Abstract
Background:
Wiskott-Aldrich syndrome (WAS) is an X-linked primary immunodeficiency disorder resulting from WASp deficiency. WAS lymphocytes manifest increased DNA damage and lymphopenia from cell death, yet how WASp influences DNA damage-linked cell survival is unknown. A recently-described mechanism promoting cell survival following irradiation (IR)-induced DNA damage involves fragmentation and dispersal of Golgi, known as the Golgi-dispersal response (GDR), which utilizes the GOLPH3•DNA-PK•MYO18A•F-actin signaling pathway.
Objective:
To define WASp role in the DNA damage-induced GDR, and its disruption as a contributor to the development of radiosensitivity-linked immunodeficiency in WAS.
Methods:
In human T helper (Th) and B cell culture systems, DNA-damage-induced GDR elicited by ionizing-radiation or radiomimetic-chemotherapy was monitored in the presence or absence of WASp or GOLPH3 alone or both together.
Results:
WASp-deficiency completely prevents the development of IR-induced GDR in human Th and B cells, this despite high DNA damage load. Loss of WASp impedes nuclear translocation of GOLPH3 and its colocalization with DNA-PKcs. Surprisingly, however, depletion of GOLPH3 alone or depolymerization of F-actin in the WASp-sufficient Th cells still allows the development of robust GDR, suggesting that WASp, but not GOLPH3, is essential for GDR and cell survival following IR-induced DNA-damage in human lymphocytes.
Conclusion:
The study identifies WASp as a novel effector of nucleus-to-Golgi, cell-survival pathway triggered by IR-induced DNA damage in the cells of the hematolymphoid lineage, and proposes impaired GDR as a new etiology in the development of a “radiosensitive” form of immune dysregulation in WAS.
Keywords: Primary immunodeficiency, Wiskott-Aldrich syndrome, Golgi morphology, DNA damage
Capsule Summary:
The study uncovers an increased susceptibility of WASp-deficient T and B lymphocytes to radiation-induced DNA damage and Golgi dysfunction, thereby proposing Wiskott-aldrich syndrome also as a “radiosensitive” form of primary immune deficiency disorder.
Introduction
Wiskott-Aldrich syndrome (WAS) is an X-linked primary immune deficiency disorder (PID) resulting from a plethora of mutations in the WAS gene (1, 2). Patients manifest a combination of symptoms, which arise from the underlying systemic immunodeficiency, circulating lymphopenia, atopy/autoimmunity, and malignancy.3–6 WAS gene encodes WAS-protein (WASp), which is both a cytoplasmic and nuclear protein. In the cytoplasm, WASp is well-known for its role in actin polymerization (F-actin generation), whereas, in the nucleus, WASp has a newly-described role in RNA Pol II-dependent gene transcription and in maintaining a stable genome.7–12 Nuclear-WASp is essential in preventing the accumulation of genome-destabilizing nucleic-acid structure known as “R loop” (RNA-DNA hybrid plus a displaced single stranded DNA).13 R loops, when marked by histone H3-phosphorylated Ser10 (H3S10p) result in double strand breaks (DSBs).14, 15 Recent evidence shows that WASp-deficiency triggers accumulation of H3S10p-marked R loops and DSBs in human T cells.13 In addition to the role of WASp in “preventing” R loop-mediated DNA DSBs, nuclear-WASp also functions to “correct” the already-sustained DNA DSBs in human B cells, by facilitating the repair of the DSB ends by the homology-directed repair (HDR) pathway.16 Accordingly, WASp has an essential nuclear function of maintaining a stable genome of human T and B lymphocytes. Consequently, many WAS patients manifest circulating lymphopenia from spontaneous, accelerated apoptosis and genome-instability,17–20, 13 which contributes to an aspect of the immunodeficient phenotype in WAS.
Recently, it was show that the irradiation (IR)-induced DNA damage response (DDR) elicited by DSBs is not delimited to the nucleus, but extends to the Golgi apparatus in the cytoplasm. Specifically, the IR-induced DDR triggers a cell-protective Golgi-dispersal response (GDR), which in the case of mammalian cells involves GOLPH3•DNA-PK•MYO18A•F-actin signaling pathway.21 Defects in the DDR-mediated GDR associates with reduced cell-survival, and hence the GDR is proposed to offer cytoprotection following DNA damage. Given that WASp-family proteins (N-WASp, WHAMM) that effect F-actin polymerization have a role in regulating Golgi morphology,22–28 we wondered if WASp, a founding member of this family, has a role in the IR-induced, DDR-mediated GDR. We hypothesized that WASp-deficiency by disrupting the GDR contributes to lymphopenia and immune dysregulation in WAS. Our study uncovers a new function for WASp that links the DDR-signaling pathway in the nucleus to the cytoplasmic organelle (Golgi) morphology and redistribution, thereby expanding WASp’s role in the “inside-out” DDR signaling that maintains cell homeostasis during IR-induced DNA damage repair. Accordingly, the study uncovers an increased susceptibility of WASp-deficient Th and B lymphocytes to radiation-induced DNA damage and organelle dysfunction, thereby proposing WAS also as a “radiosensitive” form of PID.
