Abstract
INTRODUCTION:
It is important to establish the natural history of familial frontotemporal lobar degeneration (f-FTLD) and provide clinical and biomarker data for planning these studies, particularly in the asymptomatic phase.
METHODS:
The Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects protocol was designed to enroll and follow at least 300 subjects over at least three annual visits who are members of kindreds with a mutation in one of the three most common f-FTLD genes – microtubule associated protein tau, progranulin or chromosome 9 open reading frame 72.
RESULTS:
We present the theoretical considerations of f-FTLD and the aims/objectives of this protocol. We also describe the design and methodology for evaluating and rating subjects, in which detailed clinical and neuropsychological assessments are performed, biofluid samples are collected and MRI scans are performed using a standard protocol.
DISCUSSION:
These data and samples, which are available to interested investigators worldwide, will facilitate planning for upcoming disease-modifying therapeutic trials in f-FTLD.
Keywords: frontotemporal dementia, genetics, MAPT, GRN, C9orf72, tau, TDP-43
1. Introduction
Frontotemporal lobar degeneration (FTLD) is caused by two major proteinopathies–microtubule associated protein tau and TAR DNA binding protein molecular weight 43 [1, 2]. At least 20% of all FTLD presents as a dominantly inherited familial disorder (f-FTLD), usually due to mutations in the microtubule associated protein tau (MAPT) [3], progranulin (GRN) [4], or chromosome 9 open reading frame 72 (C9orf72) [5, 6] genes, which together account for at least 50% of f-FTLD [7–9]. Because each mutation is highly predictive of a specific proteinopathy, the study of f-FTLD mutation carriers has the unique opportunity to provide specific biochemical targets in clinical drug studies. In addition, f-FTLD is currently the only practical way to identify people in asymptomatic or very early symptomatic stages of FTD, making it the best context for testing drugs aimed at delaying symptom onset. To prepare for disease-modifying trials, it is important to establish the natural history of f-FTLD and provide clinical and biomarker data for planning these studies, particularly in the asymptomatic phase.
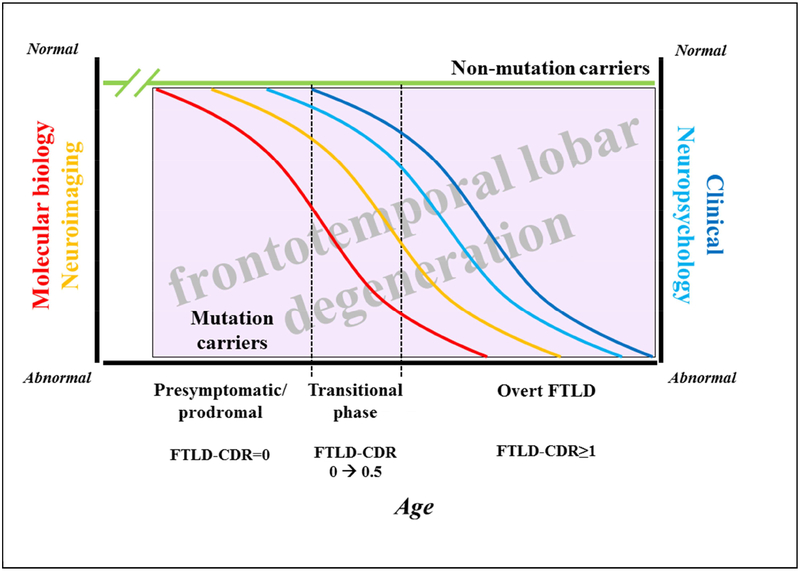
The rates of clinical and biomarker change in f-FTLD are complex and dynamic (Figure 1). The alterations in the molecular biology of tau, progranulin and the granulins, C9RAN proteins, etc., undoubtedly occur early in life during the presymptomatic phase. As neuronal and/or glial dysfunction evolves, changes in neuronal networks occur over an acceleration phase which can be demonstrated on neuroimaging measures, with functional MR changes likely preceding structural MR changes. Various other ancillary studies, including behavioral measures, neuropsychological measures, motor measures, etc., likely show the evolution of clinically silent to very minimally evident cognitive, behavioral or motor changes over the several year transitional period from presymptomatic to prodromal to minimally symptomatic phases of f-FTLD. MR-based and other imaging measures likely change over this transitional period also. Additional changes occur with the onset of overt symptoms and continue onward through the mild, moderate, severe and terminal phases of the symptomatic period – the latter aspects likely evolve in a decelerated manner. This hypothesized cascade of dynamic changes is analogous to what has been proposed in the evolution of Alzheimer’s disease [10, 11].
Figure 1. Schema and research approach of familial FTLD.
The alterations in the molecular biology (red curve) of tau, progranulin and the granulins, C9RAN proteins, etc., undoubtedly occur early in life during the presymptomatic phase. As neuronal and/or glial dysfunction evolves, changes in neuronal networks occur which can be demonstrated on neuroimaging measures (orange curve), with functional MR changes likely preceding structural MR changes. Other measures including neuropsychological measures (light blue curve) and clinical (including behavioral and motor measures, as shown in the dark blue curve) likely show the evolution of clinically silent (represented by an FTLD-CDR rating of 0) to very minimally evident cognitive, behavioral or motor changes over the several year transitional period from presymptomatic to prodromal to minimally symptomatic phases of f-FTLD (represented by an FTLD-CDR rating of 0.5). MR-based and other imaging measures likely change over this transitional period also. Additional changes occur with the onset of overt features (represented by an FTLD-CDR rating ≥1) and continue onward through the mild, moderate, severe and terminal phases of the symptomatic period. For each set of measures, there is likely a slow change phase, followed by an acceleration phase, then a deceleration phase, and then a terminal slow change phase. Those individuals who do not carry a mutation (shown as the green line) are expected to show no consistent change across these measures. This hypothesized cascade of dynamic changes is analogous to what has been proposed in the evolution of Alzheimer’s disease. See text for abbreviations.
While there is growing data that supports the cascade of events and findings just described [12–26], many of the findings are based on cross-sectional analyses, and little longitudinal data has been published thus far. Plus, many questions remain. How does one predict the onset of symptoms and rate of progression? What dictates the initial seed of neuronal dysfunction and hence the constellation of early features and evolving neuronal network dysfunction and associated clinical phenomenology over time? Why do some mutation carriers develop symptoms early in life while others never develop symptoms (i.e., incomplete penetrance)? Longitudinal evaluations of a large number of individuals in families with known mutations, followed prospectively in a standardized and comprehensive manner, offer the best hope of providing insights to these and other questions while also informing investigators how to optimally design clinical trials.
We sought to address many of these questions as part of the Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects or LEFFTDS protocol (UO1 AG045390). We describe herein the design and methodology of the LEFFTDS protocol. The initial baseline characteristics and analyses and other topics will be reported separately.
2. Objectives/Aims
The specific aims/objectives of the LEFFTDS protocol are:
To model the rates of decline in traditional measures of clinical (neuropsychological and behavioral composites) function and cortical volume on structural MRI in the symptomatic phase (+mFTLD-CDR>0) of f-FTLD.
To model the rates of decline in traditional measures of clinical (neuropsychological and behavioral composites) function and cortical volume on structural MRI in the asymptomatic phase (+mFTLD-CDR=0) of f-FTLD.
To assess the value of novel imaging and clinical measures for characterizing asymptomatic f-FTLD subjects, and identify factors predicting clinical rates of progression in each group.
To identify genetic and biofluid factors that modify rates of clinical and neuroimaging decline in the asymptomatic and symptomatic phases of f-FTLD.
These aims are shown schematically in e-figures 1–4.
Note that the term “asymptomatic” is preferred over “presymptomatic” in the context of these LEFFTDS aims since there is incomplete penetrance across all three major genetic groups.
