Abstract
Background
Ozone (O3) inhalation elicits airway inflammation and impairs treatment responsiveness in asthma. The underlying immune mechanisms have been difficult to study because of the lack of relevant experimental models. Rhesus macaques spontaneously develop asthma and have a similar immune system to humans.
Objectives
To investigate the mucosal immune changes after O3 inhalation in a clinically relevant non-human primate asthma model and study the effects of an anti-oxidant synthetic lignan (LGM2605).
Methods
A cohort of macaques (n=17) previously characterized with airway hyperreactivity to methacholine was assessed (day 1). Macaques were treated (orally) with LGM2605 (25 mg/kg) or placebo twice per day for 7 days, exposed to 0.3 ppm O3 or air for 6 hours (on day 7) and were studied 12 hours later (day 8). Lung function, blood and bronchoalveolar lavage (BAL) immune cell profile, bronchial brush and blood cell mRNA expression were assessed.
Results
O3 induced significant BAL neutrophilia and eosinophilia, increased airway hyperreactivity and expression of il6 and il25 mRNA in the airway epithelium together with increased BAL group 2 innate lymphoid cell (ILC2), CD1c+ mDC, and CD4+ T cell counts and diminished surfactant protein-D (SP-D) expression. While LGM2605 attenuated some of the immune and inflammatory changes, it completely abolished O3-induced airway hyperreactivity.
Conclusion
ILC2, CD1c+ mDC, and CD4+ T cells are selectively involved in O3-induced asthma exacerbation. The inflammatory changes were partially prevented by anti-oxidant pretreatment with LGM2605 that had an unexpectedly disproportionate protective effect on airway hyperreactivity.
Keywords: asthma, airway inflammation, air pollution, therapies (investigational), non-human primate
Capsule summary
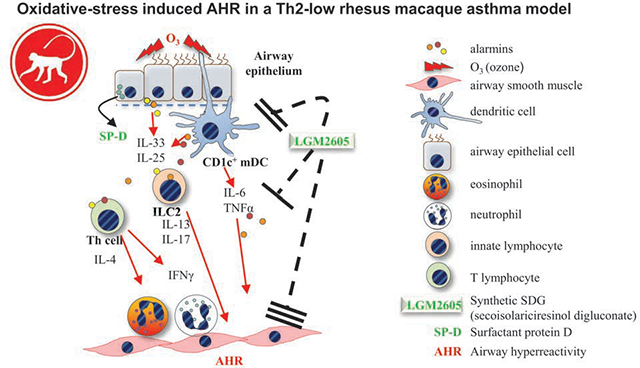
O3-induced airway inflammation and increased airway hyperreactivity, characterized by selective ILC2, CD1c+ mDC and Th cell activation in asthmatic rhesus macaques, was inhibited by pre-treatment with a synthetic lignan (LGM2605).
Graphical Abstract
INTRODUCTION
Air pollution was identified as one of the most significant contributors to prevalence, severity and chronicity of asthmatic airways inflammation. In our highly polluted neighboring San Joaquin County, the lifetime prevalence of asthma for children ages 5 to 17 was greater than 28% in a 2013 survey (https://www.cdph.ca.gov/Programs/CCDPHP/DEODC). Asthmatics are particularly susceptible to airway obstruction, inflammation and impaired responsiveness to treatment after exposure to the toxic air pollutant, O3 (1, 2). O3 induces direct oxidative modifications of biomolecules in the respiratory tract leading to proinflammatory activation of the mucosal tissue and neutrophilic airway activation (3). The pathogenic role of oxidative stress in asthma is accepted but studies that target oxidative burden using a variety of nutritional, pharmacological, and environmental approaches have been controversial (4) and the underlying immune mechanisms responsible for the airway changes remain unclear. Using mice our laboratory previously showed that airway epithelial, dendritic (DCs) natural killer (NK) (5) and group 2 innate lymphoid (ILC2) (6) cells play highly proinflammatory roles while presence of the immunoprotective surfactant protein-D (SP-D), an epithelial derived lung collectin, is important in attenuating the O3-induced inflammatory process (5–9). Establishment of the clinical relevance of these findings has been hindered due to the lack of adequate experimental systems.
We turned to study the non-human primate rhesus macaques because their genetic makeup is highly similar to that of humans with a high degree of immune crossreactivity and shared receptor characteristics (10–13). Macaques, like humans, naturally develop airway hyperreactivity (AHR) with peripheral and mucosal immunological responses demonstrating individual variability (14, 15) making these animals more suitable than rodents to study asthma [reviewed in (16)]. As a result of clinical asthma trials using Th2 targeting biologics over the last few years, it has become clear that over 50% of asthmatic patients belong to a “Th2-low” endotype (17). New therapeutic approaches are needed for these patients as they are not responsive to Th2-targeting strategies and also demonstrate poor glucocorticoid responsiveness. At the California National Primate Research Center (CNPRC), macaques that naturally developed AHR show no Th2 cell activation and have immune cells unresponsive to glucocorticoids (12). Thus, these animals may represent “Th2-low” asthmatic patients and are therefore uniquely poised for investigation of novel alternative or adjuvant approaches to glucocorticoid treatment.
LGM2605 is the synthetic version of secoisolariciresinol diglucoside, the bioactive lignan found in flaxseed, with free radical scavenging, anti-oxidant and anti-inflammatory properties (18) acting via multiple pathways in mice and in vitro inflammatory models (18–22). LGM2605 inhibited human and murine myeloperoxidase activity, induced anti-oxidant enzymes in macrophages (18), and reduced oxidative tissue damage in human precision-cut lung slices (18–22). Because of its synthetic availability, this compound can be administered at a precise, biologically relevant dose.
Based on our mouse models and the extensive studies demonstrating in vitro and in vivo anti-inflammatory potential of LGM2605, we hypothesized that O3-induced exacerbation of asthma in macaques will be associated with neutrophilic and eosinophilic airway inflammation, activation of the airway mucosal innate immune system and will be amenable to anti-oxidant targeting with LGM2605. We aimed to (1) comprehensively define lung functional and mucosal immune changes after O3 exposure in rhesus macaques and (2) assess the effects of LGM2605. We designed a longitudinal study applying minimally invasive procedures to sequentially sample animals before and after O3 exposure and LGM2605 administration.
MATERIALS & METHODS
Subjects
Two- to three-year old male and female age- and sex-matched rhesus macaques were enrolled into this study from a pre-selected cohort (previously presented with AHR within the past 12 months). Animals were relocated from outdoor field cages at the CNPRC to the experimental rooms (subject information Table E1). Macaques were randomly segregated into experimental groups (LGM2605+air, Placebo+O3, or LGM2605+O3). During acclimation indoors, macaques were trained to accept LGM2605 or placebo in small treats (peanut butter sandwiches). Before baseline measurements on day 1 [blood draw, BAL, bronchial brush biopsy, pulmonary function test (PFT)] animals were fasted overnight (Figure 1A). Animals were immobilized with an intramuscular injection of Ketamine (5–30 mg/kg), and a blood sample (up to 20 mL) was drawn from a peripheral vessel in accordance with veterinary staff guidelines. Following the blood draw, monkeys were further anesthetized using intravenous Propofol (10–30 mg/kg/hr) with the dose adjusted as deemed necessary by the attending veterinarian. Macaques were then intubated with an appropriate sized cuffed endotracheal tube. Static lung mechanisms and airway responsiveness to methacholine challenge was performed (additional details in the Online Data Repository). Figure E1 shows that 4 animals in our cohort were not displaying AHR on Day 1 while the other 13 were. Following PFT, BAL was harvested and a bronchial brush biopsy was performed (additional details in the Online Data Repository). While the animals were still sedated, a single 50 mg/kg dose of LGM2605 was administered using a syringe. Macaques were then treated twice per day 25 mg/kg LGM2605 (days 2–7). Following the final dose of LGM2605, macaques were exposed to air or 0.3 ppm O3 for 6.5 hours. 12 hours later, blood draw and bronchoscopy were repeated (day 8). Macaques then rested indoors overnight before being returned to their native outdoor field cage, or when deemed appropriate by the staff veterinarian. The University of California, Davis Institutional Animal Care and Use Committee approved the experimental protocol.
