Abstract
Background:
The retinal microvasculature provides a window to the cerebral vasculature and enables examination of changes in retinal caliber that may mimic those occurring in cerebrovascular disease. The association of central arterial stiffness and retinal vessel caliber in a population sample is not fully understood.
Methods:
In 1,706 older adults (mean age 76.3, 58.1% female) from the population-based Atherosclerosis Risk in Communities Study, we examined the cross-sectional association of central arterial stiffness (carotid-femoral pulse wave velocity (cfPWV)) with retinal vessel calibers (central retinal arteriolar equivalent (CRAE) and central retinal vein equivalent (CRVE)). We estimated the association of cfPWV with CRAE narrowing (<25th percentile) and CRVE widening (>75th percentile) after adjustment for age, sex, race-field center, body mass index, smoking, and type 2 diabetes. We tested for effect modification by sex, hypertension, and type 2 diabetes.
Results:
Carotid-femoral PWV (m/s) was not associated with the odds of CRAE narrowing (odds ratio (OR): 0.99; 95% CI: 0.95, 1.03). The association of cfPWV with CRVE widening was stronger in those without hypertension (OR: 1.10; 95% CI: 1.01, 1.20) versus those with hypertension (OR: 1.01 95% CI: 0.96, 1.05) and slightly stronger in those with type 2 diabetes (OR: 1.07; 95% CI: 1.00, 1.14) versus without type 2 diabetes (OR: 1.01; 95% CI: 0.96, 1.06).
Conclusions:
In older adults, cfPWV was associated with wider retinal venular caliber, particularly in individuals without hypertension. Central arterial stiffening may be associated with cerebral microvascular changes, as exhibited in its retinal vasculature component.
Keywords: arterial stiffness, retinal photography, small vessel disease, aging, cerebral vasculature, the ARIC study
Introduction
Due to the structural and functional similarities of the retinal and cerebral vessels, non-invasive measures of retinal vessels are of interest in the study of cerebral microvascular disease [1]. Retinal macro- and microvascular abnormalities are associated with risk of stroke [2, 3], large and small cerebral ischemic lesions on magnetic resonance imaging [4–6], and microvascular abnormalities are particularly associated with reduced cognitive function [7–10]. Thus, specific attention has focused on alterations in retinal arteriolar and retinal venule widths, or calibers, representing a window into similar alterations in the cerebral vasculature [11, 12].
Associations between central artery stiffness and various manifestations of cerebral small vessel disease have been reported, including lacunar strokes [13, 14], microbleeds [15], white matter hyperintensities [14, 16, 17], and white matter integrity [18]. Prior studies have shown that carotid arterial stiffness [19] and aortic distensibility [20] are also associated with retinal vessel calibers independent of blood pressure or hypertension in population-based studies. However, most reports evaluated the ratio of the arteriolar and venular calibers or the wall-to-lumen ratio of the retinal arteries [21, 22], rather than both calibers separately. It is now known that retinal arteriolar and venular calibers have differential associations with vascular risk factors. Narrower arteriolar caliber is strongly associated with hypertension [23], whereas wider venular caliber is associated with hyperglycemia and diabetes, higher body mass index [23, 24], and markers of inflammation [23, 25, 26]. Prior studies did not use central arterial stiffness measured by carotid-femoral pulse wave velocity (cfPWV), which is the gold standard measure of central aortic stiffness. Finally, most studies of the association between central arterial stiffness and retinal characteristics have focused on specific cohorts, such as individuals with type 2 diabetes [27, 28] or persons with stroke or other clinically-defined populations [29, 30].
A more detailed characterization of associations between central artery stiffness measured by carotid-femoral pulse wave velocity and retinal vessel diameters in a population-based study can offer insights into similar pathogenesis of cerebral vessels. Thus, the aim of this study was to quantify the cross-sectional association of central arterial stiffness with retinal vascular calibers (central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE)). We hypothesized that central arterial stiffness would be associated with narrower retinal arteriolar caliber (CRAE narrowing) and wider retinal venular caliber (CRVE widening).
Methods
Study participants
The Atherosclerosis Risk in Communities (ARIC) Study is a population-based, longitudinal study of 15,792 participants aged 45–64 years at the time of their enrollment in 1987–1989 from the following four US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Details of the baseline visit have been described [31]. This investigation included 2,044 participants who attended the visit 5 examination between 2011 and 2013 and had graded retinal fundus photography scans and measures of arterial stiffness. Participants provided written informed consent, and the ARIC study was approved by the Institutional Review Boards at all field centers and other study agencies.
We excluded participants with retinal abnormalities in both eyes (age-related macular degeneration, vein occlusions, retinopathy, macular edema, and other pathologies; n=70), body mass index (BMI) ≥40 kg/m2 (n=57) or missing BMI (n=4), major arrhythmias (Minnesota code 8-1-3, 8-3-1, and 8-3-2; (n=74), Minnesota code 8–1-2 with evidence of low quality PWV waveforms (n=9), self-reported history of aortic or peripheral revascularization or aortic graft (n=40), echocardiographic evidence of aortic stenosis or moderate or greater aortic regurgitation (n=11), or missing covariates of interest (mean arterial pressure (MAP), n=5; cigarette smoking history, n=13; hypertension status, n=15, and diabetes status, n=13), cfPWV greater than three standard deviations away from the mean (n=15), participants who self-identified as Asian or Native American from any site (n=2), and black participants from Minnesota or Maryland sites (n=10) were also excluded due to small numbers. The final analytic set included 1,706 participants after the exclusions.