Methods
Cells
CD4+ T helper (Th) cells were isolated from PBMCs by MACS-apparatus (Miltenyi) from 3 normal-donors: ND3, ND8 (both primary Th cells), and ND1 (HTLV-1 immortalized) Th cells were propagated in in vitro culture, as previously described.8–11, 13 Briefly, primary, non-immortalized, Th cells (ND3, ND8) were propagated for multiple weeks in vitro in IL2-containing culture by adding irradiated, unrelated donor PBMCs (1×106/mL) and JY, an EBV-BLCL (0.1×106/mL), and stimulating with PHA (1μg/mL). Only those cell cultures showing >90% live cells by trypan blue exclusion test were included for studies. Normal control BLCL (B lymphoblastoid cell line) expressing normal WASp (cat #: ND03179) and WAS-BLCL expressing a pathogenic WAS mutation V75M (Cat#: GM21868) that results in the lack of WASp expression were purchased from Coriell Institute (Camden, NJ).
For irradiation (IR) treatment, Th cells propagated in fresh medium at 2M/ml cell-density received 5, 10, 15, 20 Gy at the dose rate of 3.5 Gy/min using the X-Ray machine (Pantak Orthovoltage unit, Bipolar Series 2, HF 320, Pantak Inc. Connecticut). The IR-treated cells were propagated in 37°C CO2 incubator and harvested at specific time point (4, 24, 72h post IR) for downstream analyses. For F-actin depolymerization, normal Th cells were treated with 1μM of Latrunculin-A (Lat-A) for 2h. For chemical treatment, either Camptothecin (CPT) (2.5 mM) for 2h or CPT for 2hr + Lat-A (1 mM) added for an additional 10 min or dimethylsulfoxide (DMSO) (0.1% V/V) at 37°C and then harvested for assays.
Co-immunoprecipitation assay
To prepare whole cell lysates (WCL), 20–30 million cells were lysed using RIPA buffer (Sigma) containing Haul protease & phosphatase inhibitor cocktail (ThermoFisher) and Pierce Universal Nuclease (ThermoFisher) at 15 million cells/100 μl density, 4°C with constant agitation for 1 h. The supernatant was collected after centrifugation. For Co-IP, WCL was diluted with the IP buffer (150 mM NaCl, 20 mM Tris, 5 mM EDTA pH 7.5, 1% Triton X-100, 2 mM Na3VO4, 50 mM NaF and protease inhibitors cocktail (Sigma)), and incubated with 1 μg anti-GOLPH3 Ab overnight at 4 °C with constant rotation. This mixture was further reacted with 30 μl Dynabead Protein G (ThermoFiasher) for 1h at 4°C with rotation. The beads were washed with IP buffer, and mixed with 20 μl Lamemmli sample buffer (Bio-rad), incubated at 95°C for 5 min. The proteins in the suspension were separated with 4–15% SDS-PAGE and analyzed by immunoblotting. The coimmunoprecipiation (coIP) assays were performed with/without formaldehyde fixing prior to cell lysis. Cells were harvested, washed with 2X PBS, and suspended in 0.4% paraformaldehyde (or vehicle) in PBS (Sigma) at 10 M cells/ml density for 7 min and spun down at 1800X g for 3 min at room temperature. The fixed cells were further washed 2X 1.25 M glycine/PBS and stored in - 80°C.
CRISPR/Cas9-mediated knock-out and shRNA-mediated knock-down of WASp and GOLPH3 expression in human Th cells
WASp- or GOLPH3- deficient Th cells were generated from the ND1 Th cell line by CRISPR/Cas9-mediated knock-out (KO), as previously described.13 Briefly, Th cells were transfected with 2ug of WASP-CRISPR/CAS9 GFP-tagged plasmid (sc-400712-KO-2) or GOLPH3-CRISPR/CAS9 GFP-tagged plasmid (sc-412973) using Amaxa-nucleofaction. GFP+ cells were FACS-sorted, plated into 96-well plates by serial dilution, and screened by PCR to identify clones exhibiting total WASp-KO (WKO) or GOLPH3 KO (GKO). For RNA interference-mediated knock-down (KD) of the WAS gene in primary Th-cells (ND8 T cell sample), lentiviral vectors encoding GFP and shRNA-sequences targeting WAS or a control (scrambled sequence) were used as described,13 and WASp expression assessed on sorted GFP+ Th-cells. To generate WASp/GOLPH3 double KO (WGKO) T cells, we depleted GOLPH3 expression in WKO Th cells by CRISPR/Cas9 approach.
DNA-sequencing of genomic DNA, RT-PCR of mRNA and Western blot for WASp- or GOLPH3-expression were employed to verify their respective depletion.
