristics by FSC-area (FSC-A) and SSC-area (SSC-A) gating (Figure 1B) were grouped into four regions representing granulocytes, monocytes, primordial (region between lymphocytes and monocytes) and lymphocytes.43,44 After automatic fluorescent compensation with FlowJo software, dead cells were excluded from analysis with a fixable dead cell staining (Figure 1C).
Figure 1.
The gating strategy and four regions in flow cytometry analysis. A, Doublets and debris were removed. B, BM cells were divided into four regions: granulocytes, monocytes, primordial and lymphocytes. C, After fluorescence compensation, dead staining positive cells were removed.
Statistical Analysis
All values are expressed as mean ± SEM. Normality was examined by histogram and Shapiro-Wilk test. Comparison of chymase activity between BM and left ventricular tissue were analyzed by Welch’s t-test. Statistical significance between three or more groups was calculated by one-way ANOVA followed by Tukey’s post hoc test. One-way ANOVA and post-test for trend was performed to detect the increasing trends in the value of relative MFI. GraphPad PRISM Version 7.0 (GraphPad, San Diego, CA) was employed in these analyses and a P < 0.05 considered statistically significant.
Results
Ang-(1–12) Expression in the Bone Marrow
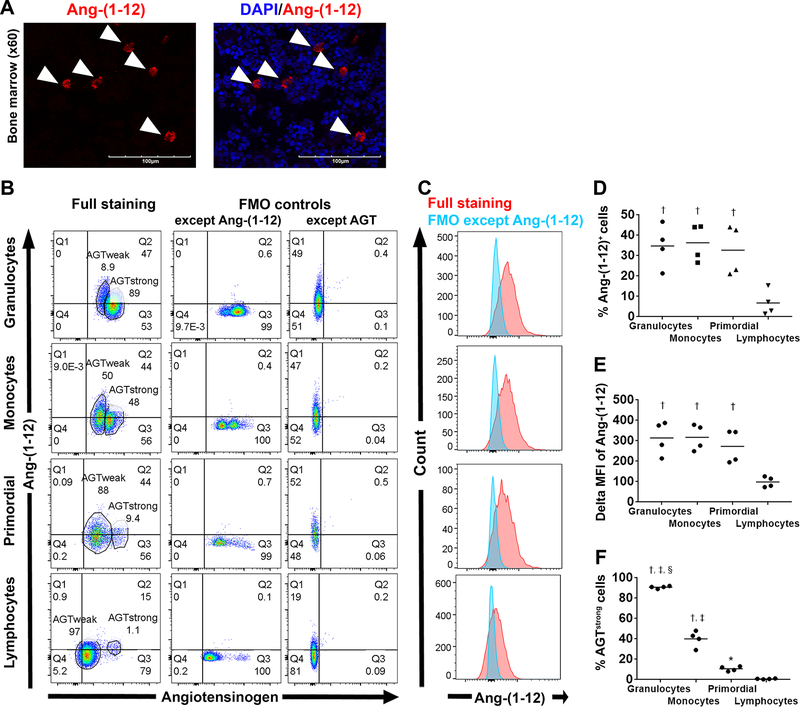
Laser scanning confocal microscopy visualized scattered Ang-(1–12) expressing cells in male rat BM tissue (Figure 2A). To exclude whether this anti-rat Ang-(1–12) antibody binds to amino acids within the N-terminus of AGT, we employed multi-color flow cytometry on single cell suspensions of BM stained with a mixture containing anti-AGT and anti-Ang-(1–12) antibodies. As expected, the gate determined by FMO controls illustrates the existence of Ang-(1–12) negative and AGT positive [Ang-(1–12)−AGT+] cells and that of Ang-(1–12)+AGT+ cells in each of the four regions (Figure 2B). These data show the absence of significant crossreactivity between the anti-rat Ang-(1–12) antibody and AGT.
Figure 2.
Ang-(1–12) expression in male SD rat bone marrow. A, Representative fluorescent photomicrographs (60× magnification) of BM tissue stained with a primary antibody against Ang-(1–12) (red, arrowhead). B-F, Flow cytometry analysis for intracellular staining of AGT and Ang-(1–12) in male SD rat BM (n = 4). Gating strategy is shown in Figure 1. B, Representative AGT/Ang-(1–12) plots in each region. Full staining includes dead cell staining, AGT and Ang-(1–12). C, Red illustrates Ang-(1–12) fluorescence intensity in cells with full staining while blue shows that with full staining except Ang-(1–12). Mean data are shown in D-F. * P<0.05 vs lymphocytes region, † P<0.001 vs lymphocyte region, ‡ P<0.01 vs primordial region, § P<0.001 vs monocyte region.
The percentage of Ang-(1–12) positive cells and delta MFI of Ang-(1–12) in cells found in the lymphocyte region were significantly lower than those in the other three regions. (Figure 2 C–E, ANOVA P<0.001). Furthermore, cells in each region could be divided into two subpopulations: cells having strong AGT intensity (AGTstrong) and those having weak AGT intensity (AGTweak). The percentage of AGTstrong cells increased progressively from the lymphocyte region to primordial, monocytes, and granulocytes regions (Figure 2F).
