Abstract
Social behavior is pervasive across the animal kingdom, and elucidating how the brain enables animals to respond to social contexts is of great interest and profound importance. Our understanding of ‘the social brain’ has been fractured as it has matured. Two drastically different conceptualizations of the social brain have emerged with relatively little awareness of each other. In this review, we briefly recount the history behind the two dominant definitions of a social brain. The divide that has emerged between these visions can, in part, be attributed to differential attention to cortical or sub-cortical regions in the brain, and differences in methodology, comparative perspectives, and emphasis on functional specificity or generality. We discuss how these factors contribute to a lack of communication between research efforts, and propose ways in which each version of the social brain can benefit from the perspectives, tools, and approaches of the other. Interface between the two characterizations of social brain networks is sure to provide essential insight into what the social brain encompasses.
Keywords: Affective / value-based processing, Cognitive Social Brain, Complex cognition, Emotion, Empathy, Judgment, Mentalizing network, Mirroring/action-observation network, Planning, Social Behavior Network, Social Decision-Making Network, Theory of mind
Graphical Abstract
1. Introduction
Social behavior is a ubiquitous feature across animals, although the degree of sociality can vary profoundly in terms of form and frequency. For as long as people have studied social behavior, an underlying goal has been to understand the mechanisms that govern them. Neuroscientists with different backgrounds and expertise have attempted to define the core neural mechanisms that underlie social behavior, but defining a ‘social brain’ is as complicated as the behaviors that are presumably under its control. This is because social behavior takes so many different forms (affiliation, aggression, approach, consolation, grouping, mating, nurturing, play, etc.), each of which involves many different cognitive processes, and behavioral elements and interactions. The neural control of social behavior is inherently even more complex than the behaviors themselves. Nevertheless, determining how and where the brain processes and shapes behavior in response to social factors is of great importance if we are to ever truly understand the nature and universality of social behaviors.
Several attempts have been made to characterize the social brain, and the most dominant views are responsible for tremendous progress toward understanding social behavior. Inertia surrounding disciplinary and motivational origins, and constraints in technical and conceptual approaches to understanding the relationship between the brain and social behavior have unfortunately led to diverging visions of the constellation of neural structures that comprise the social brain. This review will focus on two distinct characterizations of a social brain. Leslie Brothers seeded the idea of a social brain that focused on regions of the brain required for specialized social cognition in primates (Brothers, 1990), paving the way for others to develop more detailed elaborations (e.g., Adolphs, 2003, 2009; Frith, 2007). A little later, Sarah Newman independently proposed that a very different network of highly interconnected regions of the brain formed a ‘Social Behavior Network’, which collectively supports a diverse range of social behaviors in mammals (Newman, 1999). Newman’s version has been modified to encompass greater taxonomic diversity since then, and has provided a very useful framework for behavioral ecologists and neuroscientists to understand the neural mechanisms of social behavior for a wide range of animals. These two very different visions of the social brain established a foundation for their respective branches of social neuroscience, each of which has progressed steadily over the past 30 years. Both views of the social brain have effectively pursued the same larger goal: to provide a characterization of what constitutes the social brain. Unfortunately, relatively little crosstalk has occurred between the two groups. Thus, two literatures - each aspiring to provide a deeper appreciation of the social brain - have progressed relatively independently, like two ships passing in the night.
Key differences central to the divide exist between these two research areas that frequently use the phrase ‘the social brain’ to refer to very different networks. The most notable difference boils down to the sub-units of the brain that comprise each. One view of the social brain, which we will refer to collectively as the Cognitive Social Brain (CSB) for simplicity, is constructed of mostly neocortical structures, described in primates and associated with what can be referred to broadly and in over-simplified terms as ‘high-order cognitive tasks’. The Social Behavior Network’ (SBN) provides a second characterization of the social brain, which is constructed of mostly limbic forebrain and midbrain structures that are evolutionarily ancient and conserved, governed by mechanisms that are equally ancient, and that are associated with relatively ‘primitive’ social behaviors.
Cortical structures primarily drew the attention of CSB researchers during the early stages of defining their version of the social brain. This focus was due to the elaboration of cortical structures in primates, and the documented role of several cortical structures in complex cognitive tasks. In contrast, the SBN focused on interconnected subcortical regions with an established role of hormonal regulation of social (sexual and non-sexual) behaviors across vertebrate species.
The chasm that lies between the cognitive social brain (CSB) and the social behavior network (SBN) is wide, but ironically, more unites the two visions than divides them. In this review, we will discuss the major areas of division between the CSB and the SBN that present a challenge to synthesis. These areas of division include (i) historical inertia and the locations where each network resides within the brain, (ii) the disciplinary and motivational roots for studying social behavior and the distinct methods that have been used to identify the components of the social brain, (iii) the degree of incorporating comparative perspectives, and (iv) an emphasis on domain general and collective action of regions vs. specificity/modularity of regions within the social brain. Within each section we will comment on recent developments and future directions that we hope will result in increased awareness of and interaction between these two literatures and move toward an integrative approach to exploring and conceptualizing the social brain. Along the way, we will promote the idea that these two characterizations actually function together as a single larger system that enables the cognitive processing of social information to facilitate social behavior ranging from simple to more elaborate forms of social behavior and cognition.
2. What is Meant by Social Behavior
Ultimately, whatever the social brain is, its function should be to facilitate social behavior and all that is involved with it. Thus, to understand the social brain, one must have a general understanding of what is meant by the term ‘social behavior’. We note that it is not our intention to provide a deep discussion on what is and is not social behavior here. For a comprehensive review on deconstructing sociality, see Goodson (2013). However, it is important to point out that the term ‘social behavior’ can be used to refer to a number of different aspects of behavior and cognition.
In the realm of animal behavior, sociality was originally formally used to discuss group living (Alexander, 1974). Since then, it has taken on a rather broad meaning and often serves as a panacea for several different types of behaviors that are often affiliative in nature (Carter et al., 2008; Donaldson and Young, 2008; Goodson, 2013). Specific behaviors that fall under this umbrella include: group-size preference, pairbonding, parental and alloparental care, and affiliative contact or huddling. At first glance, referring to these individual behaviors generally as social does not seem overly problematic. Yet, this ignores the fact that behaviors that involve social interactions, regardless of their positive or negative valence, are equally important for discussions of sociality. For example, the deterrence of social interactions seems equally qualified to be considered social behavior as prosocial behaviors. We suggest that ‘social behavior’ is best thought of as a multifaceted complex of several behaviors and processes that span the domain of pro- and anti-social interactions (see Kelly & Ophir, 2015), and extend beyond observable behaviors to include the cognitive processes that govern them.
In strict terms, behavior refers to observable motor responses. However, the use of the term social behavior can be used heuristically to capture something more general: the cognitive processes that ultimately influence behavioral output in social domains. For instance, social recognition or discrimination are great examples of relatively simple cognitive tasks that are nearly ubiquitous across animals, and often referred to as a form of social behavior. But should the term ‘social cognition’ refer to the social behavior it produces? Whether the terms social cognition and social behavior should be equated could come down to how one views the cognitive process and the motor output that results.
We consider cognition to be the neuronal processes associated with acquisition of, processing of, and ability to use, store, and retain information (c.f., Shettleworth, 2010; Dukas & Ratcliffe 2009). To extend this to the social domain, Weitekamp and Hofmann (2014) define social cognition as the ability to flexibly respond during social interactions by integrating the behavior of others with memories of past interactions and predictions of future behavior in real-time. This definition of social cognition closely overlaps with a definition offered by Brothers (1990), in which she characterized primate social behavior as the processing of any information that culminates in the accurate perception of the dispositions and intentions of other individuals. These definitions imply that social cognition is part and parcel of the behaviors that result from social interactions. Supporting this view, Redish (2017) has argued that ‘deliberation’ and cognitive updating in decision-making happens in real-time as the animal is actively behaving. With all of this in mind, it is understandable why the terms social cognition and social behavior are sometimes conflated.
If the cognitive processing of information is happening as motor responses are occurring, then it follows that social cognition is, itself, a central part of the expression of social behavior, even if it is not a measurable motor output, per se. Thus, a simplistic view could be that the neural processing of agonistic signals from conspecifics to inform the decision to fight, submit, or run, for example, qualifies as cognition, whereas the acts of fighting, submitting, or running (i.e., the specific outcomes of the ultimate decision-making process) are the behaviors. Similarly, theory of mind, for example, often refers to a ‘higher order’ cognitive process that may, or may not, result in observable motor outputs, and thus is exclusively ‘cognitive’. But at some level theory of mind processing will impact measurable social behaviors (like approach or avoidance) that result. Indeed, the distinction between where cognition ends and behavior begins is tricky, and it is easy to get lost between these two terms.
If one is attempting to evaluate what constitutes the social brain, as we are here, then we must primarily focus on the neural machinery that enables the processing of social information to inform social reactions. Therefore, in the sections that follow, when we talk about the neural processing of social information that results in social behavior, however simple or elaborate that processing might be, we are talking about social cognition. When we use the term social behavior, we can be referring to the specific pattern of motor outputs that are easily observed or quantified, but we might also refer to the combination of social cognition that works in tandem with the expression of that behavior, for the reasons explained above. We do not claim that these are the best characterizations of these terms, but we believe they are largely consistent with other views that have been developed beyond what we have discussed here and that they can be useful to discuss the topic at the heart of this review: what constitutes the social brain? We provide relatively brief characterizations of two prominent ways in which the term ‘social brain’ has been used in the following two sections. In both cases, the networks of structures are thought to be responsible for the processing of social information and either directly or indirectly lead to social interactions.
3. The ‘Cognitive Social Brain’ (CSB)
The first definitions of a social brain were inspired by neural mechanisms believed to support the unique social cognition of humans and other primates. Since then, researchers in this area have largely been concerned with trying to understand the kinds of complex sociality found in some primates (and occasionally other mammals) that tend to be relatively rare and/or unique across taxa. Several variations of this view have been developed (see below), but for ease we refer to these collectively as the cognitive social brain (or CSB for short).
Leslie Brothers is often credited with providing the first formalized definition of this view of the social brain (Brothers, 1990). She defined social cognition as “the processing of any information which culminates in the accurate perception of the dispositions and intentions of other individuals” and she proposed that a social brain was developed to support this cognition in primates. At a time before widespread availability of advanced neuroimaging technology, Brothers’ social brain was founded on the results of lesion studies, electro-stimulation studies, and single-unit recordings of neurons in the primate brain. Brothers proposed identifiable core operations supporting social cognition within the amygdala (AMG), temporal cortex (i.e., inferotemporal cortex and superior temporal sulcus), and orbital frontal cortex (OFC). She claimed that the primate amygdala, when compared to that of other species, supports more varied social affects (e.g., shame, triumph, jealousy, parental tenderness, romantic love) that are required for the complex social life experienced by primates. Brothers also noted the discovery of face-selective neurons in the amygdala (Leonard et al., 1985), and the inferotemporal cortex and superior temporal sulcus (Perrett et al., 1992). These neurons are proposed to be components of a system evolved to enable primates to interpret information about other individuals.
In the years that followed, Robin Dunbar and colleagues offered evolutionary explanations for the uniquely sizable neocortex in primates. Dunbar’s ‘social brain hypothesis’ suggested that primates evolved a large neocortex to cope with their unusually complex social lives (Dunbar, 1993, 1998, 2009; Dunbar and Shultz, 2007). This was supported by findings that, in primates alone, brain size is positively correlated with group size (but see DeCasien and Higham, 2019). In other taxonomic groups (e.g., birds, ungulates, carnivores), large brains were strictly associated with the presence of pair bonding behavior. Dunbar proposed that the social bonds between many members of a primate group were qualitatively similar to pair bonds observed strictly between mates in other species, and for this reason, group size was positively correlated with several measures of brain size exclusively in primates.