3. Study Design
3.1. Overview
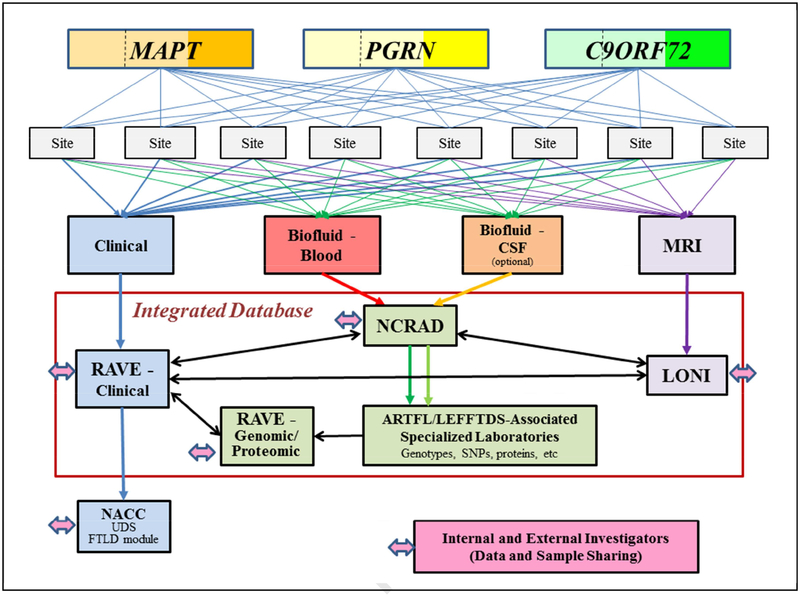
The overall schema for the LEFFTDS protocol is shown in Figure 2. The project is designed to enroll at least 300 subjects from families with f-FTLD into a longitudinal clinical and biomarker study. The subjects are recruited based on interest of potential participants, with the expectation that enrollment will transpire in an approximately even fashion across kindreds with mutations in three most common genes associated with f-FTLD: MAPT (n=100), GRN (n=100) and C9orf72 (n=100). Our goal was to recruit approximately equal numbers of symptomatic mutation carriers, asymptomatic mutation carriers, and non-carriers (i.e., familial controls). At least three annual assessments (henceforth termed “visits”) for each subject are planned over this five year phase of the study. Each visit includes a clinical assessment, biofluid sampling with blood and CSF collection, and MRI; CSF collection is optional. The clinical data is entered into an electronic data capture system (via the iMedidata RAVE system, Houston, TX). The majority of the data is collected using measures in the Uniform Data Set (UDS) and Frontotemporal Lobar Degeneration (FTLD) Module of the National Alzheimer’s Coordinating Center (NACC), and these data are uploaded to NACC at the University of Washington. Blood and CSF are collected and sent to the National Cell Repository for Alzheimer’s Disease (NCRAD) at Indiana University. Aliquots of DNA, plasma and CSF are sent to LEFFTDS-associated laboratories for genotyping and protein quantification. Brain MRI is performed using a standardized protocol similar to the Alzheimer’s Disease Neuroimaging version 3 (ADNI-3) protocol. The data is transferred to the Laboratory of Neuroimaging (LONI) at the University of Southern California, and downloaded and assessed at Mayo Clinic Rochester for quality review. All data and samples are available to internal and external investigators. The overarching design of the LEFFTDS protocol is to address the specific aims and to provide clinical, biofluid and neuroimaging samples and data to investigators. More details on this infrastructure and procedures are described below.
Figure 2. Protocol Schema.
Three hundred subjects (100 among kindreds with a mutation in MAPT, 100 among kindreds with a mutation in PGRN, and 100 among kindreds with the C9orf72 mutation) are enrolled and followed at one of the eight sites. Within each gene, approximately 1/3 are symptomatic (reflected by darker shades of orange, yellow or green) whereas 2/3 are asymptomatic (reflected by lighter shades of the colors). The lighter shade areas are divided by a dashed line, which reflects one half of the asymptomatic are non-mutation carrier/family controls while the other half are mutation carriers. Each subject can participate in four research arms – clinical, biofluid-blood, biofluid-CSF and MRI; the CSF arm is optional. Each subject can also participate in a 5th arm (not shown) in which clinical genetic counseling and testing can be performed. The clinical data is entered into an electronic data capture system (RAVE), and the majority of this data is uploaded to the NACC. Biofluid samples are submitted to NCRAD for processing and storage. See text for abbreviations.
3.2. Recruitment
The subjects are recruited from subjects/kindreds already identified at the collaborating centers. In addition, referrals are solicited from other centers interested in f-FTLD, the Association for Frontotemporal Degeneration (www.theaftd.org), and the ClinicalTrials.gov websites for LEFFTDS (https://clinicaltrials.gov/show/NCT02372773) and a closely related protocol known as the Advancement in Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) (https://www.rarediseasesnetwork.org/cms/artfl/). Interested subjects and clinicians are welcome to contact any of the individuals listed on this ClinicalTrials.gov websites. A website for the LEFFTDS protocol is under development at the time of this writing.
3.3. Inclusion/exclusion criteria
Subjects are eligible for enrollment if they are members of families with a known mutation in one of the three major FTLD related genes: MAPT, GRN, and C9orf72 and is age 18 or older, and preferably above age 30. Other inclusion criteria include: the predominant phenotype in the kindred should be cognitive/behavioral (ie, kindreds in whom bvFTD or PPA is the predominant clinical phenotype among affected relatives is favored over parkinsonism or ALS, although all phenotypes are eligible for enrollment), a reliable informant who personally speaks with or sees that subject at least weekly, subject is sufficiently fluent in English to complete all measures, willing and able to consent to the protocol and undergo yearly evaluations, willing and able to undergo neuropsychological testing (at least at the baseline visit), and no contraindication to MRI imaging. Exclusion criteria include: absence of a known mutation in MAPT, GRN or C9orf72 in the subject or family, presence of a structural brain lesion (eg, tumor, cortical infarct), presence of another neurologic disorder which could impact findings (eg, multiple sclerosis), unwillingness to return for follow-up yearly, unwillingness to undergo neuropsychological testing and MR imaging, and no reliable informant.
Individuals are not required to know or learn their own genetic status, but all are offered the option of determining the mutation carrier status via genetic testing after genetic counseling. Genetic results are confirmed by a CLIA approved lab before disclosure. Counseling and testing services are paid for by the study.
3.4. Ethics
This protocol has been reviewed and approved by all local institutional review boards.
4. Procedures
4.1. Overview
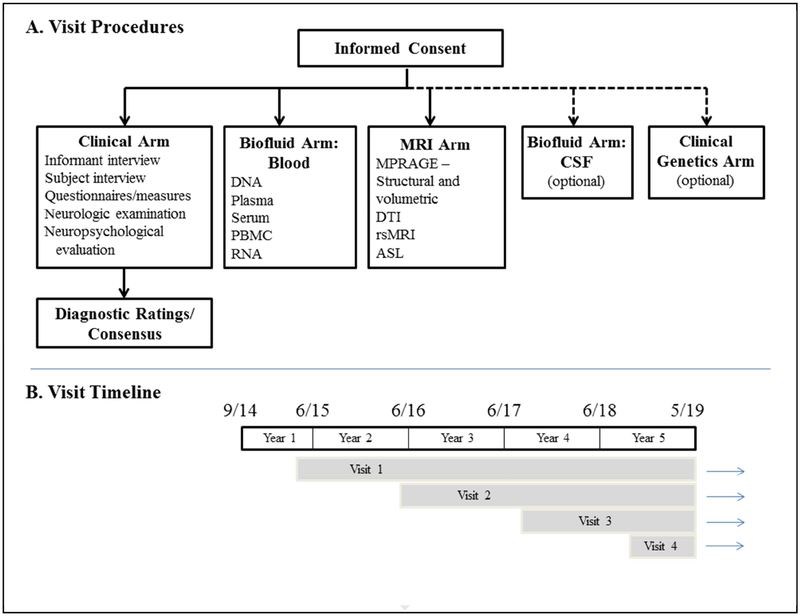
A summary of the procedures for each visit is shown in Figure 3. The procedures can be viewed as five arms – clinical, biofluid-blood, MRI, biofluid-CSF and genetic testing. Each visit includes, at a minimum, the clinical evaluation, blood draw as well as MRI scanning; the CSF arm and genetic testing arms are optional. A genetic evaluation for counseling with our without genetic testing is available for any subject who desires it.
Figure 3. Study Procedures and Timeline.
See text in Section 4 for details and abbreviations.
4.2. Enrollment
Each presumed asymptomatic subject reviews and provides written consent. Each subject also identifies a reliable informant to provide collateral history – typically a spouse, sibling, parent or adult child. For symptomatic subjects, the person provides written consent if deemed to have capacity; for those who are not viewed as having capacity, the informant is the proxy who provides written consent and the participant provides written assent.
4.3. Clinical arm
All subjects undergo a detailed interview, examination and neuropsychological assessment. Each informant is also interviewed. The data, measures [27–63] and databases where the data are stored are summarized in Table 1.
Table 1.