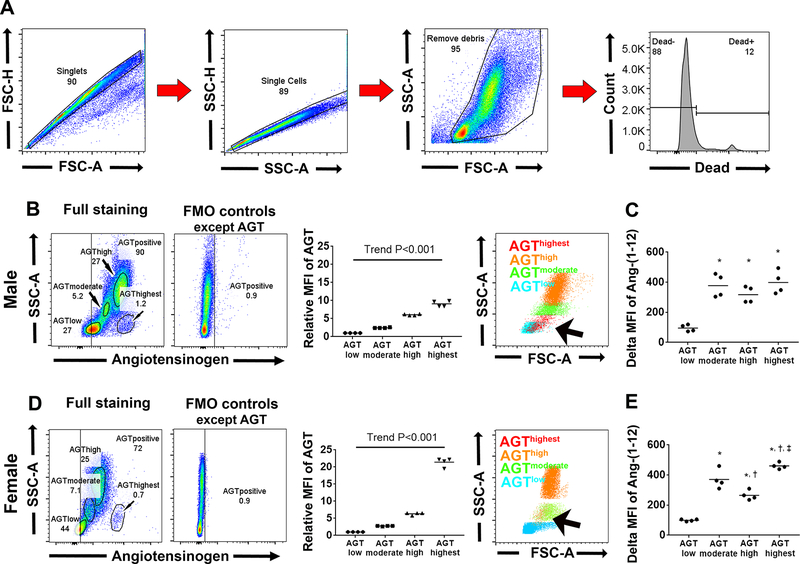
Cells in Primordial Region have the Highest Angiotensinogen Intensity in Bone Marrow
To evaluate which cell expressed more AGT, we analyzed whole BM single cell suspensions except doublet, debris and dead staining positive cells according to the gating strategy illustrated in Figure 3A. Four subsets were identified by AGT/SSC plots according to their AGT fluorescence intensity: AGThighest, AGThigh, AGTmoderate and AGTlow, and backgated onto FSC-A/SSC-A plot (Figure 3B). The AGThighest subset was visualized in the primordial region (red on Figure 3B, arrow) while the AGThigh and AGTmoderate subsets were identified in granulocytes (orange) and monocytes regions (green), respectively. The AGTlow subset was localized in the lymphocyte region (blue on Figure 3B); this region had significantly lower Ang-(1–12) intensity compared to other subsets (Figure 3C). No overt sex differences are found in flow cytometry findings (Figure 3D–E and Figure S1 in online-only Data Supplement).
Figure 3.
Back gating analysis for intracellular staining of AGT and Ang-(1–12) on flow cytometry. A, The employed gating strategy. B-C, Experiments conducted on male SD rats. B, Representative AGT/SSC-A plot and quantitative analysis divide whole BM cells into four subsets: AGTlow, AGTmoderate, AGThigh and AGThighest. Right panel shows subsets were backgated onto FSC-A/SSC-A plot. Arrow indicates AGThighest subset. C, Delta MFI of Ang-(1–12) in each subset. D-E, Experiments were repeated in female SD rats. * P<0.001 vs AGTlow, † P<0.05 vs AGTmoderate, ‡ P<0.001 vs AGThigh.
A Selective Chymase Inhibitor Eliminates Ang II Production in Bone Marrow Tissue
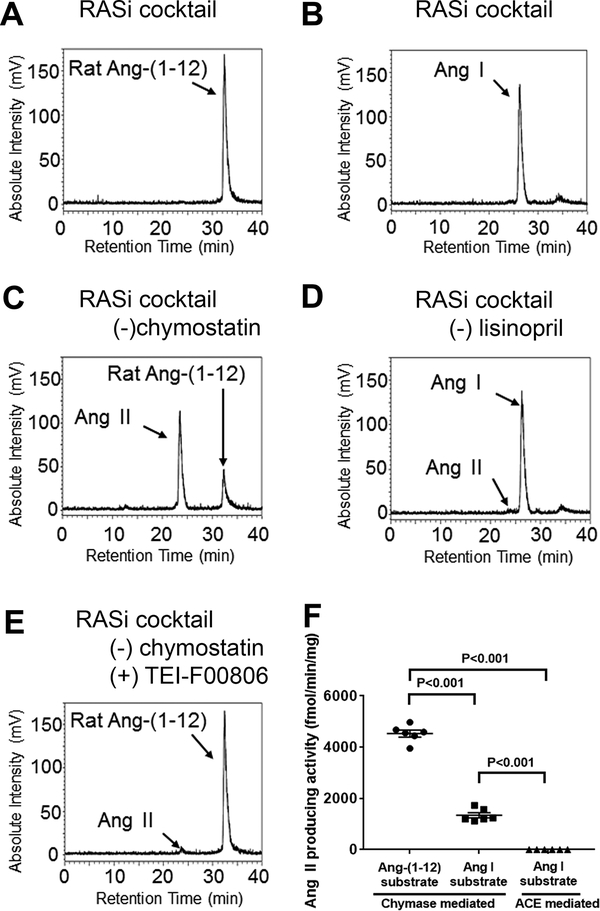
To determine whether chymase showed higher Ang II forming activity over ACE, radiolabeled 125I-Ang-(1–12) or 125I-Ang I substrate was incubated with plasma membranes obtained from BM tissues under different combinations of RAS inhibitors (Table S1); 125I-metabolic products were analyzed by HPLC. In the presence of all RAS inhibitors, no metabolic products were generated from either 125I-Ang-(1–12) or 125I-Ang I (Figure 4A and B, respectively). A large peak of 125I-Ang II was generated from the 125I-Ang-(1–12) substrate when chymostatin was withdrawn from the inhibitor cocktail (Figure 4C) while a tiny 125I-Ang II peak was detected from 125I-Ang I in the absence of lisinopril only (Figure 4D). The specificity of the chymostatin inhibitory activity was verified by demonstrating an almost complete inability of 125I-Ang-(1–12) to be degraded into 125I-Ang II when chymostatin was replaced by the selective chymase inhibitor TEI-F0806 (Figure 4E).45,46 BM plasma membranes chymase activity averaged 4,531 ± 137 fmol/min/mg when 125I-Ang-(1–12) was used as a substrate, which was 3.4 fold higher compared to 1,340 ± 101 fmol/min/mg when 125I-Ang I was the substrate (Figure 4F, n=6, P<0.001). These data show that Ang-(1–12) is the preferable substrate compared to Ang I. ACE activity in BM plasma membranes based on the amounts of 125I-Ang II production from 125I-Ang I substrate averaged 4.2 ± 0.3 fmol/min/mg. This value is approximately 1,000 fold lower than the corresponding chymase activity values employing 125I-Ang-(1–12) as a substrate (Figure 4F, n=6, P<0.001). More surprisingly, the BM chymase activity was approximately 280-fold higher than left ventricle chymase activity from 125I-Ang-(1–12) of 16.3 ± 1.7 fmol/min/mg in the same rats (P<0.001). In female rats, BM chymase activity averaged 4,295 ± 183 fmol/min/mg when 125I-Ang-(1–12) was used as a substrate, which was 3,800 fold higher than ACE activity averaging 1.14 ± 0.04 fmol/min/mg (Figure S2A–D illustrates the representative chromatograms, P<0.001).