The early perspectives on the CSB provided a target for social neuroscience research on human and non-human primates. Over the course of the 1990’s and beyond, the CSB was developed to include other areas of the brain, or more specific sub-regions of areas that were previously identified. Similarly, the functions of this network were expanded, and new neural mechanism were identified. For example, the first mirror neurons in the premotor cortex and inferior parietal cortex of macaques were discovered in the early- to mid 1990’s (di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti et al., 1996). The discovery of ‘mirror neurons’ in the primate brain, and mirror systems for emotion in humans (Bastiaansen et al., 2009) served as a major milestone towards the proposal of specialized networks for perspective taking and other-oriented information. Around this time, positron emission tomography (PET) scanning in humans explored the neural correlates of theory of mind (ToM), defined as the unique human ability to attribute independent mental states to self and others in order to explain and predict behavior (Fletcher et al., 1995). Behavioral tasks that challenged ToM abilities in humans activated the dorsomedial prefrontal cortex, posterior cingulate cortex, temporal poles, and the posterior superior temporal sulcus (pSTS). The CSB became further defined with the widespread application of functional magnetic resonance imaging (fMRI) techniques to social cognitive neuroscience. Neuroimaging with fMRI identified a role of the temporoparietal junction (TPJ) in ToM (Saxe and Kanwisher, 2003). The access to better imaging tools led to a deeper understanding of the CSB. For example, several researchers used fMRI or PET scanning to demonstrate the role of the OFC in attributing emotional salience and reward/motivational value to social stimuli (Hynes et al., 2006; Moll et al., 2002; O’Doherty et al., 2003; Royet et al., 2000), and to show that the anterior cingulate cortex (ACC) was important for empathy (de Vignemont and Singer, 2006; Singer et al., 2004, 2006). Similarly, the fusiform face area was identified as a region selectively activated for faces in humans (Kanwisher et al., 1997). A major focus during this period of development of the CSB was to identify domain-specific regions responsible for well-defined aspects of social information processing or cognition.
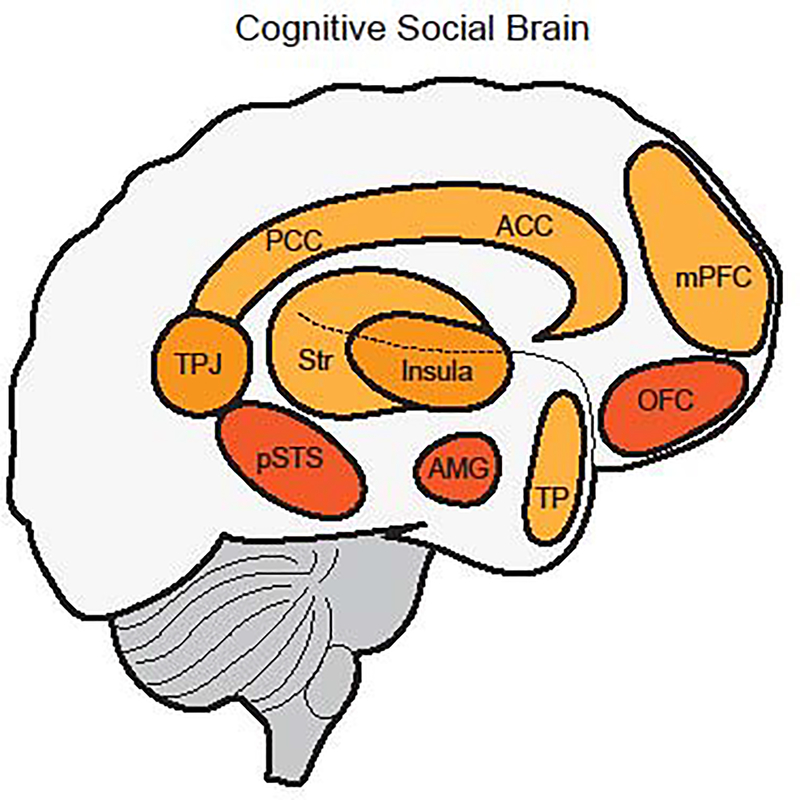
The cognitive social brain would be revisited and modified to account for additional areas of interest brought to light by these new methods and an increasing interest in social neuroscience as a subdiscipline (Adolphs, 2003; Frith, 2007; Lieberman, 2007; Ochsner and Lieberman, 2001). This updated cognitive social brain elaborated on the roles of the pSTS, the AMG, and the OFC (regions originally proposed by Brothers), and now included the ACC, TPJ, temporal poles, and the medial prefrontal cortex (mPFC) (Adolphs, 2003; Frith, 2007; Lieberman, 2007). The striatum and insula were also gaining notoriety for their roles in social cognition (Aharon et al., 2001; Kampe et al., 2001; Rilling et al., 2002; de Vignemont and Singer, 2006; Olausson et al., 2002; Winston et al., 2002). Taken together, the prevailing view of what we refer to here as the cognitive social brain network encompasses cortical structures (i.e., ACC, pSTS, mPFC, and OFC) working together with support from other cortical and non-cortical areas of the brain (i.e., AMG, TPJ, insular cortex, posterior cingulate cortex, striatum, and temporal poles) to regulate complex social behavior and decision-making (see figure 1).
Figure 1.
A simplified characterization of the Cognitive Social Brain (CSB). Brain areas shaded in dark (pSTS, AMG, OFC) represent the core, or original, components of the CSB, and lighter shaded brain areas (TPJ, PCC, ACC, mPFC, Str, TP, and insula) represent elaborations of the CSB that have been pervasively described as important for higher-order processing of social behavior. Abbreviations: pSTS: posterior superior temporal sulcus; AMG: amygdala; OFC: orbital frontal cortex; TPJ: temporoparietal junction; PCC: posterior cingulate cortex; ACC: anterior cingulate cortex; mPFC: medial prefrontal cortex; Str: striatum; TP: temporal poles.
Over the last decade, research has examined the social functions of core and associated regions at a finer spatial resolution (Adolphs and Tusche, 2017; Frith and Frith, 2010; Kennedy and Adolphs, 2012; Platt et al., 2016). The extensive neurophysiological recordings that have delineated social functions in macaques at the neuronal level provide just one example (Apps et al., 2016; Chang et al., 2013, 2015; Haroush and Williams, 2015; Klein and Platt, 2013). This research has revealed that social function can be highly specific to subpopulations of neurons within regions of the cognitive social brain. Another development of note is the delineation of specific networks for different aspects of human social cognition (Kennedy and Adolphs, 2012). For instance, a ‘mentalizing network’ comprised of the TPJ and dorsal mPFC (Lee and Seo, 2016), a ‘mirror neuron system’ (a.k.a, an ‘ action-observation network’) comprised of the ventral premotor cortex, inferior parietal lobule, and the STS (Kilner, 2011; Rizzolatti and Craighero, 2004), a ‘network for affective and value-based processing’ comprised of the amygdala, ventral mPFC, orbitofrontal cortex, and striatum (Ruff and Fehr, 2014), and a ‘network for empathy’ comprised of the ACC and anterior insula (de Vignemont and Singer, 2006) have all been built upon the foundation of the broader vision of the cognitive social brain outlined above.
Of the aforementioned networks and systems falling under the CSB umbrella, the mirror neuron system and the mentalizing network have received particular attention recently, and the two differ based on their distinct and critical roles during social interactions. The mirror neuron system consists of regions of premotor and parietal cortex that contain neurons with mirroring properties that, through association with visual processing areas (i.e., the STS), facilitate the processing of social signals (Rizzolatti and Craighero, 2004). In contrast, the mentalizing network facilitates the ability to predict and explain the behaviors of others (i.e., theory of mind; Lee and Seo, 2016). Due to their respective functions in ‘social detection’ and ‘social evaluation’, the mirror neuron system and the mentalizing network together might constitute the core elements involved in social information processing during interactions between humans (Vogeley, 2017). These two networks and their associated functions also sit at the heart of a debate around whether an understanding of others is achieved by automatic simulations (i.e., through the mirror neuron system) or by abstract inferences (i.e., through the mentalizing network) (Alcalá-López et al., 2019).
Advances in neuroimaging, which now allows for simultaneous imaging of two subjects, have begun to deepen our understanding of structure-function relationships in the CSB (reviewed in Schilbach et al., 2013). Such ‘hyperscanning’(Montague et al. 2002) techniques have identified brain areas that are recruited when a participant engages in tasks ranging from gaze following and joint attention (Schilbach et al., 2006, 2010) to social interactions during competitive games (Rilling et al., 2004; Haitu et al., 2017). For example, when subjects were told a ‘virtual other’ was controlled by a second participant, joint attention towards a visual stimulus led to recruitment of regions associated with the mentalizing system (i.e., mPFC and the posterior cingulate cortex; Schilbach et al., 2010). Furthermore, the ventral striatum was recruited when subjects initiated the gaze leading to joint attention, whereas responding to the gaze of the virtual other resulted in recruitment specific to the anterior mPFC (Schilbach et al., 2010). Interestingly, Sliwa and Freiwald (2017) identified social-specific activation within ventrolateral and medial prefrontal cortex in monkey-monkey interactions, suggesting evolutionary precursors to human mentalizing using similar scanning methods. Moreover, Saito et al. (2010) applied hyperscanning to two human subjects engaged in paired gaze following of a visual target on a monitor and discovered inter-subject synchronization of neural activity (i.e., fMRI BOLD signal) in the inferior frontal gyrus, a region of the mirror neuron system.
The emerging definition of a cognitive social brain placed a focus on mechanisms supporting the exceptional cognition of primates, cortical structures, and a search for regions with functions specific to a social domain. We acknowledge that not all concepts of what we have collectively referred to as the CSB are entirely consistent with or derived from the Brothers view. Reviews comparing and contrasting some of the different ways in which the networks and systems contributing to the cognitive social brain will offer a more detailed account than we are able to outline here (see Lieberman, 2012; Singer, 2012). Taken together in broad strokes, the CSB (i) has been largely based on data collected from human and non-human primates, (ii) is primarily (but not exclusively) located in the neocortex and therefore can be viewed as a relatively evolutionarily derived feature, and (iii) speaks to regulating behaviors that are primarily important for social decision-making, planning, emotion, judgment, and complex or ‘higher order’ cognition. An important theme underlying much of the research on the cognitive social brain has been to uncover the elements of the brain that enable a few extraordinary species (mostly primate) to accomplish the socially complex tasks that differentiate them from other animals and why these feats require so much neural real estate and the computing power to fuel it.
4. The Social Behavior Network (SBN) and the Social-Decision-Making Network (SDMN)
We acknowledge that the primate cortex is an astonishing evolutionary development that has enabled species such as our own to leverage and exploit a tremendous number of social and ecological aspects of our world. But the behavioral ecology literature has provided abundant examples of extraordinarily complex social behavior in animals that do not possess a neocortex comparable to the primate brain, if they contain one at all (Simons and Tibbets, 20019). Examples such as these clearly suggest that an exceptionally developed neocortex cannot alone account for the expression of complex social behavior.
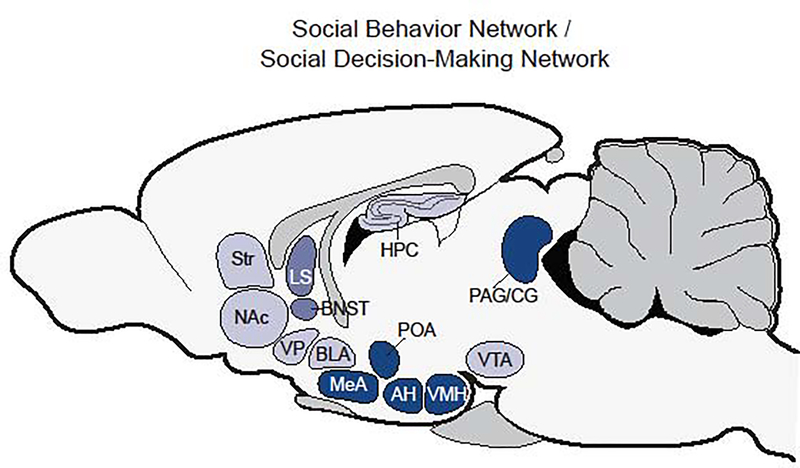
While the enterprise to define the CSB was well underway, Sarah Newman took a far different approach to outlining the neural substrates of social behavior. Newman’s Social Behavior Network (SBN) defined a circuit of subcortical brain regions conserved across mammals that are repeatedly and reliably implicated in governing a range of social behaviors (Newman, 1999). The circuit included the preoptic area (POA) of the hypothalamus, the medial extended amygdala (which is comprised by the medial amygdala (MeA) and the bed nucleus stria terminalis (BNST)), the lateral septum (LS), the anterior hypothalamus (AH), the ventromedial hypothalamus (VMH), and the periaqueductal grey (PAG) and the central grey (CG) (originally discussed vaguely as the midbrain) (see figure 2). The SBN was inspired by findings across the field of neuroethology that repeatedly supported these regions as regulators of male and female mating behavior, parental behavior, and various forms of aggression (Albert and Chew, 1980; Kollack-Walker and Newman, 1995; Lehman et al., 1980; Numan and Sheehan, 1997; Powers et al., 1987). According to Newman, these brain regions meet specific standards to qualify for inclusion in the SBN: Each region (1) must support more than one social behavior, (2) is reciprocally connected, and (3) contains sex steroid hormone receptors. Taken together, the SBN represents a subcortical limbic network that supports expression of the entire spectrum of sex-steroid modulated social behaviors.