Data and Measures
| Data | Measure | Database | |
|---|---|---|---|
| Standard History and Physical Examination | |||
| Demographic | A/L, UDS form | A/L, UDS | |
| History | Clinical Global Impression [27] | A/L | |
| Medications | A/L, UDS form | A/L, UDS | |
| Past medical history | A/L, UDS form | A/L, UDS | |
| Family history | A/L, UDS form | A/L, UDS | |
| Physical examination | A/L, UDS form | A/L, UDS | |
| Neurologic examination | Neurologic examination form | A/L | |
| Functional/Clinical Status/Quality of Life | |||
| Global/functional | Modified Clinical Dementia Rating Scale (mCDR) [28] | A/L, UDS | |
| Activities of daily living | Functional Assessment Questionnaire (FAQ) [29] | A/L, UDS | |
| Schwab and England Activities of Daily Living (SEADL) [30] | A/L | ||
| Clinical severity/change | Clinical Global Impression - Severity and Change [27] | A/L | |
| Quality of life | Dementia Quality of Life - subject and informant [31] | A/L | |
| Caregiver burden | Zarit Burden Interview [32] | A/L | |
| Cognitive/Neuropsychologic | |||
| Global intellectual function | Montreal Cognitive Assessment (MoCA)[33] | A/L, UDS | |
| Executive | Number Span - forward and backward [34] | A/L, UDS | |
| Trails A & B [35] | A/L, UDS | ||
| *EXAMINER battery [36] | A/L, FTLD | ||
| Language | Semantic Fluency - fruits and vegetables [34] | A/L, UDS | |
| Verbal Fluency - Phonemic Test [34] | A/L, UDS | ||
| Multilingual Naming Test (MINT) [37] | A/L, UDS | ||
| *Semantic Associates (Northwestern Naming Battery) | A/L, FTLD | ||
| *Regular and Irregular Word Reading (Hopkins Experimental Battery) | A/L, FTLD | ||
| *Action Naming (Northwestern Naming Battery) | A/L, FTLD | ||
| *Northwestern Anagram test | A/L, FTLD | ||
| *Sentence reading (Hopkins Experimental Battery) | A/L, FTLD | ||
| *Sentence repetition (Hopkins Experimental Battery) | A/L, FTLD | ||
| Learning and memory | Craft Story [34] | A/L, UDS | |
| California Verbal Learning Test [38] | A/L, FTLD | ||
| Benson figure recall [39] | A/L, UDS | ||
| Visuospatial | Benson figure copy [39] | A/L, UDS | |
| Behavioral measures | |||
| Depression | Geriatric Depression Scale [40] | A/L, UDS | |
| Neuropsychiatric | Neuropsychiatric Inventory Q [41] | A/L, UDS | |
| Social | *Behavioral Inhibition Scale [42] | A/L, FTLD | |
| *Interpersonal Reactivity Index [43] | A/L, FTLD | ||
| *Revised Self-monitoring Scale [44] | A/L, FTLD | ||
| *Social Norms Questionnaire | A/L, FTLD | ||
| *Social Behavior Observer Checklist | A/L, FTLD | ||
| Neurologic Disorder-Focused | |||
| Parkinsonism | UPDRS - Motor subtest [45] | A/L | |
| PSP | PSP Rating Scale [46] | A/L | |
| ALS | ALS Functional Rating Scale - Revised [47] | A/L | |
Most of the subject- and informant-based questionnaires/measures are administered by a trained study coordinator who is experienced in assessing FTLD subjects. All measures are completed in-person with subjects and informants whenever possible; when this is not feasible (ie, insufficient time during the scheduled in-person visit, informant not present), then measures are completed by telephone. The standard medical/neurologic interview and examination is completed face-to-face by a clinician experienced in FTLD, which is usually the site PI. The clinician interviews the informant in person or by telephone whenever feasible. The neuropsychological battery is administered by a trained psychometrist who is experienced in assessing FTLD subjects.
Most of the data are collected using measures developed by the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS) Task Force, which comprise the UDS version 3.0 (UDS 3.0) [48]. While the UDS measures have been applicable to subjects with normal cognition, mild cognitive impairment and a variety of dementia syndromes, the focus over many years was on subjects with MCI and AD dementia. In order to support FTLD research, the NIA and NINDS jointly funded the FTLD Module Task Force which led to the creation of the measures expanding the characterization of the cognitive, behavioral, language and motor features typical of FTLD spectrum disorders. More information on UDS 3.0, the FTLD Module and other aspects of NACC can be found at: https://www.alz.washington.edu/.
Both the LEFFTDS and ARTFL protocols were designed to develop methodology and infrastructure to prepare for therapeutic trials in FTLD. Some measures were therefore added to both protocols to supplement the UDS and FTLD Module – these are designated as being only in the ARTFL/LEFFTDS (A/L) database in Table 1.
4.4. Clinical ratings and diagnoses
4.4.1. Overview
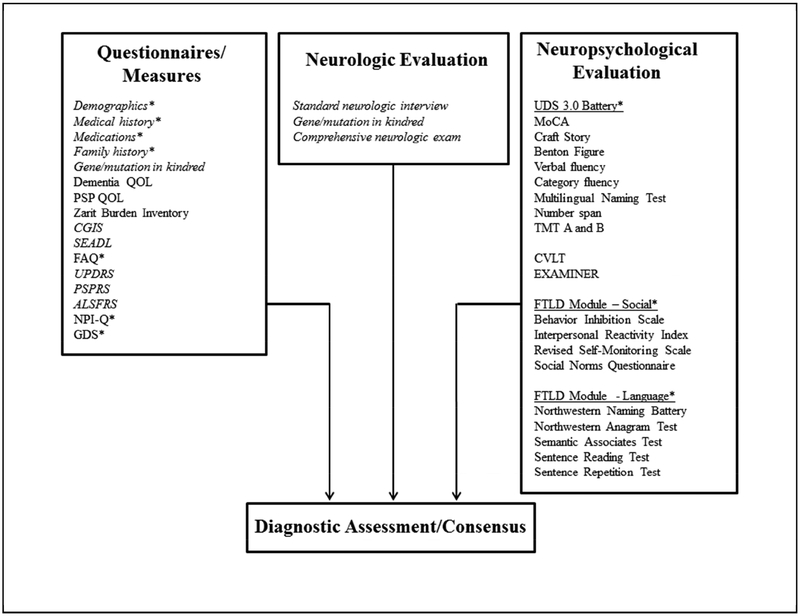
The key ratings for the assessment team include the CDR® and the NACC FTLD Module scale [64] (which is a modification of the standard CDR® Dementia Staging Instrument, [65] – more details on these scales are below), neuropsychological data, the consensus clinical diagnostic assessment and the confidence rating. The measures used in the diagnostic ratings and assessment are shown in Figure 4 and Tables 2 and 3.
Figure 4. Diagnostic Assessment Scheme.
*measures from the NACC UDS version 3 and FTLD Module; additional references: [39, 49–63].
See text in Section 4.4 for details and abbreviations.
Table 2.
Rating and Diagnosis Measures
| Rating/Diagnosis | Measure/Description | Database |
|---|---|---|
| Clinical Rating Scores | ||
| Standard CDR - Subject | Standard 6 domain CDR with Global and Sum of the Boxes measures | A/L |
| FTLD domains - Subject | Supplemental Behavior/Comportment and Language domains | A/L |
| FTLD-CDR - Subject | Global and Sum of the Boxes measures rating based on all subject data | A/L |
| Standard CDR - Informant | Standard 6 domain CDR with Global and Sum of the Boxes measures | A/L |
| FTLD domains - Informant | Supplemental Behavior/Comportment and Language domains | A/L |
| FTLD-CDR - Informant | Global and Sum of the Boxes measures rating based on all informant data | A/L |
| Standard CDR - Neuropsych | Key domains on CDR with Global and Sum of the Boxes measures | A/L |
| FTLD domains - Neuropsych | Supplemental Behavior/Comportment and Language domains | A/L |
| UDS Neuropsych Rating | Cognitive domain and global rating based on all UDS neuropsych data | A/L |
| FTLD-CDR - Neuropsych | Global and Sum of the Boxes measures rating based on all neuropsych data | A/L |
| Consensus Clinical Dementia Rating | ||
| Standard CDR - Consensus | Standard 6 domain CDR with Global and Sum of the Boxes measures | A/L, UDS |
| FTLD-CDR - Consensus | Global and Sum of the Boxes measures based on all data | A/L, UDS |
| Consensus Clinical Diagnosis | ||
| Primary clinical diagnosis | Primary clinical diagnosis | A/L, UDS |
| Confidence rating | Confidence in the rating of the primary clinical diagnosis | A/L |
| Secondary clinical diagnosis | Secondary clinical diagnosis, if applicable | A/L |
| Tertiary clinical diagnosis | Tertiary clinical diagnosis, if applicable | A/L |
A/L = ARTFL/LEFFTDS database in RAVE; UDS = National Alzheimer’s Coordinating Center Uniform Data Set
Table 3.