Study staff asked participants to bring all prescription and nonprescription medications taken within the prior two weeks, to not consume food or drinks, and to refrain from tobacco and vigorous physical activity after midnight prior to the clinic visit or for 8 hours prior to the visit. Participants underwent a blood draw, B-mode scan of the abdominal aorta, standard 12-lead electrocardiogram, anthropometry, assessment of functional abilities, and interviewer-administered questionnaires to obtain medical history and lifestyle information. Body weight was measured to the nearest 0.1 kilogram and height was recorded to the nearest centimeter. Three seated blood pressure measurements were obtained after a five-minute rest using an oscillometric automated sphygmomanometer (Omron HEM-907 XL, Omron Co. Ltd., Kyoto, Japan) and the average of the last two measurements was used.
Hypertension was defined as systolic blood pressure (SBP) ≥140 mm/Hg, diastolic blood pressure (DBP) ≥90 mm/Hg, or anti-hypertensive medication use. Diabetes was defined as fasting glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, anti-diabetic medication use, or self-reported physician diagnosis of diabetes. Prevalent coronary heart disease (CHD) and stroke were defined by baseline status, ARIC cohort surveillance data, and adjudication through 2013, where the event occurred prior to the participant’s visit 5 examination. Standard resting 12-lead ECGs were digitally acquired using a GE MAC 1200 electrocardiograph (GE, Milwaukee, WI) at 10 mm/mV calibration and a speed of 25 mm/s. ECGs were centrally processed using GE 12-SL Marquette Version 2001 (GE, Milwaukee, Wisconsin) at the Epidemiological Cardiology Research Center at the Wake Forest School of Medicine.
Retinal Characteristics
Digital, non-mydriatic retinal fundus photographs were taken and evaluated by the Ocular Epidemiology Reading Center at the University of Wisconsin-Madison. CRAE and CRVE were calculated using a semiautomatic method used previously by ARIC [32, 33]. Data from a randomly selected eye were included (block randomization); if missing data, the other eye was selected (n=745).
Pulse wave velocity
Details of the PWV methodology used in ARIC have been reported [34]. Briefly, technicians measured PWV using the automated waveform analyzer VP-1000 Plus (Omron Co., Ltd., Kyoto, Japan) [35] after participants were supine for 5 to 10 minutes. Carotid and femoral arterial pressure waveforms were acquired by applanation tonometry sensors on the left common carotid artery and left common femoral artery. Bilateral brachial and posterior-tibial arterial pressure waveforms were detected by plethysmographic and an oscillometric pressure sensor wrapped on both arms and ankles. Higher values of cfPWV indicate arterial stiffness.
PWV was calculated as distance divided by transit time. Distance for carotid femoral PWV (cfPWV) was measured with a segmometer (Rosscraft, Surray, Canada) and calculated as the carotid to femoral distance minus the distance between the suprasternal notch to carotid. Technicians obtained two measurements and the results were averaged. Outliers, defined as values three standard deviations above or below the mean, were excluded for the analyses. Repeat visits were conducted for a subset of participants at each field center approximately 4 to 8 weeks later (n = 79; mean age 75.7 years; 46 females). The intra-class correlation coefficients and 95% confidence intervals (95% CIs) for single measurements were 0.70 (0.59, 0.81) for cfPWV [36] and approximately 0.82 for averaged cfPWV measurements, according to the Spearman-Brown formula.
Statistical analysis
Participant characteristics overall and by CRAE narrowing and CRVE widening were estimated as means and standard deviations or frequencies and percent, where appropriate. We used analysis of covariance to estimate adjusted means and the 95% confidence intervals (CI) of PWV by quartiles of CRAE and CRVE adjusted for age and sex.
Multivariable logistic regression analysis was used to separately evaluate the association of cfPWV with CRAE narrowing (<25th percentile) or CRVE widening (>75th percentile). The lower 25th percentile cut point was 132.6 µm for CRAE and the upper 75th percentile cut point was 219.7 µm for CRVE. We modeled cfPWV as a continuous variable and as a standard deviation change (per 3.00 m/s). Analyses were adjusted for age, sex, race-field center, body mass index, smoking (current, former, and never as the referent category), and type 2 diabetes. In a second model, we additionally adjusted for hypertension and/or MAP. As a priori hypotheses, we tested for interactions by sex, hypertension, and type 2 diabetes. P-values were two-sided with statistical significance of P<0.05 (SAS, version 9.2, SAS Institute, Inc., Cary, NC). We conducted a sensitivity analysis excluding participants with coronary heart disease and adjusting for inflammation (high-sensitivity C-reactive protein (hs-CRP), which is associated with cerebral venular widening.
Results
Participant characteristics
Among the 1.706 study participants, 58.1% were female and 21% were black (Table 1). The mean age was 76.3 years and the mean BMI was 27.6 kg/m2. Compared to those without CRAE narrowing, those with CRAE narrowing had, on average, a higher MAP, SBP, and DBP, and a lower prevalence of current smokers and type 2 diabetes. A different pattern was seen with CRVE widening. Compared to those without CRVE widening, those with CRVE widening had a higher prevalence of type 2 diabetes. They also had lower MAP, SBP, and DBP, higher fasting glucose, and a higher prevalence of current smokers, hypertension, and black participants.
Table 1.