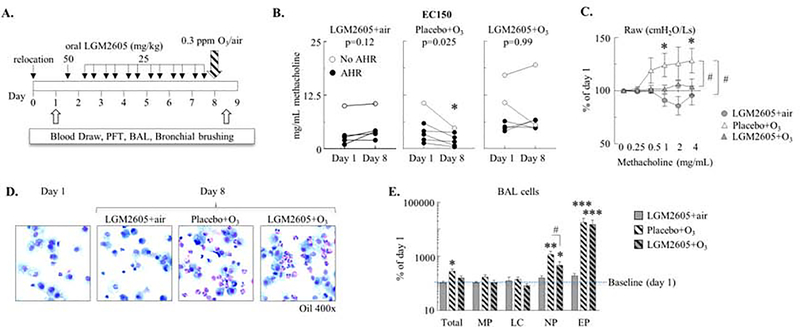
Figure 1. O3-induced increases in AHR were prevented by LGM2605 treatment.
(A) Study time line: Baseline measurements were performed on selected macaques following relocation indoors (day 1). Animals were given LGM2605 orally (as a small treat, in a peanut butter sandwich) daily for 7 days, then exposed to 0.3 ppm O3 or air for 6.5 hours. 12 hours post exposure blood draw and bronchoscopy were repeated (day 8). BAL: bronchoalveolar lavage, PFT: pulmonary function test. Experimental groups: LGM2605+air, Placebo+O3, and LGM2605+O3. (B) Effective concentration of methacholine that raised airway resistance (Raw) by 150% (EC150) on days 1 and 8 (mg/mL). Mean±SEM of n=5–6, *p<0.05 (day 1 vs. day 8, Student’s paired t-test). (C) Methacholine dose response (Raw; % of day 1; each macaque served as its own control). (D) Representative photomicrographs of Kwik-Diff stained cytospins from day 1 and day 8 BAL indicating neutrophil and eosinophil influx in O3-exposed animals. (E) Macrophages (MP), lymphocytes (LC), neutrophils (NP), and eosinophils (EP) were differentially counted on cytospins (the absolute cell counts are expressed as % of day 1; each macaque served as its own control). (C, E): Mean±SEM of n=5–6; *p<0.05, **p<0.01, ***p<0.001 [day 1 vs. day 8, or vs. 0 mg/mL methacholine (C); Two-way ANOVA with Bonferroni’s multiple comparisons] #p<0.05; between groups; (Two-way ANOVA).
This design was performed 6 times. Each time three macaques were relocated indoors and randomly assigned to one of the three experimental groups. This ensured that if any outdoor air quality events would impact the results of the study, they would be evenly distributed among the experimental groups. Between December 2017 and February 2018, when the study was performed, there were no significant outdoor air quality events at the CNPRC.
Source of LGM2605
Synthetic secoisolariciresinol diglucoside (LGM2605), a lignan originally derived from flaxseed, was synthesized and supplied by LignaMed, LLC. The lyophilized product was dissolved in sterile water (at 0.62 mg/mL) and stored at −80°C. The stock was used to make up the compound for treatment freshly, every day.
O3 exposure
O3 exposures were conducted in 5.1 cubic meter stainless steel and glass, whole-body inhalation chambers described before (23). O3 was produced from a vaporized liquid, medical grade oxygen by electric discharge ozonizers. O3 was mixed with HEPA and activated carbon filtered dilution air and pumped into the inlet air-flow of the exposure chamber with 30 air changes per hour for an exposure concentration of 0.3 ppm that was actively maintained with a proportional control system. We selected to use exposure to 0.3 ppm O3 for 6.5 hours because studies conducted in healthy young subjects showed respiratory effects between 0.04–0.6 ppm, during physical activity (24). Further, in highly polluted urban, suburban and rural areas in China, the hourly and 8 hourly maximum concentration of ambient O3 and O3 precursor levels are frequently between 0.1–0.3 ppm (25). We reasoned that an exposure to 0.3 ppm O3 for a prolonged period (over 6 hours) would simulate the effects of a poor-air quality day outdoors._O3 concentration, airflow, temperature, pressure, and humidity were monitored throughout the exposure. HEPA and activated carbon filtered air was injected into the inlet air-flow of the exposure chamber with 30 changes per hour for the LGM2605+air control group which maintained O3 levels below 0.005ppm.
Peripheral blood mononuclear cell isolation
Blood was layered over Lymphoprep (STEMCELL Technologies, Vancouver, Canada), centrifuged at 20°C for 20 minutes, brake and acceleration speed set to 0. Cells were harvested from the interphase layer and washed twice with ice cold PBS. Cells were counted via the Countess® Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA) then apportioned for downstream flow cytometry and fluorescent activated cell sorting (FACS) experiments.
Bronchoalveolar lavage cells and supernatant
Total cells recovered in the BAL were counted using the Countess® Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA). 50,000 cells were cytospun and stained with Shandon™ Kwik-Diff™ Stain (Thermo Fisher Scientific, Waltham, MA). Differential cell count was performed at 400x magnification under a light microscope to determine the proportion and absolute number of macrophages, lymphocytes, neutrophils, and eosinophils. The remaining cells were apportioned for flow cytometry experiments. In the text and figures we report the absolute count for each cell type in the BAL (cells/mL BAL).
Immune and inflammatory cell morphology appeared normal in the BAL samples and macrophages displayed inflammatory alterations in response to ozone as expected. We noted a few small dark blue inclusions in some of the macrophage cytoplasms across all experimental groups and time points, the source and nature of which is unclear. We speculate that these inclusions could come from wildfire smoke-derived particles as the macaques are housed in outdoor corrals but since there was no wildfire smoke exposure within one year before our study this would be an unlikely source (unless the macrophages with inclusions persisted over a year after the actual exposure occurred). The inclusions may also be of microbial origin through infection (although comprehensive medical assessment of our cohort did not reveal any signs of infection), or, through oropharyngeal contamination of the BAL samples during collection. Further investigation of this interesting phenomenon was outside of the scope of the present study.
Bronchial Brush Biopsy
Bronchial brush biopsies were obtained from six sites per animal at airway generations from 2 to 5 using 1mm x 6mm brushes and deposited into sample tubes containing PBS. The harvested cells were then aliquoted, snap frozen, and stored at −80°C in TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA).
Flow cytometry, qPCR, and western blot
Flow cytometry, qPCR, and western blot protocols are in the Online Data Repository.
Culture of human cell lines in vitro
To test the mechanism of action of LGM2605 in vitro, primary human airway smooth muscle (hASM) and papilloma virus-immortalized human bronchial epithelial (HBE1) cells were kindly donated by Dr. Yang Kevin K. Xiang (University of California, Davis) and Dr. J Yankaskas (University of North Carolina), respectively. hASM cells were maintained in F12 (Ham) Nutrient, 1% Pen-Strep, and 200 mM L-Glutamine (Thermo Fisher Scientific, Waltham, MA), 1M HEPES, CaCl2, and NaOH (Sigma-Aldrich, St. Louis, MO), an 10% Fetal Bovine Serum (Hyclone, Logan, UT) while HBE1 cells were maintained as previously described (26). Cells were grown to confluency in T-75 flasks, then split into 12-well plates. At 0 hour medium was replaced with fresh medium containing 0, 12.5, 25, or 50 μM LGM2605. 10 hours later, TBHP or control H2O was added to bring the final concentration to 0.05 or 0 mM, respectively. At the 12 hour time point, the TBHP-containing medium was replaced with fresh medium containing LGM2605. At the 24 hour time point, cells were harvested in TRIzol. Details of RNA extraction and qPCR are in the Online Data Repository.