Figure 2.
A simplified characterization of the Social Behavior Network (SBN) and Social Decision-Making Network (SDMN). Brain areas shaded in dark (LS, BNST, POA, MeA, AH, VMH, PAG/CG) represent the SBN. Brain areas shaded lightly (NAc, Str, VP, BLA, HPC, VTA) represent the additional structures that comprise the SDMN, in which the SBN was modified to include core structures from the mesolimbic reward system toward understanding how the brain integrates valence with processing of social information. The LS and BNST have intermediate shading to indicate that they are included in both the SBN and the mesolimbic reward system. Abbreviations: LS: lateral septum; BNST: bed nucleus of the stria terminalis; POA: preoptic area of the hypothalamus; MeA: medial amygdala; AH: anterior hypothalamus; VMH: ventromedial hypothalamus; PAG/CG: periaqueductal grey/ central grey; NAc: nucleus accumbens; Str: striatum; VP: ventral pallidum; BLA: basolateral amygdala; HPC: hippocampus; VTA: ventral tegmental area.
This view of the social brain was largely inspired by early research in hamsters, wherein lesions to different sub-regions of the MeA and BNST resulted in specific sexual behavior deficits (Lehman et al., 1980). Further, analysis of expression of the immediate early gene c-fos demonstrated that the sub-regions of the MeA and BNST in male and female hamsters are selectively active during both sexual behavior and aggressive encounters (Joppa et al., 1995; Kollack-Walker and Newman, 1995; Wood and Newman, 1993). Other research that contributed to the development of the SBN utilized discrete lesions, electrical stimulation, local hormonal or neuropharmacological manipulations, and immediate early gene (IEG) expression to demonstrate that interconnected limbic regions of the SBN (i.e., LS, mPOA, AH, and VMH), and the midbrain, mediate a range of sexual, parental, and territorial behaviors in mammals (Goodson and Kabelik, 2009).
Newman (1999) emphasized that separate social behaviors are an emergent property of specific, dynamic, temporal patterns of activity across the SBN, and that no single node of the SBN initiates or exterminates a given behavior. Rather, Newman (1999) proposed that social behavior is regulated by a collective tuning of activity between the six nodes of the network, which mediate the emergence of one social behavior over another. Specifically, this view argues that the activation of each subunit of the SBN relative to each other provides nuanced differences in overall neural patterning that can lead to behavioral outcomes that differ based on social context. The overarching pattern could take the form that Newman, Goodson, and Crews have depicted with different nodes peaking or being silenced under different social contexts (Crews, 2003, 2008; Goodson, 2005; Newman, 1999). Alternatively, the patterns of activation in different social contexts could produce subtler differences. For example, the overarching patterns might look largely the same but differ by magnitude (e.g., having the same relative pattern but all nodes showing more activation by one or more orders of magnitude in one context compared to another). The collective patterns of neural activation appear to differentiate courtship, copulation, attacks, submissiveness, territorial marking, nest building, nursing, mate guarding, protection of young, and other social behaviors (Newman, 1999). In this way, Newman (1999) avoided strong claims of domain-specificity for any particular brain region.
Newman’s social behavior network established a framework developed in a rodent upon which behavioral neuroscientists and neuroendocrinologists could interpret their findings in other taxa. One of the early applications of the SBN demonstrated the variable increases in metabolic activity (via cytochrome oxidase histochemistry) across the network following sexual experience, and as a function of early life experience (i.e., incubation temperature on aggression in geckos or family structure in mice) (Crews, 2003, 2008). Jim Goodson emphasized that this circuit was highly conserved across vertebrate taxa, and synthesized literature on homologous neural circuits in teleost fish and birds that mediate social behavior (Goodson, 2005). For example, activity of a homologous SBN in midshipman fish mediates this species’ intra- and intersexually dimorphic vocalization behavior, which is utilized for both courtship and agonistic interactions (Goodson and Bass, 2000). Neurophysiological recordings of sonic motor neurons, which fire in a one-to-one manner in relation to fundamental frequency and duration of calling behavior, allowed Goodson and Bass (2000) to investigate the contributions of SBN activity to these very specific and acutely measurable social phenomena. Tract tracing confirmed a connection between vocally active regions of the midshipman forebrain that are homologous to those proposed in the SBN: the POA, AH, and a VMH homolog (anterior tuberal nucleus) (Forlano et al., 2005). In birds, patterns of IEG induction across the SBN during agonistic and sexual interactions are highly similar to those found in mammals (Goodson, 2005; Goodson and Evans, 2004). The LS emerges as a particularly interesting region within the SBN, wherein Goodson and colleagues identified several LS subdivisions in which responses to social stimuli and aggressive behavior occur in a subregion-specific manner (Goodson et al., 2004, 2005), and a BNST-LS sub-circuit appears to modulate social grouping across gregarious and territorial finch species (Goodson et al., 2012; Kelly et al., 2011). It is important to note that both Newman and Goodson acknowledged that the SBN should only be regarded as the ‘core’ of the social brain, and not the social brain in toto; both acknowledged additional basal forebrain regions that regulate stress and reward processes, and cortical areas that serve executive functions. Ultimately, the following common themes united the original research inspired by the SBN: (i) an emphasis on comparative perspectives of a conserved network, (ii) the importance of neuromodulatory sex steroids in dictating social functions within the network, and (iii) the emergence of social behavior from collective action of the network nodes.
In 2011, Lauren O’Connell and Hans Hofmann proposed an extended network that incorporated the SBN with the mesolimbic reward system. O’Connell and Hofmann (2011) proposed that the mesolimbic reward system could function to evaluate the salience of an external stimuli (whether social or asocial), but that its interface with the SBN provided the motivation and valence to functionally evaluate and engage in social behavior. Like the SBN, the mesolimbic reward system is a forebrain-midbrain network of interconnected brain regions. The major sub-units of the mesolimbic reward system include the nucleus accumbens (NAc) and the ventral tegmentum area (VTA), however it also includes the LS and BNST (which serve as crucial relay points between the reward system and the SBN), the striatum (Str), the ventral pallidum (VP), the basolateral amygdala (BLA), and the hippocampus (HPC) (see figure 2). O’Connell and Hofmann (2011) argued that the SBN and the mesolimbic reward system were best viewed as two sub-networks that together worked in coordination to form what they called the ‘Social Decision-Making Network’ (SDMN). The SDMN, in their view, is responsible for regulating and implementing adaptive behavioral outputs in response to salient environmental challenges and opportunities (O’Connell and Hofmann, 2011). They went on to demonstrate the deep homology of the SDMN in each major class of vertebrate. For example, the full suite of components of the SDMN are found across all vertebrate taxa with only a few exceptions (e.g., absence of homologs for VP and MeA in teleost fish). O’Connell and Hofmann (2011) acknowledge previous recognition of the prefrontal cortex (PFC) as a region in the mammalian reward system, however an unclear homology of the PFC between vertebrates makes it a particularly difficult region to include in a conserved SDMN (Reiner, 1986). This is an important point considering that the PFC is a critical area of interest for people who research the cognitive social brain and could suggest that the role of an elaborated cortex in social behavior began earlier in evolutionary history than at the point of divergence between primates and other vertebrates.
Upholding the comparative tradition of the SBN, O’Connell and Hofmann (2012) mapped expression profiles for gene products across the SDMN of 88 species representing five major lineages of vertebrates (mammals, amphibians, reptiles, birds, and teleost fish). They found that across nodes of the SDMN, neurochemical profiles associated with dopamine, sex steroid, and nonapeptide systems are remarkably conserved between the major vertebrate lineages. In particular, the distribution of receptors for these neurochemical systems across the SDMN shows remarkably little taxonomic variation. Accordingly, species differences in social behavior between or within major vertebrate lineages may emerge from selection pressures on either the sites of ligand production or the relative densities of receptors across the SDMN (O’Connell and Hofmann, 2012).
The more recent work on these networks drew strong attention to the fact that the roles of nonapeptides were extremely important to the function of the SBN and the SDMN. Indeed, nonapeptides (i.e., vasopressin/vasotocin, and oxytocin/mesotocin/isotocin, etc.) have long been known for their importance in modulating a spectrum of social behaviors, and their rich evolutionary history in doing so (see Goodson and Thompson, 2010). For example, oxytocin and vasopressin might be most popularly recognized for their roles in modulating mating behavior in monogamous and non-monogamous species of voles (Johnson and Young, 2017; Walum and Young, 2018; Young and Wang, 2004), but oxytocin/vasopressin-like peptides also impact mating decisions in the worm, Caenorhabditis elegans (Garrison et al., 2012). Furthermore, the roles nonapeptides play in social behavior extend well beyond reproduction and the mating context and are crucial for social grouping (solitary or gregarious behavior), social approach, play, social cognition (e.g., social recognition and discrimination), consolation/altruism, and numerous other non-reproductive social behaviors (e.g., Bredewold et al., 2014; Kelly and Goodson, 2013; Kelly et al., 2018; Goodson & Thompson 2010; Burkett et al., 2016; Choleris et al., 2013; Young, 2002). Indeed, nonapeptide system variation between nodes of the SBN serve as a mechanism of socio-behavioral variation both within and between species and have been implicated in many of the aforementioned behaviors (Albers, 2012; Goodson and Bass, 2001). These signaling molecules were not included as a centerpiece of Newman’s original criteria, but they are now explicitly touted as fulfilling a prominent role within the context of the SBN and/or the SDMN function (Johnson and Young, 2015; O’Connell and Hofmann, 2011, 2012; Ophir, 2017; Zheng et al., 2013).
The SBN and SDMN, which we will primarily refer to collective as the SBN for simplicity, offer a useful foundation upon which studying the mechanisms of social behavior from a comparative perspective is possible and speak to the evolutionarily conserved nature of the neural mechanisms that govern social behavior (e.g., Cummings and Ramsey, 2015; DiBenedictis et al., 2017; Smith et al., 2017; Teles et al., 2015; Zheng et al., 2013). Considering the deep evolutionary roots and ubiquity of social behavior across animals, it is not surprising to find ancient neural structures governed by ancient signaling molecules like steroid hormones or nonapeptides. The SBN emphasizes domain general properties of regions of the brain that serve to collectively process social information and shape social behavior.
5. Contrasting views of the social brain
In characterizing the CSB and SBN, we have alluded to some key differences central to the divide between the research areas that use the phrase ‘the social brain’ to refer to very different networks. Despite the overarching differences in where the social brain can be found, some elements of the brain have been implicated in both characterizations of it. For example, both networks include the amygdala. The role of the amygdala in attributing emotional salience to social information seems consistent between both CSB and SBN perspectives, resulting in a range of social behaviors related to fear, anxiety, and aggression across vertebrates (Costafreda et al., 2008; Newman, 1999; Phelps and LeDoux, 2005). Expanded definitions of both social brain theories also address the role of the striatum in social behavior (Adolphs, 2003; Frith and Frith, 2010; O’Connell and Hofmann, 2011). Generally, the striatum is known for its role in attribution of reward value to social information and regulating the motivation for different forms of social interaction (Báez-Mendoza and Schultz, 2013; Izuma et al., 2008; Klein and Platt, 2013; Wake and Izuma, 2017). The striatum and amygdala serve as relay points between socially relevant sensory information and cortical processing, and therefore represent convergence points between the CSB and the SBN.
The lateral septum is another core region of the SBN (Goodson, 2005; Goodson et al., 2004; O’Connell and Hofmann, 2011) that has become a centerpiece toward understanding the neuromodulation of social behavior. Indeed, several social functions have been associated with the LS, including pair bonding (Liu et al., 2001), aggression (Leroy et al., 2018; Wong et al., 2016), sociality/gregariousness (Goodson et al., 2009), and maternal care (Champagne et al., 2009; Curley et al., 2012). In his 2013 book ‘Social’, Matthew Lieberman highlights the lateral septum as the most unjustifiably ignored region of the brain by people interested in the CSB (Lieberman, 2013). Lieberman believes this oversight is due to the relatively small size of the structure when compared to the cortical regions that were of primary focus and more detectable by early fMRI. He cites relatively recent findings that support the role of the LS in human empathy. For example, the LS was the only region identified in an fMRI study that was commonly activated across empathy for pain, anxiety, and happiness (Morelli et al., 2014). Moll et al. (2011) highlights damage to the LS as a mediator of impairments to prosocial sentiments in subjects with a behavioral variant of frontotemporal dementia. At the time of publishing his book, Lieberman predicted the LS would be the “hot area of study in the next ten years” (Lieberman, 2013). Considering the vast involvement of the LS in so many forms of social behavior across taxonomic groups (e.g., O’Connell and Hofmann, 2011), and its extensive connections throughout the brain and the aforementioned areas implicated in social behavior in particular (Reichard et al., 2017; Risold and Swanson, 1997; Vertes, 2004), we echo the hope expressed by Lieberman (2013) that increased focus will be placed on the role of the LS in primate social cognition, and facilitate bridges between the CSB and the SBN across vertebrate taxa.