Participant characteristics overall and by CRAE narrowing and CRVE widening in the Atherosclerosis Risk in Communities study (n=1,706)
| All |
CRAE narrowing (<25th percentile) |
CRVE widening (>75th percentile) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n=1,706 | No (n=1,245) | Yes (n=414) | No (n=1,243) | Yes (n=414) | ||||||
| Characteristic | n | % or mean (SD) | n | % or mean (SD) | n | % or mean (SD) | n | % or mean (SD) | n | % or mean (SD) |
| Age (years) | 1706 | 76.3 (5.2) | 1245 | 76.1 (5.2) | 414 | 77.1 (5.2) | 1243 | 76.6 (5.2) | 414 | 75.6 (5.1) |
| Female | 991 | 58.1 | 750 | 60.2 | 217 | 52.4 | 718 | 57.8 | 246 | 59.4 |
| Black | 359 | 21.0 | 265 | 21.3 | 76 | 18.4 | 190 | 15.3 | 162 | 39.1 |
| Body Mass Index (kg/m2) | 1706 | 27.6 (4.4) | 1245 | 27.8 (4.5) | 414 | 27.1 (4.3) | 1243 | 27.5 (4.4) | 414 | 28.1 (4.5) |
| Mean arterial pressure (mmHg) | 1706 | 87.5 (11.2) | 1245 | 87.0 (11.1) | 414 | 88.9 (11.2) | 1243 | 87.8 (11.3) | 414 | 86.9 (10.8) |
| Systolic blood pressure (mmHg) | 1706 | 130.6 (17.4) | 1245 | 130.1 (17.6) | 414 | 132.1 (16.9) | 1243 | 131.2 (17.4) | 414 | 128.7 (17.1) |
| Diastolic blood pressure (mmHg) | 1706 | 66.0 (10.1) | 1245 | 65.5 (10.1) | 414 | 67.2 (10.3) | 1243 | 66.1 (10.2) | 414 | 65.9 (9.6) |
| LDL cholesterol (mg/dL) | 1696 | 105.3 (34.9) | 1239 | 105.3 (34.7) | 411 | 104.8 (33.8) | 1237 | 105.5 (34.5) | 410 | 106.1 (35.8) |
| HbA1c (%) | 1699 | 5.9 (0.8) | 1240 | 5.9 (0.8) | 412 | 5.8 (0.7) | 1238 | 5.9 (0.7) | 412 | 6.0 (0.9) |
| Fasting glucose (mg/dL) | 1701 | 111.8 (27.1) | 1242 | 112.3 (27.2) | 412 | 109.9 (26.6) | 1240 | 111.3 (27.0) | 412 | 113.9 (28.1) |
| High Sensitive C-Reactive Protein (mg/L) | 1703 | 3.5 (6.0) | 1243 | 3.5 (6.1) | 413 | 3.3 (5.9) | 1241 | 3.4 (5.7) | 413 | 3.8 (6.8) |
| cfPWV (m/s) | 1706 | 11.7 (3.0) | 1245 | 11.7 (3.0) | 414 | 11.6 (3.0) | 1243 | 11.6 (2.9) | 414 | 12.0 (3.1) |
| CRAE (µm) | 1657 | 205.0 (23.4) | 1205 | 210.0 (21.8) | 405 | 189.3 (20.8) | 1243 | 194.9 (16.2) | 414 | 235.1 (14.1) |
| CRVE (µm) | 1659 | 142.5 (15.5) | 1245 | 149.1 (11.2) | 414 | 122.8 (8.2) | 1212 | 139.3 (14.7) | 398 | 151.8 (14.1) |
| Cigarette smoking | ||||||||||
| Current | 82 | 4.8 | 68 | 5.5 | 11 | 2.7 | 47 | 3.8 | 33 | 8.0 |
| Former | 805 | 47.2 | 575 | 46.2 | 203 | 49.0 | 581 | 46.7 | 199 | 48.1 |
| Never | 819 | 48.0 | 602 | 48.4 | 200 | 48.3 | 615 | 49.5 | 182 | 44.0 |
| Education | ||||||||||
| < High school | 216 | 12.7 | 169 | 13.6 | 35 | 8.5 | 131 | 10.5 | 78 | 18.9 |
| High school | 584 | 34.3 | 437 | 35.2 | 129 | 31.2 | 439 | 35.3 | 133 | 32.2 |
| > High school | 904 | 53.1 | 637 | 51.2 | 250 | 60.4 | 672 | 54.1 | 202 | 48.9 |
| Diabetes | 523 | 30.7 | 399 | 32.0 | 102 | 24.6 | 363 | 29.2 | 150 | 36.2 |
| Hypertension | 1240 | 72.7 | 908 | 72.9 | 298 | 72.0 | 890 | 71.6 | 312 | 75.4 |
| Prevalent coronary heart disease | 229 | 13.7 | 169 | 13.9 | 54 | 13.2 | 173 | 14.2 | 47 | 11.5 |
| Prevalent stroke | 52 | 3.0 | 33 | 2.7 | 16 | 3.9 | 39 | 3.1 | 12 | 2.9 |
cfPWV: carotid-femoral pulse wave velocity; CRAE: central retinal arteriolar equivalent; CRVE: central retinal vein equivalent; SD: standard deviation
Association between age- and sex-adjusted mean cfPWV by retinal vessel calibers quartiles
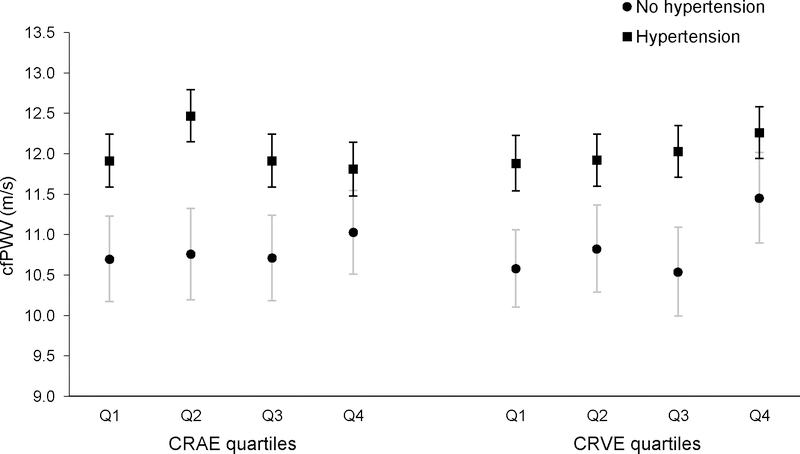
The age- and sex-adjusted mean cfPWV did not significantly differ across CRAE and CRVE quartiles (Figure 1). Although not statistically significant, the mean cfPWV was higher in the second CRAE quartile (12.05 m/s) compared to the other CRAE quartiles (range: 11.57 to 11.59 m/s), and the mean cfPWV was higher in the fourth quartile of CRVE (12.07 m/s) compared to the other CRVE quartiles (range: 11.44 to 11.65 m/s, in Q1 and Q3, respectively). Among those with hypertension, the association of the mean cfPWV with CRAE quartiles was similar to the overall results, whereas among those without hypertension, the mean cfPWV was similar across CRAE quartiles (Figure 2). For CRVE, the pattern of mean cfPWV values by CRVE quartiles was similar in both those with and without hypertension, although the increase in mean cfPWV from the first to fourth CRVE quartile was more prominent in those without hypertension.