Data analysis
Statistical analysis was performed using Prism v7 software (GraphPad Inc., La Jolla, CA). Data are expressed as mean±SEM unless otherwise specified and are representative of 6 independent experiments (n=5–6 macaques per group). Two-way ANOVA with Bonferroni’s multiple comparison’s test was used to compare experimental groups. When each animal served as its own control (% change or fold change), Student’s paired t-test was used [day 1 (before) vs. day 8 (after)]. Asterisks (*) denote statistically significant changes within experimental groups (ie. day 1 vs. day 8) while hash marks (#) show significance between experimental groups (ie. day 8 placebo+O3 vs. day 8 LGM2605+O3). A p-value less than 0.05 was considered significant.
RESULTS
O3-induced increases in AHR were prevented by anti-oxidant (LGM2605) treatment
We selected 17 age and sex-matched macaques in the present study from one of our previously characterized cohorts of animals (13) that displayed AHR within the past year. The baseline (day 1) lung function measurements showed that 13 macaques still had “AHR” (effective concentration of methacholine that raised lung resistance by 150% [EC150]<8 mg/mL) and 4 had “no AHR” (Figure E1A–B). None of the 17 animals had any remarkable airway inflammation at baseline (day 1, Figure 1D).
To investigate the effects of O3 on the lung function of macaques with preexisting AHR and to study whether anti-oxidant treatment would affect the O3-induced changes we developed a longitudinal experimental design. This design allowed for a “before and after” comparison of parameters in the same individual macaques to control for genetic heterogeneity and baseline variability of AHR in the outbred macaque cohort. After the baseline measurements (day 1; D1), macaques underwent 7 days of oral treatment with LGM2605 [a synthetic lignan compound with potent anti-oxidant properties (18–22)] or placebo, before exposure to O3 and a repeat of measurements (day 8; D8) (Figure 1A). The mechanism of the LGM2605 action in protecting from oxidative damage is thought to involve both free-radical scavenging and induction of anti-oxidant enzymes (18–22, 27, 28). To investigate the effects of LGM2605 we used a different model of radiation lung injury (described in the Online Repository Materials). LGM2605 treatment of irradiated rhesus macaques induced dose-dependent increases in multiple Nrf2 target anti-oxidant gene expression in the lung (Figure E2).
Airway resistance (Raw) to methacholine inhalation was dose-dependent (Figure E1B) and O3 exposure significantly increased it after placebo but not LGM2605 treatment when day 8 data were expressed as a function of day 1 data in the individual macaques (Figure 1C). Remarkably, when monkeys were exposed to O3, their EC150 on day 8 was significantly decreased compared to day 1 in the placebo but not LGM2605 treated group (Figure 1B). Of note, our sample size design was powered for paired (before and after) statistics. Therefore, while the original Raw values show similar trends when depicted as day 1 and day 8 group averages (Figure E3), without controlling for individual variability, no statistically significant differences could be demonstrated, highlighting the importance of the macaques’ variability in their airway responsiveness. Further, in animals exposed to air and treated with LGM2605, there was a trend for increased EC150 and decreased Raw (Figure 1C) suggesting that this anti-oxidant treatment may also improve baseline airway obstruction in asthmatic macaques. Taken together, these data indicated that O3 exposure significantly increased airway responsiveness to methacholine in macaques and that anti-oxidant LGM2605 treatment prevented such increase.
We previously showed in allergen sensitized and challenged mice that O3 exposure markedly amplified airway neutrophil and eosinophil counts (6, 9, 29). Similarly in the macaques here there were highly significant increases of the total cell counts as well as the proportions of both neutrophils and eosinophils in the airways after O3 exposure (Figure 1D–E) when data were expressed either as a % of the day 1 value (Figure 1E) or as absolute counts (Figure E4). LGM2605 (but not placebo), had a significant, selective inhibitory effect on BAL neutrophilia but eosinophils were unaffected after O3 exposure (Figure 1E and E4). In our studies on mouse models of allergen or O3-induced airway inflammation the extent of eosinophil (30) and neutrophil (unpublished observations) influx, respectively, strongly correlated with changes in lung function. The weak correlation we saw between the EC150 methacholine concentrations and the BAL eosinophil or neutrophil cell counts of O3-exposed macaques were therefore unexpected (Figure E4B). These results suggested that LGM2605 eliminated the O3 effects on AHR even in the presence of large numbers of eosinophils, raising a question about the role of these cells in airway physiology.
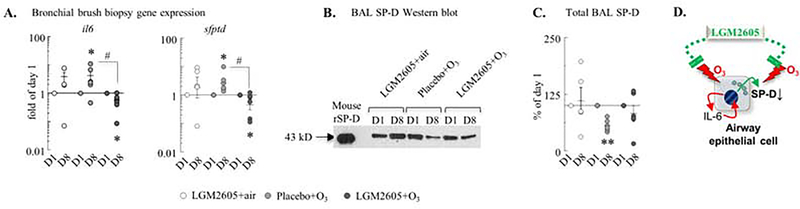
O3 induced il6 and sftpd mRNA expression in airway epithelial cells but diminished SP-D protein levels in the BAL. LGM2605 attenuated these effects
To study the molecular mechanisms involved in O3-induced inflammation and AHR, we investigated surfactant protein D (SP-D) expression at the mRNA and protein level in the bronchial brush biopsies and the BAL supernatant, respectively, of the macaques. We also studied expression of il6 mRNA because this is a major cytokine induced by O3 and because we previously showed in mice that IL-6 can stimulated SP-D gene expression. In the airway epithelium, O3 induced il6 and sfptd mRNA. This effect was reversed by LGM2605 (Figure 2A). Native gel electrophoresis showed that O3 exposure did not change the oligomeric SP-D structure in macaque BAL (Figure E5). However, O3 significantly reduced the total BAL SP-D protein concentration. This effect was also prevented by LGM2605 (Figure 2B–C). Thus, O3 induced sfptd gene activation likely through autocrine effects of IL-6 on airway epithelial cells. Oxidative stress diminished SP-D protein levels that was reversed by LGM2605 (Figure 2D).
Figure 2. O3 induced il6 and sftpd mRNA expression in airway epithelial cells but diminished SP-D protein expression in the BAL. LGM2605 alleviated these effects.
(A) RNA was extracted from bronchial brush biopsies, and expression of the il6 and sfptd genes were measured by qPCR. ΔΔCt (gapdh and rpl32), fold of baseline/day 1; each macaque served as its own control. (B) Representative reducing SDS-page western blot detecting non-human primate BAL SP-D. Mouse (and human; not shown) recombinant SP-D (rSP-D) was used as a loading control (C) Densitometric values (reducing SDS-page gels, ImageJ, (% of day 1; each macaque served as its own control). (D) Schematic depiction of the dual antagonistic effects of O3 and LGM2605. O3 induces IL-6 production in an autocrine manner that increases SP-D synthesis. O3-induced oxidative damage diminishes released SP-D. LGM2605 interacts with these events. Mean±SEM of n=5–6; *p<0.05 (within groups: day 1 vs. day 8; Student’s paired t-test) #p<0.05 (between groups; Two-way ANOVA).