Confocal Microscopy and Western blot
Immunofluorescence imaging was performed using Zeiss LSM710 integrated with Zen software, and z-stack images acquired at 0.3μM step-size at 63X magnification for analyses, as we described previously.8–11, 13 FIJI-image analysis software (NIH) was used for all signal quantifications and statistical analyses employing the Mann-Whitney nonparametric algorithm to calculate p values. Signal saturation of γH2A.X and DNA-PKcs in the nucleus was calculated using “3D-Object counter” and “Coloc-2” functions (Image-J software). Automated quantification of the GM130 immunofluorescence signal area in an individual cell and GOLPH3 foci in each nucleus (DAPI stained area) were performed using “Analyze Particles” function in FIJI software.
Western blots were performed as we described previously,8–11, 13 using commercial reagents shown in Supplemental table-1. For studies on the cytoplasmic and nuclear subcellular fractions, extracts were prepared from 20–30 million cells using N-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher), as per the commercial protocol. The proteins in extractions based on the equal cell number preparation were further separated by 10% SDS-PAGE. Western Blots were performed with Trans-Blot Turbo system (Bio-Rad). Proteins in SDS-PAGE were transferred to PVDF membrane, and then probed by immunoblotting using standard procedures. The primary and secondary antibodies conjugated with horseradish peroxidase are as listed in Supplemental table-1. Chemiluminescence was visualized with Clarity Western ECL substrate (Bio-Rad) with ChemiDoc-It2 Imager (UVP) within the linear rage of detection. The intensity of protein bands for analysis purpose was quantified using FIJI software (NIH).
Cell proliferation/survival assay and cell cycle analyses by flow cytometry
MTS assay using CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (MTS) (Promega) was employed to asses cell proliferation. Briefly, 3 × 104 cells (in 100ul growth medium/well) were plated into 96-well plates (Costar, Cambridge, MA) and incubated for 24, 48 or 72h. 20ul MTS/PMS solution (Promega G5421) was added to each well 4 hours before measurement at the absorbance at 490 nm using a Multiskan Spectrum 96-well plate spectrophotometer (Thermo Scientific, Hudson, NH). Annexin V plus Propidium Iodide (PI) staining was employed to asses cell apoptosis. Briefly, 5 × 105 cells were resuspended with 100ul of 1× Annexin V binding buffer (Invitrogen V13246), incubated at room temperature for 15 min after adding 5ul of Annexin V, Alexa Fluor™ 488 conjugate (Invitrogen A13201), 10ul of PI (50ug/ml), and then analyzed by flow cytometer. For cell cycle analysis, 2 × 106 cells were resuspended by vortex after adding 1ml hypotonic PI staining solution (1mg sodium citrate, 0.1 ml lysis buffer [1mM Tris, 0.1mM EDTA, 0.1% Triton X-100], 0.9ml ddH2O, 2.5ul PI stock solution [20mg/ml in 1× PBS]), incubated on ICE for at least 15 min and then analyzed by flow cytometer.
Results
WASp is required to maintain the physiological Golgi ribbon morphology in human Th and B lymphocytes.
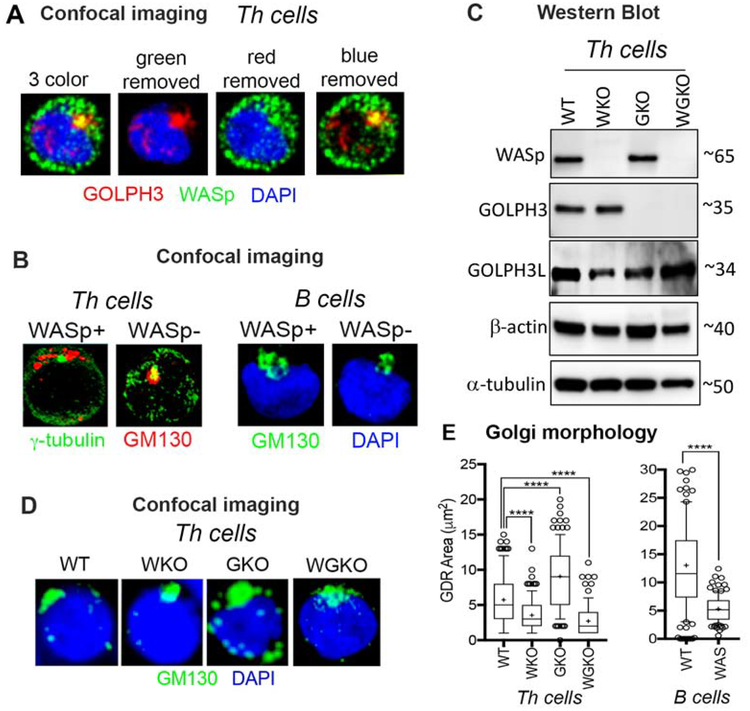