Figure 4.
Representative chromatograms of 125I-angiotensin products from male BM tissue. A, 125I-Ang II metabolic products from 125I-Ang-(1–12) in the presence of all inhibitors. B, 125I-Ang II metabolic products from 125I-Ang I in the presence of all inhibitors. C, 125I-Ang II metabolic products from 125I-Ang-(1–12) in the presence of all inhibitors without chymostatin. D, 125I-Ang II metabolic products from 125I-Ang I in the presence of all inhibitors without lisinopril. E, 125I-Ang II metabolic products from 125I-Ang-(1–12) in the presence of all inhibitors without chymostatin but including TEI-F00806. F, The values of chymase- or ACE-mediated ANG II production activities in male rat BM.
Chymase Expressing Cells in Rat Bone Marrow are Not Mature Mast Cells but CD68 Positive Cells
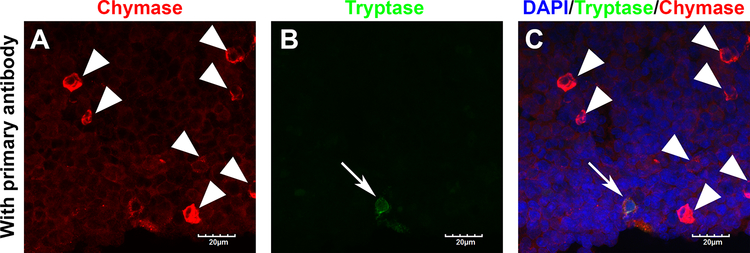
Mast cells (MCs), which contain proteases such as chymase and tryptase, are considered to play an important role in immediate hypersensitivity and inflammatory reactions in various peripheral organs.47 MCs arising from HSC in BM enter the circulation as progenitor cells and migrate into all vascularized tissues where they complete their maturation.48 To determine whether mature MCs are detected in rat BM, double immuno-fluorescence staining was performed. Almost all BM cells demonstrated red chymase fluorescence in slides stained with the primary antibody (Figure 5A); background autofluorescence was not detected on slides stained without the primary antibody in the same laser setting (Figure S3A–C). In addition, scattered strong chymase positive cells were illustrated in BM tissue (Figure 5A, arrowhead) while few tryptase expressing cells were found (Figure 5B, arrow). The merged image shows chymase positive and tryptase negative cells are the most dominant (Figure 5C), implying that scattered strong chymase expressing cells in rat BM are not mature MCs.
Figure 5.
Representative immunofluorescence photomicrographs (100×) of bone marrow tissue showing chymase (red), tryptase (green) and nuclei (DAPI, blue) A, Red channel. Arrowheads indicate chymase strongly positive cells. B, Green channel. Arrow indicates tryptase positive cell. C, Merged image. Scale bar indicates 20 μm.