Just as non-cortical areas of the brain are tied to the CSB, the SBN is not completely independent of cortex. Several studies have implicated the medial prefrontal cortex as critical in many social behaviors described in rodents (Ko, 2017). Indeed, these studies are gradually exposing compelling parallels between the cortical circuitry mediating social cognition in rodents and humans (Bicks et al., 2015). For example, neurons in the mPFC of mice fire faster during social investigation than novel object investigation (Lee et al., 2016). Neurons in the mPFC of socially high-ranking mice exhibit stronger excitatory synaptic inputs when compared to their subordinate cage mates, and manipulations that increase or decrease the synaptic efficacy in these neurons, respectively, increase or decrease a mouse’s social rank (Wang et al., 2011). Optogenetic stimulation of mPFC neurons in mice that normally express antisocial symptoms of depression following chronic social defeat stress demonstrate normal social interaction (Covington et al., 2010). Exploring the relationship between these findings and the well-documented role of the dorsal mPFC in more complex human social cognition (e.g., mentalizing) is an exciting direction for future study (Amodio and Frith, 2006).
The anterior cingulate cortex has also emerged as a region of great interest for social cognition in both rodents (Burkett et al., 2016; Jeon et al., 2010) and primates (Apps et al., 2016; Chang et al., 2013; Rudebeck et al., 2006). Primate research has highlighted the importance of the ACC gyrus (ACCg) as a subregion with functions specific to social cognition (Rudebeck et al., 2006). For example, compared to other regions of the ACC, a relatively large proportion of neurons in the ACCg of macaques respond solely to the reward outcome of others in a modified dictator game (Chang et al., 2013). These unique social functions of the ACCg may be supported by its unique connectivity profile placing the ACCg at the nexus between the mentalizing network (i.e., TPJ and dorsal mPFC), mirror neuron system (i.e., ventral premotor and inferior parietal cortices), and amygdala network (i.e., amygdala and ventral mPFC) (see above; Apps et al., 2016). In rodent studies, the ACC has been implicated in empathy related behaviors, including observational fear learning in mice (Jeon et al., 2010) and consolation behavior towards social partners in prairie voles (Burkett et al., 2016). Research on the ACC has resulted in including it and the anterior insula in an ‘empathy network’ (de Vignemont and Singer, 2006). We will expand on comparative considerations of the ACC’s role in empathy in the next section. Taken together, the commonality of sub-cortical and cortical structures in each version of the social brain highlights the value in potentially broadening each network to incorporate more limbic and cortical areas. Indeed, the extent to which the CSB has historically underappreciated subcortical structures has been matched by the degree to which the SBN has offered insufficient perspective on the role of the cortex in social behavior (e.g., Rogers-Carter and Christianson, 2019). Bridging this division of regional focus is a major step towards providing a cohesive portrayal of the social brain. We propose that ongoing study of the potential parallel functions found in the amygdala, striatum, medial prefrontal cortex, anterior cingulate cortex, insular cortex, and lateral septum across vertebrate taxa is particularly relevant to this goal.
Just as research toward developing each vision of the social brain has begun to identify the same regions of interest, a focus on the signaling molecules that operate within them has pointed toward exciting points of convergence. For example, as mentioned above, nonapeptides have become a centerpiece toward understanding the SBN (e.g., Goodson, 2005), but the role of oxytocin (and its homologs in non-mammalian vertebrates, OT for simplicity) has become increasingly prominent in studies that bridge both the CSB and SBN. Social bonding takes many forms (e.g., parental, peer, pairbonding with mates, etc.), at least some of which can be found in most vertebrates to some degree. The prefrontal cortex and the nucleus accumbens / ventral striatum are sites within the rodent brain where manipulating the oxytocin receptor facilitates or prevents pair bond formation with a mating partner (Young and Wang, 2004). Parallels between species differences in nonapeptide function in social bonding between vole species and between ape species have been proposed as potential evidence for convergent evolution for the neural modulation of social bonding (Donaldson and Young, 2005), and social bonding serves as a cornerstone for some hypotheses that explain the evolution of large group size and how advanced social cognition evolved in humans and other primates (Dunbar and Schultz, 2007). It is important to note, however, that the social functions of OT extend well beyond bonding. For example, in humans OT has a long-established history in maternal care beyond bonding and, more recently, many varieties of more complex versions of social behavior including trust, affiliation, alterations in social salience, ingroup-outgroup discrimination, and ‘mind-reading’ (Domes et al., 2007; Feldman, 2012; Feldman et al., 2011; Kosfeld et al., 2005; Shamay-Tsoory and Abu-Akel, 2016; Zhang et al., 2019). As research on the CSB becomes increasingly invested in the use of intranasal oxytocin to study social cognition in humans (Baumgartner et al., 2008; Churchland and Winkielman, 2012; Van IJzendoorn and Bakermans-Kranenburg, 2012), insights surrounding the function of nonapeptides in the SBN offer a resource for the study of conserved or specialized roles of oxytocin in the human social brain. Furthermore, the SBN could be used as a platform for understanding the neural basis of simpler forms of social behavior in humans, such as aggression and affiliation and the many other signaling and neuromodulatory molecules that govern these and other social behaviors. Indeed, the neuromodulatory action of nonapeptides and gene-regulatory actions of steroid hormones in the brain and their implications for social behavior are becoming increasingly appreciated (reviewed in McCall and Singer 2012; Bos et al. 2012). Despite this growing knowledge, precise localization of these mechanisms in a manner compatible with the SBN has not been explored to our knowledge. Moving forward, insights from the SBN and CSB can serve as alternative roadmaps of locations in the brain where a variety of neural mechanisms are likely influencing social behavior in both humans and non-humans.
6. Different emphasis resulting from different tool-kits and techniques
Distinct methodological approaches may further bias the perceived importance of cortical or subcortical structures to the social brain. For example, the CSB was founded on evidence supported by lesion studies and single-unit recordings in non-human primates, and shortly thereafter neuroimaging techniques such as PET scanning and fMRI were applied to localizing social cognition in the human and non-human primate brain. Indeed, early forms of fMRI and other neuroimaging and neurorecording technology may have unintentionally emphasized the role of cortical regions during behavioral tasks due to easy access of the outer cortical layers and because large areas of activity and/or dense vascularization in these brain regions produced interesting signals (Menon, 2012). In contrast, a descriptive and functional definition of the various nodes of the SBN was established using a variety of ex vivo histological imaging methods, like immunocytochemistry (ICC), qPCR, in situ hybridization, autoradiography, and more recently genomic and transcriptomic assessments of gene-mRNA-protein expression across the brain. For example, the original characterization of the SBN was derived from studies that relied on ICC quantification of the IEG c-fos as an indirect marker of neuronal activation. IEG ICC is superficially comparable to fMRI in that it can produce high fidelity comprehensive snapshots of neural activation. Yet, despite these face-value similarities in approaches, the tool-kits that have been used to characterize each version of the social brain are fundamentally different in timescale, repeatability, reach, and species for with they can be used.
For obvious reasons, the ability to conduct ex vivo brain studies or manipulate the brain with transient or permanent lesions, pharmacological or transgenic manipulations, optogenetics, and chemogenetics is much more practical when studying non-human and non-primate species. The ability to describe the extent of gene or protein expression in human or non-human primate brains, as has been done in other species, is relatively limited. Nevertheless, advances in these techniques has begun to allow comparable characterizations of brain areas important for social behavior in humans and other primates (Freeman et al., 2014a, 2014b, 2016, 2018). Refinement of the SBN has been achieved through precise manipulation of its interconnected sub-units and the determination of the molecular signaling and modulating factors that operate within them. For example, optogenetic manipulation of specific subpopulations of galanin-expressing neurons in the POA was shown to dictate parental care, aggression towards pups, and mating behavior in both virgin male and female mice (Wu et al., 2014). Although these techniques are not available for human research, cognitive neuroscientists can use non-invasive techniques, including rapid transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), to safely manipulate components of the social brain (Buckholtz et al., 2015; Knoch et al., 2006; Ruff, 2018). Similarly, applying rTMS in conjunction with fMRI imaging in awake rodents is a recently developed extension to this approach that holds great promise (Seewoo et al., 2018a, 2018b).
Although IEG ICC affords exquisite visualization of neuron-level activity in the brain, it comes at the cost of temporal resolution because it is limited to labeling neurons that were active within a specific window of time before sacrificing an animal. Snapshots of IEG expression fail to represent the progression of neuronal activity patterns over time, in real-time, and/or over repeated behavioral testing that fMRI offers. Recent technological developments such as serial two-photon tomography enable whole-brain IEG activity maps from behaving rodents (Kim et al., 2015; Renier et al., 2016). Although still relatively limited in temporal resolution, the images produced by this technique offer an unprecedented spatial representation of neuronal activity, and have the potential to compare descriptive images of brain activation across vertebrate species in a manner that is more compatible with fMRI. Similarly, calcium imaging and fiber photometry offer the ability to visualize neuronal activation at specific brain sites (in cortex or deep brain targets) in real-time that allow unprecedented assessment of brain activity than ever before (e.g., Murugan et al., 2017; Remedios et al., 2017; Liang et al., 2018; Kingsbury et al., 2019; Wang et al., 2019). These techniques are currently unable to assess activation across the entire brain, although new innovations are moving toward multiple site monitoring (de Groot et al., 2019). The extent to which fMRI has been directly used to image whole brain activity of awake non-primates is limited, and even less common for questions of social behavior. However, this is changing too (Bajic et al., 2017; Madularu et al., 2017; Yee et al., 2016). For example, Kenkel et al., (2016) used fMRI to identify neural responses in transgenic Fragile X versus wild-type rats when exposed to social and non-social odors.
The clear strength of fMRI and PET neuroimaging is that they offer the benefit of widespread visualization of neural activity during real-time social behavior. Furthermore, using fMRI in primate experiments can assess relatively advanced social behavior because complex tasks can be implemented using subjects that have been instructed or trained to respond to visual stimuli such as videos of conspecific social interactions using touchscreens, buttons, or other manipulanda. More recently a ‘two-person’ approach to describing the cognitive social brain has established an improved ethologically relevant means of neuroimaging during social interaction (reviewed in Schilbach et al., 2013). In this approach participants have their neural activity measured as they either interact with virtual others or a second live participant (i.e., ‘hyperscanning’) (Montague et al., 2002). This form of fMRI has been successfully implemented in non-human primates (e.g., Sliwa and Freiwald, 2017) and promises to significantly extend what we know about how the brain functions under interactive social contexts.
On the other hand, fMRI and PET neuroimaging has been criticized for not easily generating reproducible or precise localization of function (Logothetis, 2008; Bennett and Miller, 2010; Eklund et al., 2016). However, it is important to emphasize that many of the core social brain claims, such as the existence of a ToM network, have replicated many times. Further, recent technical advances have improved fine-scale detection of changes with fewer pixels and computational correction of vascularization (Kalcher et al., 2015). Other challenges of neuroimaging in non-primate species will also limit the extent to which fMRI can be used. For example, assessing behavior in awake rodents under fMRI is particularly challenging because they are less amenable to training paradigms that measure behavioral responses to videos when restrained for imaging purposes. Indeed, these imaging techniques largely preclude the kinds of social behaviors such as grouping, grooming, approach, aggression, etc. often measured in awake behaving terrestrial animals (e.g., rodents or birds) and are simply not possible in aquatic animals. Nevertheless, when effort has been made, such work has provided new insights into the functioning social brain (e.g., Van Ruijssevelt et al., 2017).