Multiple F-actin nucleation-promoting factors (NPFs) including the members of WASp-family (WHAAM, N-WASp, Wave) are located at the Golgi and are involved in Golgi biology.22–28 We now show that WASp, the founding member of the WASp-family, is also located at Golgi in human primary CD4 Th cells, evidenced by the colocalization of a portion of WASp with GOLPH3 (Golgi phosphoprotein 3) by confocal immunofluorescence imaging (Figure 1A). To investigate the role of WASp in Golgi biology, we first asked how the presence or absence of WASp influences Golgi ribbon morphology.29 In normal human Th cells under basal conditions, the Golgi ribbon monitored by GM130 (a marker of cis-Golgi) is clustered loosely around γ-tubulin (a marker of MTOC) at one end of the nucleus (Figure 1B, left panel), as previously reported in Jurkat T cell leukemia line.30 In normal B cells, Golgi is similarly clustered at one end of the nucleus (Figure 1B, right panel). Notably, loss of WASp by CRISPR/Cas9-mediated knock-out (hereinafter WKO) in human Th cells (Figure 1C) caused tight condensation of Golgi (Figures 1B, D, E). Patient-derived WAS B cells lacking WASp expression from a pathogenic mutation also show an abnormal condensation of Golgi (Figures 1B, E). Such condensed Golgi morphology was seen also in cells lacking the expression of another F-actin modifier, ROCK1.31, 32 In contrast, loss of GOLPH3 expression by CRISPR/Cas9-mediated knock-out (hereinafter GKO) (Supplemental Figure 1, Figure 1C) in the same donor Th cells caused spontaneous dispersal of Golgi (i.e., Golgi disassembly) (Figures 1D, E), the latter phenotype seen also with the deficiency of another Golgi protein, GM130.33 Of note, GOLPH3L (a GOLPH3 paralog) and WASp expression in the GKO Th cells are unaffected, just as the expression of GOLPH3 is unaffected in WKO Th cells (Figure 1C). Similarly, the expression of other WASp-family proteins, N-WASp and WAVE2, are also unaffected by the loss of WASp or GOLPH3 (Supplemental Figure 2A). Our findings in the GKO Th cells, therefore, suggest that GOLPH3 functions to prevent spontaneous Golgi dispersal in human Th cells, which contrasts with its effect in the adherent epithelial and malignant cells (HeLa, HEK293, NIH3T3), where the transient reduction of GOLPH3 (by siRNA-mediated knock-down) caused Golgi condensation rather than dispersal.21, 34 Although the reasons for the contrasting findings are unclear, we speculate that the differences in the cellular context and/or GOLPH3 depletion methodologies (transient versus durable and/or partial versus complete) might contribute to the differing phenotypes. At the minimum, the data show that WASp is present at the Golgi and is required for maintaining the pericentriolar Golgi ribbon morphology in human Th and B cells.
Figure 1. WASp is required to maintain the physiological Golgi morphology in T and B cells.
(A) Confocal immunofluorescence images of the human, primary Th cells triple-labelled with the indicated reagents. Displayed images are the collapsed composites of 20–30 z-stack images acquired at x63 magnification, and are representative of at least 50 cells for each condition. (B) confocal images of Th cells and B cells (WASp+, WASp−) dual-labelled with the indicated reagents showing Golgi morphology under basal, steady-state conditions. (C) Western blot showing the presence or absence of the indicated protein in 4 isogenic Th cell lines lacking either WASp (WKO), GOLPH3 (GKO), both WASp and GOLPH3 (WGKO), or wild-type (WT). (D) Confocal immunofluorescence images of the Golgi morphology in the same 4 Th cell lines shown in panel C and B cells (WT and WAS). (E) Box-and-whisker plots (whiskers@10–90%, horizontal bar denotes median, “+” denotes mean) generated from 3D (z-stack) images of at least n=100 cells from n=3 independent experiments. p**** ≤ 0.0001, Mann-Whitney nonparametric.
Ionizing radiation (IR) and camptothecin (CPT) induced DNA-damage triggers the cell protective Golgi dispersal response (GDR) in human Th and B lymphocytes.