Clinical Phenotypes and Confidence Rating
| Clinical Phenotypes (primary, and secondary and tertiary if applicable) | |
|---|---|
| Normal neurologic functioning | |
| Behavioral variant frontotemporal dementia | |
| Primary progressive aphasia – agrammatic/nonfluent variant subtype | |
| Primary progressive aphasia – semantic variant subtype | |
| Primary progressive aphasia – logopenic variant subtype | |
| Corticobasal syndrome - typical or variant | |
| Progressive supranuclear palsy/Richardson’s syndrome | |
| Amyotrophic lateral sclerosis | |
| FTD/ALS | |
| MCI - cognitive variants: aMCIsd, aMDmd, naMCIsd, naMCImd | |
| MCI - behavior | |
| MCI - language | |
| MCI - unknown | |
| Alzheimer’s disease dementia | Confidence in Primary Clinical Phenotype Diagnosis |
| Dementia with Lewy bodies | 100% (extremely confident) |
| Parkinson’s disease | 90% |
| Parkinson’s disease with dementia | 80% |
| Multiple system atrophy | 70% |
| Posterior cortical atrophy | 60% |
| Primary psychiatric disorder - Mood | 50% |
| Primary psychiatric disorder - Thought | 40% |
| Primary psychiatric disorder - Personality | 30% |
| Other, specify | 20% |
| 10% | |
| 0% (not confident at all) | |
To broaden the utility of the Clinical Dementia Rating scale (which is now known as the CDR® Staging Instrument and will be abbreviated as CDR® hereafter), into FTLD spectrum disorders, the Behavior/Comportment/Personality and Language domains were added to the CDR® to form the 8-domain “FTLD-CDR”. The older terminology “FTLD-CDR” represented the exact same group of measures now used by the updated name of “CDR® Dementia Staging Instrument PLUS National Alzheimer Coordinating Center (NACC) Frontotemporal Lobar Degeneration (FTLD Module Behavior & Language Domains (CDR® plus NACC FTLD). Since the CDR® is now trademarked, this updated abbreviation for the 8-domain ratings was proposed by the developers of CDR® and the NACC FTLD Module, and all references to this combination of measures will be abbreviated “CDR® plus NACC FTLD” henceforth in this manuscript.
A foundation of the LEFFTDS protocol is the rating of each subject as normal or not, and if not normal, how severely abnormal (questionable, mild, moderate, severe) each subject is. The CDR® scale, adapted more for FTLD spectrum cases to represent the CDR® plus NACC FTLD scale, was determined to be the initial benchmark.
The six domain CDR® has functioned very well in the Alzheimer’s disease clinical spectrum. Two additional domains were added as part of the FTLD Module – behavior/comportment and language – but these ratings are viewed separately from the six domains and have not been incorporated into a FTLD-specific global score. Motor dysfunction as seen in FTD with parkinsonism, PSP, CBS and ALS is also important in the clinical and functional assessment of FTLD subjects, requiring a motor domain to be designed – this is under development at the time of this writing.
Another key aspect of FTLD characterization, particularly in the early phenoconversion transition from normal to minimally symptomatic, is to determine the drivers of change. One could hypothesize that for some syndromes (eg, bvFTD), the data from the informant’s interview may be more informative than the data from the subject’s interview and neurologic exam and the traditional neuropsychological data. For other syndromes (eg, PPA), the data from the subject’s interview and exam as well as the language-based neuropsychological data would be most informative. To capture these scenarios in order to test hypotheses, it would be important to analyze data based on 1) interactions with and measures completed by the clinician with the subject, 2) interactions with and measures completed by the informant, and 3) the neuropsychological assessment. Furthermore, attempts should be made to complete these ratings as independently as is feasible. Finally, a consensus rating considering all data would be the key classification for determining the clinical status at any given visit. It would also be possible for investigators to go back and analyze data from different rating streams to earlier visits to determine which data was optimally predictive of phenoconversion.
4.4.2. CDR® plus NACC FTLD
There are two scores that are generated as part of the CDR® plus NACC FTLD scoring system – the global CDR® plus NACC FTLD score and the CDR® plus NACC FTLD Sum of the Boxes (SB). For the CDR® plus NACC FTLD SB score, the value is determined by simply adding all 8 of the domain scores.
The global CDR® plus NACC FTLD is more complex. First, it does NOT follow the standard CDR® algorithm. For example, if there are one or more scores of 0.5 in the non-memory domains of the standard CDR®, the global score may still be 0; this standard CDR® algorithm was developed to emphasize the importance of at least mild memory impairment for the global value of the standard CDR® to be >0 for those with suspected Alzheimer’s disease pathology. Since the earliest manifestations of evolving FTLD are usually non-memory, the CDR® plus NACC FTLD does not require memory impairment to score >0 – any domain score >0 will result in a global score >0. The guidelines of the CDR® plus NACC FTLD global score are therefore as follows:
If all domains are 0, the global CDR® plus NACC FTLD score is 0.
If the maximum domain score is 0.5, the global CDR® plus NACC FTLD score is 0.5.
- If the maximum domain score is above 0.5 in any domain, then the following applies:
- If the maximum domain score is 1 and all other domains are 0, the global CDR® plus NACC FTLD score is 0.5.
- If the maximum domain score is 2 or 3 and all other domains are 0, the global CDR® plus NACC FTLD score is 1.
- If the maximum domain score occurs only once, and there is another rating besides zero, the global CDR® plus NACC FTLD score is one level lower than the level corresponding to maximum impairment (e.g. if maximum = 2, and there is another rating besides zero, the global CDR® plus NACC FTLD score is 1; if maximum = 1, and there is another rating besides zero, the global CDR® plus NACC FTLD score is 0.5).
- If the maximum domain score occurs more than once (e.g. 1 in 2 domains, 2 in 2 domains), then the global CDR® plus NACC FTLD score is that maximum domain score.
4.4.3. ARTFL-LEFFTDS (A/L) FTLD-CDR classification
The A/L FTLD-CDR classification was developed in an attempt to categorize subjects for purposes of comparison similar to the manner with which the classic CDR® score has served the aging and Alzheimer’s disease field for many years; the Behavior/Comportment and Language domains were added to the classic CDR® in an attempt to capture similar degrees of neurologic impairment among the phenotypic variability inherent to f-FTLD. Note that the “CDR” in this A/L FTLD-CDR classification scheme represents a more broad clinical dementia rating perspective. The criteria for this A/L FTLD-CDR classification are as follows:
A/L FTLD-CDR=0 - Asymptomatic: These subjects have a) normal cognitive, behavioral/comportment and language functioning based on the absence of subjective complaints of cognitive, behavioral, and language changes from their baseline, b) a global CDR® plus NACC FTLD score of 0, and c) cognitive/behavioral/language functioning based on a normal neurologic examination and performance on neuropsychological and behavioral measures within normal limits.
A/L FTLD-CDR=0.5 – Questionably/Minimally Symptomatic: These subjects generally have a) a questionable or mild change in cognitive, behavioral, or language functioning based on the subject and/or informant, b) a global CDR® plus NACC FTLD score of 0.5, c) a mild degree of impairment in cognitive/behavioral/language functioning based on neurologic examination and/or neuropsychological and behavioral measures, and d) does not fulfill established criteria for probable bvFTD, PPA, or another defined neurodegenerative disorder.
A/L FTLD-CDR=1 – Definitely and mildly symptomatic: These subjects generally have a) at least mild change in cognitive, behavioral, or language functioning based on the subjects and/or informant, b) a global CDR® plus NACC FTLD score of 1, and c) at least mild degree of impairment in cognitive/behavioral/language functioning based on neurologic examination and/or neuropsychological and behavioral measures, and d) does fulfill established criteria bvFTD, PPA, or another defined neurodegenerative disorder.