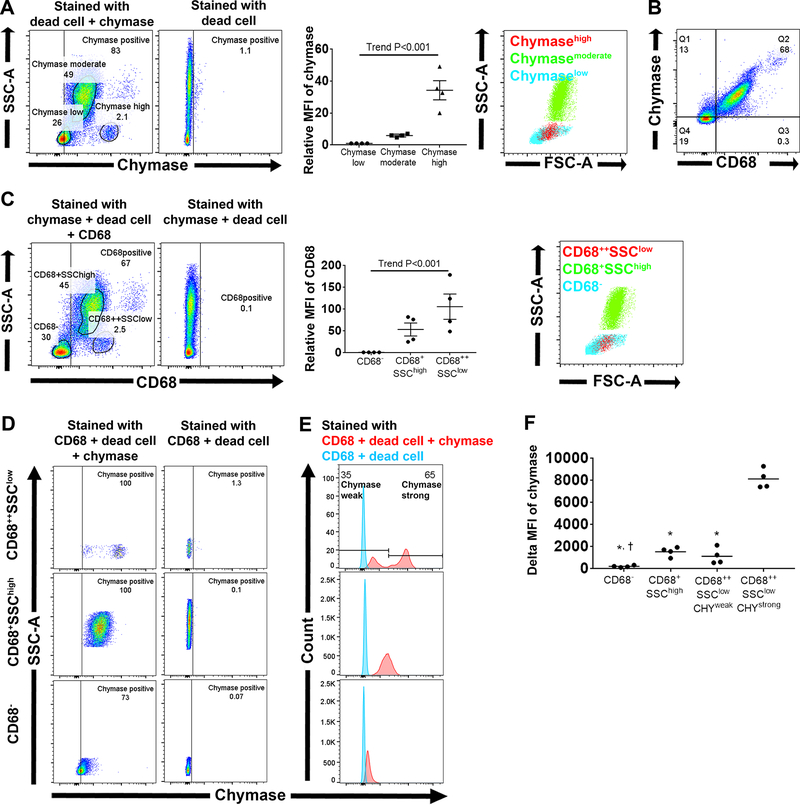
To determine what cells in BM express chymase, we employed flow cytometry analysis on BM cells stained with anti-chymase antibody. Chymase/SSC plots revealed that 78 ± 3 % of whole BM cells except doublets and dead cells expressed chymase (Figure 6A). In addition, these cells could be divided into 3 different subsets according to their relative MFI of chymase; chymaselow, chymasemoderate and chymasehigh (Figure 6A). Chymasehigh and chymasemoderate subsets were mainly located in primordial and granulocyte/monocyte regions, respectively, while the chymaselow subset resided in both lymphocyte and primordial regions. To determine whether myeloid lineage cells express more chymase than lymphoid lineage, we stained BM cells with a mixture containing anti-chymase and anti-CD68 antibodies. CD68 is commonly used as a monocyte/macrophage marker in peripheral organs;49 however, its expression has been reported in granulocytes in BM50 and peripheral blood.51 CD68/Chymase plots shows almost all CD68+ cells express chymase while 41 ± 11 % of CD68− cells express chymase (Figure 6B). In addition, delta MFI of chymase in CD68+ cells were significantly higher than that in CD68− cells (1,546 ± 157 a.u. and 222 ± 48 a.u., respectively, P=0.0021). CD68/SSC-A plots and relative MFI of CD68 divided whole BM cells into 3 different subsets according to their CD68 fluorescence intensity; CD68−, CD68+SSChigh and CD68++SSClow (Figure 6C). CD68+SSChigh subset is located in the granulocytes/monocytes region while CD68++SSClow subset is located in the primordial region. Since CD68 expression starts very early during myeloid differentiation and it is strongly upregulated in early myeloperoxidase (MPO) positive myeloid progenitors,50 CD68++SSClow are considered to contain myeloid progenitors that differentiate into monocytes/granulocytes. On the other hand, cells in the CD68− subset were in both the lymphocyte and primordial region (Figure 6C), implying that this subset contains mature lymphocytes, lymphoid progenitors and HSC. According to the chymase expression, CD68+SSChigh and CD68− subsets had one population while the CD68++SSClow subset could be divided into two subpopulations (Figure 6D and E); cells having strong chymase intensity (CHYstrong) and those having weak chymase intensity (CHYweak). Delta MFI of chymase in the CD68++SSClowCHYstrong subset was significantly higher than that of the CD68++SSClowCHYweak, CD68+SSChigh or CD68− subsets (Figure 6F), indicating that scattered strong chymase expressing cells in immunohistochemistry were in the CD68++SSClow subset. Besides, delta MFI of chymase in CD68+SSChigh was significantly higher than that in CD68− subset (Figure 6F). Figure S4 shows female flow cytometry experiments. CD68++SSClow subset could also be divided into two subpopulations; however, CHYweak subpopulation was the majority of CD68++SSClow subset in females (Figure S4E) while CHYstrong was in males (Figure 6E).
Figure 6.
Flow cytometry analysis for intracellular staining of chymase and CD68 (n = 4) in male SD rat bone marrow. Data are shown for whole BM cells except doublets and dead cell stained cells according to the gating strategy shown in Figure 3A. A, The representative chymase/SSC-A plot and quantitative analysis. Right panel shows subsets according to chymase fluorescence were backgated onto FSC-A/SSC-A plot. B, Representative CD68/chymase plot. C, CD68/SSC plot and quantitative analysis. Right panel shows subsets according to CD68/SSC-A plots were backgated onto FSC-A/SSC-A plot. D, Representative chymase/SSC-A plot. E, Red illustrates chymase fluorescence intensity in cells with full staining while blue shows those with full staining except chymase. F, Delta MFI of chymase in each subset. * P<0.001 vs CD68++SSClowCHYstrong subset, † P<0.05 vs CD68+SSChigh subset.
Discussion
We identify for the first time immunoreactive-Ang-(1–12) expressing cells in rat BM and demonstrate thousands-fold higher chymase-dependent Ang II generation than ACE-dependent. This conclusion is based on: a)- the detection of the rat sequence of Ang-(1–12) in BM cells by immunohistochemistry and flow cytometry; b)- chromatographic evidence for the direct conversion of 125I-Ang-(1–12) into Ang II by chymase in BM tissues; c)- a primacy of chymase catalytic activity over ACE as the principal source for Ang II production from either radiolabeled Ang-(1–12) or Ang I; d)- an exceedingly high BM Ang II-forming chymase activity when compared to that in left ventricle; e)- identification of CD68+ cells, especially CD68++ SSClow cells, as the source for the high chymase expression. Female study reenacted the main findings shown in male rats.
Although BM-derived inflammatory cells synthesize AGT protein52 and express a considerable amount of Ang II15,17 there are no previous studies comparing the catalytic activity of chymase versus ACE in Ang II production in BM tissue. The comparative data obtained by determining chymase and ACE catalytic activity in Ang II generation from either 125I-Ang-(1–12) or 125I-Ang I confirmed a primacy of chymase over ACE in Ang II production. This finding agrees with our previous demonstration that chymase exhibits a 20-fold higher catalytic activity for the conversion of Ang I to Ang II compared to ACE.34 That chymase was responsible for Ang-(1–12) conversion into Ang II was further established by the nature of the metabolic products generated in the absence and presence of chymostatin or the chymase selective inhibitor TEI-F00806.45,46 While both agents inhibited Ang II production from radiolabeled Ang-(1–12), the effects obtained with TEI-F00806 excluded the potential hydrolytic activity of cathepsins and tryptases from accounting for Ang II production.