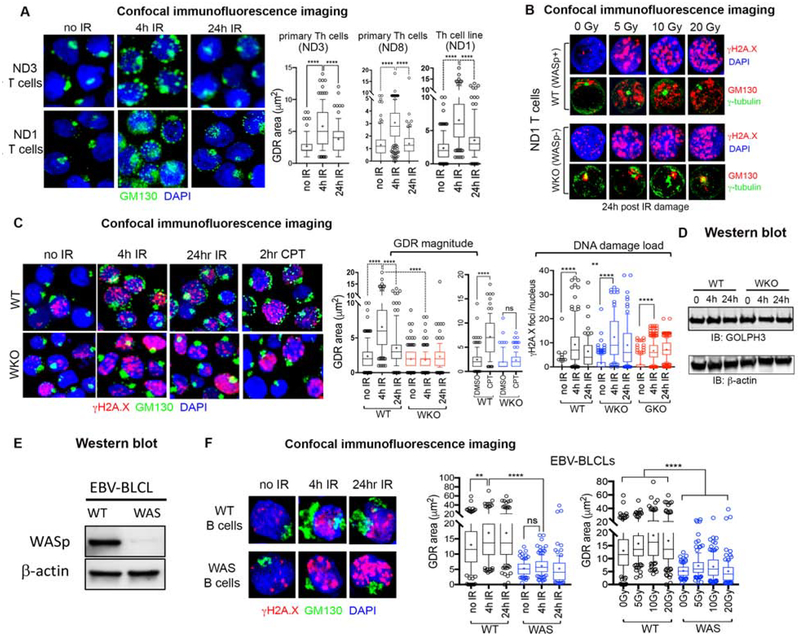
GDR from IR- or CPT-induced DNA double strand break (DSB) damage was first reported in malignant and nonmalignant adherent, mammalian cells.21 We asked if DNA DSBs inflicted by ionizing radiation (X-Ray, IR) or radiomimetic drug (camptothecin, CPT) can elicit GDR also in human Th and B lymphocytes (non-adherent cells). Using confocal immunofluorescence imaging of wild-type (WT) Th cells (primary Th cells from 2 donors, ND3, ND8; and a Th cell line from a 3rd donor ND1) triple-labelled with GM130 (a marker of Golgi), γH2A.X (a marker of DSBs), and DAPI (a marker of nucleus), we found that GDR (reported by GM130 redistribution) was captured at 4hr post-IR (Figure 2A). GDR was elicited at multiple genotoxic doses of IR (5, 10, 20 Gy) in normal Th cells, contemporaneously with an increase in the DSB DNA damage load reported by γH2A.X immunofluorescence foci (Figure 2B). GDR was elicited also by CPT (analyzed at 2hr post-treatment) (Figure 2C, WT panel). However, unlike in malignant cells or adherent epithelial cells reported previosly,21 the GDR in human Th lymphocytes is ill-sustained, often decreasing (but not absent) by 24h post-IR (Figures 2A), contemporaneously with the decrease also in the DSB DNA damage load reported by γH2A.X immunofluorescence foci/signal enrichment in the nucleus (Figure 2C, WT panel). This implies that in human Th cells, Golgi reorganizes/reassembles rapidly after the DNA damage load is reduced (i.e., the damaged DNA is repaired). In WT Th cells, this is reflected in cell survival and proliferation measured by the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay at 4h, 24h, 48h, and 72h post damage induction (Supplemental Figure 3A). Cell apoptosis as reported by the fraction of cells in the propidium iodide/annexin V flow cytometric analyses show ~2% apoptotic cells (Supplemental Figure 3B). Similarly, the % of cells in the subG0/G1 phase of the cell-cycle (monitors apoptotic cells) by flow cytometry was also nominal (0.74%- no IR control; 1.11% 4h post) (Supplemental Figure 3C). These data together suggest that the GDR captured at 4h post-IR damage in human Th cells is not due to increased apoptosis. Furthermore, because GDR is typically irreversible in apoptotic cells,32, 35 the reversibility of GDR in our Th cells further diminishes the possibility of apoptosis contributing to the observed GDR phenotype in Th cells. Moreover, the 4hr GDR is captured in cells that are predominantly in their G1 or S cell-cycle phases (~80%) with a smaller fraction (~20%) in G2/M-phase (Supplemental Figure 1C), suggesting that the observed GDR is also not secondary to the G2/M-phase arrest, the latter known to cause Golgi dispersal.36 In human B cells, however, the GDR captured at 4h is sustained at 24h post-IR (Figure 2F, WT B cell panel), suggesting that the longevity of the GDR phenotype is cell-type specific. Together, we conclude that Golgi disassembly and dispersal are features of the DNA damage and repair (DDR) signaling elicited by ionizing radiation or radiomimetic chemotherapy in human Th and B lymphocytes.
Figure 2. WASp is required to generate the DNA damage-induced Golgi dispersal response in Th and B cells.