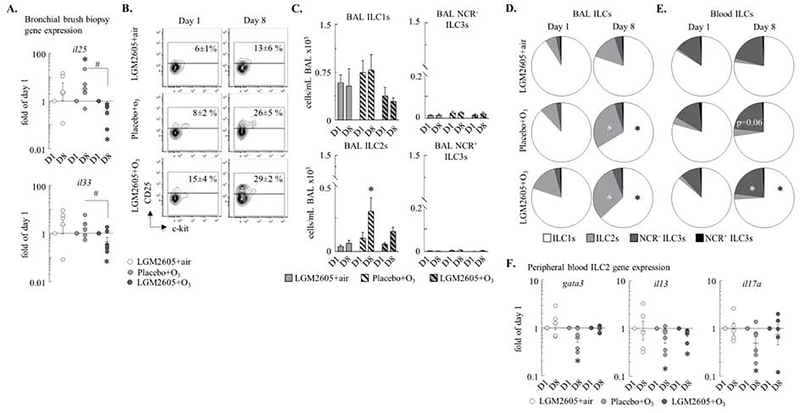
O3 increased ILC2 counts in the BAL were attenuated by LGM2605
We hypothesized that the O3 effects would involve activation of ILC2s, a previously undescribed cell population in the macaques (Figure E6). Expression of the ILC2 activating cytokine genes, Il25 and Il33 were induced by O3 in airway epithelial cells although changes in Il33 mRNA did not reach statistical significance (Figure 3A). LGM2605 significantly inhibited both Il25 and Il33 genes. O3 increased both the proportion and the absolute count of BAL ILC2s in placebo but not LGM2605 treated macaques (Figure 3B–C). This effect was selective because other ILC counts did not change upon O3 exposure or anti-oxidant treatment. The pie charts show that the most abundant ILC in the macaques was ILC1 followed by ILC2 and then NCR3− and NCR3+ ILC3 in the airways. In the peripheral blood there were more NCR3-ILC3s than ILC2s (Figure 3D). O3 increased the ILC2 proportion in the BAL and the NCR3-ILC3 proportion in the peripheral blood (Figure 3D). Further, qPCR showed that O3 reduced gene expression of FACS isolated ILC2 for gata3, il13 and the neutrophilia-inducing pro-inflammatory cytokine Il17a (Figure 3F) and that these changes were not affected by anti-oxidant treatment (Figure 3F).
Figure 3. O3 increased ILC2 count in the BAL and NCR− ILC3 count in the peripheral blood.
(A) RNA was extracted from bronchial brush biopsies, and expression of the il25 and il33 genes were measured by qPCR. ΔΔCt (gapdh and rpl32), fold of the baseline/day 1 values in each individual monkey. (B) Blood and BAL ILCs: live CD90+Lineage−CD127+ cells. ILC2s: CD25+c-kitvar ILCs. Numbers show mean±SEM of the % of ILCs that were CD25+ (ILC2s). (C) ILC1s, ILC2s, NCR− ILC3s, and NCR+ ILC3s were quantified (cells/mL BAL ×103). ILC1s: c-kit−NKp44− ILCs; NCR+ ILC3s: NKp44+c-kit+ ILCs; NCR− ILC3s: NKp44−c-kit+ ILCs. (D-E) Pie charts: the relative proportions of the ILC subsets on day 1 and day 8. (F) RNA was extracted from blood ILC2s (live Lineage−CD90+CD25+c-kitvar cells) and expression of gata3, il13, and il17a was measured by qPCR. ΔΔCt (gapdh), expressed as fold of baseline/day 1; each macaque served as its own control. Mean±SEM of n=5–6; *p<0.05 (within groups: day 1 vs. day 8; A, D-F: Student’s paired t-test; C: Two-way ANOVA); #p<0.05 (between groups, Two-way ANOVA).
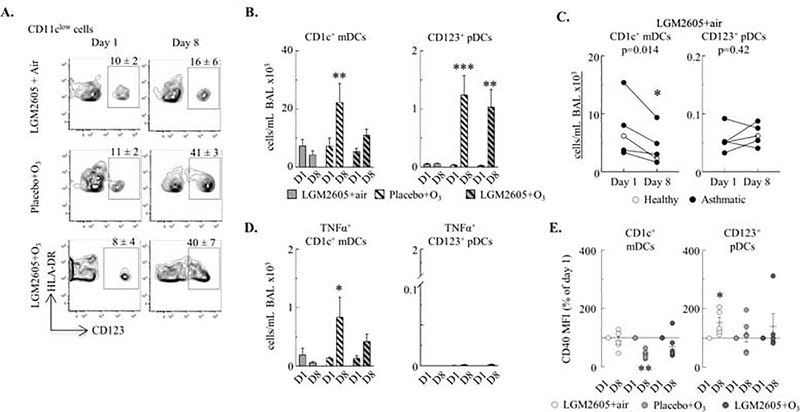
O3-induced pro-inflammatory mDC accumulation and activation was inhibited by LGM2605 in the BAL
Although it is not known how exactly DCs may regulate the inflammatory airway response, different DC subsets have specific functions (5) (Figure E7). We studied the myeloid CD1+ and CD16+ and the plasmacytoid CD123+ dendritic cell populations. We found the most striking changes in the absolute BAL count of CD1c+ mDCs and CD123+ pDCs in response to O3 (Figure 4A) while there were no significant changes in CD16+ mDCs from day 1 to day 8 (data not shown). Anti-oxidant LGM2605 treatment not only prevented the O3-induced increases in CD1c+ mDCs (Figure 4B) but it also reduced the number of these cells in air exposed macaques (Figure 4C). Further, the number of highly activated pro-inflammatory TNFα+ CD1c+ mDCs (but not the tolerogenic CD123+ pDCs), was significantly increased in the BAL following O3 exposure in placebo but not LGM2605-treated animals (Figure 4D). Expression of the membrane marker CD40 (involved in antigen presentation and other cell functions) (31), was decreased by O3 in CD1c+ mDCs but increased by anti-oxidant treatment in CD123+ pDCs (Figure 4E). These findings were specific to the airways because LGM2605 treatment or O3 exposure had no significant effects on DCs recovered from the peripheral blood of macaques (Figure E8).
Figure 4. O3-induced pro-inflammatory mDC accumulation and activation and increased the antigen-presenting pDC counts in the BAL.
(A) CD123+ pDCs: live CD45+CD11clowHLA-DR+CD123+ cells. Mean±SEM of CD11clowHLA-DR+ cells that were CD123+ (pDCs). (B) BAL CD1c+ mDCs (live CD45+CD11c+HLA-DR+CD1c+ cells) and CD123+ pDCs were quantified (cells/mL BAL ×103). (C) BAL CD1c+ mDCs and CD123+ pDCs were quantified in LGM2605+air treated macaques (cells/mL BAL ×103). (D) TNFα+ CD1c+ mDCs and CD123+ pDCs (cells/mL BAL ×103). (E) CD40 expression (MFI) on DCs in the BAL (% of day 1; each macaque served as its own control). Mean±SEM of n=5–6; *p<0.05, **p<0.01, ***p<0.001 (within groups: day 1 vs. day 8; C, E: Student’s paired t-test, B, D: Two-way ANOVA).
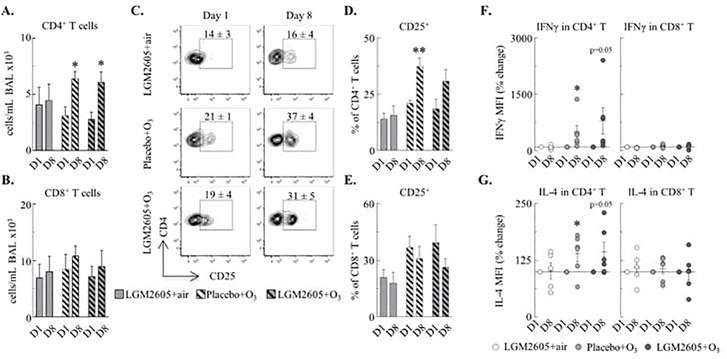
O3 activated BAL CD4+ T cells
Because we found increased numbers of ILC2s and DCs in response to O3 exposure in the macaques we wanted to know if T cells (Figure E9) would be involved in the O3 response. O3 exposure increased the absolute CD4+ T cell (Figure 5A–B) and activated (CD25+) CD4+ T cell count in the BAL but not CD8+ T cell numbers or activation when macaques were treated with placebo (Figure 5C–E). Expression (MFI) of the Th1 and Th2-type cytokines (IFNγ and IL-4, respectively) were increased in CD4+ but not in CD8+ T cells by O3 regardless of LGM2605 treatment (Figure 5F–G). In the peripheral blood, no changes were found in CD4+ or CD8+ T cells numbers (Figure E10). Collectively, these data demonstrate that O3 activated CD4+ (not CD8+) T cells in the BAL but not in the circulation. Th cell activation was not affected by LGM2605 in the macaques.