More surprisingly, Ang II producing activity by chymase in BM was several orders of magnitude higher than that in the left ventricle of the same rats. We previously reported four times higher Ang II producing activity by chymase compared to ACE in heart tissue while seven times lower values were found in lung tissue.34 We now show that chymase-dependent Ang II generating activity from Ang-(1–12) in rat BM is literally orders of magnitude higher than in other organs. Based on these findings, it is tempting to conclude that the BM may be included as one of the main Ang II producing organs.
Immunohistochemistry and flow cytometry demonstrate the existence of Ang-(1–12), the preferable substrate for generating Ang II via chymase in BM tissue. Flow cytometry revealed that cells in the lymphocyte region displayed lower Ang-(1–12) and AGT fluorescence intensity than cells in other regions. In addition, CD68+ cells expressed more chymase than CD68− cells; this finding suggests that BM tissue RAS may be predominantly expressed in cells of myelogenic origin (i.e., cells arising from progenitor cells for granulocytes and monocytes).53 Because chronic Ang II infusion leads to HSC proliferation and myeloid biased differentiation,24 we suggest that chymase, by augmenting Ang II production, may constitute a positive-feedback mechanism augmenting myeloid lineage cell proliferation and function.
The current findings clearly show that chymase is preferentially expressed in CD68+ cells (Figure 6B). Further, immunohistochemistry demonstrate that MCs were not the chymase strong positive cells in BM (Figure 5). Flow cytometry revealed that the chymasehigh subset appeared not in the high SSC region, where MCs should exist because of their high granularity, but in the primordial region, which has low SSC and are considered as the “mother-region” for all leukocyte subsets in BM.43 Combined with the report by Strobl et al.50 that CD68 expression is strongly up-regulated in early myeloperoxidase positive precursor cells and decreased according to the differentiation into granulocytes, we conclude that CD68++ SSClow subset, which has the highest chymase expression compared to other subsets, should contain myeloid precursors and monocytes. From the fact that CD68++SSClow cells contain CHYstrong and CHYweak subpopulations (Figure 6D and E), chymase expression may originate after CD68 expression and decrease with granulocytic maturation. This result is consistent with the findings by Shimizu et al.54 showing that CD34+CD117− BM monocyte/macrophage progenitors express chymase while CD34+CD117+ MC progenitors do not in humans. Given that MCs complete their maturation not in the BM but in peripheral tissue,48 it can be concluded that MCs are not the main source of chymase in BM.
The primacy of chymase in BM Ang II formation and myeloid biased chymase expression were reenacted in female rat experiments. The number of CHYstrong cells in female (Figure S4D–E) looks smaller than that in male (Figure 6D–E) while chymase activity in female rats averaged 5% less than activity values in males (p > 0.05).
The remarkable chymase-mediated Ang II producing activity in BM myeloid lineage cells and the evidence that BM-derived cells can infiltrate into other organs suggest that BM-derived cells regulate chymase-mediated Ang II activity in various tissues in response to inflammatory stimuli. Gomez et al.52 described a similar concept as the “mobile angiotensin-generating system”, that is, an efficient AGT delivering mechanism by blood-borne cells. This concept52 is supported by studies showing that circulating leukocytes, especially monocytes, express a considerable amount of Ang II15 and that macrophages in atherosclerotic vessels express Ang II16 and ACE23. The functional importance of BM Ang II producing pathway is highlighted by a reduction in atherosclerosis in chimeric mice with BM ACE deficiency.23 The current study identifies the primacy of chymase, which is implicated in various low-grade inflammatory diseases including CVD, cancers and auto-immune diseases,42 over ACE as an Ang II producing enzyme in BM.
Okamura et al.55 demonstrated the augmented chymase-dependent Ang II forming activity in circulating mononuclear leukocytes in patients with acute myocardial infarction, implying BM chymase activity may be augmented in CVD.42 CVD risk factors including aging, loss of estrogen56 or diabetes mellitus augment tissue chymase activity in heart56,57 and kidney.58 An attractive hypothesis links neuroinflammatory mechanisms to BM dysfunction and microbiota in hypertension.59–61 Therefore, further studies addressing the specific role of the Ang-(1–12)/chymase axis in mediating BM Ang II dependent mechanisms are warranted.
Perspectives
Knowledge of the biochemical and functional mechanisms constituting the RAS has progressively evolved to organ-based systems performing paracrine/intracrine functions.32 Since the first complete identification of RAS components in rat and monkey BM,17,19 a strong literature points toward a critical modulatory role of BM-borne Ang II in hematopoiesis, pan lineage mitogen, myeloid differentiation, and macrophage production. Identification of a non-canonical mechanism for Ang II generation based on a very high chymase affinity for Ang-(1–12) establishes new research horizons linking the BM chymase/Ang-(1–12) axis to inflammatory processes associated with the pathophysiology of low-grade inflammatory diseases including hypertension, atherosclerosis, heart failure, diabetes mellitus, and renal diseases. The demonstration that CD68+ myeloid lineage cells, especially CD68++SSClow cells, are the major source of the high enzymatic activity of BM chymase unveils the relevance of chymase-mediated Ang II production in BM in stem cell-based therapy.
Supplementary Material
Novelty and Significance:
What Is New?