A/L FTLD-CDR>1 - Definitely and moderately to severely symptomatic: Subjects with moderate severity dementia (or moderate degree of neurologic impairment associated with another defined neurodegenerative disorder) plus a moderate degree of dependency on caregivers +/− devices would be classified as FTLD-CDR=2, and those with severe dementia (or severe degree of neurologic impairment associated with another defined neurodegenerative disorder) plus near complete or complete dependency on caregivers +/− devices would be classified as FTLD-CDR=3.
Atypical cases: Any subjects who do not fulfill any set of criteria as stated above are classified in the most appropriate A/L FTLD-CDR category (e.g. a subject who fulfills criteria a and b for A/L FTLD-CDR=0.5 but has a normal neurologic examination and normal neuropsychological scores will be classified as A/L FTLD-CDR=0.5 since this designation best approximates the criteria.
4.4.4. Clinicogenetic classification
The clinical classification scheme described above was designed to be paired with the genetic status of any subjects, such that the presence (+m) or absence (−m) of a mutation is followed by the clinical code (e.g., +mFTLD-CDR=0 for asymptomatic mutation carrier). This is described in more detail below.
4.4.5. ARTFL/LEFFTDS Clinical Diagnosis and Confidence Rating Form
This form (Table 3) permits the consensus committee to render primary (and secondary and tertiary, when appropriate) diagnoses – including mild features outside of the amnestic and non-amnestic categories of MCI – as well as make a confidence rating (0–100% confident) in the primary diagnosis.
MCI-behavior.
In the FTLD field, it is not uncommon for patients to have mild behavioral changes that are definitely a change from baseline, but these changes are not of sufficient severity to warrant a diagnosis of dementia, or more specifically, behavioral variant FTD. Some investigators have applied the term “mild behavioral impairment or MBI” for this phenotype, but this terminology and its interpretation are increasingly being used in the setting of Alzheimer’s disease and/or Lewy body disease and not necessarily applicable to FTLD. We have therefore chosen to use the term “MCI-behavior” and purposefully use this diagnosis loosely, since there are no operational criteria for this presumed intermediate stage in the normal to bvFTD evolution.
A reasonable application of the MCI-behavior diagnosis would be in the setting of any patient who exhibits features and findings consistent with clinically possible bvFTD using the Consensus criteria (see below) [66]. In other words, the presence of one or more of the following would be fitting for MCI-behavior:
Disinhibition: Socially inappropriate behavior; loss of manners or decorum; impulsive, rash, or careless actions
Apathy or inertia: Loss of interest, drive, and motivation; decreased initiation of behavior
Loss of sympathy/empathy: Diminished response to other people’s needs or feelings; diminished social interest, interrelatedness, or personal warmth
Ritualistic/compulsive behavior: Simple repetitive movements or complex compulsive or ritualistic behaviors
Hyperorality and appetite changes: Altered food preferences, binge eating, increased consumption of alcohol or cigarettes, oral exploration or consumption of inedible objects
Importantly, particularly in familial FTD, there are circumstances in which delusions, hallucinations, and other forms of odd behavior may be part of the evolving behavioral phenotype. Therefore, the diagnosis of MCI-behavior is a loosely-defined clinical diagnosis which will be operationalized with more rigor in the future after more data is gathered and analyzed.
Accessing data.
The procedures for accessing data from the clinical arm are described in the Accessing Data and Samples section below.
4.5. Biofluid arm - Blood
The procedures involved in acquiring, processing, storing and accessing biofluid samples are described in detail on the National Cell Repository for Alzheimer’s Disease (NCRAD) website (https://ncrad.iu.edu/). Briefly, each subject undergoes a blood draw in the morning after an overnight fast. The blood is obtained to collect DNA, plasma, serum (serum collection began in mid-2016), RNA and PBMC. The blood is collected in appropriate tubes and processed; the tubes designed for PBMC generation are submitted overnight express to NCRAD, and the others are submitted later as batch shipments to NCRAD.
ARTFL/LEFFTDS blood biofluid sample processing.
Several key analyses on biofluid samples are carried out internally in specialized laboratories within the ARTFL/LEFFTDS consortium so that the specific aims can be addressed. Aliquots of blood for each subject are sent from NCRAD to UCLA (Giovanni Copolla, PhD, site PI for genomic analyses) for DNA analysis (see additional description below), and to Mayo Clinic Florida (Rosa Rademakers, PhD, site PI for genomic/proteomic analyses) for genotyping of modifier genes such as TMEM106B. An aliquot of plasma is also sent to Dr. Rademakers’s laboratory for progranulin quantification. The results from these laboratories are then submitted securely to a separate ARTFL/LEFFTDS Genomic/Proteomic database within the RAVE system that is purposefully housed separate from the clinical data. Access to this database is password-protected and only accessible by a few key staff.
Accessing data/samples.
The procedures for accessing data from the biofluid-blood arm within the ARTFL/LEFFTDS Genomic/Proteomic database are described in the Accessing Data and Samples section below.
4.6. Biofluid arm - CSF
All participants are asked to undergo CSF collection, and this arm is optional. The procedures involved in acquiring, processing, storing and accessing CSF samples are described in detail on NCRAD website (https://ncrad.iu.edu/). Briefly, CSF is collected via standard lumbar puncture procedures in polypropylene tubes and aliquoted.
ARTFL/LEFFTDS CSF biofluid sample processing.
Several key analyses on CSF samples will be carried out internally in specialized laboratories within the ARTFL/LEFFTDS consortium; these samples will be analyzed at periodic intervals to permit standardization across measures. Additional information is provided below. The results from these laboratories are then submitted securely to the ARTFL/LEFFTDS Genomic/Proteomic database.
Accessing data/samples.
The procedures for accessing data from the biofluid-CSF arm within the ARTFL/LEFFTDS Genomic/Proteomic database are described in the Accessing Data and Samples section below.
4.7. MRI arm
Acquisition.
Images are acquired at all centers at 3Tesla using sequences that are harmonized across multiple vendors (i.e. GE, Siemens, and Phillips), and similar to those employed in the Alzheimer’s Disease Neuroimaging Initiative – versions 2 and 3 (ADNI-2 and ADNI-3 basic) protocols (see: http://adni.loni.usc.edu/methods/documents/mri-protocols/). Scanning begins with a three-planar localizer scan and an auto-alignment scout scan which yields orthogonal orientation and AC-PC alignment followed by MRI sequences as follows. 1) T1-weighted MPRAGE with parameters: TR/TE/TI = 2300//900 ms, flip angle of 9 degrees, a bandwidth of 240 Hz/pixel, sagittal orientation with a FOV = 256 × 240 mm with slices. Time is min. 2) T2-weighted FLAIR: TR/TE/TI = 6000/390/2100 ms with a 800 ms long turbo spin echo readout train, 750 Hz/pixel bandwidth with 3mm. slice thickness. Time is 7 min. 3) Diffusion Tensor imaging (DTI): A 2D single-shot gradient echo sequence with TR/TE = 7200/56 ms, a 232 × 232 base matrix, 2.0 mm slices yielding 2.0 × 2.0 × 2.0 mm isotropic resolution [67, 68]. The sequence is augmented by diffusion encoding gradients and incorporates two refocusing pulses to reduce distortions from eddy-currents. Diffusion-weighting gradients will be applied along 48 directions with b=1000 mm2/s. Time is 7:30 min. 4) Arterial Spin Labeled (ASL) perfusion imaging: The ASL protocol consists of a 3D (FSE) pseudo-continuous ASL sequence with an interleaved stack-of-spiral readout and background suppression on GE scanners [69]. The imaging parameters used were, a labeling duration of 1450 ms, post labeling delay of 2025 ms, repetition time/echo time of 4800/10 milliseconds, refocusing flip angle 111 degrees, FOV 240 mm, acquisition matrix 512/8 samples re-gridded to a 128 × 128 matrix with an in-plane reconstructed resolution of 1.875 × 1.875 cm2; 40 slices with slice thickness 4mm, no gap. 3 excitation averages of label and control volume pairs are acquired, as well as a proton density (PD) weighted volume using the same readout scheme, resulting in an overall scan duration of 4:30 min. 5) Intrinsic Connectivity Network functional MRI (ICN fMRI): A T2*-weighted gradient echo–echo planar sequence with TR/TE = 3000/30 ms, flip angle 90°; FOV = 210 × 210 mm; matrix size: 64 × 64; 3.3 mm slices with 2.5 × 2.5 mm in-plane resolution. Subjects are instructed to keep their eyes open. Time is 10 min.
Processing.