Unfortunately, much like some fMRI experiments used in human research, these studies can be criticized for lacking ecological validity, because they are classically centered on imaging isolated subjects in relatively passive spectator roles. But as just discussed, modern studies employing an interactive two-person approach to neuroimaging are making huge strides to provide more nuanced designs that occur in increasingly ethologically relevant contexts to provide a better understanding of how the social brain functions in humans and non-humans. A particularly interesting example of such work used high-resolution fMRI to image the midbrain of human subjects as they played the ultimatum game with what they thought was another participant (in reality, they were responding to offers made by a computer) (Hetu et al., 2017). This study identified that significant activation of the ventral tegmental area (VTA) is associated with assessment of social norm violations associated with the perceived fairness of monetary offers (Hetu et al., 2017). Notably, the VTA is a principle component of the mesolimbic reward pathway and part of the SBN (specifically the SDMN). This study represents an example of how improved spatial resolution to subcortical structures (via improved fMRI techniques) and utilization of an ethologically relevant paradigm of social interaction (via a two-person approach) can reveal that regions traditionally associated with the SBN are highly relevant to human/primate social cognition.
Although the gap between matching approach and species is beginning to narrow, the technical challenges are sure to persist. Nevertheless, increased common ground will be achieved with attempts to use comparable methodology in untraditional contexts. The outcomes of such efforts are sure to increase appreciation for the role of sub-cortical regions in primate social cognition and cortical structures in non-primates (e.g., Duchemin et al., 2017; Ortiz et al., 2018; Yee et al., 2016), helping to establish bridges between the two conceptualizations of the social brain.
7. Comparative considerations of social cognition and social behavior
The two approaches to the social brain adopt distinct comparative perspectives. The CSB defined neural mechanisms that support the exceptional degree of primate social cognition. These unique aspects of primate cognition include elaboration of social emotions, theory of mind, and mentalizing. This created a legacy for cognitive neuroscientists to focus nearly exclusively on the social brain of primates. However, more recent notions of social cognition used by CSB researchers have expanded to include a larger range of processes, many of which are shared across a broader array of species. For example, Kennedy and Adolphs (2012) define social cognition as any cognitive processing (e.g., perception, reasoning, memory, attention, motivation, and decision-making) that underlies a social ability or social behavior, but that is to some degree distinct from broader, nonsocial abilities and behaviors. According to this definition, elements of both the SBN and CSB should be required to support social cognition. With this evolution of thought, the distinction between how the CSB community placed great emphasis on ‘advanced’ social cognition and the SBN community placed on relatively ‘simple’ social cognition has inched closer together.
Adding to these reimagined views of social cognition is the mounting evidence suggesting that many non-primate species are capable of social abilities previously thought to be exclusive to primates (Emery and Clayton, 2009; Weitekamp and Hofmann, 2014). Weitekamp and Hofmann (2014) offer several examples to make the case that non-primate species living in social groups engage in ‘advanced’ social cognition. For example, mate-choice copying occurs when animals (usually females) alter their preferences for non-preferred mates based on their assessment that other individuals (i.e., females) have selected those mates. This effect has been described in taxa as diverse as fish, birds, and humans (Kavaliers et al., 2017), and references therein) and has been attributed to an animal’s ability to use public information to assess the preferences of others to inform their own decisions. Moreover, Ophir and Galef (2003) demonstrated that female Japanese quail could use video images of males to update and modify their preferences for the live male the images represented, a cognitive ability that had not been demonstrated in birds before. Cichlid fishes provide another set of thought-provoking results that that hint at the reach of some rather complex cognitive abilities. For instance, subordinate males will increase aggressive displays when dominant males in a community are not paying attention to them (Desjardins et al., 2012) and can deduce relationships of social rank between two other fish using the transitive property of inequality from known relationships (Grosenick et al., 2007).
The study of empathy across vertebrate species represents another promising arena to bridge between the SBN and CSB research (Meyza et al., 2017; Meyza and Knapska, 2018). Empathy has been proposed to occur on incremental orders of complexity across different species (Panksepp and Panksepp, 2013). A promising report on empathy in prairie voles shows that consolation behavior towards a stressed partner is governed by oxytocin receptors in the ACC (Burkett et al., 2016). As discussed previously, the ACC, and specifically the ACCg, seem to be equipped with uniquely social functions (Apps et al., 2016) and are central to the CSB. If the conversations between the SBN and CSB communities were better developed, there would be opportunities to focus on the study of specific subregions of interest in non-primate vertebrates where more directly descriptive techniques are available.
Comparative research on theory of mind (ToM) is another promising direction for social brain research. Indeed, flexibility and deception in food caching behavior of corvids has provided an exciting opportunity to extend the current understanding of social cognition (Bugnyar, 2007, 2013; Clayton et al., 2007). Pilfering the cached seeds of others has placed a strong social pressure on Western scrub jays to process what conspecifics ‘know’ and ‘do not know’ about their caching habits. In the presence of observing conspecifics, scrub jays will cache food out of sight of the observer, build false caches to deceive observers, and specifically recache food to a new location if they were aware they were observed caching previously (Emery and Clayton, 2009). Neuroimaging studies employing video playback of caching behavior and possibly virtual food caching interactions would elucidate the neural basis of social cognition in this ToM-like behavior in birds, and offer an opportunity to better understand the function of the social brain. Although fMRI in scrub jays would certainly present methodological challenges, fMRI has been used in awake birds before (e.g., Van Ruijssevelt et al., 2017), indicating such work might be feasible. Work such as this is significant because some researchers, particularly those interested in the CSB have argued that non-human animals are incapable of theory of mind (Penn and Povinelli, 2007; Saxe, 2006). In response to this, comparative ethologists have made convincing arguments against this position by sub-dividing ToM into its constituent behavioral parts and exploring three classes of ToM: perceptual (understanding of seeing and attention), motivational (understanding of goals and intentions), and informational (understanding of knowledge and beliefs) (Emery and Clayton, 2009). Similar to the comparative study of empathy, there are promising opportunities to explore the requisite neural components for each level of ToM, which might yield exciting breakthroughs for comparative perspectives on the neural basis of social cognition.
On first glance, discovering and characterizing more examples of non-primate species engaging in ‘advanced’ forms of social cognition potentially chisels away at the question: what on earth IS different in primates (and a handful of other mammals) that they need so much computing power? This question has rested at the center of what has motivated some to study the CSB. However, we believe progress toward identifying similarities and parallels between primate social brain function and non-primate social brain function does not undercut this important question. In fact, we believe it extends this question in compelling and interesting ways, to ask: what are the neural substrates that account for social behavior from its simplest forms to its most advanced, and could a unified neural system, fractured across two views of the social brain, account for the variance in forms and functions of social behavior?
It is certainly not our intention to advocate for the idea that all animals have the same behavioral or cognitive capacities to express social cognition or behavior across the broad repertories found in animals. To be sure, we would never claim that a chickadee, for example, has the capacity for theory of mind (or if this species somehow did, that it approximates the extent of theory of mind seen in humans) any more than we would suggest that humans have the capacity for spatial memory to the extent of a chickadee. Evolution has clearly acted on each species in ways that maximally prepare them to fit their environments, social or otherwise. When social complexity is great, innovations within the neural tissue and network of substructures that govern the processing of social information should adapt to accommodate such processing, and such adaptation could be purely unique to a specialized species or clade or could be partially or universally shared across species with extreme exaggeration in some species over others. We also acknowledge that although many aspects of social behaviors appear to be universal, similarities can be superficial or misleading. Avoiding modern versions of Clever Hans, while also not failing to appreciate examples that extend our knowledge of the evolution of social complexity is crucial (Trestman, 2015). It goes without saying that there are some aspects of primate cognition and social behavior that make them stand out relative to other species. These characteristics are either unique or extremely different and profoundly more developed when compared to the vast majority of social animals. However, we challenge the reader to consider that the neural architecture that underlies the processing of such complex behaviors must have neural tissue that evolved from preexisting structures, connected to a preexisting network of structures in the brain. In other words, it is important to think beyond the cortex and its size (Finlay 2019). We simultaneously argue that those with interest in ‘simpler’ forms of social behavior such as mating decisions, agonistic interactions, and other forms of social cognition that are more common across species should not ignore the cortex simply because it does not appear to be as large or complex as it is in other species that clearly demonstrate ‘higher order’ social behavior and cognition. We have provided evidence and justification for the notion that the components of the CSB commonly associated with ‘advanced’ social behavior are plugged in to components of the SBN that are more generally associated with social cognition and behavior across all species, and that these systems almost certainly work in tandem to process social information at all levels of complexity. Our goal is to spotlight potential areas of brain-behavior convergence that are overlooked by scientists that tend to focus on either of the two concepts of the social brain discussed here to push each group to think more deeply about what is meant by the term the social brain.
8. Generality vs specificity of function
Discussion of domain generality vs specificity is a popular topic of debate across the study of neuroscience, and we note that such divisions are rarely productive. The differences between the CSB and SBN represent a pathway toward such a rift that we caution should be avoided. Nevertheless, the CSB tends to place a strong emphasis on domain specificity in regions specialized for the aspects of social cognition that distinguish human and non-human primates from other vertebrates. This contrasts with the relatively more domain general perspective inherent in the SBN proposal, wherein a given social behavior manifests according to the concerted and relative activation between regions of the network. This is not to say that domain general arguments are absent from the cognitive neuroscience approach to the social brain. Rather, an argument for domain generality can often be found in opposition to claims of specificity within the CSB. For example, proponents of ‘simulation theory’ argue that processing of others’ actions and emotions is performed by a mirrored simulation of those actions and emotions from a self-perspective. Rather, the so-called ‘theory theory’ has argued for the existence of regions within the cognitive social brain that understand others’ actions via inferential processes that do not require mirroring. The extent of this debate is beyond the scope of this review, but detail can be found elsewhere (Apperly, 2008; Brass et al., 2007; Gallese and Goldman, 1998). A similar debate emerged regarding the specificity of face-selective regions in the human brain (e.g., fusiform face area (FFA)), leading to alternative theories of more general function, such as the expert hypothesis and the individuation hypothesis (Kanwisher et al., 1997; Kanwisher and Yovel, 2006). Across the CSB literature one will find a dizzying number of contradicting reports claiming the domain specificity or generality of particular regions. Different reports claim the unique social specificity for the TPJ (Carter et al., 2012; Lee and Seo, 2016; Platt et al., 2016; Scholz et al., 2009), mPFC (Lee and Seo, 2016; Sliwa and Freiwald, 2017), ACC gyrus (Apps et al., 2016; Platt et al., 2016; Rudebeck et al., 2006), dorsal ACC (Haroush and Williams, 2015), and areas of STS (Deen et al., 2015). Taken together, there seems to be little consensus about where domain specificity truly exists across the CSB.
Spunt and Adolphs (2017) offer a much needed recontextualization of domain specificity in the CSB. They distinguish between different forms of domain specificity that can exist in the social brain. A domain can exhibit input specificity, arising from the restriction of sensory inputs to the domain. Input specificity in the social brain is best exemplified by the vomeronasal organ in non-human vertebrates, transmitting information from particular pheromone molecules to elicit specific behaviors (Dulac and Torello, 2003; Francia et al., 2014). Central specificity refers to the specificity of computations internal to a central module. Spunt and Adolphs (2017) highlight the FFA as an exemplar of central specificity while acknowledging the debate surrounding the bounds of this specificity (Kanwisher et al., 1997; Kanwisher and Yovel, 2006). On the other hand, Spunt and Adolphs (2017) describe the dorsal mPFC as being domain general despite the region’s prominent role in mentalizing. They propose that social cognition is best characterized by multiple components that comprise functional circuits, and that these components do not need to be individually domain specific when operating together to produce a specific social behavior (Spunt and Adolphs, 2017). This concept is strongly aligned with the conceptual approach taken by the SBN.
Although the SBN was portrayed as a collection of nodes working in a relatively general fashion to process social information, it would be misleading to say that the research on the specific nodes of the SBN have universally been portrayed as fundamentally domain general. For example, the anterior hypothalamus has often been described as an area closely associated with regulating various forms of aggression (Ferris et al., 1997). Similarly, the POA is often tied to maternal care and bonding, but is also well known for its importance in sexual behavior (Champagne et al., 2003; Paredes et al., 1993). The medial extended amygdala (MeA and BNST) is often regarded as modulating social approach and grouping (Hiura et al., 2018; Kelly and Goodson, 2013; Newman, 1999), but like the POA it is also important for reproductive behavior. Yet, as Newman (1999) highlighted, most studies only occasionally analyze the same brain area in more than one social context, and these and other structures are usually participants in the activation or modulation of multiple behaviors and the information processing involved. For example, the lateral septum is involved in shaping mating behavior (Kollack-Walker and Newman, 1997), aggression (Wong et al., 2016), social communication of dominance (Ferris et al., 1990), social aggregation (Kelly et al., 2011) pair bonding (Liu et al., 2001), social recognition (Ferguson et al., 2002), and a number of other behaviors. Similar breadth for behavioral function can be found for the other structures that are parts of the SBN. Recognizing this bias and taking a more inclusive view of the functional breadth of each node of the SBN laid the foundation for the argument that the nodes of the SBN (and likely all networks important for regulating social behavior) work in unison to produce emergent social behavior properties. The potential tension between specificity and generality may still exist to some extent among those interested in the collective functioning of the SBN or those with a specific focus on its components. Nevertheless, this re-framing of these sub-units as parts of a larger system has led to a potentially more constructive view of the way the brain processes social behavior. Perspectives like this, can only be accomplished by approaching the network as serving a general function toward processing social behavior. Because of its resistance to embracing a functionally reductionist view of the brain, the SBN framework has been very useful to the scientists that have used it to understand the development and expression of social behavior across species.