Ang-(1–12) [angiotensin-(1–12)], the preferable substrate for direct Ang II (angiotensin II), generation is now revealed in the bone marrow (BM) of rats.
Chymase-mediated Ang II production in BM was thousands-fold higher than ACE-mediated and 280-fold higher than that in the heart.
CD68 positive myeloid lineage cells, especially myeloid progenitors, have higher chymase expression than CD68 negative lymphoid lineage cells in BM.
What Is Relevant?
This study identifies BM, the origin of inflammatory cells and the source of stem cell-based therapy, as one of the main Ang II producing organs.
Source(s) and Funding
This study was supported by the National Heart, Blood, Lung Institute of the NIH through HL-051952 grant (CMF) and Department of Surgery, Wake Forest School of Medicine (TY).
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Aparicio-Vergara M, Shiri-Sverdlov R, Koonen DP, Hofker MH. Bone marrow transplantation as an established approach for understanding the role of macrophages in atherosclerosis and the metabolic syndrome. Curr Opin Lipidol. 2012;23:111–121. doi: 10.1097/MOL.0b013e3283508c4f [DOI] [PubMed] [Google Scholar]
- 2.Fujimiya M, Nagaishi K, Yamashita T, Ataka K. Bone marrow stem cell abnormality and diabetic complications. Anat Rec (Hoboken). 2012;295:917–921. doi: 10.1002/ar.22445 [DOI] [PubMed] [Google Scholar]
- 3.Soler MJ, José Tomas OP. Stem cells in kidney diseases. J Stem Cells. 2012;7:245–259. doi: jsc.2013.7.4.245. [PubMed] [Google Scholar]
- 4.Yamashita T, Fujimiya M, Nagaishi K, Ataka K, Tanaka M, Yoshida H, Tsuchihashi K, Shimamoto K, Miura T. Fusion of bone marrow-derived cells with renal tubules contributes to renal dysfunction in diabetic nephropathy. FASEB J. 2012;26:1559–1568. doi: 10.1096/fj.11-183194 [DOI] [PubMed] [Google Scholar]
- 5.de Macedo Braga LM, Lacchini S, Schaan BD, Rodrigues B, Rosa K, De Angelis K, Borges LF, Irigoyen MC, Nardi NB. In situ delivery of bone marrow cells and mesenchymal stem cells improves cardiovascular function in hypertensive rats submitted to myocardial infarction. J Biomed Sci. 2008;15:365–374. doi: 10.1007/s11373-008-9237-z [DOI] [PubMed] [Google Scholar]
- 6.Yao L, Heuser-Baker J, Herlea-Pana O, Barlic-Dicen J. Bone marrow endothelial progenitors in atherosclerotic plaque resolution. Organogenesis. 2013;9:29–33. doi: 10.4161/org.24433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63 [DOI] [PubMed] [Google Scholar]
- 8.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep. 2016;6:34842. doi: 10.1038/srep34842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bochon B, Kozubska M, Surygala G, Witkowska A, Kuzniewicz R, Grzeszczak W, Wystrychowski G. Mesenchymal Stem Cells-Potential Applications in Kidney Diseases. Int J Mol Sci. 2019;20:2462. doi: 10.3390/ijms20102462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haznedaroglu IC, Beyazit Y. Local bone marrow renin-angiotensin system in primitive, definitive and neoplastic haematopoiesis. Clin Sci (Lond). 2013;124:307–323. doi: 10.1042/CS20120300 [DOI] [PubMed] [Google Scholar]
- 11.Rodgers KE, Dizerega GS. Contribution of the Local RAS to Hematopoietic Function: A Novel Therapeutic Target. Front Endocrinol (Lausanne). 2013;4:157. doi: 10.3389/fendo.2013.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durik M, Seva Pessoa B, Roks AJ. The renin-angiotensin system, bone marrow and progenitor cells. Clin Sci (Lond). 2012;123:205–223. doi: 10.1042/CS20110660 [DOI] [PubMed] [Google Scholar]
- 13.Griffing GT, Melby JC. Enalapril (MK-421) and the white cell count and haematocrit. Lancet. 1982;1:1361. doi: 10.1016/s0140-6736(82)92430-8 [DOI] [PubMed] [Google Scholar]
- 14.Studer A, Vetter W. Reversible leucopenia associated with angiotensin-converting-enzyme inhibitor MK 421. Lancet. 1982;1:458. doi: 10.1016/s0140-6736(82)91672-5 [DOI] [PubMed] [Google Scholar]
- 15.Kitazono T, Padgett RC, Armstrong ML, Tompkins PK, Heistad DD. Evidence that angiotensin II is present in human monocytes. Circulation. 1995;91:1129–1134. doi: 10.1161/01.cir.91.4.1129 [DOI] [PubMed] [Google Scholar]
- 16.Potter D,D, Sobey C,G, Tompkins P,K, Rossen JD, Heistad DD. Evidence that macrophages in atherosclerotic lesions contain angiotensin II. Circulation. 1998;98:800–807. doi: 10.1161/01.cir.98.8.800 [DOI] [PubMed] [Google Scholar]
- 17.Strawn WB, Richmond RS, Ann Tallant E, Gallagher PE, Ferrario CM. Renin-angiotensin system expression in rat bone marrow haematopoietic and stromal cells. Br J Haematol. 2004;126:120–126. doi: 10.1111/j.1365-2141.2004.04998.x [DOI] [PubMed] [Google Scholar]
- 18.Ferrario CM, Richmond RS, Smith R, Levy P, Strawn WB, Kivlighn S. Renin-angiotensin system as a therapeutic target in managing atherosclerosis. Am J Ther. 2004;11:44–53. [DOI] [PubMed] [Google Scholar]
- 19.Richmond RS, Tallant EA, Gallagher PE, Ferrario CM, Strawn WB. Angiotensin II stimulates arachidonic acid release from bone marrow stromal cells. J Renin Angiotensin Aldosterone Syst. 2004;5:176–182. doi: 10.3317/jraas.2004.037 [DOI] [PubMed] [Google Scholar]