Quality control (QC) measures are performed at Mayo Clinic Rochester, which permits analytic integrity across centers and MRI scanner manufacturers. All scan data along with QC data for each scan are uploaded to the Laboratory of Neuroimaging (LONI), Arthur Toga, PhD, site PI (website: http://www.loni.usc.edu/).
4.8. Clinical genetics arm
Each subject enrolled in LEFFTDS has a personal history or family history of a known mutation in MAPT, GRN or C9orf72. Each subject who is not already aware of their mutation status (positive versus negative), whether asymptomatic or symptomatic, is offered the opportunity to undergo genetic counseling and genetic testing (which includes assessment of psychologic/psychiatric status to determine mental readiness and psychological fitness). In those who are deemed appropriate for genetic testing, a clinical blood sample is collected (usually via cryopreservation) and then a portion of this sample is submitted to a CLIA-approved laboratory for testing; most sites use Mayo Medical Laboratories for this purpose (https://www.mayomedicallaboratories.com/). Results of genetic testing are then provided at least three weeks later according to site-specific procedures.
The specific procedures for psychologic/psychiatric assessment, genetic counseling, genetic testing and results disclosure are according to clinical practice guidelines at each site.
4.9. Research genetic and proteomic analyses
Individuals recruited into this study are part of known MAPT, GRN or C9ORF72 mutation families. For each family member we therefore specifically sequence the exon harboring the known MAPT, GRN or C9ORF72 mutation observed in that family using previously published protocols[4, 5, 70]; sequencing is also performed to detect variants in the following genes: TARDBP, PSEN1, PSEN2, APP; For individuals from C9ORF72 mutation families, the presence of an expanded GGGGCC hexanucleotide repeat is considered likely pathogenic if the characteristic stutter amplification pattern is present on the electropherogram [5]. For all expanded repeat carriers the approximate length of the repeat in blood is determined using Southern blot analysis as published[5]. Taqman SNP genotyping assays are further used to genotype rs5848 (GRN), rs1990622 (TMEM106B) and rs1799990 (PRNP) as well as the extended MAPT H1 and H2 haplotypes (rs1052553) and APOE genotypes (rs7412 and rs429358) in all individuals. Progranulin expression levels are measured in human plasma samples using the human Progranulin ELISA kit (Adipogen Inc., Seoul, Korea) using a 1:100 dilution of plasma samples and undiluted CSF samples.
Aliquots of CSF for each subject are sent from NCRAD to University of Pennsylvania (Les Shaw, PhD, site PI for CSF proteomic analyses) for quantification of AB42, total tau and phospho-tau; and to Mayo Clinic Florida (Rosa Rademakers, PhD, for progranulin quantification and to Len Petrucelli, PhD, for C9RAN translation quantification). Progranulin expression levels are measured in CSF samples using the human Progranulin ELISA kit (Adipogen Inc., Seoul, Korea) using a 1:100 dilution of plasma samples and undiluted CSF samples. C9RAN protein is quantified as previously described [71]. Samples are run in duplicate and six interplate control samples will be used to adjust for plate-to-plate variation.
4.10. Clinicogenetic characterization
Many scientific questions are anchored on the clinical plus genetic status of subjects. For example, are there differences in clinical, biofluid or imaging measures in f-FTLD between asymptomatic mutation and non-mutation carriers? What is the temporal course of change on clinical, biofluid and imaging measures of mutation carriers as they transition from the asymptomatic to the symptomatic state? Therefore, a clinicogenetic characterization system was developed so that each subject is assigned to one of the following categories: asymptomatic non-mutation carriers (−mFTLD-CDR=0), asymptomatic mutation carriers (+mFTLD-CDR=0), and symptomatic mutation carriers (+mFTLD-CDR>0). Depending on the analysis of interest, the +mFTLD-CDR>0 group can be further subclassified as +mFTLD-CDR=0.5, +mFTLD-CDR=1, +mFTLD-CDR=2 and +mFTLD-CDR=3.
An obvious tenet to the LEFFTDS approach is ensuring confidentiality and blinding. For those subjects who undergo the clinical genetic testing arm of the protocol, while they can share the results of testing with any of the research staff with whom they come in contact, this process is not encouraged so as to promote blinding of the study staff. For those subjects who do not wish to undergo clinical genetic testing, all research staff who may interact with the subjects or their relatives must remain blind to the genetic test results which are performed for the purposes of the protocol. The research genetic testing is performed at UCLA, and the results for each subject based on their subject code (but not name or other identifying information) are uploaded into a secure database.
4.11. Accessing data and/or samples
The schema for accessing data and/or samples is shown in Figure 2. The clinical data (which includes neuropsychological data) can be accessed via two mechanisms – the ARTFL/LEFFTDS database management system (RAVE) or the NACC; note that the data in the ARTFL/LEFFTDS RAVE system includes more measures, and can also potentially be attached to genetic and biofluid data if desired. The process for requesting data is explained at the website: https://ucsf.co1.qualtrics.com/jfe/form/SV_e4BBGMiXV7HRTg1. The data requests are reviewed by committee and adjudicated in a timely fashion, and data is provided in a secure manner after all vetting and confidentiality measures are satisfied. Clinical data focused on UDS 3.0 and FTLD Module measures can be requested at NACC at the website: https://www.alz.washington.edu/.
Samples can be requested by accessing the NCRAD website and following all procedures as described at the website: https://ncrad.iu.edu/accessing_data.html. A minimum data set of clinical and genetic information can be attached to each sample. If more detailed clinical and/or imaging data is desired in addition to samples, then this website should also be accessed: https://ucsf.co1.qualtrics.com/jfe/form/SV_e4BBGMiXV7HRTg1. The sample request and approval processes are similar to those regarding the clinical +/− genetic data. Samples are provided to investigators in a blinded manner, in which the investigator is expected to submit all findings derived from his/her analyses for incorporation into the full ARTFL/LEFFTDS database. Additional aspects on this process are explained at these websites and associated links.
MR imaging scan data can be accessed as described on the LONI website: http://www.loni.usc.edu/. There is a quality control file associated with each scan. A minimum data set of clinical and genetic information is also attached to each scan. If more detailed clinical and/or genetic data is desired in addition to scans, then this website should also be accessed: https://ucsf.co1.qualtrics.com/jfe/form/SV_e4BBGMiXV7HRTg1. Additional aspects on this process are explained at these websites and associated links.
5. Future considerations
The methods and processes described herein will surely undergo evolution in the future. Updates will be maintained in the website (under construction). The LEFFTDS protocol is also similar in many ways to the Genetic Frontotemporal Dementia Initiative (GENFI) study in Europe and Canada, and attempts to harmonize many aspects of LEFFTDS and GENFI will continue over the years ahead.
Supplementary Material
Research in Context.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations.
Interpretation: Our methodology provides details on the recruitment scheme, evaluation and rating procedures, and processes for accessing data and samples.
Future directions: The manuscript proposes a framework for the considering the dynamic processes associated with familial frontotemporal lobar degeneration evolution. The data and samples collected in this protocol, which are available to interested investigators worldwide, will be used to test this framework as well as facilitate planning for upcoming disease-modifying therapeutic trials in f-FTLD.
Highlights.
It is important to establish the natural history of familial frontotemporal lobar degeneration (f-FTLD) and provide clinical and biomarker data for planning these studies, particularly in the asymptomatic phase.
The Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS) protocol was designed to enroll and follow members of kindreds with a mutation in one of the three most common f-FTLD genes – microtubule associated protein tau, progranulin or chromosome 9 open reading frame 72.
The theoretical considerations of f-FTLD, aims/objectives of the LEFFTDS protocol, design and methodology for evaluating and rating subjects, and scheme for collecting biofluid samples and performing MRI scans are described.
These data and samples, which are available to interested investigators worldwide, will facilitate planning for upcoming disease-modifying therapeutic trials in f-FTLD.
Acknowledgements
We extend our appreciation to Drs. John Hsiao and Dallas Anderson from the National Institute on Aging, Drs. Marg Sutherland and Codrin Lungu from the National Institute of Neurological Disorders and Stroke, the staff of all centers, and particularly to our patients and their families for their participation in this protocol.
Funding
This work is supported by the National Institutes of Health [grants U01 AG045390, U54 NS092089, U24 AG021886 and U01 AG016976].