As is all too often the case with dichotomous thinking, the truth lies somewhere in between. It is very likely that elements of the CSB and SBN have domain specific responsibilities when processing social behavior. It is also, very likely that the second order effects from the interactions between subunits work cohesively to produce a coalescence of processing of social information, which is then translated into social behaviors. We are hopeful that developing nuanced perspectives on domain specificity and generality with respect to the social brain will facilitate more direct comparisons between the CSB and the SBN and enrich the ways each is thought about and used to advance our understanding of social behavior.
9. What is the social brain?
It is clear that organisms with little to no cortex have the capacity for engaging in social behavior, and often this can be rather complex social behavior (Simons and Tibbets, 2019). It is also clear that the emphasis on an exclusively cortex-based social brain underestimates the extensive reach of socially acting animals without these more derived brain structures or elaborations therein. On the other hand, a large cortex is clearly associated with relatively advanced social cognition that is not typically seen in animals with a comparatively smaller cortex. So what functions does the SBN and CSB support and how do they work together if they work together at all?
The current views of each might lead one to consider the possibility that there are two social brains - one evolutionarily primitive and one evolutionarily derived, each with different domains of control and that attract different sets of scientific questions. It would follow from this point of view that the work of processing social information, particularly ‘higher order’ information, might have been off-loaded to the derived social brain as selective pressures created increasingly complex social contexts that required more intricate planning and perspective taking (e.g., Dunbar, 1993, 1998, 2009; Dunbar and Shultz, 2007; but see DeCasien and Higham, 2019). This view raises the important question: what happened to the SBN in these animals? Were its functions to process social information completely taken over by the CSB, and if so, was it coopted for new functions? Was it compartmentalized and relegated to processing the ‘simpler’ forms of social processing allowing for the CSB to take on the more demanding tasks of social information processing? Or have the CSB and SBN (in one form or another) coexisted and intimately worked together to process social information of all levels of complexity from the earliest moments in the social brain’s evolution?
It strikes us as unlikely that elements of the brain that are so foundational to the evolution of neural control of social information processing could be functionally labile enough to remain present, but no longer continue to serve their original purpose. It also strikes us as redundant and unnecessarily costly to think that animals with a large cortex have two systems that work fairly independently, one dedicated to higher order cognition and one to more or less do the same kind of thing but for other contexts like mating and fighting. We have emphasized that the SBN is highly conserved across animals, including in primates, and that when its components have received attention in animals with relatively elaborate cortex, these ancestral areas of the brain continue to have important contributions to both advanced and simple forms of social cognition (see above). We have also discussed examples in which many of the components of the CSB appear to be conserved in both form and function. Therefore, in our view, it is much more plausible that the components of the SBN work in primates and in non-primates in largely the same ways. The innovation of the cortex and all that it offers in terms of computational power has certainly set the animals with the derived characters apart, but with respect to processing social information, it is probably best to view this as a significant modification of the ability of the entire social brain to process and react to social contexts. If this is true, then the most parsimonious conclusion we could offer is that the SBN and the CSB are two parts of the same larger social brain. Rather than brushing aside the functions of either the CSB or the SBN, it might be worthwhile to ask how the parts of each function together to shape ‘higher order’ or relatively ‘simple’ social cognition across vertebrates, primates or otherwise.
If we truly want to understand the full extent of what the social brain actually is, it will require an analysis that focuses on how the CSB and SBN are intertwined and interactive. For example, Preston (2013) advocated for a network of neural structures that work cohesively to promote caregiving, and ultimately lays the foundation for the evolution of altruistic behavior. Interestingly the network of brain structures she characterized with this purpose in mind pulls from key players found in the CSB (e.g., OFC, mPFC, ACCg), the SBN (POA, AH, PAG, VTA, NAc, VP), or both (AMG). Similarly, Rogers-Carter and Christianson (2019) argued that an important component of the CSB, the insular cortex, is anatomically and functionally positioned to work directly with the SBN to integrate the socio-sensory cues to facilitate context-appropriate social decision-making. Furthermore, Tremblay et al. (2017) discussed the neural components of social decision-making from a cross-species perspective, in part considering the conserved social functions of oxytocin in both the CSB and the SBN, also argued that the SBN and CSB deserve consideration in light of each other. These compelling examples underscore the importance of bridging the divide between the CSB and the SBN to provide a more accurate and nuanced account of the social brain. Like Tremblay et al. (2017), we encourage the identification of common ground between these two rather different and isolated conceptions of the social brain. This is because it is highly likely that they should be collectively considered together as one, and doing so will lead to highly fruitful advances and exciting discoveries.
The CSB and SBN are concepts of the social brain embedded in distinct methodology, regional focus, comparative approaches, and perspectives on domain specificity vs. generality that have superficially focused on different aspects of sociality. If social behavior reflects the underlying cognitive processes that interpret social information and in turn inform how animals should best interact with each other, then identifying the complete set of neural substructures that are responsible for this processing will ultimately provide a better appreciation for the evolution and expression of social behavior. We have addressed several issues that speak to the similarities and differences between two concepts of the social brain in the hopes that doing so will inspire more collaboration and thoughtful consideration between the researchers that are continuing to define the concept of what the social brain actually is. Through a consideration of the deep homology in the social brain, we have attempted to push back against the inertia fueling a lack of crosstalk in hopes of inspiring a broader characterization of what the social brain is, and what it is doing. We hope that future work will expand discussion of the SBN and CSB in a way that is mindful of the historical underpinnings, regional and methodological limitations, and distinct perspectives that each vision of the social brain has added to the conversation, and that this will lead to a more inclusive and cohesive concept of what makes ‘the social brain’.
Highlights.
Two fundamentally different concepts of the social brain exist
One view of the social brain centers on higher-order cognition in primates
One view emphasizes a network supporting commonalities across vertebrate sociality
Despite sharing the same ultimate goal, little awareness of the two views exists
Crosstalk is essential to achieve a comprehensive understanding of the social brain
Acknowledgements
We are grateful to Barb Finlay for her constructive comments, advice, and insights that helped develop this review. We also wish to acknowledge the memory of Jim Goodson, a dear friend whose exuberance and advocation for understanding the social brain was an inspiration to us and many others.
Funding
This work would not be possible without the generous funding from the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development HD079573), and the National Science Foundation (IOS-1354760).
Footnotes
Conflict of interest statement
The work detailed herein was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R (2003). Cognitive neuroscience of human social behaviour. Nat. Rev. Neurosci 4, 165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R (2009). The social brain: Neural basis of social knowledge. Annu. Rev. Psychol 60, 693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, and Tusche A (2017). From faces to prosocial behavior: Cues, tools, and mechanisms. Curr. Dir. Psychol. Sci 26, 282–287. doi: 10.1177/0963721417694656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, and Breiter HC (2001). Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32, 537–51. [DOI] [PubMed] [Google Scholar]
- Albers HE (2012). The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm. Behav 61, 283–92. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Albert DJ, and Chew GL (1980). The septal forebrain and the inhibitory modulation of attack and defense in the rat. A review. Behav. Neural Biol 30, 357–88. [DOI] [PubMed] [Google Scholar]
- Alcalá-López D, Vogeley K, Binkofski F, and Bzdok D (2019). Building blocks of social cognition: Mirror, mentalize, share? Cortex 118, 4–18. doi: 10.1016/j.cortex.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Amodio DM, and Frith CD (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci 7, 268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Apperly IA (2008). Beyond simulation-theory and theory-theory: why social cognitive neuroscience should use its own concepts to study “theory of mind”. Cognition. 107, 266–83. doi: 10.1016/j.cognition.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Apps MAJ, Rushworth MFS, and Chang SWC (2016). The anterior cingulate gyrus and social cognition: Tracking the motivation of others. Neuron. 90, 692–707. doi: 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, and Schultz W (2013). The role of the striatum in social behavior. Front. Neurosci 7, 233. doi: 10.3389/fnins.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic D, Craig MM, Mongerson CRL, Borsook D, and Becerra L (2017). Identifying rodent resting-state brain networks with independent component analysis. Front. Neurosci 11. doi: 10.3389/fnins.2017.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen JACJ, Thioux M, and Keysers C (2009). Evidence for mirror systems in emotions. Philos. Trans. R. Soc. Lond. B. Biol. Sci 364, 2391–404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, and Fehr E (2008). Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58, 639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bennett CM and Miller MB (2010). How reliable are the results from functional magnetic resonance imaging? Ann. N.Y. Acad. Sci, 1191, 133–155 [DOI] [PubMed] [Google Scholar]
- Bicks LK, Koike H, Akbarian S, and Morishita H (2015). Prefrontal cortex and social cognition in mouse and man. Front. Psychol doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthé R-M, and van Honk, J. (2012). Acute effects of steroid hormones and neuropeptides on human social–emotional behavior: A review of single administration studies. Front. Neuroendocrinol 33, 17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Brass M, Schmitt RM, Spengler S, and Gergely G (2007). Investigating action understanding: Inferential processes versus action simulation. Curr. Biol 17, 2117–21. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Bredewol R., Smit CJW., Dumai KM., Veenem AH. (2014) Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L (1990). The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1, 27–51. [Google Scholar]
- Buckholtz JW, Martin JW, Treadway MT, Jan K, Zald DH, Jones O, et al. (2015). From blame to punishment: Disrupting prefrontal cortex activity reveals norm enforcement mechanisms. Neuron. 87, 1369–1380. doi: 10.1016/j.neuron.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T 2007. An integrative approach to the study of “theory of mind”-like abilities in ravens. Jap. J. Anim. Psychol 57:15–27 [Google Scholar]
- Bugnyar T (2013). Social cognition in ravens. Comp. Cogn. Behav. Rev 8, 1–12. doi: 10.3819/ccbr.2013.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, and Young LJ (2016). Oxytocin-dependent consolation behavior in rodents. Science 351, 375–8. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Bowling DL, Reeck C, and Huettel SA (2012). A distinct role of the temporal-parietal junction in predicting socially guided decisions. Science. 337, 109–111. doi: 10.1126/science.1219681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP, Swaney WT, Hasen NS, and Keverne EB (2009). Paternal influence on female behavior: The role of Peg3 in exploration, olfaction, and neuroendocrine regulation of maternal behavior of female mice. Behav. Neurosci 123, 469–480. doi: 10.1037/a0015060. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Sharma S, and Meaney MJ (2003). Natural variations in maternal care are associated with estrogen receptor α expression and estrogen sensitivity in the medial preoptic area. Endocrinology 144, 4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Fagan NA, Toda K, Utevsky AV, Pearson JM, and Platt ML (2015). Neural mechanisms of social decision-making in the primate amygdala. Proc. Natl. Acad. Sci 112, 16012–16017. doi: 10.1073/pnas.1514761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Gariépy J-F, and Platt ML (2013). Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci 16, 243–250. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Pfaff DW, Kavaliers M (2013). Oxytocin, vasopressin and related peptides in the regulation of behavior. Cambridge University Press. [Google Scholar]
- Churchland PS, and Winkielman P (2012). Modulating social behavior with oxytocin: How does it work? What does it mean? Horm. Behav 61, 392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton NS, Dally JM, and Emery NJ (2007). Social cognition by food-caching corvids. The western scrub-jay as a natural psychologist. Philos. Trans. R. Soc. Lond. B. Biol. Sci 362, 507–22. doi: 10.1098/rstb.2006.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, and Fu CHY (2008). Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res. Rev 58, 57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. (2010). Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci 30, 16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D (2003). The development of phenotypic plasticity: Where biology and psychology meet. Dev. Psychobiol 43, 1–10. doi: 10.1002/dev.10115. [DOI] [PubMed] [Google Scholar]