- 20.Beyazit Y, Purnak T, Guven GS, Haznedaroglu IC. Local bone marrow Renin-Angiotensin system and atherosclerosis. Cardiol Res Pract. 2010;2011:714515. doi: 10.4061/2011/714515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda D, Sata M, Ishizaka N, Nagai R. Critical role of bone marrow angiotensin II type 1 receptor in the pathogenesis of atherosclerosis in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:90–96. doi: 10.1161/ATVBAHA.107.152363 [DOI] [PubMed] [Google Scholar]
- 22.Yokoi H, Yamada H, Tsubakimoto Y, et al. Bone marrow AT1 augments neointima formation by promoting mobilization of smooth muscle progenitors via platelet-derived SDF-1{alpha}. Arterioscler Thromb Vasc Biol. 2010;30:60–67. doi: 10.1161/ATVBAHA.109.192161 [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Lu H, Zhao M, Tashiro K, Cassis LA, Daugherty A. Contributions of leukocyte angiotensin-converting enzyme to development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:2075–2080. doi: 10.1161/ATVBAHA.113.301777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, Zingler M, Harrison JK, Scott EW, Cogle CR, Luo D, Raizada MK. Angiotensin II Regulation of Proliferation, Differentiation, and Engraftment of Hematopoietic Stem Cells. Hypertension. 2016;67:574–584. doi: 10.1161/HYPERTENSIONAHA.115.06474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swirski FK, Nahrendorf M. Bone Marrow Takes Center Stage in Cardiovascular Disease. Circ Res. 2016;119:701–703. doi: 10.1161/CIRCRESAHA.116.309584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Tang Y, Wang Y, Tascau L, Balcerek J, Tong W, Levine RL, Welch C, Tall AR, Wang N. LNK/SH2B3 Loss of Function Promotes Atherosclerosis and Thrombosis. Circ Res. 2016;119:e91–e103. doi: 10.1161/CIRCRESAHA.116.308955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strawn WB, Ferrario CM. Angiotensin II AT1 receptor blockade normalizes CD11b+ monocyte production in bone marrow of hypercholesterolemic monkeys. Atherosclerosis. 2008;196:624–632. doi: 10.1016/j.atherosclerosis.2007.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, Li W, Santisteban MM, Yuan W, Febo M, Mocco J, Feng Y, Scott E, Baekey DM, Raizada MK. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63:542–550. doi: 10.1161/HYPERTENSIONAHA.113.02722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zubcevic J, Santisteban MM, Pitts T, Baekey DM, Perez PD, Bolser DC, Febo M, Raizada MK. Functional neural-bone marrow pathways: implications in hypertension and cardiovascular disease. Hypertension. 2014;63:e129–139. doi: 10.1161/HYPERTENSIONAHA.114.02440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dell’Italia LJ, Ferrario CM. The never-ending story of angiotensin peptides: beyond angiotensin I and II. Circ Res. 2013;112:1086–1087. doi: 10.1161/CIRCRESAHA.113.301246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, Dell’italia LJ. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond). 2014;126:461–469. doi: 10.1042/CS20130400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, Collawn JF, Dell Italia LJ. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol. 2016;311:H404–414. doi: 10.1152/ajpheart.00219.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146 [DOI] [PubMed] [Google Scholar]
- 34.Ahmad S, Varagic J, VonCannon JL, Groban L, Collawn JF, Dell’Italia LJ, Ferrario CM. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1–12) metabolizing enzyme. Biochem Biophys Res Commun. 2016;478:559–564. doi: 10.1016/j.bbrc.2016.07.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes. PLoS One. 2011;6:e15759. doi: 10.1371/journal.pone.0015759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad S, Wei CC, Tallaj J, Dell’Italia LJ, Moniwa N, Varagic J, Ferrario CM. Chymase mediates angiotensin-(1–12) metabolism in normal human hearts. J Am Soc Hypertens. 2013;7:128–136. doi: 10.1016/j.jash.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Mello WC, Dell’Itallia LJ, Varagic J, Ferrario CM. Intracellular angiotensin-(1–12) changes the electrical properties of intact cardiac muscle. Mol Cell Biochem. 2016;422:31–40. doi: 10.1007/s11010-016-2801-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrario CM, VonCannon J, Jiao Y, Ahmad S, Bader M, Dell’Italia LJ, Groban L, Varagic J. Cardiac angiotensin-(1–12) expression and systemic hypertension in rats expressing the human angiotensinogen gene. Am J Physiol Heart Circ Physiol. 2016;310:H995–1002. doi: 10.1152/ajpheart.00833.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H2614–2618. doi: 10.1152/ajpheart.91521.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50. doi: 10.1093/cvr/cvp003 [DOI] [PubMed] [Google Scholar]
- 41.Caughey GH. Chymase. In: Rawlings ND, Salvesen G, eds. Handbook of Proteolytic Enzymes. Vol. 3 3rd ed. Amsterdam, Netherlands: Elsevier; 2013: 2675–2683. doi: 10.1016/B978-0-12-382219-2.00590-1 [DOI] [Google Scholar]