Figure 1.
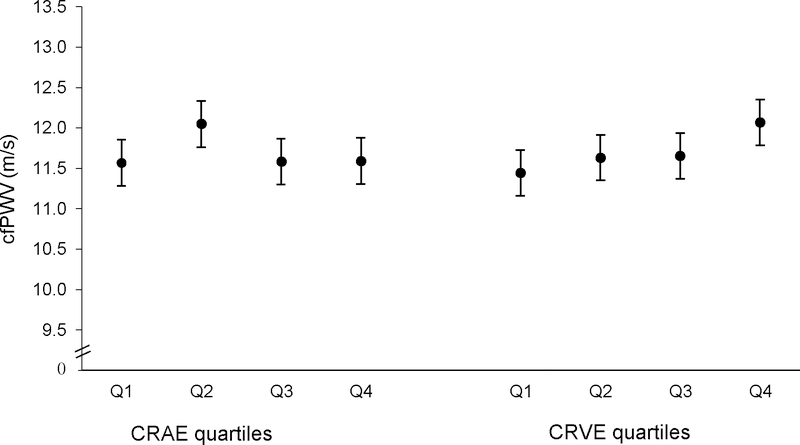
Age- and sex-adjusted mean carotid-femoral pulse wave velocity (cfPWV, m/s) by quartiles of central retinal arteriolar equivalent (CRAE) and central retinal vein equivalent (CRVE) with 95% confidence limits. The age- and sex-adjusted mean cfPWV did not significantly differ across CRAE and CRVE quartiles. Although not statistically significant, the mean cfPWV was higher in the second CRAE quartile compared to the other CRAE quartiles, and the mean cfPWV was higher in the fourth quartile of CRVE compared to the other CRVE quartiles.
Figure 2.
Age- and sex-adjusted mean carotid-femoral pulse wave velocity (cfPWV, m/s) by quartiles of central retinal arteriolar equivalent (CRAE) and central retinal vein equivalent (CRVE) with 95% confidence limits in those with hypertension (square, black lines) and without hypertension (circles, light gray lines). In those with hypertension, the association of the mean cfPWV with CRAE quartiles was similar to the overall results, whereas in those without hypertension, the mean cfPWV was similar across CRAE quartiles (Figure 2). For CRVE, the pattern of mean cfPWV values by CRVE quartiles was similar in both those with and without hypertension, although the increase in mean cfPWV from the first to fourth CRVE quartile was more prominent in those without hypertension.
Association of cfPWV with CRAE narrowing and CRVE widening
In multivariable logistic regression, cfPWV (per m/s) was not associated with the odds of CRAE narrowing (odds ratio (OR) 0.99; 95% confidence interval (CI): 0.95, 1.03) after adjusting for age, sex, smoking status, BMI, type 2 diabetes, and race-center (Table 2). Results were similar after adjusting for hypertension and MAP. Each m/s increase in cfPWV was associated with a 1.03 higher odds of CRVE widening (95% CI: 0.99, 1.07) after adjusting for age, sex, smoking status, BMI, type 2 diabetes, and race-center. Associations were stronger after additional adjustment for hypertension and MAP (OR per m/s: 1.05; 95% CI: 1.00, 1.09).
Table 2.