- Crews D (2008). Epigenetics and its implications for behavioral neuroendocrinology. Front. Neuroendocrinol 29, 344–357. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings ME, and Ramsey ME (2015). Mate choice as social cognition: predicting female behavioral and neural plasticity as a function of alternative male reproductive tactics. Curr. Opin. Behav. Sci 6, 125–131. doi: 10.1016/j.cobeha.2015.10.001. [DOI] [Google Scholar]
- Curley JP, Jensen CL, Franks B, and Champagne FA (2012). Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav 61, 454–61. doi: 10.1016/j.yhbeh.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasien AR, and Higham JP (2019) Primate mosaic brain evolution reflects selection on sensory and cognitive specialization. Nature Ecology & Evolution. 3, 1483–1493. [DOI] [PubMed] [Google Scholar]
- de Groot A, van den Boom BJG, van Genderen RM, Coppens J, van Veldhuijzen J, Bos J, Hoedemaker H, Negrello M, Wiluhn I, De Zeeuw CI, Hoogland TM, (2019). NINscope: a versatile miniscope for multi-region circuit investigations. bioRxiv doi: 10.1101/685909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vignemont F, and Singer T (2006). The empathic brain: How, when and why? Trends Cogn. Sci 10, 435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, and Saxe R (2015). Functional organization of social perception and cognition in the superior temporal sulcus. Cereb. Cortex 25, 4596–4609. doi: 10.1093/cercor/bhv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Hofmann HA, and Fernald RD (2012). Social context influences aggressive and courtship behavior in a cichlid fish. PLoS One 7, e32781. doi: 10.1371/journal.pone.0032781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, and Rizzolatti G (1992). Understanding motor events: A neurophysiological study. Exp. brain Res 91, 176–80. [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, and Veenema AH (2017). Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J. Comp. Neurol 525, 2549–2570. doi: 10.1002/cne.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC (2007). Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 61, 713–733. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, and Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 322, 900–904. [DOI] [PubMed] [Google Scholar]
- Duchemin A, Seelke AMH, Simmons TC, Freeman SM, and Bales KL (2017). Localization of oxytocin receptors in the prairie vole (Microtus ochrogaster) neocortex. Neuroscience 348, 201–211. doi: 10.1016/j.neuroscience.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukas R, Ratcliffe JM (2009) Cognitive Ecology II. Chicago: University of Chicago Press. [Google Scholar]
- Dulac C, and Torello AT (2003). Molecular detection of pheromone signals in mammals: From genes to behaviour. Nat. Rev. Neurosci 4, 551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM (1993). Coevolution of neocortical size, group size and language in humans. Behav. Brain Sci 16, 681–694. doi: 10.1017/S0140525X00032325. [DOI] [Google Scholar]
- Dunbar RIM (1998). The social brain hypothesis. Evol. Anthropol 6, 178–190. doi:. [DOI] [Google Scholar]
- Dunbar RIM (2009). The social brain hypothesis and its implications for social evolution. Ann. Hum. Biol 36, 562–572. doi: 10.1080/03014460902960289. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, and Shultz S (2007). Evolution in the social brain. Science. 317, 1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, and Knutsson H (2016) Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. PNAS, 113, 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, and Clayton NS (2009). Comparative social cognition. Annu. Rev. Psychol 60, 87–113. doi: 10.1146/annurev.psych.60.110707.163526. [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Oxytocin and social affiliation in humans. Horm. Behav 61, 380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, and Zagoory-Sharon O (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev. Sci 14, 752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, and Insel TR (2002). The neuroendocrine basis of social recognition. Front. Neuroendocrinol 23, 200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Gold L, De Vries GJ, and Potegal M (1990). Evidence for a functional and anatomical relationship between the lateral septum and the hypothalamus in the control of flank marking behavior in golden hamsters. J. Comp. Neurol 293, 476–485. doi: 10.1002/cne.902930310. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, and Delville Y (1997). Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci 17, 4331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL (2019). Human exceptionalism, our ordinary cortex and our research futures. Developmental Psychobiology. 61, 317–322. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, et al. (1995). Other minds in the brain: A functional imaging study of ‘theory of mind’ in story comprehension. Cognition 57, 109–28. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, and Bass AH (2005). Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J. Comp. Neurol 483, 91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Francia S, Pifferi S, Menini A, and Tirindelli R (2014). Vomeronasal receptors and signal transduction in the vomeronasal organ of mammals in Neurobiology of Chemical Communication. Boca Raton (FL: ): CRC Press/Taylor & Francis. doi: 10.1201/b16511. [DOI] [PubMed] [Google Scholar]
- Frith CD (2007). The social brain? Philos. Trans. R. Soc. Lond. B. Biol. Sci 362, 671–8. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, and Frith C (2010). The social brain: Allowing humans to boldly go where no other species has been. Philos. Trans. R. Soc. B Biol. Sci 365, 165–76. doi: 10.1098/rstb.2009.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, and Rizzolatti G (1996). Action recognition in the premotor cortex. Brain 119, 593–609. [DOI] [PubMed] [Google Scholar]
- Gallese V, and Goldman A (1998). Mirror neurons and the simulation theory of mind-reading. Trends Cogn. Sci 2, 493–501, doi: 10.1016/S1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, and Bargmann CI (2012). Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science. 338, 540–543. doi: 10.1126/science.1226201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson J, Evans A, and Lindberg I (2004). Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J. Comp. Neurol 473, 293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL (2005). The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav 48, 11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, and Bass AH (2000). Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 403, 769–72. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, and Bass AH (2001). Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Rev 35, 246–65. doi: 10.1016/S0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, and Evans AK (2004). Neural responses to territorial challenge and nonsocial stress in male song sparrows: Segregation, integration, and modulation by a vasopressin V 1 antagonist. Horm. Behav 46, 371–81. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, and Soma KK (2005). Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport. 16, 1719–23. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, and Kabelik D (2009). Dynamic limbic networks and social diversity in vertebrates: From neural context to neuromodulatory patterning. Front. Neuroendocrinol 30, 429–441. doi: 10.1016/J.YFRNE.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kelly AM, and Kingsbury MA (2012). Evolving nonapeptide mechanisms of gregariousness and social diversity in birds. Horm. Behav 61, 239–250. doi: 10.1016/j.yhbeh.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, and Kingsbury MA (2009). Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science 325, 862–6. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, and Thompson RR (2010). Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol 20, 784–794. doi: 10.1016/J.CONB.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, and Fernald RD (2007). Fish can infer social rank by observation alone. Nature 445, 429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Haroush K, and Williams ZM (2015). Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell. 160, 1233–1245. doi: 10.1016/j.cell.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hétu S, Luo Y, D’Ardenne K, Lohrenz T, and Montague PR (2017). Human substantia nigra and ventral tegmental area involvement in computing social error signals during the ultimatum game. Soc. Cogn. Affect. Neurosci 12, 1972–1982. doi: 10.1093/scan/nsx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiura LC, Kelly AM, and Ophir AG (2018). Age-specific and context-specific responses of the medial extended amygdala in the developing prairie vole. Dev. Neurobiol 78, 1231–1245. doi: 10.1002/dneu.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, and Grafton ST (2006). Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia 44, 374–383. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, and Sadato N (2008). Processing of social and monetary rewards in the human striatum. Neuron 58, 284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin S-Y, et al. (2010). Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci 13, 482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, and Young LJ (2017). Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev 76, 87–98. doi: 10.1016/j.neubiorev.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, and Young LJ (2015). Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci 3, 38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joppa MA, Meisel RL, and Garber MA (1995). c-Fos expression in female hamster brain following sexual and aggressive behaviors. Neuroscience. 68, 783–792. doi: 10.1016/0306-4522(95)00179-M. [DOI] [PubMed] [Google Scholar]
- Kalcher K, Boubela RN, Huf W, Našel C, and Moser E (2015). Identification of voxels confounded by venous signals using resting-state fMRI functional connectivity graph community identification. Front. Neurosci 9, 472. doi: 10.3389/fnins.2015.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Dolan RJ, and Frith U (2001). Reward value of attractiveness and gaze. Nature 413, 589–589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, and Chun MM (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci 17, 4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, and Yovel G (2006). The fusiform face area: A cortical region specialized for the perception of faces. Philos. Trans. R. Soc. B Biol. Sci 361, 2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Matta R, Choleris E (2017). Mate-choice copying, social information processing, and the roles of oxytocin. Neuroscience and Biobehavioral Reviews. 72, 232–242. [DOI] [PubMed] [Google Scholar]
- Kelly AM, and Goodson JL (2013). Behavioral relevance of species-specific vasotocin anatomy in gregarious finches. Front. Neurosci 7, 242. doi: 10.3389/fnins.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, et al. (2011). Vasotocin neurons and septal V1a-like receptors potently modulate songbird flocking and responses to novelty. Horm. Behav 60, 12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, and Ophir AG, (2015) Compared to what: What can we say about nonapeptide function and social behavior without a frame of reference? Current Opinion in Behavioral Sciences. 6, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Saunders AG, Ophir AG (2018) Mechanistic substrates of a life history transition in male prairie voles: Developmental plasticity in affiliation and aggression corresponds to nonapeptide neuronal function. Hormones and Behavior. 99, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Yee JR, Moore K, Madularu D, Kulkarni P, Gamber K, et al. (2016). Functional magnetic resonance imaging in awake transgenic fragile X rats: Evidence of dysregulation in reward processing in the mesolimbic/habenular neural circuit. Transl. Psychiatry 6, e763–e763. doi: 10.1038/tp.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, and Adolphs R (2012). The social brain in psychiatric and neurological disorders. Trends Cogn. Sci 16, 559–72. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM (2011). More than one pathway to action understanding. Trends Cogn. Sci 15, 352–357. doi: 10.1016/j.tics.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, et al. (2015). Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell Rep. 10, 292–305. doi: 10.1016/j.celrep.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury L, Huang S, Wang J, Gu K, Golshani P, Wu YE, Hong W (2019). Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell. 178, 429–446.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JT, and Platt ML (2013). Social information signaling by neurons in primate striatum. Curr. Biol 23, 691–6. doi: 10.1016/j.cub.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, and Fehr E (2006). Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 314, 829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Ko J (2017). Neuroanatomical substrates of rodent social behavior: The medial prefrontal cortex and its projection patterns. Front. Neural Circuits 11, 41. doi: 10.3389/fncir.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack-Walker S, and Newman S (1995). Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience 66, 721–736. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, and Newman SW (1997). Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J. Neurobiol 32, 481–501. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, and Fehr E (2005). Oxytocin increases trust in humans. Nature 435, 673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lee D, and Seo H (2016). Neural basis of strategic decision making. Trends Neurosci. 39, 40–48. doi: 10.1016/j.tins.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Rhim I, Lee JW, Ghim J-W, Lee S, Kim E, et al. (2016). Enhanced neuronal activity in the medial prefrontal cortex during social approach behavior. J. Neurosci 36, 6926–6936. doi: 10.1523/JNEUROSCI.0307-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, and Powers JB (1980). Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 210, 557–560. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Rolls ET, Wilson FAW, and Baylis GC (1985). Neurons in the amygdala of the monkey with responses selective for faces. Behav. Brain Res 15, 159–176. doi: 10.1016/0166-4328(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, et al. (2018). A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 564, 213–218. doi: 10.1038/s41586-018-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Zhang L, Barbera G, Fang W, Zhang J, Chen X, Chen R, Li Y, Lin D-T (2018). Distinct and dynamic ON and OFF neural ensembles in the prefrontal cortex code social exploration. Neuron. 100, 700–714.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD (2007). Social cognitive neuroscience: A review of core processes. Annu. Rev. Psychol 58, 259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lieberman MD (2012). A geographical history of social cognitive neuroscience. Neuroimage. 61, 432–436. doi: 10.1016/j.neuroimage.2011.12.089. [DOI] [PubMed] [Google Scholar]
- Lieberman MD (2013). Social: Why our brains are wired to connect. 1st ed. NewYork: Broadway Books. [Google Scholar]
- Liu Y, Curtis JT, and Wang Z (2001). Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav. Neurosci 115, 910–9. [DOI] [PubMed] [Google Scholar]