(A) Confocal immunofluorescence images of the human Th cells (wild-type, WT) from normal donors (WASp-sufficient) dual-labelled with the indicated reagents at 4h and 24hr after 5Gy X-Ray treatment, or no X-Ray control. Displayed images are the collapsed composites of 20–30 z-stack images acquired at x63 magnification, and are representative of at least 100 cells for each sample. Box-and-whisker plots (whiskers@10–90%, horizontal bar denotes median, “+” denotes mean) generated from 3D (z-stack) images of at least n=100 cells from n=3 independent experiments for each biological sample (ND1, ND3, ND8). p**** ≤ 0.0001, Mann-Whitney nonparametric. ND3 and ND8 are primary Th cells, ND1 is HTLV-1 immortalized Th cell line. (B) Confocal immunofluorescence images of the human Th cells, WT (WASp-sufficient) or WKO (WASp-deficient), isogenic pair from ND1 donor, triple-labelled with the indicated reagents at 24hr after 5Gy, 10Gy, and 20Gy X-Ray treatment, or no X-Ray (0 Gy) control. Displayed images, acquired at 24h post IR treatment, are collapsed composites of 20–30 z-stack images acquired at x63 magnification, and are representative of at least 100 cells for each cell type. (C) Confocal immunofluorescence images of the human Th cells, WT (WASp-sufficient) or WKO (WASp-deficient) triple-labelled with the indicated reagents after IR- or CPT-induced DNA damage. The accompanying box-and-whisker plots display the magnitude of GDR, induced by 5Gy IR or CPT, relative to the DNA damage load. Other descriptions of the images, plots, and p- values are as per panel A. Similar findings of impaired IR-induced GDR was captured also in WASp-deficient (WKD) ND8 Th cells relative to WT control (data not shown). (D) Western blot depicting GOLPH3 expression level relative to the loading control (β-actin) in the whole cell extracts of the indicated Th cells at the indicated times after 5Gy of IR treatment. GOLPH3-knock out (GKO) data is included in this panel to allow direct comparison of the DNA damage loads between WT, WKO and GKO T cells. The corresponding GKO T cell images are shown in Figure 4. (E) Western blot depicting WASp expression level relative to the loading control (β-actin) in the whole cell extracts of the indicated B cells, WT and WAS. (F) Confocal immunofluorescence images of the human B cells (EBV-immortalized), WT (WASp-sufficient, normal donor) or WAS (WASp-deficient, patient-derived) triple-labelled with the indicated reagents after IR treatment for the duration indicated. The accompanying box-and-whisker plots display the magnitude of GDR induced by IR at the set done of 5Gy (on left) and increasing IR doses (on the right). p- values per Mann-Whitney nonparametric ****<0.0001, **<0.001, ns: nonsignificant. Other descriptions of the images and plots as per panel A.
DNA damage-induced GDR is impaired in WASp-deficient human Th and B lymphocytes.
Because, loss of WASp causes increased DNA damage,13 decreased DNA damage repair,16 decreased cell proliferation, and lymphocytopenia even when F-actin cytoskeleton is normal,3, 17, 18 we asked if the DNA damage-linked role of WASp in the nucleus extends to regulating DNA damage-linked GDR in the cytoplasm. We compared GDR in the wild-type (WT) and WASp-deficient human Th cell isogenic pairs from 2 donors (ND1, ND8), and found significant impairment of the GDR in WASp-deficient Th cells subjected to IR- or CPT-induced DNA damage (Figures 2B, C, WKO panels). The magnitude of DNA damage in WASp-deficient Th cells was high (γH2A.X immunofluorescence foci), and yet the GDR was significantly blunted (Figure 2C). GDR could not be induced even at increasing doses of IR (up to 20 Gy) in WASp-deficient Th cells (Figure 2B, WKO panel). Of note, WASp depletion did not affect the expression of GOLPH3 protein in WKO Th cells, at steady state (0 hr) or after IR-induced damage (Figure 2D), which rules out the possibility for any GOLPH3-expression changes contributing to the GDR phenotype in WKO Th cells. In addition to the WASp-deficient Th cells, GDR is impaired also in WAS patient-derived B cells lacking WASp expression from a pathogenic mutation (Figures 2E, F, WAS B cell panel), and similar to WKO Th cells, GDR in WAS B cells cannot be induced even at increasing doses of IR (up to 20 Gy) (Figure 2F, bar graph on extreme right). We therefore conclude that WASp is necessary for the development of IR-mediated, DNA damage-induced GDR in human Th cells and B lymphocytes, and that endogenous GOLPH3 expression in WASp-deficient Th and B cells does not rescue the GDR defect.
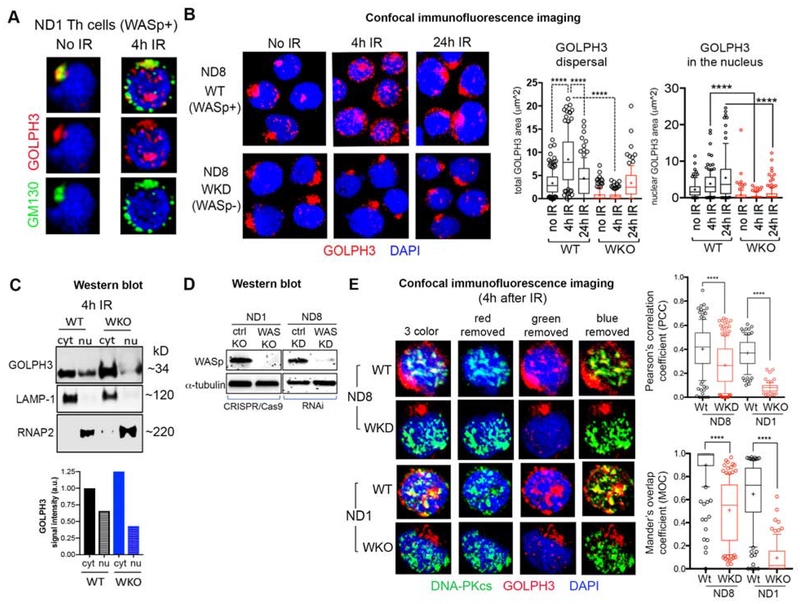
Loss of WASp impedes GOLPH3 nuclear localization and its colocalization with DNA-PKcs.