Association (prevalence odds ratio) of carotid-femoral pulse wave velocity (cfPWV) and covariates with central retinal arteriolar equivalent (CRAE) narrowing (<25th percentile) in the Atherosclerosis Risk in Communities study (n=1,659)
| Model 1 | Model 1 + HTN and MAP | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| cfPWV, m/s | 0.99 (0.95, 1.03) | 0.97 (0.93, 1.01) |
| Age, years | 1.04 (1.01, 1.06) | 1.04 (1.02, 1.07) |
| Female | 0.69 (0.55, 0.88) | 0.69 (0.55, 0.87) |
| Former smoker (vs. never smoker) | 1.00 (0.79, 1.26) | 1.02 (0.81, 1.29) |
| Current smoker (vs. never smoker) | 0.45 (0.23, 0.87) | 0.47 (0.24, 0.92) |
| Body mass index, kg/m2 | 0.98 (0.95, 1.00) | 0.97 (0.95, 1.00) |
| Diabetes | 0.73 (0.56, 0.96) | 0.77 (0.59, 1.01) |
| Hypertension | 0.93 (0.71, 1.23) | |
| Mean arterial pressure, mmHg | 1.02 (1.01, 1.03) |
Also adjusted for race-center; bold indicates p<0.05; HTN: hypertension; MAP: mean arterial pressure; OR: odds ratio
We detected evidence of effect modification by hypertension (interaction p=0.04) and modest effect modification by type 2 diabetes (interaction p=0.12) in the association between cfPWV and CRVE. We did not find evidence of effect modification for cfPWV and CRAE. In those without hypertension, cfPWV was associated with a higher odds of CRVE widening (OR: 1.10; 95% CI: 1.01, 1.20) with adjustment for age, sex, smoking status, BMI, type 2 diabetes, and race-center (Table 3). The magnitude of the association was stronger after additional adjustment for MAP. In those with hypertension, the association between cfPWV and CRVE widening was weaker for cfPWV (OR 1.01; 95% CI: 0.96, 1.05) and was stronger after adjustment for MAP, but not statistically significant. In those with type 2 diabetes, cfPWV was associated with a 1.07 times higher odds of CRVE widening (95% CI: 1.00, 1.14) and was statistically significant after additional adjustment for hypertension and MAP (Supplemental Table 1). In contrast, in those without type 2 diabetes, the association of cfPWV and CRVE was weaker (OR per m/s: 1.01; 95% CI: 0.96, 1.06) and did not change when additionally adjusting for hypertension and MAP.
Table 3.
Association (prevalence odds ratio) of carotid-femoral pulse wave velocity (cfPWV) and covariates with central retinal vein equivalent (CRVE) widening (>75th percentile) by hypertension status (p-value for interaction = 0.04) in the Atherosclerosis Risk in Communities study (n=1,657)
| No Hypertension, n=455 | Yes Hypertension, n=1,202 | |||
|---|---|---|---|---|
| Model 1 | Model 1 + MAP | Model 1 | Model 1 + MAP | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| cfPWV, m/s | 1.10 (1.01, 1.20) | 1.15 (1.05, 1.26) | 1.01 (0.96, 1.05) | 1.02 (0.97, 1.07) |
| Age, years | 0.93 (0.89, 0.98) | 0.91 (0.87, 0.96) | 0.98 (0.96, 1.01) | 0.98 (0.95, 1.01) |
| Female | 0.67 (0.41, 1.09) | 0.66 (0.40, 1.09) | 1.30 (0.98, 1.73) | 1.31 (0.99, 1.76) |
| Former smoker (vs. never smoker) | 1.23 (0.74, 2.02) | 1.30 (0.78, 2.15) | 1.33 (1.00, 1.77) | 1.29 (0.97, 1.72) |
| Current smoker (vs. never smoker) | 2.46 (0.95, 6.40) | 2.13 (0.79, 5.74) | 2.83 (1.55, 5.17) | 2.75 (1.50, 5.05) |
| Body mass index, kg/m2 | 1.03 (0.97, 1.09) | 1.04 (0.98, 1.10) | 1.01 (0.98, 1.04) | 1.01 (0.98, 1.04) |
| Diabetes | 0.65 (0.35, 1.21) | 0.55 (0.29, 1.06) | 1.29 (0.97, 1.72) | 1.22 (0.91, 1.63) |
| Mean arterial pressure, mmHg | 0.95 (0.92, 0.98) | 0.98 (0.97, 1.00) | ||
Additionally adjusted for race-center; bold indicates p<0.05; cfPWV: carotid-femoral pulse wave velocity; CI: confidence interval; OR: odds ratio; MAP: mean arterial pressure
In a sensitivity analysis, the results did not change after adjusting for high-sensitivity C-reactive protein. After excluding participants with prevalent coronary heart disease (n=240), the association of cfPWV and CRAE narrowing was the same, but the association of cfPWV and CRVE widening was stronger. Results were also the same in a complete case analysis (i.e. only including those with both CRAE and CRVE measures, N=1,610). Lastly, the results were consistent when analyzing CRAE and CRVE as continuous variables.
Discussion
In this study of older adults, higher cfPWV was associated with CRVE in those without hypertension, but was not significantly associated with CRAE narrowing. Additionally, the association between cfPWV and CRVE widening varied by hypertension and diabetes status, which was not the case for CRAE narrowing. When additionally adjusting for hypertension and/or MAP, the results did not change for CRAE, but were stronger for CRVE. Different associations between the cfPWV and the type of vessels suggest that retinal arteriolar and venular caliber abnormalities are influenced by different vascular risk factors, possibly reflecting a different underlying pathogenesis.
Few studies of arterial stiffness and retinal vessel calibers included the standard PWV measurement for arterial stiffness, and their results are not consistent. In a study of hypertensive individuals (n=181; mean age 54 years), cfPWV was negatively associated with CRAE and positively associated with CRVE [37]. However, in another study of hypertensive individuals (n=88; mean age 54 years), cfPWV was negatively associated with both CRAE and CRVE, but the association was not robust to additional covariate adjustment [38]. In 223 normotensive and newly diagnosed hypertensive adults, cfPWV was associated with narrower CRAE, but not with CRVE [29]. Similar results were shown in ischemic stroke patients where cfPWV was associated with narrower CRAE, although CRVE was not included in the analysis [30]. These studies included participants with hypertension or stroke, and in fairly small numbers, in contrast to this population-based study of much older adults. Additionally, few studies included both CRAE and CRVE measurements, which were included in this study.