Figure 5. O3 activated BAL CD4+ T cells.
(A-B) CD4+ T cells (live CD45+CD3+CD4+CD8− cells) and CD8+ T cells (live CD45+CD3+CD4−CD8+ cells) were quantified (cells/mL BAL ×103). (C-E) Proportion of CD4+ and CD8+ T cells that were CD25+ (mean±SEM in flow plots and bar graphs). (F-G) IFNγ and IL-4 expression (MFI) was measured in CD4+ and CD8+ T cells (% of day 1; each macaque served as its own control). Mean±SEM of n=5–6; *p<0.05, **p<0.01 (within groups: day 1 vs. day 8, F-G: Student’s paired t-test; A-E: Two-way ANOVA).
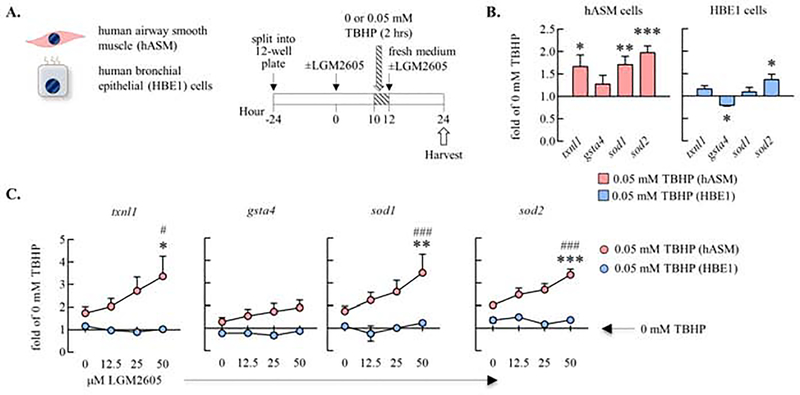
LGM2605 induced anti-oxidant gene expression in the presence (but not in the absence) of TBHP in human airway smooth muscles in vitro
Our prior work suggested that the mechanism of action of LGM2605 was induction of anti-oxidant gene expression in cells derived from bronchial brush biopsy. In the present study, treatment of macaques with LGM2605 prevented O3 induced increases in expression of the il6, sfptd, and il25 genes in cells derived from bronchial brush biopsy. Since the bronchial brush biopsy is comprised of a mix of airway epithelial, immune, and airway smooth muscle cells, among others, we tested the effect of LGM2605 on anti-oxidant gene expression (by qPCR)_in human airway smooth muscle (hASM) and immortalized human bronchial epithelial (HBE1) cells. We utilized tert-butyl hydroperoxide (TBHP) to simulate O3 exposure as TBHP is a stable form of hydrogen peroxide, a major chemical product of the inhalation of O3. hASM and HBE1 cells were treated with 0, 12.5, 25, or 50 μM LGM2605 for 24 hours total, while TBHP or control H2O was added to the culture medium from hour 10 to 12 (Figure 6A). In response to TBHP exposure, the anti-oxidant genes txnl1, sod1, and sod2 were significantly upregulated in hASM cells (Figure 6B). In HBE1 cells, sod2 was upregulated and gsta4 was downregulated by exposure to TBHP (Figure 6B). Txnl1, sod1, and sod2 expression was heightened in hASM, but not HBE1 cells, when LGM2605 was added to the culture supernatant pre- and post-exposure to TBHP (Figure 6C). Together, these data suggest that hASM are a unique target of LGM2605 heightened, TBHP-induced anti-oxidant expression.
Figure 6. LGM2605 induced anti-oxidant gene expression in the presence (but not in the absence) of TBHP in human airway smooth muscles in vitro.
(A) Human airway smooth muscle (hASM) and human bronchial epithelial (HBE1) cells were cultured in 12-well plates (−24h to 0h). At the 0h time point, fresh medium containing 0, 12.5, 25, or 50 μM LGM2605 was added. tert-Butyl hydroperoxide (TBHP) or control H2O was added to the wells (final concentration 0.05 or 0 mM, respectively) 10h later and cells were incubated for 2h. At the 12h time point the medium containing TBHP was replaced with fresh medium containing LGM2605 and cells were harvested in TRIzol 12h later. (B) Effect of TBHP on txnl1, gsta4, sod1, and sod2 antioxidant gene expression in hASM and HBE1 cells (qPCR). ΔΔCt (actb), expressed as fold of 0 mM TBHP. Mean±SEM of n=3–6 (hASM); n=2–3 (HBE1); *p<0.05, **p<0.01, ***p<0.001 (0 mM TBHP vs. 0.05 mM TBHP; Student’s unpaired t-test; (C) Dose dependent effects of LGM2605 on TBHP-induced txnl1, gsta4, sod1, and sod2 antioxidant gene expression. Mean±SEM of n=3–6 (hASM); n=2–3 (HBE1); *p<0.05, **p<0.01, ***p<0.001 (vs. 0 μM LGM2605; Two-way ANOVA) #p<0.05, ##p<0.01, ###p<0.001 (vs. 0 mM TBHP; Two-way ANOVA).
DISCUSSION
Our goals were to characterize the airway immune responses and their significance in lung function changes upon O3 inhalation in asthmatic macaques, and to assess the role of oxidative stress by treating the animals with synthetic secoisolariciresinol diglucoside, the anti-oxidant lignan LGM2605. We demonstrate here that O3 significantly augmented AHR and induced airway neutrophilia and eosinophilia, increased the number and activation of airway ILC2s, TNFα+CD1c+mDCs, CD123+pDCs and CD4+ T cells in macaques. LGM2605 had a selective inhibitory effect on airway neutrophil, ILC2 and TNFα+CD1c+mDC counts in response to O3 inhalation. The present work did not address if LGM2605 would facilitate inflammatory resolution after exposure to O3. Asthmatic macaques not exposed to O3, had their BAL total and TNFα+ mDC counts reduced and showed a trend for a reduction in AHR after one week of LGM2605 treatment suggesting the importance of oxidative stress in Th2-low AHR and that this drug could be a potential therapeutic modality in asthma. LGM2605 treatment did not affect O3-induced airway eosinophilia, however it remarkably prevented increases in AHR raising questions both about the role of eosinophils in mediating O3-induced AHR and a potential specific effect of LGM2605 on airway smooth muscle function. Using in vitro human cell lines we confirmed that in comparison with airway epithelial cells, smooth muscles were indeed highly responsive to oxidative stress as well as to treatment with LGM2605. This is the first comprehensive description of the airway mucosal immune players involved in the non-human primate repsonse to O3 and the effects of LGM2605 on AHR (graphical abstract).
We previously showed that when naïve mice are treated with O3 alone they develop a predominantly neutrophilic airway inflammation (6) but when they were sensitized and challenged with an allergen before O3 exposure, O3 induced an eosinophil predominant inflammatory response (29). These and other investigations in mice, non-human primates and humans suggested that O3 affects the healthy and asthmatic lungs in a highly differential manner (1, 32, 33). In the macaque studies however, there were some discrepancies to resolve. For example, acute exposure to 0.4 or 1 ppm O3 induced airway neutrophilia and eosinophilia, but not AHR (34–36) while repeated exposures to 0.5 ppm O3 in 11 two-week cycles elicited BAL eosinophilia and AHR to histamine, but not neutrophilia (37–40). To reconcile these disparate findings we therefore carefully selected our study animals from a previously characterized age and sex matched cohort that displayed AHR within the last 12 months. In addition, we developed a longitudinal design to account for individual genetic differences and variability in AHR in this outbred and heterogeneous population of macaques. This way we saw significant individual airway changes in response to a single exposure to O3 at an environmentally relevant level that wasn’t apparent previously. Further, the use of methacholine and not histamine for the induction of lung resistance revealed highly significant changes in AHR that previous studies may have missed (38, 39).