To obtain molecular insights into why GDR is impaired in WASp-deficient cells, we focused on defining the relationship of WASp with the signaling pathway (GOLPH3•MYO18A•DNA-PK•F-actin), pivotal for the development of DNA damage-mediated GDR in the adherent/malignant cells.21 To this end, we first tested GOLPH3 activity, because GOLPH3 was shown to be a critical driver of GDR.21 In normal (WT) Th cells, GOLPH3 colocalizes with GM130 (a marker of cis-Golgi) in the steady state (no IR control) (Figure 3A). Upon IR-induced DNA-damage, GOLPH3 disperses around the cytoplasm, tracking with dispersed GM130 (Figures 3A, B). Notably, a fraction of GOLPH3 translocates to the nucleus (Figures 3A–C), where it colocalizes partially with DNA-PKcs in both primary Th cells (ND8) and a Th line (ND1) subjected to IR damage (Figure 3E). Significantly, loss of WASp in ND8 and ND1 Th cells (Figure 3D) resulted in decreased nuclear translocation of GOLPH3 (Figures 3B, C) and its co-localization with DNA-PKcs (Figure 3E, Pearson’s correlation coefficient and Mander’s overlap coefficient). This reduced colocalization of GOLPH3 with DNA-PKcs may occur in part from abnormal GOLPH3 redistribution due to increased Golgi condensation in WKO T cells. These imaging findings insert WASp into the previously proposed signaling pathway for GDR, in which DNA-damage triggers GOLPH3 post-translational modification by DNA-PKcs (the afferent loop), which in turn augments the binding of GOLPH3 to MYO18A required for generating the tensile force at Golgi (the efferent loop).21 Alternatively, WASp effect on GOLPH3 nuclear localization could impact cell function in a GDR-independent manner. To further explicate how WASp might affect GOLPH3 nuclear localization, we asked if WASp physically associates with GOLPH3, and if this protein-protein association is constitutive or induced by IR-mediated DNA damage. First, we show that WASp, predominantly a cytosol-resident protein in the steady-state, is translocated to the nucleus upon IR treatment (Supplemental Figure 2B). Thus, apart from the Th1-activation stimulus, which also induces WASp nuclear translocation,8 DNA damage draws WASp into the nucleus. Using “native” coimmunoprecipitation (no formaldehyde cross-linking), we show that GOLPH3 does not physically associate with WASp in either Th or B cells, in multiple assays (Supplemental Figure 2C). However, employing in vivo formaldehyde cross-linking in intact T cells prior to lysis, we captured WASp:GOLPH3 association from the same donor T cells (Supplemental Figure 2C). Together, these findings suggest that the WASp:GOLPH3 interaction induced by DNA damage is of low affinity and/or is transient.
Figure 3. WASp deficiency impairs nuclear translocation of GOLPH3 and its colocalization with DNA-PKcs.
(A, B) Confocal immunofluorescence images of the human Th cells (WASp+ or WASp−) dual-labelled with the indicated reagents at 4h and 24hr after X-Ray (IR) treatment, or no IR control. Displayed images are the collapsed composites of 20–30 z-stack images acquired at x63 magnification, and are representative of at least 100 cells for each cell type (ND1, ND8). Box-and-whisker plots depict GOLPH3 dispersal area throughout the cell (on left) and the magnitude of GOLPH3 nuclear localization (on the right) at the indicated time points after IR treatment, in WT and WKO Th cells. (C) Western blot depicting GOLPH3 expression levels in the nuclear (nu) and cytoplasmic (cyt) fractions of ND1 Th cell pair (WT and WKO) at 4 h after IR treatment. LAMP-1 (Lysosomal-associated membrane protein 1- a marker of cytoplasmic fraction) and RNAP2 (RNA Pol II- a marker of nuclear fraction) monitored the purity of cell extracts. GOLPH3 signal intensity quantified by gel densitometry is shown under the Western blot images. (D) Western blot depicting WASp expression level relative to the loading control (α-tubulin) in the whole cell extracts of the indicated Th cells (ND1 and ND8) after knocking out (KO by CRISPR/Cas9) or knocking-down (KD by RNA interference) or their respective control/scrambled KO/KD. (E) Confocal immunofluorescence images of the human Th cells (WASp+ or WASp−) triple-labelled with the indicated reagents at 4h after X-Ray (IR) treatment showing the degree of colocalization between GOLPH3 and DNA-PKcs fluorophores was quantified by the Pearson correlation coefficient (PCC) and the Mander’s overlap coefficient (MOC), in which the scores reach 0 only when the 2 fluorophore signals are completely mutually exclusive and reach 0.99 when the 2 signals are completely overlapping. Other descriptions of the images, plots, and p-values as per Figure 2A.