Beyond cfPWV, other markers of arterial stiffness and vascular disease are associated with retinal diameters. In a prior ARIC report, pulsatile carotid arterial diameter change was associated with the arteriole-to-venule ratio, though these calibers were not included individually in the analysis [19]. This association was independent of hypertension status. However, in this study, we observed effect modification by hypertensive status. In the Multi-Ethnic Study of Atherosclerosis, reduced aortic distensibility was associated with retinal arteriolar narrowing, but not with venular caliber [20]. Another MESA study showed that reduced brachial large-artery compliance, a peripheral measure of arterial stiffness, was associated with smaller retinal arteriolar caliber, whereas small-artery compliance was associated with larger venular caliber [39]. Both MESA study results were independent of blood pressure and hypertension medications and the population was younger than this study. Indicators of atherosclerosis, higher ankle-arm index and carotid plaque score, were associated with wider CRVE in the Rotterdam study, and estimates were stronger when accounting for blood pressure [24]. Similarly, our study showed arterial stiffness was associated with larger venular caliber and the associations were stronger after accounting for hypertension status and MAP. Our study and others suggest the association of arterial stiffness with CRAE and CRVE represent different pathophysiologic mechanisms, and hypertension status and blood pressure affects these associations.
Historically, studies typically evaluated the association of blood pressure with arteriole-to-venule ratio because it was thought that venular vessel calibers are unaffected by blood pressure. Now, the focus has shifted to both retinal arteriolar and venular calibers as important and distinct markers of disease [24, 40]. CRVE widening predicts hypertension [41, 42], stroke [43–45], and dementia [46]. Further, blood pressure level is consistently associated with CRAE narrowing [23], whereas hyperglycemia, obesity [23, 24], and inflammation [23, 25] are associated with CRVE widening. We observed these distinct profiles of covariates for the arteriolar and venular vessels, and our results also suggest a stronger association of cfPWV with venular diameters versus arteriolar diameters in older age.
Arterial stiffness is increased in persons with diabetes and the association is stronger in those with microvascular complications [47]. In studies of participants with type 2 diabetes, cfPWV [28] and heart-femoral PWV [27] were associated with retinopathy. However, these studies did not include retinal vessel calibers. In the present study, there was a modest effect modification where the association of arterial stiffness and retinal venular widening was stronger among those with diabetes compared to those without diabetes. In diabetes, the arterial stiffening and microvasculature complications may be exacerbated by hypertrophic remodeling seen in diabetes [48, 49] and elevated advanced glycosylation end-products (AGEs) [50, 51], which cross-link with collagen and elastin resulting in decreased vascular elasticity. Altogether, this suggests that shared mechanisms could magnify the association between arterial stiffness and retinal venular widening in those with type 2 diabetes.
Given the established association of blood pressure with CRAE, we expected to observe an association of CRAE with arterial stiffness. The lack of association between cfPWV and CRAE may reflect the older age of our examinees. In a meta-analysis of individual data, CRAE narrowing was associated with incident hypertension in participants <60 years old, but not in participants ≥70 years old [41]. This was not observed with CRVE widening. Similarly, in the Rotterdam study, the association of arteriolar diameters and blood pressure was stronger in younger participants and not significant after 80 years old, whereas the association of venular diameters and blood pressure was weaker and there was no association when stratified by age [24]. Although we did not observe an association between cfPWV and CRAE, we observed differences in the association between cfPWV and CRVE by hypertension status where the effect was stronger among those without hypertension. The lack of association in hypertension may be due to the older age of the participants, consistent with the prior studies of blood pressure.
Arteries stiffen with age and exposure to risk factors. Elevated arterial stiffness is accompanied by high pulse pressure that is transmitted to the microvasculature leading to hypoperfusion and vascular remodeling [52], with detrimental effects on end-organs, particularly the brain. Arterial stiffness has emerged as a risk factor for adverse morphologic and functional changes in the brain, but the expense and burden of magnetic resonance imaging, alternate non-invasive methods to study this association are needed, such as the quantification of retinal vessels characteristics. The retinal microvasculature serves as an index of cerebral small vessel pathologies, supported by evidence that retinal microvascular abnormalities are associated with lower cognitive function [7, 8] and cerebral ischemic lesions on magnetic resonance imaging [4–6]. The relation of retinal venular widening with cerebral small vessel disease is not clear, and indeed difficult to explain since wide venules should not impair blood flow. However, studies have shown venular widening is associated with lower cerebral oxygen supply [53], stroke [3], progression of cerebral small vessel disease [54], and vascular dementia [46], suggesting that venular widening could relate to cerebral hypoperfusion and ischemia in the brain through associated processes such as inflammation, and they could also be related to vascular risk factors such as arterial stiffness. Thus, the relationship between central arterial stiffness and retinal vascular calibers may indicate other mechanisms underlying the observed associations between cfPWV and cerebral ischemic abnormalities on magnetic resonance imaging.
There are limitations of the current study to consider. The cross-sectional design precludes the assessment of temporality and causality in the observed associations. Some PWV and retinal measurements were not collected due to technical limitations, low quality retinal images, participant factors, and scheduling conflicts. We excluded retinal measures with certain pathologies that would prevent obtaining a good measure of the arterioles/veins. Additionally, we did not include ungraded retinal measures (e.g. issue with focus, washed out, or disk head not visible) or measures with less than six arteries or venues graded to address potential concerns with the focus of the retinal image using fundus photography. Another limitation to consider is the potential for survival bias due to attrition over the course of >25 years of follow-up. The surviving cohort members likely are healthier and more vigorous than those who did not take part in the examination, which would tend to attenuate the observed associations. The internal validity of the associations estimated in this study is supported by our multivariable statistical adjustment for participant characteristics that influenced attrition, and we deem our results from this population-based cohort to be generalizable to other populations of older adults of comparable demographic characteristics.