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ARTFL
Advancing Research and Treatment for Frontotemporal Lobar Degeneration
- bvFTD
behavioral variant frontotemporal dementia
- C9ORF72
gene encoding chromosome 9 open reading frame 72
- CDR®
CDR® Dementia Staging Instrument
- CDR® plus NACC FTLD
CDR® Dementia Staging Instrument plus NACC FTLD Behavior and Language Domains
- CSF
cerebrospinal fluid
- DNA
deoxyribonucleic acid
- FTD
frontotemporal dementia
- f-FTLD
familial frontotemporal lobar degeneration
- LEFFTDS
Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects
- MAPT
gene encoding microtubule associated protein tau
- MRI
magnetic resonance imaging
- NACC
National Alzheimer’s Coordinating Center
- NCRAD
National Cell Repository for Alzheimer’s Disease
- PBMC
peripheral blood mononuclear cells
- GRN
gene encoding progranulin
- PPA
primary progressive aphasia
- RNA
ribonucleic acid
- −mFTLD-CDR=0
asymptomatic non-mutation carriers (familial controls)
- +mFTLD-CDR=0
asymptomatic mutation carriers
- +mFTLD-CDR>0
symptomatic mutation carriers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Boeve B – has served as an investigator for clinical trials sponsored by GE Healthcare and Axovant. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program and the Little Family Foundation.
Rosen H – has received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from NIH.
Forsberg L – receives research support from NIH.
Boxer A – receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer’s Drug Discovery Foundation and the Alzheimer’s Association. He has served as a consultant for Aeton, Abbvie, Alector, Amgen, Arkuda, Ionis, Iperian, Janssen, Merck, Novartis, Samumed, Toyama and UCB, and received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche and TauRx.
Heuer H – receives research support from NIH.
Bove J – nothing to disclose
Brannelly P – employed by the Rainwater Charitable Foundation
Brushaber D – nothing to disclose
Coppola G – receives research support from NIH.
Dheel C – nothing to disclose
Dever R – nothing to disclose
Dickerson B – receives research support from NIH.
Dickinson S – on staff at the Association for Frontotemporal Degeneration and a member of the National Institute for Neurological Disorders and Stroke Advisory Council.
Faber K – receives research support from NIH
Fields J – receives research support from NIH
Fong J – nothing to disclose
Foroud T – receives research support from NIH
Gavrilova R – receives research support from NIH
Gearhart D – nothing to disclose
Ghoshal N - has participated or is currently participating in clinical trials of anti-dementia drugs sponsored by the following companies: Bristol Myers Squibb, Eli Lilly/Avid Radiopharmaceuticals, Janssen Immunotherapy, Novartis, Pfizer, Wyeth, SNIFF (The Study of Nasal Insulin to Fight Forgetfulness) study, and A4 (The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease) trial. She receives research support from Tau Consortium and Association for Frontotemporal Dementia and is funded by the NIH.
Goldman, JS – is serving as a consultant to the Novartis Alzheimer’s Prevention Advisory Board. She receives research support from NIH, HDSA, New York State Department of Health (RFA #1510130358)
Graff-Radford J – receives research support from the NIH.
Graff-Radford N – receives royalties from UpToDate, has participated in multicenter therapy studies by sponsored by Biogen, TauRx, AbbVie, Novartis and Lilly. He receives receives research support from NIH.
Grossman M - receives grant support from NIH, Avid and Piramal; participates in clinical trials sponsored by Biogen, TauRx, and Alector; serves as a consultant to Bracco and UCB; and serves on the Editorial Board of Neurology.
Haley D – nothing to disclose
Hsiung G-Y – has served as an investigator for clinical trials sponsored by AstraZeneca, Eli Lilly, and Roche / Genentech. He receives research support from Canadian Institutes of Health Research and the Alzheimer Society of British Columbia.
Huey E – receives research support from NIH
Irwin D – receives support from NIH, Brightfocus Foundation and Penn Institute on Aging.
Jones D- – receives research support from NIH and the Minnesota Partnership for Biotechnology and Medical Genomics
Jones L – nothing to disclose
Kantarci K - served on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc.; data monitoring boards of Pfizer and Janssen Alzheimer Immunotherapy; research support from the Avid Radiopharmaceuticals, Eli Lilly, the Alzheimer’s Drug Discovery Foundation and NIH
Karydas A – nothing to disclose
Knopman D - serves on the DSMB of the DIAN-TU study, is a site PI for clinical trials sponsored by Biogen, Lilly and the University of Southern California, and is funded by NIH.
Kornak J - – has provided expert witness testimony for Teva Pharmaceuticals in Forest Laboratories Inc. et al. v. Teva Pharmaceuticals USA, Inc., Case Nos. 1:14-cv-00121 and 1:14-cv-00686 (D. Del. filed Jan. 31, 2014 and May 30, 2014) regarding the drug Memantine; for Apotex/HEC/Ezra in Novartis AG et al. v. Apotex Inc., No. 1:15-cv-975 (D. Del. filed Oct. 26, 2015, regarding the drug Fingolimod. He has also given testimony on behalf of Puma Biotechnology in Hsingching Hsu et al, vs. Puma Biotechnology, INC., et al. 2018 regarding the drug Neratinib. He receives research support from the NIH.
Kramer J – receives research support from NIH.
Kraft R – nothing to disclose
Kremers W - receives research funding from AstraZeneca, Biogen, Roche, DOD and NIH.
Kukull W – receives research support from NIH.
Lapid M – nothing to disclose
Lucente D - receives research support from NIH
Lungu C – honoraria for editorial work from Elsevier, Inc.
Mackenzie I – receives research funding from Canadian Institutes of Health Research.
Manoochehri M – nothing to disclose
McGinnis S – has served as an investigator for clinical trials sponsored by AbbVie, Allon Therapeutics, Biogen, Bristol-Myers Squibb, C2N Diagnostics, Eisai Inc., Eli Lilly and Co., Genentech, Janssen Pharmaceuticals, Medivation, Merck, Navidea Biopharmaceuticals, Novartis, Pfizer, and TauRx Therapeutics. He receives research support from NIH.
Miller B – receives research support from NIH.
Pearlman R – employed by The Bluefield Project.
Petrucelli L – receives research support from NIH.
Potter M – receives research support from NIH.
Rademakers R – receives research funding from NIH and the Bluefield Project to Cure Frontotemporal Dementia.
Ramos E – nothing to disclose
Rankin K – receives research support from NIH.
Rascovsky K – receives research support from NIH.
Sengdy P – nothing to disclose
Shaw L – receives research support from NIH.
Syrjanen J – nothing to disclose
Tatton N – employed by the Association for Frontotemporal Degeneration
Taylor J – nothing to disclose
Toga A – receives research support from the NIH and the Alzheimer’s Association
Trojanowski J – may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is coinventor and he received revenue from the sale of Avid to Eli Lily as coinventor on Aβ amyloid imaging–related patents submitted by the University of Pennsylvania. He receives research support from the NIH and several nonprofits.
Weintraub S – receives research support from the NIH
Wong B – receives research support from the NIH
Wszolek Z - supported by the NIH, Mayo Clinic Center for Regenerative Medicine, the gift from Carl Edward Bolch, Jr., and Susan Bass Bolch, The Sol Goldman Charitable Trust, and Donald G. and Jodi P. Heeringa. He has also received grant funding support from Allergan, Inc. (educational grant), and Abbvie (medication trials).