Our studies however posed some new questions related to a disconnect between the changes in O3-induced airway eosinophilia and the exacerbation of AHR. Our cohort of macaques was designated “asthmatic,” with the caveat that we did not assess the reversibility of airway obstruction and these animals did not present with a Th2-high inflammation at the baseline of the study. The etiology of “Th2-low asthma” is complex and possibly driven by multiple factors (41). The present cohort of macaques belonged to a larger population behaviorally investigated for manifestations of psychosocial stress, both a comorbidity and a risk factor for asthma (12, 42, 43). Indeed approximately 60% of stressed macaques in this cohort had AHR in the absence of atopy [by skin test to common aeroallergens (12)] or T cell activation in the periphery (12). AHR in these animals is thought to be driven by autonomic neuronal dysregulation of airways (12, 13, 44) leading to development of Th2-low asthma (41, 45). Social stress and AHR have been also associated with glucocorticoid receptor dysfunction significantly contributing to the airway changes and complicating therapeutic efforts in Th2-low asthma (46–48). Lastly, oxidative pathways maybe active in the absence of Th2-type inflammation driving cellular and molecular components of AHR (4).
To study the role of oxidative stress in the O3-induced airway changes we investigated LGM2605, a synthetic lignan secoisolariciresinol diglucoside (19–22). LGM2605 dose-dependently increased the expression of anti-oxidant genes in the lung tissue of macaques in vivo and in airway smooth muscle in vitro. We tested the hypothesis that this compound will protect from the effects of O3 inhalation. Interestingly, asthmatic macaques (with AHR) given LGM2605 showed a trend for improved lung function even without O3 exposure corroborating the involvement of oxidative stress in the pathogenesis and potential therapeutic effects on Th2-low asthma and especially on AHR. This was supported by the fact that LGM2605 had a disproportionately greater protective effect on lung function than on airway inflammation in response to O3 inhalation. In fact, this is the first study demonstrating the specific efficiency of an anti-oxidant lignan compound on alleviating AHR. The anti-inflammatory effects of LGM2605 were also selective and affected BAL neutrophil counts but not eosinophils in O3-exposed macaques. This observation will require further clarifications of its significance and mechanisms as it raises uncertainty in regards to the role of eosinophils in AHR. Using human airway epithelial (HBE1) and smooth muscle (ASM) cells in vitro we confirmed that LGM2605 specifically affects airway smooth muscles. In hASM, but not HBE1 cells LGM2605 highly enhanced TBHP-induced anti-oxidant gene expression. txnl1 and sod2 in fact were significantly upregulated by LGM2605 both in hASM cells in vitro and in macaques in vivo, suggesting these genes to be specifically important in mediating the effects of LGM2605.
Given the importance of oxidative stress in mediating the O3 effects, glutathione-related pathways may play a significant role. Glutathione-S-transferase (GST) gene variants for example, were suggested to influence the effects of O3 inhalation in asthmatic children (49). 15 nonsynonymous variants and 1 nonsense variant in Glutathione-S-transferase theta (GSTT)1 and 15 nonsynonymous variants in GSTT2 found in macaques were associated with GSTT-dependent individual drug responses (50) suggesting a role of GST and other antioxidant gene polymorphisms in air pollutant-induced asthma and may determine potential responsiveness to antioxidant treatment.
To investigate the possible molecular mechanisms involved in O3-induced inflammation and AHR, we studied the lung collectin surfactant protein D (SP-D). This immune protective airway epithelial molecule is constitutively expressed in the lung but during an inflammatory response its production is highly amplified. SP-D suppresses pro-inflammatory activation of immune and inflammatory cells in the lung (8, 51). We previously showed that oxidative damage to the quaternary structure of SP-D (9) or genetic deficiency of the SP-D gene (sfptd) conveys a heightened susceptibility to inflammatory changes (5, 7). Interleukin (IL)-6, a cytokine essential in eliciting neutrophilia and AHR in response to O3, is also important for inducing SP-D mRNA activation (7, 52). In the macaque airway epithelium, O3 induced both the il6 and sfptd genes and this effect was reversed by LGM2605. While O3 did not specifically alter the oligomeric SP-D structure in macaques it significantly reduced SP-D protein concentration and this effect was also prevented by LGM2605. Thus, even though O3 induced sfptd gene activation likely through autocrine effects of IL-6 on airway epithelial cells, it destroyed the released SP-D protein, known to be susceptible to oxidative damage (9, 53). Alleviation of oxidative stress by LGM2605 prevented these changes.
Innate lymphocytes (ILCs) are mucosal resident cells that are among the first to respond to foreign substances and pathogens, especially in the lung. We previously showed in mice that O3-induced AHR required a cooperation between airway epithelial cells, dendritic cells and ILCs (5–7, 9). ILC2s were indeed essential to mediate O3-induced airway inflammation and AHR (6). We hypothesized here that the O3 effects would involve activation of ILC2s in the macaques. Studies of ILCs in rhesus macaques are limited and to our knowledge, ILC2s have never been examined in this species. We developed a flow cytometry method based on studies in humans to examine these elusive cells (54). CD25 was used to define ILC2s in contrast to more specific ILC2 markers due to availability of commercial reagents, though prior studies in humans showed that of the ILC subsets, CD25 is expressed most highly on ILC2s (55). The genes for the ILC2 activating cytokines il25 and il33 in airway epithelial cells and the number of ILC2s in the BAL were markedly increased by O3, but not when animals were treated with LGM2605. Changes in ILC2 were selective and localized to the airways, revealing that O3 had compartment-specific effects on ILC populations. Intriguingly, altered expression of gata3, il13, and il17a mRNA in blood ILC2s from O3 exposed macaques suggest potential involvement in airway neutrophilia and hyperreactivity. We were surprised to find the expression of il17a altered in blood ILC2s since this is not a canonical cytokine for this population, though mouse models have shown that IL-25 signaling can induce IL-17A+ ILC2s (56).
We previously demonstrated the involvement of proinflammatory myeloid (m)DCs in the pathogenesis of O3- and allergen- induced airway inflammation in mouse models (5, 57, 58) and found that O3 increased the number of DCs in the airways, due in part to decreased recirculation to the mediastinal lymph nodes (5, 31). It was hypothesized that DC activation and trapping in the lung contribute to injury in response to O3. Our macaque study corroborates this hypothesis and shows that the proinflammatory myeloid CD1c+ DC population with increased TNF-α and decreased CD40, was accumulated in the airways of O3-exposed macaques. We also found that anti-oxidant treatment alleviated these changes and intriguingly inhibited CD1c+ DC count in the asthmatic macaques even without O3 exposure suggesting a role of oxidative stress in activation of these cells but not in CD123+ pDCs. That O3 would increase CD123+ pDC count in the airways was previously unknown and this finding will require further investigation especially because of the significance of these cells in anti-viral immunity (59). The reduction in the TNFα+ mDCs by LGM2605 treatment in O3 exposed macaques correlated to the reduction in AHR and BAL neutrophilia in the same animals highlighting the potential pathogenic role of these cells in mediating the O3 effects. Further, these results complemented our findings on ILC2s prompting us to speculate that mDCs and ILC2s may cooperate and instruct adaptive immune cells during O3-induced airway inflammation (60). Indeed, a recent study demonstrated that IL-33 derived from DCs induced ILC2 activation to promote airway inflammation (61).