- 42.Dell’Italia LJ, Collawn JF, Ferrario CM. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling. Circ Res. 2018;122:319–336. doi: 10.1161/CIRCRESAHA.117.310978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnoulet C, Bene MC, Durrieu F, Feuillard J, Fossat C, Husson B, Jouault H, Maynadie M, Lacombe F. Four- and five-color flow cytometry analysis of leukocyte differentiation pathways in normal bone marrow: a reference document based on a systematic approach by the GTLLF and GEIL. Cytometry B Clin Cytom. 2010;78:4–10. doi: 10.1002/cyto.b.20484 [DOI] [PubMed] [Google Scholar]
- 44.Bjorklund E, Gruber A, Mazur J, Martensson A, Hansson M, Porwit A. CD34+ cell subpopulations detected by 8-color flow cytometry in bone marrow and in peripheral blood stem cell collections: application for MRD detection in leukemia patients. Int J Hematol. 2009;90:292–302. doi: 10.1007/s12185-009-0389-z [DOI] [PubMed] [Google Scholar]
- 45.Ansary TM, Urushihara M, Fujisawa Y, Nagata S, Urata H, Nakano D, Hirofumi H, Kitamura K, Kagami S, Nishiyama A. Effects of the selective chymase inhibitor TEI-F00806 on the intrarenal renin-angiotensin system in salt-treated angiotensin I-infused hypertensive mice. Exp Physiol. 2018;103:1524–1531. doi: 10.1113/EP087209 [DOI] [PubMed] [Google Scholar]
- 46.Maeda Y, Inoguchi T, Takei R, Sawada F, Sasaki S, Fujii M, Kobayashi K, Urata H, Nishiyama A, Takayanagi R. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. Am J Physiol Renal Physiol. 2010;299:F1328–1338. doi: 10.1152/ajprenal.00337.2010 [DOI] [PubMed] [Google Scholar]
- 47.Reid AC, Silver RB, Levi R. Renin: at the heart of the mast cell. Immunol Rev. 2007;217:123–140. doi: 10.1111/j.1600-065X.2007.00514.x [DOI] [PubMed] [Google Scholar]
- 48.Arock M Mast cell differentiation: still open questions? Blood. 2016;127:373–374. doi: 10.1182/blood-2015-12-686592 [DOI] [PubMed] [Google Scholar]
- 49.Ferenbach D, Hughes J. Macrophages and dendritic cells: what is the difference? Kidney Int. 2008;74:5–7. doi: 10.1038/ki.2008.189 [DOI] [PubMed] [Google Scholar]
- 50.Strobl H, Scheinecker C, Csmarits B, Majdic O, Knapp W. Flow cytometric analysis of intracellular CD68 molecule expression in normal and malignant haemopoiesis. Br J Haematol. 1995;90:774–782. doi: 10.1111/j.1365-2141.1995.tb05195.x [DOI] [PubMed] [Google Scholar]
- 51.Amanzada A, Malik IA, Blaschke M, Khan S, Rahman H, Ramadori G, Moriconi F. Identification of CD68(+) neutrophil granulocytes in in vitro model of acute inflammation and inflammatory bowel disease. Int J Clin Exp Pathol. 2013;6:561–570. [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez R,A, Norling L,L, Wilfong N, Isakson P, Lynch K,R , Hock R, Quesenberry P Leukocytes synthesize angiotensinogen. Hypertension. 1993;21:470–475. doi: 10.1161/01.hyp.21.4.470 [DOI] [PubMed] [Google Scholar]
- 53.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimizu Y, Suga T, Maeno T, Tsukagoshi H, Kawata T, Narita T, Takahashi T, Ishikawa S, Morishita Y, Nakajima T, Hara F, Miura T, Kurabayashi M. Detection of tryptase-, chymase+ cells in human CD34 bone marrow progenitors. Clin Exp Allergy. 2004;34:1719–1724. doi: 10.1111/j.1365-2222.2004.02105.x [DOI] [PubMed] [Google Scholar]
- 55.Okamura K, Okuda T, Shirai K, Urata H. Increase of chymase-dependent angiotensin II-forming activity in circulating mononuclear leukocytes after acute myocardial infarction chymase activity after acute myocardial infarction. Heart Vessels. 2019;34:1148–1157. doi: 10.1007/s00380-019-01352-x [DOI] [PubMed] [Google Scholar]
- 56.Ahmad S, Sun X, Lin M, Varagic J, Zapata-Sudo G, Ferrario CM, Groban L, Wang H. Blunting of estrogen modulation of cardiac cellular chymase/RAS activity and function in SHR. J Cell Physiol. 2018;233:3330–3342. doi: 10.1002/jcp.26179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Froogh G, Pinto JT, Le Y, Kandhi S, Aleligne Y, Huang A, Sun D. Chymase-dependent production of angiotensin II: an old enzyme in old hearts. Am J Physiol Heart Circ Physiol. 2017;312:H223–H231. doi: 10.1152/ajpheart.00534.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cristovam PC, Carmona AK, Arnoni CP, Maquigussa E, Pereira LG, Boim MA. Role of chymase in diabetic nephropathy. Exp Biol Med (Maywood). 2012;237:985–992. doi: 10.1258/ebm.2012.011356 [DOI] [PubMed] [Google Scholar]
- 59.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117:178–191. doi: 10.1161/CIRCRESAHA.117.305853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-Gut-Bone Marrow Axis: Implications for Hypertension and Related Therapeutics. Circ Res. 2016;118:1327–1336. doi: 10.1161/CIRCRESAHA.116.307709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santisteban MM, Zubcevic J, Baekey DM, Raizada MK. Dysfunctional brain-bone marrow communication: a paradigm shift in the pathophysiology of hypertension. Curr Hypertens Rep. 2013;15:377–389. doi: 10.1007/s11906-013-0361-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.