- Logothetis NK (2008) What we can do and what we cannot do with fMRI. Nature. 453, 869–878 [DOI] [PubMed] [Google Scholar]
- Madularu D, Mathieu AP, Kumaragamage C, Reynolds LM, Near J, Flores C, et al. (2017). A non-invasive restraining system for awake mouse imaging. J. Neurosci. Methods 287, 53–57. doi: 10.1016/j.jneumeth.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C, and Singer T (2012). The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat. Neurosci 15, 681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- Menon RS (2012). The great brain versus vein debate. Neuroimage 62, 970–974. doi: 10.1016/j.neuroimage.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Meyza K, and Knapska E (2018). What can rodents teach us about empathy? Curr. Opin. Psychol 24, 15–20. doi: 10.1016/j.copsyc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Meyza KZ, Bartal IBA, Monfils MH, Panksepp JB, and Knapska E (2017). The roots of empathy: Through the lens of rodent models. Neurosci. Biobehav. Rev 76(Pt B), 216–234. doi: 10.1016/j.neubiorev.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Bramati IE, and Grafman J (2002). Functional networks in emotional moral and nonmoral social judgments. Neuroimage 16, 696–703. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Bramati IE, Krueger F, Tura B, et al. (2011). Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. Neuroimage. 54, 1735–1742. doi: 10.1016/j.neuroimage.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Berns GS, Cohen JD, McClure SM, Pagnoni G, Dhamala M, Wiest MC, Karpov I, King RD, Apple N, Fisher RE (2002). Hyperscanning: Simultaneous fMRI during linked social interactions. Neuroimage 16, 1159–64. [DOI] [PubMed] [Google Scholar]
- Morelli SA, Rameson LT, and Lieberman MD (2014). The neural components of empathy: Predicting daily prosocial behavior. Soc. Cogn. Affect. Neurosci 9, 39–47. doi: 10.1093/scan/nss088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan M, Jang HJ, Park M, Miller EM, Cox J, Taliaferro JP, Parker NF, Bhave V, Hur H, Liang Y, Nectow AR, Pillow JW, Witten IB (2017). Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell. 171, 1663–77.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW (1999). The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Ann. N. Y. Acad. Sci 877, 242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Numan M, and Sheehan TP (1997). Neuroanatomical circuitry for mammalian maternal behavior. Ann. N. Y. Acad. Sci 807, 101–25. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, and Hofmann HA (2011). The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J. Comp. Neurol 519, 3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, and Hofmann HA (2012). Evolution of a vertebrate social decision-making Network. Science. 336, 1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, and Dolan RJ (2003). Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 41, 147–55. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, and Lieberman MD (2001). The emergence of social cognitive neuroscience. Am. Psychol 56, 717–34. [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, et al. (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci 5, 900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Ophir AG, and Galef BG Jr. (2003). Female Japanese quail affiliate with live males that they have seen mate on video. Anim Behav. 66, 369–375. [Google Scholar]
- Ophir AG (2017). Navigating Monogamy: Nonapeptide Sensitivity in a Memory Neural Circuit May Shape Social Behavior and Mating Decisions. Front. Neurosci 11, 397. doi: 10.3389/fnins.2017.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz JJ, Portillo W, Paredes RG, Young LJ, and Alcauter S (2018). Resting state brain networks in the prairie vole. Sci. Rep 8, 1231. doi: 10.1038/s41598-017-17610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, and Panksepp JB (2013). Toward a cross-species understanding of empathy. Trends Neurosci. 36, 489–496. doi: 10.1016/J.TINS.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Highland L, and Karam P (1993). Socio-sexual behavior in male rats after lesions of the medial preoptic area: Evidence for reduced sexual motivation. Brain Res. 618, 271–6. [DOI] [PubMed] [Google Scholar]
- Penn DC, and Povinelli DJ (2007). On the lack of evidence that non-human animals possess anything remotely resembling a “theory of mind”. Philos. Trans. R. Soc. Lond. B. Biol. Sci 362, 731–44. doi: 10.1098/rstb.2006.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Hietanen JK, Oram MW, and Benson PJ (1992). Organization and functions of cells responsive to faces in the temporal cortex. Philos. Trans. R. Soc. London. Ser. B Biol. Sci 335, 23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- Phelps EA, and LeDoux JE (2005). Contributions of the amygdala to emotion processing: From animal Models to human behavior. Neuron 48, 175–187. doi: 10.1016/J.NEURON.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Platt ML, Seyfarth RM, and Cheney DL (2016). Adaptations for social cognition in the primate brain. Philos. Trans. R. Soc. B Biol. Sci 371, 20150096. doi: 10.1098/rstb.2015.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers JB, Newman SW, and Bergondy ML (1987). MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav. Brain Res 23, 181–95. [DOI] [PubMed] [Google Scholar]
- Preston SD (2013) The origins of altruism in offspring care. Psychological Bull. 139, 1305–1341. doi: 10.1037/a0031755 [DOI] [PubMed] [Google Scholar]
- Redish AD (2016) Vicarious trial and error. Nature Reviews Neuroscience. 17, 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard RA, Subramanian S, Desta MT, Sura T, Becker ML, Ghobadi CW, et al. (2017). Abundant collateralization of temporal lobe projections to the accumbens, bed nucleus of stria terminalis, central amygdala and lateral septum. Brain Struct. Funct 222, 1971–1988. doi: 10.1007/s00429-016-1321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A (1986). Is prefrontal cortex found only in mammals? Trends Neurosci. 9, 298–300. doi: 10.1016/0166-2236(86)90086-X. [DOI] [Google Scholar]
- Remedios R, Kennedy A, Zelikowsky M, Grewe BF, Schnitzer MJ, Anderson DJ (2017). Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature. 550, 388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, et al. (2016). Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165, 1789–1802. doi: 10.1016/J.CELL.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, and Kilts C (2002). A neural basis for social cooperation. Neuron 35, 395–405. [DOI] [PubMed] [Google Scholar]
- Rilling J, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD (2004). The neural correlates of theory of mind within interpersonal interactions. NeuroImage. 22, 1694–1703. [DOI] [PubMed] [Google Scholar]
- Risold P, and Swanson L (1997). Connections of the rat lateral septal complex. Brain Res. Rev 24, 115–195. doi: 10.1016/S0165-0173(97)00009-X. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, and Craighero L (2004) The mirror-neuron system. Annu Rev Neurosci. 27, 169–92. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, and Fogassi L (1996). Premotor cortex and the recognition of motor actions. Brain Res. Cogn. Brain Res 3, 131–41. [DOI] [PubMed] [Google Scholar]
- Rogers-Carter MM, and Christianson JP (2019). An insular view of the social decision-making network. Neurosci Biobehav Rev. 130, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, et al. (2000). Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: A positron emission tomography study. J. Neurosci 20, 7752–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, and Rushworth MFS (2006). A role for the macaque anterior cingulate gyrus in social valuation. Science. 313, 1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- Ruff CC (2018). Brain stimulation studies of social norm compliance: Implications for personality disorders? Psychopathology. 51, 105–109. doi: 10.1159/000486898. [DOI] [PubMed] [Google Scholar]
- Ruff CC, and Fehr E (2014). The neurobiology of rewards and values in social decision making. Nat. Rev. Neurosci 15, 549–562. doi: 10.1038/nrn3776. [DOI] [PubMed] [Google Scholar]
- Saito DN, Tanabe HC, Izuma K, Hayashi MJ, Morito Y, Komeda H, Uchiyama H, Kosaka H, Okazawa H, Fujibayashi Y Sadato N (2010). “Stay tuned”: Inter-individual neural synchronization during mutual gaze and joint attention. Front. Integr. Neurosci 4, 127. doi: 10.3389/fnint.2010.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R (2006). Uniquely human social cognition. Curr. Opin. Neurobiol 16, 235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, and Kanwisher N (2003). People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 19, 1835–1842. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K (2013). Toward a second-person neuroscience. Behav. Brain Sci 36, 393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K (2010). Minds made for sharing: Initiating joint attention recruits reward-related neurocircuitry. J. Cogn. Neurosci 22, 2702–2715. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wohlschlaeger AM, Kraemer NC, Newen A, Shah NJ, Fink GR, Vofeley K (2006). Being with virtual others: Neural correlates of social interaction. Neuropsychologia 44, 718–730. doi: 10.1016/j.neuropsychologia.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, and Saxe R (2009). Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 4, e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seewoo BJ, Etherington SJ, Feindel KW, and Rodger J (2018a). Combined rTMS/fMRI studies: An overlooked resource in animal models. Front. Neurosci 12, 180. doi: 10.3389/fnins.2018.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seewoo BJ, Feindel KW, Etherington SJ, and Rodger J (2018b). Resting-state fMRI study of brain activation using low-intensity repetitive transcranial magnetic stimulation in rats. Sci. Rep 8, 6706. doi: 10.1038/s41598-018-24951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, and Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry. 79, 194–202. [DOI] [PubMed] [Google Scholar]
- Shettleworth S (2010) Cognition, evolution, and behavior. New York, New York: Oxford University Press. [Google Scholar]
- Simons M, and Tibbetts E (2019) Insects as models for studying the evolution of animal cognition. Curr Op Insect Sci. 34, 117–122. [DOI] [PubMed] [Google Scholar]
- Singer T (2012). The past, present and future of social neuroscience: A European perspective. Neuroimage. 61, 437–449. doi: 10.1016/j.neuroimage.2012.01.109. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, and Frith CD (2004). Empathy for pain involves the affective but not sensory components of pain. Science. 303, 1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, and Frith CD (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature 439, 466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa J, and Freiwald WA (2017). A dedicated network for social interaction processing in the primate brain. Science. 356, 745–749. doi: 10.1126/science.aam6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJW, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, and Veenema AH (2017). Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct. Funct 222, 981–1006. doi: 10.1007/s00429-016-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, and Adolphs R (2017). A new look at domain specificity: Insights from social neuroscience. Nat. Rev. Neurosci. 18, 559–567. doi: 10.1038/nrn.2017.76. [DOI] [PubMed] [Google Scholar]
- Teles MC, Almeida O, Lopes JS, and Oliveira RF (2015). Social interactions elicit rapid shifts in functional connectivity in the social decision-making network of zebrafish. Proc. R. Soc. B Biol. Sci 282, 20151099. doi: 10.1098/rspb.2015.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Sharika KM, and Platt ML (2017). Social decision-making and the brain: A comparative perspective. Trends Cogn. Sci 21, 265–276. doi: 10.1016/j.tics.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trestman M (2015). Clever Hans, Alex the parrot, and Kanzi: What can exceptional animal learning teach us about human cognitive evolution? Biological Theory. 10, 86–99. [Google Scholar]
- Van IJzendoorn MH, and Bakermans-Kranenburg MJ (2012). A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology 37, 438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Van Ruijssevelt L, Hamaide J, Van Gurp MT, Verhoye M, Van der Linden A (2017). Auditory evoked BOLD responses in awake compared to lightly anaesthetized zebra finches. Scientific Reports. 7, 13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogeley K (2017). Two social brains: Neural mechanisms of intersubjectivity. Philos. Trans. R. Soc. B Biol. Sci 372, 20160245. doi: 10.1098/rstb.2016.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake SJ, and Izuma K (2017). A common neural code for social and monetary rewards in the human striatum. Soc. Cogn. Affect. Neurosci 12, 1558–1564. doi: 10.1093/scan/nsx092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, and Young LJ (2018). The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci 19, 643–654. doi: 10.1038/s41583-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, and Hu H (2011). Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 334, 693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wang L, Talwar V, Osakada T, Kuang A, Guo Z, Yamaguchi T, Lin D (2019). Hypothalamic control of conspecific self-defense. Cell Reports. 26, 1747–1758.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitekamp CA, and Hofmann HA (2014). Evolutionary themes in the neurobiology of social cognition. Curr. Opin. Neurobiol 28:22–27. doi: 10.1016/j.conb.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, and Dolan RJ (2002). Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat. Neurosci 5, 277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Wong L, Wang L, D JA, Feng JE, Froemke RC, Lin Correspondence D, et al. (2016). Effective modulation of male aggression through lateral septum to medial hypothalamus projection. Curr. Biol 26, 1–12. doi: 10.1016/j.cub.2015.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, and Newman SW (1993). Mating activates androgen receptor-containing neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 614, 65–77. doi: 10.1016/0006-8993(93)91019-O. [DOI] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, and Dulac CG (2014). Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JR, Kenkel WM, Kulkarni P, Moore K, Perkeybile AM, Toddes S, et al. (2016). BOLD fMRI in awake prairie voles: A platform for translational social and affective neuroscience. Neuroimage 138, 221–232. doi: 10.1016/j.neuroimage.2016.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ (2002). The neurobiology of social recognition, approach, and avoidance. Biological Psychiatry. 51, 18–26. [DOI] [PubMed] [Google Scholar]
- Young LJ, and Wang Z (2004). The neurobiology of pair bonding. Nat. Neurosci 7, 1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gross J, De Dreu C, Ma Y (2019). Oxytocin promotes coordinated out-group attack during intergroup conflict in humans. eLife. 8:e40698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D-J, Larsson B, Phelps SM, and Ophir AG (2013). Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behav. Brain Res 246, 139–47. doi: 10.1016/j.bbr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]