The role of T cells in O3-induced airway inflammation and hyperreactivity is controversial. Acute exposure of humans to O3 increased or decreased activated (CD25+) CD4+ T cells recovered from the BAL (62). When CD4+ T cells were depleted from mice prior to O3 exposure, inflammation was either enhanced or reduced depending on the strain, while IL-4 consistently suppressed O3-induced inflammation (63). Our laboratory showed that CD4+ T cells were not activated upon O3 inhalation in Balb/c mice within the first 12 hours (6). In the present study, we showed that O3 increased IFNγ and IL-4 expression in CD4+ and CD4+CD25+ T cells independent of antioxidant treatment, albeit some of these changes were marginally significant (p=0.05). On the other hand, decreased expression of CD40 on DCs after O3 and increased number of CD4+CD25+ T cells suggests a tolerogenic phenotype (64). Because LGM2605 did not prevent increases in the numbers of these cells, it is unlikely that they play any role in improved AHR, similarly to our CD123+ pDC findings. Since neutrophils (Th1 or Th17-induced) and eosinophils and eosinophils (Th2-induced) were in the airways following O3, it is likely that this mixed inflammation was driven by mixed activation of Th-type cells. Taken together, our data on ILCs, DCs, and T cells suggest that O3 activates various arms of the immune system, likely Th1, Th2 and Th-17-type responses that play competing roles in mediating AHR and inflammation in asthma.
Our study demonstrates the role of mucosal immunity in the pathophysiology of O3-induced AHR and reveals the significance of oxidative stress in rhesus macaques (graphical abstract). We propose the therapeutic potential of LGM2605 as an adjuvant treatment for AHR and airway neutrophilia in asthmatics.
Supplementary Material
Clinical Implication.
Our study highlights the role of selective immune pathways and oxidative stress in a non-human primate model of asthma exacerbation by ozone inhalation. LGM2605 may have a clinical potential as an adjuvant asthma therapy
ACKNOWLEDGEMENTS
The authors thank Paul-Michael Sosa and Sarah Davis of the UC Davis CNPRC for their assistance in planning and executing this work. We thank the UC Davis air pollution journal club led by Laura Van Winkle for their critical reading of the manuscript. We acknowledge Dr. Angela Linderholm and Lisa Franzi for sharing their expertise in culture of hASM and HBE1 cells.
Support: T32ES007059 & T32 HL007013 (C.H.F); P30ES023513 pilot grant & R21AI116121 (A.H.); 1R41AI132012–01 (A.H. and T.S.) H12.01-8754 (T.S. and M.C.S); R24OD010962 (J.C.); CNPRC base operating grant P51OD011107 (Prasant Mohapatra).
Abbreviations
- (O3)
Ozone
- (LGM2605)
Synthetic secoisolariciresinol diglucoside
- (AHR)
Airway hyperreactivity
- (BAL)
Bronchoalveolar lavage
- (parts per million)
ppm
- (ILC)
Innate lymphoid cell
- (DC)
Dendritic cell
- (mDC)
myeloid DC
- (pDC)
plasmacytoid DC
- (CNPRC)
California National Primate Research Center
- (FACS)
Fluorescent activated cell sorting
- (qPCR)
Quantitative real time polymerase chain reaction
- (SP-D)
Surfactant protein-D
- (Raw)
airway resistance to methacholine)
- (IL-)
interleukin-
- (Nrf2)
nuclear factor-like 2
Footnotes
Conflict of Interest Statement: T.S. is the Chief Scientific Officer at LignaMed, LLC. M.C.S. has patents No. PCT/US2015/033501, PCT/US2016/049780, PCT/US17/35960, PCT/US2014/041636, No. PCT/US15/22501 pending and has a founders equity position in LignaMed, LLC. The other authors report no conflicts.
Additional materials can be found in the Online Repository Materials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kreit JW, Gross KB, Moore TB, Lorenzen TJ, D’Arcy J, Eschenbacher WL. Ozone-induced changes in pulmonary function and bronchial responsiveness in asthmatics. J Appl Physiol (1985). 1989;66(1):217–22. [DOI] [PubMed] [Google Scholar]
- 2.Chen LL, Tager IB, Peden DB, Christian DL, Ferrando RE, Welch BS, et al. Effect of ozone exposure on airway responses to inhaled allergen in asthmatic subjects. Chest. 2004;125(6):2328–35. [DOI] [PubMed] [Google Scholar]
- 3.Peden DB. The role of oxidative stress and innate immunity in O(3) and endotoxin-induced human allergic airway disease. Immunol Rev. 2011;242(1):91–105. [DOI] [PubMed] [Google Scholar]
- 4.Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O. Oxidative stress in asthma: Part of the puzzle. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2018;29(8):789–800. [DOI] [PubMed] [Google Scholar]
- 5.Ge MQ, Kokalari B, Flayer CH, Killingbeck SS, Redai IG, MacFarlane AWt, et al. Cutting Edge: Role of NK Cells and Surfactant Protein D in Dendritic Cell Lymph Node Homing: Effects of Ozone Exposure. J Immunol. 2016;196(2):553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Q, Ge MQ, Kokalari B, Redai IG, Wang X, Kemeny DM, et al. Group 2 innate lymphoid cells mediate ozone-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2016;137(2):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kierstein S, Poulain FR, Cao Y, Grous M, Mathias R, Kierstein G, et al. Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D. Respir Res. 2006;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haczku A Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J Allergy Clin Immunol. 2008;122(5):861–79; quiz 80–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousefi S, Sharma SK, Stojkov D, Germic N, Aeschlimann S, Ge MQ, et al. Oxidative damage of SP-D abolishes control of eosinophil extracellular DNA trap formation. J Leukoc Biol. 2018;104(1):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991;87(4):1470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golub MS, Hogrefe CE, Germann SL, Capitanio JP, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicology and teratology. 2006;28(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capitanio JP, Miller LA, Schelegle ES, Mendoza SP, Mason WA, Hyde DM. Behavioral inhibition is associated with airway hyperresponsiveness but not atopy in a monkey model of asthma. Psychosom Med. 2011;73(4):288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun K, Miller LA, Schelegle ES, Hyde DM, Capitanio JP. Behavioral inhibition in rhesus monkeys (Macaca mulatta) is related to the airways response, but not immune measures, commonly associated with asthma. PloS one. 2013;8(8):e71575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson R, Harris KE. IgE-mediated rhesus monkey asthma: natural history and individual animal variation. Int Arch Allergy Immunol. 1992;97(2):154–9. [DOI] [PubMed] [Google Scholar]
- 15.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990;247(4941):456–9. [DOI] [PubMed] [Google Scholar]
- 16.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, et al. Why primate models matter. American journal of primatology. 2014;76(9):801–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. 2015;135(2):299–310; quiz 1. [DOI] [PubMed] [Google Scholar]
- 18.Pietrofesa RA, Velalopoulou A, Albelda SM, Christofidou-Solomidou M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605). Int J Mol Sci. 2016;17(3):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrofesa RA, Woodruff P, Hwang WT, Patel P, Chatterjee S, Albelda SM, et al. The Synthetic Lignan Secoisolariciresinol Diglucoside Prevents Asbestos-Induced NLRP3 Inflammasome Activation in Murine Macrophages. Oxidative medicine and cellular longevity. 2017;2017:7395238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velalopoulou A, Chatterjee S, Pietrofesa RA, Koziol-White C, Panettieri RA, Lin L, et al. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage. Int J Mol Sci. 2017;18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra OP, Popov AV, Pietrofesa RA, Nakamaru-Ogiso E, Andrake M, Christofidou-Solomidou M. Synthetic secoisolariciresinol diglucoside (LGM2605) inhibits myeloperoxidase activity in inflammatory cells. Biochim Biophys Acta Gen Subj. 2018;1862(6):1364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrofesa RA, Chatterjee S, Park K, Arguiri E, Albelda SM, Christofidou-Solomidou M. Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605) Reduces Asbestos-Induced Cytotoxicity in an Nrf2-Dependent and -Independent Manner. Antioxidants (Basel, Switzerland). 2018;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herring MJ, Putney LF, St George JA, Avdalovic MV, Schelegle ES, Miller LA, et al. Early life exposure to allergen and ozone results in altered development in adolescent rhesus macaque lungs. Toxicol Appl Pharmacol. 2015;283(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonnell WF, Stewart PW, Smith MV, Kim CS, Schelegle ES. Prediction of lung function response for populations exposed to a wide range of ozone conditions. Inhal Toxicol. 2012;24(10):619–33. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Xue L, Brimblecombe P, Lam YF, Li L, Zhang L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci Total Environ. 2017;575:1582–96. [DOI] [PubMed] [Google Scholar]