Conclusions
Understanding the association of central arterial stiffness and retinal characteristics enables testable hypotheses regarding the pathogenesis of cerebral abnormalities of vascular origin. In a population-based study with a gold-standard measure of central arterial stiffness, we found central arterial stiffness to be associated with CRVE widening in those without hypertension, suggesting that macrovascular arterial stiffness may play a role in or be an indicator of microvascular retinal changes that mimic cerebral vascular disease. The different associations of cfPWV and CRAE and CRVE suggest that diverse pathogenic processes are involved in macrovascular and microvascular changes that should be investigated further; both CRAE and CRVE deserve attention as disease-related phenotypes. Central arterial stiffening may be associated with cerebral microvascular changes, as exhibited in its retinal vasculature component.
Supplementary Material
Acknowledgments
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. The authors thank the staff and participants of the ARIC study for their important contributions. MLM was supported by the Building Interdisciplinary Research Careers in Women’s Health (5K12HD001441). PP was supported by grant K99-AG-052830 from the National Institute of Aging.
Footnotes
Conflict of interest: None declared
References
- 1.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J Anat 2005; 206:319–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: The Atherosclerosis Risk in Communities Study. Lancet 2001; 358:1134–1140. [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: The Cardiovascular Health Study. Arch Intern Med 2006; 166:2388–2394. [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 2002; 288:67–74. [DOI] [PubMed] [Google Scholar]
- 5.Sharrett AR. A review of population-based retinal studies of the microvascular contribution to cerebrovascular diseases. Ophthalmic Epidemiol 2007; 14:238–242. [DOI] [PubMed] [Google Scholar]
- 6.Heringa SM, Bouvy WH, van den Berg E, Moll AC, Kappelle LJ, Biessels GJ. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: A systematic review. J Cereb Blood Flow Metab 2013; 33:983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong TY, Klein R, Sharrett AR, Nieto FJ, Boland LL, Couper DJ, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: The Atherosclerosis Risk in Communities Study. Stroke 2002; 33:1487–1492. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Patton N, Deary IJ, Strachan MW, Fowkes FG, Mitchell RJ, et al. Retinal microvascular abnormalities and cognitive dysfunction: A systematic review. Br J Ophthalmol 2008; 92:1017–1025. [DOI] [PubMed] [Google Scholar]
- 9.Shalev I, Moffitt TE, Wong TY, Meier MH, Houts RM, Ding J, et al. Retinal vessel caliber and lifelong neuropsychological functioning: Retinal imaging as an investigative tool for cognitive epidemiology. Psychol Sci 2013; 24:1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei FF, Raaijmakers A, Zhang ZY, van Tienoven TP, Huang QF, Yang WY, et al. Association between cognition and the retinal microvasculature in 11-year old children born preterm or at term. Early Hum Dev 2018; 118:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol 2004; 3:179–183. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CY, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res 2017; 57:89–107. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility--Reykjavik Study. Brain 2011; 134:3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 2013; 81:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song TJ, Kim J, Kim YD, Nam HS, Lee HS, Nam CM, et al. The distribution of cerebral microbleeds determines their association with arterial stiffness in non-cardioembolic acute stroke patients. Eur J Neurol 2014; 21:463–469. [DOI] [PubMed] [Google Scholar]
- 16.van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: A systematic review and meta-analysis. Neurosci Biobehav Rev 2015; 53:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosano C, Watson N, Chang Y, Newman AB, Aizenstein HJ, Du Y, et al. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension 2013; 61:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, et al. Effects of arterial stiffness on brain integrity in young adults from the Framingham Heart Study. Stroke 2016; 47:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao D, Wong TY, Klein R, Jones D, Hubbard L, Sharrett AR. Relationship between carotid artery stiffness and retinal arteriolar narrowing in healthy middle-aged persons. Stroke 2004; 35:837–842. [DOI] [PubMed] [Google Scholar]
- 20.Cheung N, Sharrett AR, Klein R, Criqui MH, Islam FM, Macura KJ, et al. Aortic distensibility and retinal arteriolar narrowing: The Multi-Ethnic Study of Atherosclerosis. Hypertension 2007; 50:617–622. [DOI] [PubMed] [Google Scholar]
- 21.Ott C, Raff U, Harazny JM, Michelson G, Schmieder RE. Central pulse pressure is an independent determinant of vascular remodeling in the retinal circulation. Hypertension 2013; 61:1340–1345. [DOI] [PubMed] [Google Scholar]
- 22.Salvetti M, Agabiti Rosei C, Paini A, Aggiusti C, Cancarini A, Duse S, et al. Relationship of wall-to-lumen ratio of retinal arterioles with clinic and 24-hour blood pressure. Hypertension 2014; 63:1110–1115. [DOI] [PubMed] [Google Scholar]
- 23.Liew G, Sharrett AR, Wang JJ, Klein R, Klein BE, Mitchell P, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: The Atherosclerosis Risk in Communities Study. Arch Ophthalmol 2008; 126:1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 2004; 45:2129–2134. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol 2006; 124:87–94. [DOI] [PubMed] [Google Scholar]