References
- [1].Bang J, Spina S, Miller B. Frontotemporal dementia. Lancet. 2015;386:1672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nature reviews Neurology. 2012;8:423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hutton M. Missense and splice site mutations in tau associated with FTDP-17: multiple pathogenic mechanisms. Neurology. 2001;56(suppl 4):S21–S5. [DOI] [PubMed] [Google Scholar]
- [4].Baker M, Mackenzie I, Pickering-Brown S, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. [DOI] [PubMed] [Google Scholar]
- [5].DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rohrer JD, Guerreiro R, Vandrovcova J, Uphill J, Reiman D, Beck J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73:1451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van Swieten J, Spillantini MG. Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol. 2007;17:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van Swieten JC, Heutink P. Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet neurology. 2008;7:965–74. [DOI] [PubMed] [Google Scholar]
- [10].Jack CJ, Knopman D, Jagust W, Shaw L, Aisen P, Weiner M, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jack C Jr., Knopman D, Jagust W, Petersen R, Weiner M, Aisen P, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Knopman DS, Boeve BS, Caselli RJ, Graff-Radford NR, Kramer JH, Mendez MF, et al. Longitudinal Tracking of FTLD: Toward Developing Clinical Trial Methodology. Alzheimer Dis Assoc Disord. 2007;21:S58–S63. [DOI] [PubMed] [Google Scholar]
- [13].Knopman DS, Jack CR Jr., Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, et al. Brain and ventricular volumetric changes in frontotemporal lobar degeneration over 1 year. Neurology. 2009;72:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Whitwell J, Weigand S, Boeve B, Senjem M, DeJesus-Hernandez M, Baker M, et al. Neuroanatomical Signature of C9ORF72: A Comparison to MAPT, Progranulin and Sporadic FTD (IN9–2.004). Neurology. 2012;78:IN9–2.004. [Google Scholar]
- [15].Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Whitwell JL, Boeve BF, Weigand SD, Senjem ML, Gunter JL, Baker MC, et al. Brain atrophy over time in genetic and sporadic frontotemporal dementia: a study of 198 serial magnetic resonance images. European journal of neurology. 2015;22:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. [DOI] [PubMed] [Google Scholar]
- [18].Rosen HJ, Kramer JH, Gorno-Tempini ML, Schuff N, Weiner M, Miller BL. Patterns of cerebral atrophy in primary progressive aphasia. American Journal of Geriatric Psychiatry. 2002;10:89–97. [PubMed] [Google Scholar]
- [19].Rohrer JD, Ahsan RL, Isaacs AM, Nielsen JE, Ostergaard L, Scahill R, et al. Presymptomatic generalized brain atrophy in frontotemporal dementia caused by CHMP2B mutation. Dement Geriatr Cogn Disord. 2009;27:182–6. [DOI] [PubMed] [Google Scholar]
- [20].Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. The Lancet Neurology. 2015;14:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rohrer JD, Warren JD. Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol. 2011;24:542–9. [DOI] [PubMed] [Google Scholar]
- [22].Rohrer JD, Warren JD, Barnes J, Mead S, Beck J, Pepple T, et al. Mapping the progression of progranulin-associated frontotemporal lobar degeneration. Nat Clin Pract Neurol. 2008;4:455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rohrer JD, Warren JD, Fox NC, Rossor MN. Presymptomatic studies in genetic frontotemporal dementia. Rev Neurol (Paris). 2013;169:820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Borroni B, Alberici A, Cercignani M, Premi E, Serra L, Cerini C, et al. Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol Aging. 2012;33:2506–20. [DOI] [PubMed] [Google Scholar]
- [25].Borroni B, Alberici A, Premi E, Archetti S, Garibotto V, Agosti C, et al. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res. 2008;11:585–95. [DOI] [PubMed] [Google Scholar]
- [26].Borroni B, Benussi A, Premi E, Alberici A, Marcello E, Gardoni F, et al. Biological, Neuroimaging, and Neurophysiological Markers in Frontotemporal Dementia: Three Faces of the Same Coin. J Alzheimers Dis. 2018;62:1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 Suppl 2:S22–32. [DOI] [PubMed] [Google Scholar]
- [28].Knopman D, Weintraub S, Pankratz V. Language and behavior domains enhance the value of the clinical dementia rating scale. Alz & Dem. 2011;7:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–9. [DOI] [PubMed] [Google Scholar]
- [30].Schwab R, England A. Projecton technique for evaluating surgery in Parkinson’s disease. Edinburgh, Scotland: ES Livingston; 1969. [Google Scholar]
- [31].Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess. 2005;9:1–93, iii–iv. [DOI] [PubMed] [Google Scholar]
- [32].Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–55. [DOI] [PubMed] [Google Scholar]
- [33].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Amer Geriatr Soc 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- [34].Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, et al. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord. 2018;32:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reitan R Validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- [36].Kramer J, Mungas D, Possin K, Rankin K, Boxer A, Rosen H, et al. NIH EXAMINER: Conceptualization and Development of an Executive Function Battery. J Int Neuropsychol Soc. 2013:October 8:1–9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ivanova I, Salmon DP, Gollan TH. The multilingual naming test in Alzheimer’s disease: clues to the origin of naming impairments. J Int Neuropsychol Soc. 2013;19:272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test--second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21:413–20. [DOI] [PubMed] [Google Scholar]
- [39].Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cog & Behav Neurol. 2003;16:211–8. [DOI] [PubMed] [Google Scholar]
- [40].Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version In: Brink T, editor. Clinical Gerontology: A Guide to Assessment and Intervention. Binghamton, NY: Haworth Press Inc; 1986. p. 165–73. [Google Scholar]
- [41].Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. Journal of Neuropsychiatry & Clinical Neurosciences. 2000;12:233–9. [DOI] [PubMed] [Google Scholar]
- [42].Carver CS, Pozo-Kaderman C, Harris SD, Noriega V, Scheier MF, Robinson DS, et al. Optimism versus pessimism predicts the quality of women’s adjustment to early stage breast cancer. Cancer. 1994;73:1213–20. [DOI] [PubMed] [Google Scholar]
- [43].Davis MC, Matthews KA. Do gender-relevant characteristics determine cardiovascular reactivity? Match versus mismatch of traits and situation. J Personal Social Psychol. 1996;71:527–35. [DOI] [PubMed] [Google Scholar]
- [44].Lennox RD, Wolfe RN. Revision of the self-monitoring scale. J Personal Social Psychol. 1984;46:1349–64. [DOI] [PubMed] [Google Scholar]
- [45].Fahn S, Elton R, Committee MotUD. Unified Parkinson’s Disease Rating Scale In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent developments in Parkinson’s disease. Florham Park, NJ: MacMillan; 1987. p. 153–63. [Google Scholar]
- [46].Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain : a journal of neurology. 2007;130:1552–65. [DOI] [PubMed] [Google Scholar]
- [47].Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- [48].Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Boxer AL, Rankin KP, Miller BL, Schuff N, Weiner M, Gorno-Tempini ML, et al. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Arch Neurol. 2003;60:949–56. [DOI] [PubMed] [Google Scholar]
- [50].Rankin KP, Rosen HJ, Kramer JH, Schauer GF, Weiner MW, Schuff N, et al. Right and left medial orbitofrontal volumes show an opposite relationship to agreeableness in FTD. Dement Geriatr Cogn Disord. 2004;17:328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kramer JH, Yaffe K, Lengenfelder J, Delis DC. Age and gender interactions on verbal memory performance. J Int Neuropsychol Soc. 2003;9:97–102. [DOI] [PubMed] [Google Scholar]
- [52].Rankin KP, Baldwin E, Pace-Savitsky C, Kramer JH, Miller BL. Self awareness and personality change in dementia. Journal of neurology, neurosurgery, and psychiatry. 2005;76:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36. [DOI] [PubMed] [Google Scholar]
- [54].Rankin KP, Salazar A, Gorno-Tempini ML, Sollberger M, Wilson SM, Pavlic D, et al. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. NeuroImage. 2009;47:2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rankin KP, Santos-Modesitt W, Kramer JH, Pavlic D, Beckman V, Miller BL. Spontaneous social behaviors discriminate behavioral dementias from psychiatric disorders and other dementias. J Clin Psychiatry. 2008;69:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain : a journal of neurology. 2006;129:2945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Amer J Alz Dis Other Dem. 2009;24:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76:1804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sepelyak K, Crinion J, Molitoris J, Epstein-Peterson Z, Bann M, Davis C, et al. Patterns of breakdown in spelling in primary progressive aphasia. Cortex. 2011;47:342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Crinion J, Holland AL, Copland DA, Thompson CK, Hillis AE. Neuroimaging in aphasia treatment research: Quantifying brain lesions after stroke. NeuroImage. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tsapkini K, Hillis AE. Spelling intervention in post-stroke aphasia and primary progressive aphasia. Behav Neurol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Faria AV, Crinion J, Tsapkini K, Newhart M, Davis C, Cooley S, et al. Patterns of dysgrap hia in primary progressive aphasia compared to post-stroke aphasia. Behav Neurol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Knopman DS, Weintraub S, Pankratz VS. Language and behavior domains enhance the value of the clinical dementia rating scale. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- [66].Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chang H, Fitzpatrick JM. A technique for accurate magnetic resonance imaging in the presence of field inhomogeneities. IEEE Trans Med Imaging. 1992;11:319–29. [DOI] [PubMed] [Google Scholar]
- [68].Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20:870–88. [DOI] [PubMed] [Google Scholar]
- [69].Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. [DOI] [PubMed] [Google Scholar]
- [71].Gendron TF, van Blitterswijk M, Bieniek KF, Daughrity LM, Jiang J, Rush BK, et al. Cerebellar c9RAN proteins associate with clinical and neuropathological characteristics of C9ORF72 repeat expansion carriers. Acta Neuropathologica. 2015;130:559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.