- 26.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-Differentiated Human Airway Epithelial Cell Cultures In: Picot J, editor. Human Cell Culture Protocols. Totowa, NJ: Humana Press; 2005. p. 183–206. [DOI] [PubMed] [Google Scholar]
- 27.Velalopoulou A, Tyagi S, Pietrofesa RA, Arguiri E, Christofidou-Solomidou M. The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage. Int J Mol Sci. 2015;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrofesa RA, Velalopoulou A, Arguiri E, Menges CW, Testa JR, Hwang WT, et al. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis. 2016;37(2):177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kierstein S, Krytska K, Sharma S, Amrani Y, Salmon M, Panettieri RA Jr., et al. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergen-sensitized mice. Allergy. 2008;63(4):438–46. [DOI] [PubMed] [Google Scholar]
- 30.Haczku A, Atochina EN, Tomer Y, Chen H, Scanlon ST, Russo S, et al. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol. 2001;25(1):45–50. [DOI] [PubMed] [Google Scholar]
- 31.Brand JD, Ballinger CA, Tuggle KL, Fanucchi MV, Schwiebert LM, Postlethwait EM. Site-specific dynamics of CD11b+ and CD103+ dendritic cell accumulations following ozone exposure. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, et al. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis. 1989;139(2):407–15. [DOI] [PubMed] [Google Scholar]
- 33.Torres A, Utell MJ, Morow PE, Voter KZ, Whitin JC, Cox C, et al. Airway inflammation in smokers and nonsmokers with varying responsiveness to ozone. Am J Respir Crit Care Med. 1997;156(3 Pt 1):728–36. [DOI] [PubMed] [Google Scholar]
- 34.Hyde DM, Hubbard WC, Wong V, Wu R, Pinkerton K, Plopper CG. Ozone-induced acute tracheobronchial epithelial injury: relationship to granulocyte emigration in the lung. Am J Respir Cell Mol Biol. 1992;6(5):481–97. [DOI] [PubMed] [Google Scholar]
- 35.Plopper CG, Hatch GE, Wong V, Duan X, Weir AJ, Tarkington BK, et al. Relationship of inhaled ozone concentration to acute tracheobronchial epithelial injury, site-specific ozone dose, and glutathione depletion in rhesus monkeys. Am J Respir Cell Mol Biol. 1998;19(3):387–99. [DOI] [PubMed] [Google Scholar]
- 36.Hyde DM, Miller LA, McDonald RJ, Stovall MY, Wong V, Pinkerton KE, et al. Neutrophils enhance clearance of necrotic epithelial cells in ozone-induced lung injury in rhesus monkeys. Am J Physiol. 1999;277(6):L1190–8. [DOI] [PubMed] [Google Scholar]
- 37.Crowley CM, Fontaine JH, Gerriets JE, Schelegle ES, Hyde DM, Miller LA. Early life allergen and air pollutant exposures alter longitudinal blood immune profiles in infant rhesus monkeys. Toxicol Appl Pharmacol. 2017;328:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore BD, Hyde D, Miller L, Wong E, Frelinger J, Schelegle ES. Allergen and ozone exacerbate serotonin-induced increases in airway smooth muscle contraction in a model of childhood asthma. Respiration. 2012;83(6):529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, et al. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol. 2003;191(1):74–85. [DOI] [PubMed] [Google Scholar]
- 40.Chou DL, Gerriets JE, Schelegle ES, Hyde DM, Miller LA. Increased CCL24/eotaxin-2 with postnatal ozone exposure in allergen-sensitized infant monkeys is not associated with recruitment of eosinophils to airway mucosa. Toxicol Appl Pharmacol. 2011;257(3):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright RJ. Psychological stress: a social pollutant that may enhance environmental risk. Am J Respir Crit Care Med. 2011;184(7):752–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haczku A, Panettieri RA Jr. Social stress and asthma: the role of corticosteroid insensitivity. J Allergy Clin Immunol. 2010;125(3):550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller BD, Wood BL. Psychophysiologic reactivity in asthmatic children: a cholinergically mediated confluence of pathways. J Am Acad Child Adolesc Psychiatry. 1994;33(9):1236–45. [DOI] [PubMed] [Google Scholar]
- 45.Hargreave FE, Dolovich J, O’Byrne PM, Ramsdale EH, Daniel EE. The origin of airway hyperresponsiveness. J Allergy Clin Immunol. 1986;78(5 Pt 1):825–32. [DOI] [PubMed] [Google Scholar]
- 46.Stokes JR, Casale TB. Characterization of asthma endotypes: implications for therapy. Ann Allergy Asthma Immunol. 2016;117(2):121–5. [DOI] [PubMed] [Google Scholar]
- 47.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, et al. Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J Immunol. 2009;182(12):7888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam T, Berhane K, McConnell R, Gauderman WJ, Avol E, Peters JM, et al. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax. 2009;64(3):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uno Y, Murayama N, Kato M, Tanaka S, Ohkoshi T, Yamazaki H. Genetic Variants of Glutathione S-Transferase GSTT1 and GSTT2 in Cynomolgus Macaques: Identification of GSTT Substrates and Functionally Relevant Alleles. Chem Res Toxicol. 2018;31(10):1086–91. [DOI] [PubMed] [Google Scholar]
- 51.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5(1):58–68. [DOI] [PubMed] [Google Scholar]
- 52.Kierstein S, Cao Y, Yang X, Tliba O, Amrani Y, Salmon M, et al. IL-6 Induces Surfactant Protein D (SP-D) in Alveolar Type II Cells by the Stat-3 Pathway. Am J Respir Crit Care Med. 2005;171(7):A483. [Google Scholar]
- 53.Starosta V, Griese M. Oxidative damage to surfactant protein D in pulmonary diseases. Free radical research. 2006;40(4):419–25. [DOI] [PubMed] [Google Scholar]
- 54.Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014;124(5):700–9. [DOI] [PubMed] [Google Scholar]
- 55.Simoni Y, Fehlings M, Kloverpris HN, McGovern N, Koo SL, Loh CY, et al. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2017;46(1):148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359(6371):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hortobagyi L, Kierstein S, Krytska K, Zhu X, Das AM, Poulain F, et al. Surfactant protein D inhibits TNF-alpha production by macrophages and dendritic cells in mice. J Allergy Clin Immunol. 2008;122(3):521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koziol-White C, Forbes LR, Ducka B, Fehrenbach M, Kierstein S, Sharma SK, et al. Ozone-Induced Exacerbation Of Allergic Airway Inflammation Is Associated With Altered Surfactant Protein-D (SP-D) Structure And Activation Of Macrophages And Dendritic Cells Of The Airways. American Journal of Respiratory and Critical Care Medicine. 2010;181:A1151. [Google Scholar]
- 59.Jakab GJ, Spannhake EW, Canning BJ, Kleeberger SR, Gilmour MI. The effects of ozone on immune function. Environ Health Perspect. 1995;103 Suppl 2(Suppl 2):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tjota MY, Hrusch CL, Blaine KM, Williams JW, Barrett NA, Sperling AI. Signaling through FcRgamma-associated receptors on dendritic cells drives IL-33-dependent TH2-type responses. J Allergy Clin Immunol. 2014;134(3):706–13 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krishna MT, Madden J, Teran LM, Biscione GL, Lau LC, Withers NJ, et al. Effects of 0.2 ppm ozone on biomarkers of inflammation in bronchoalveolar lavage fluid and bronchial mucosa of healthy subjects. Eur Respir J. 1998;11(6):1294–300. [DOI] [PubMed] [Google Scholar]
- 63.Chen X, Gavett SH, Wills-Karp M. CD4+ T lymphocyte modulation of ozone-induced murine pulmonary inflammation. Am J Respir Cell Mol Biol. 1995;12(4):396–403. [DOI] [PubMed] [Google Scholar]
- 64.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Seminars in immunology. 2009;21(5):265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.