- 26.Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: The Multi-Ethnic Study of Atherosclerosis (MESA). Invest Ophthalmol Vis Sci 2006; 47:2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim WJ, Park CY, Park SE, Rhee EJ, Lee WY, Oh KW, et al. The association between regional arterial stiffness and diabetic retinopathy in type 2 diabetes. Atherosclerosis 2012; 225:237–241. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso CR, Ferreira MT, Leite NC, Barros PN, Conte PH, Salles GF. Microvascular degenerative complications are associated with increased aortic stiffness in type 2 diabetic patients. Atherosclerosis 2009; 205:472–476. [DOI] [PubMed] [Google Scholar]
- 29.Triantafyllou A, Anyfanti P, Gavriilaki E, Zabulis X, Gkaliagkousi E, Petidis K, et al. Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals. Am J Hypertens 2014; 27:1472–1478. [DOI] [PubMed] [Google Scholar]
- 30.De Silva DA, Woon FP, Manzano JJ, Liu EY, Chang HM, Chen C, et al. The relationship between aortic stiffness and changes in retinal microvessels among asian ischemic stroke patients. J Hum Hypertens 2012; 26:716–722. [DOI] [PubMed] [Google Scholar]
- 31.The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC investigators. Am J Epidemiol 1989; 129:687–702. [PubMed] [Google Scholar]
- 32.Parr JC, Spears GF. Mathematic relationships between the width of a retinal artery and the widths of its branches. Am J Ophthalmol 1974; 77:478–483. [DOI] [PubMed] [Google Scholar]
- 33.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999; 106:2269–2280. [DOI] [PubMed] [Google Scholar]
- 34.Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, et al. Correlates of segmental pulse wave velocity in older adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens 2015; [DOI] [PMC free article] [PubMed]
- 35.Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 2003; 91:1519–1522, A1519. [DOI] [PubMed] [Google Scholar]
- 36.Meyer ML, Tanaka H, Palta P, Patel MD, Camplain R, Couper D, et al. Repeatability of central and peripheral pulse wave velocity measures: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens 2016; 29:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aissopou EK, Argyris AA, Nasothimiou EG, Konstantonis GD, Tampakis K, Tentolouris N, et al. Ambulatory aortic stiffness is associated with narrow retinal arteriolar caliber in hypertensives: The Safar Study. Am J Hypertens 2016; 29:626–633. [DOI] [PubMed] [Google Scholar]
- 38.Daien V, Granados L, Kawasaki R, Villain M, Ribstein J, Du Cailar G, et al. Retinal vascular caliber associated with cardiac and renal target organ damage in never-treated hypertensive patients. Microcirculation 2017; 24 [DOI] [PubMed] [Google Scholar]
- 39.Cheung N, Islam FM, Jacobs DR Jr., Sharrett AR, Klein R, Polak JF, et al. Arterial compliance and retinal vascular caliber in cerebrovascular disease. Ann Neurol 2007; 62:618–624. [DOI] [PubMed] [Google Scholar]
- 40.Liew G, Sharrett AR, Kronmal R, Klein R, Wong TY, Mitchell P, et al. Measurement of retinal vascular caliber: Issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci 2007; 48:52–57. [DOI] [PubMed] [Google Scholar]
- 41.Ding J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, et al. Retinal vascular caliber and the development of hypertension: A meta-analysis of individual participant data. J Hypertens 2014; 32:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawasaki R, Cheung N, Wang JJ, Klein R, Klein BE, Cotch MF, et al. Retinal vessel diameters and risk of hypertension: The multiethnic Study of Atherosclerosis. J Hypertens 2009; 27:2386–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doubal FN, MacGillivray TJ, Hokke PE, Dhillon B, Dennis MS, Wardlaw JM. Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology 2009; 72:1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, et al. Retinal vessel diameters and risk of stroke: The Rotterdam Study. Neurology 2006; 66:1339–1343. [DOI] [PubMed] [Google Scholar]
- 45.Dumitrascu OM, Demaerschalk BM, Valencia Sanchez C, Almader-Douglas D, O’Carroll CB, Aguilar MI, et al. Retinal microvascular abnormalities as surrogate markers of cerebrovascular ischemic disease: A meta-analysis. J Stroke Cerebrovasc Dis 2018; 27:1960–1968. [DOI] [PubMed] [Google Scholar]
- 46.de Jong FJ, Schrijvers EM, Ikram MK, Koudstaal PJ, de Jong PT, Hofman A, et al. Retinal vascular caliber and risk of dementia: The Rotterdam Study. Neurology 2011; 76:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia 2008; 51:527–539. [DOI] [PubMed] [Google Scholar]
- 48.Sonoyama K, Greenstein A, Price A, Khavandi K, Heagerty T. Vascular remodeling: Implications for small artery function and target organ damage. Ther Adv Cardiovasc Dis 2007; 1:129–137. [DOI] [PubMed] [Google Scholar]
- 49.Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation 2001; 103:1238–1244. [DOI] [PubMed] [Google Scholar]
- 50.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006; 114:597–605. [DOI] [PubMed] [Google Scholar]
- 51.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 2008; 93:1143–1152. [DOI] [PubMed] [Google Scholar]
- 52.Laurent S, Briet M, Boutouyrie P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension 2009; 54:388–392. [DOI] [PubMed] [Google Scholar]
- 53.de Jong FJ, Vernooij MW, Ikram MK, Ikram MA, Hofman A, Krestin GP, et al. Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters. The Rotterdam Study. Ophthalmology 2008; 115:887–892. [DOI] [PubMed] [Google Scholar]
- 54.Ikram MK, De Jong FJ, Van Dijk EJ, Prins ND, Hofman A, Breteler MM, et al. Retinal vessel diameters and cerebral small vessel disease: The Rotterdam Scan Study. Brain 2006; 129:182–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.