Loss of GOLPH3 does not prevent DNA damage-induced GDR in human Th lymphocytes
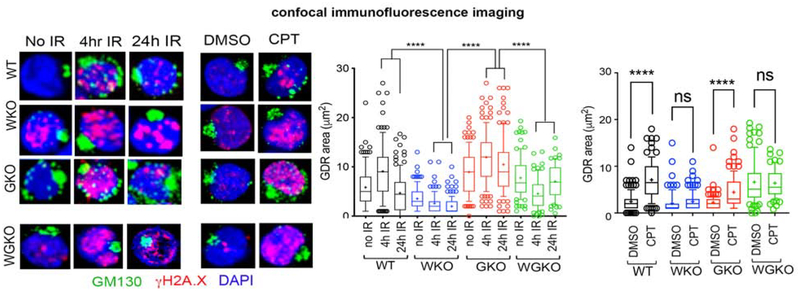
The above findings raised the question: to what extent is GOLPH3-activity required for the DNA damage-mediated GDR in WASp-sufficient Th cells? To directly address this question, we generated a stable GOLPH3 KO (GKO) Th cell line by CRISPR/Cas9 approach from the same normal donor (ND1) wild-type Th cells (WT) used for generating WAS KO (WKO) Th cell line (Supplemental Figure 1). Thus, our approach of testing all GDR-related events in the isogenic set of triplet Th cell lines (ND1-WT, ND1-WKO and ND1-GKO) mitigates any salutatory/or deleterious effects of disparate genetic backgrounds, HTLV1-mediated immortalization (in the case of ND1 Th cells), or in vitro cell growth differences on the GDR development. First, we verified that GOLPH3 KO was specific, and that it did not cause an off-target depletion of GOLPH3L- a paralog of GOLPH3 (Figure 1C). We then tested how the complete loss of GOLPH3 expression influences the development of GDR induced by CPT- and IR-mediated DNA damage in WASp-sufficient, GOLPH3-deficient Th cells (GKO). Surprisingly, we found that the magnitude of GDR in both IR-and CPT-treated GKO Th cells was comparable to that in WT Th cells (Figure 4). Accordingly, cell-survival quantified by flow cytometry using propidium iodide/annexin V staining showed minimal cell-apoptosis (2.3%) in GKO Th cells, which was comparable to the WT Th cells (1.97%) (Supplemental Figure 3B). Cell proliferation in the GKO Th cells at 24h, 48h, and 72h after X Ray damage (quantified by the MTS assay) also showed only a modest decrease relative to WT control (Supplemental Figure 3A). Although a previous study similarly demonstrated that acute GOLPH3 depletion was not universally detrimental to cell proliferation and survival,37 the slower cell proliferation in GKO Th cells could also be due to non-GDR effects of GOLPH3-deficiency, such as from downmodulating mTOR/AKT activation.37 Interestingly, depleting WASp expression in the GKO Th cells (i.e., double WAS and GOLPH3 KO by CRISPR/Cas9: aka, WGKO Th cells) (Supplemental Figure 1A), prevents GDR in response to IR- or CPT- induced DNA damage (Figure 4). Accordingly, the combined loss of WASp and GOLPH3 (WGKO) in the same Th cells causes increased cell necrosis (~52% compared to ~5% in GKO and 10% in WKO). Significantly, cell proliferation was dramatically reduced in WGKO Th cells relative to GKO Th cells (Supplemental Figure 3A), contemporaneously with an increase also in the percentage of G2/M arrested cells (35% compared to 20% in WT Th cells at 4h post-IR) (Supplemental Figure 3C). Together, WASp-deficiency causes profound impairment of GDR and reduced cell proliferation compared to an isolated GOLPH3-deficiency in human Th cells, which proposes that the DNA damage signals to the Golgi funnel through WASp.
Figure 4. GOLPH3 is nonessential for generating the DNA damage-induced Golgi dispersal response in WASp-sufficient Th cells.
Confocal immunofluorescence images of the human Th cells, GOLPH3-deficient (GKO) or WASp and GOLPH3 double-deficient (WGKO) isogenic pair from the ND1 donor triple-labelled with the indicated reagents at 4h and 24hr after X-Ray (IR) treatment, or no IR control, as well as after CPT or DMSO control treatments. Box-and-whisker plots depict the magnitude of GDR under IR treatment (on left) and CPT treatment (on right) comparing the differences between GDR phenotypes in GKO, WGKO Th cells relative to WKO Th cells. (Note: The GDR data for WKO Th cells was shown also in Figure 2C).
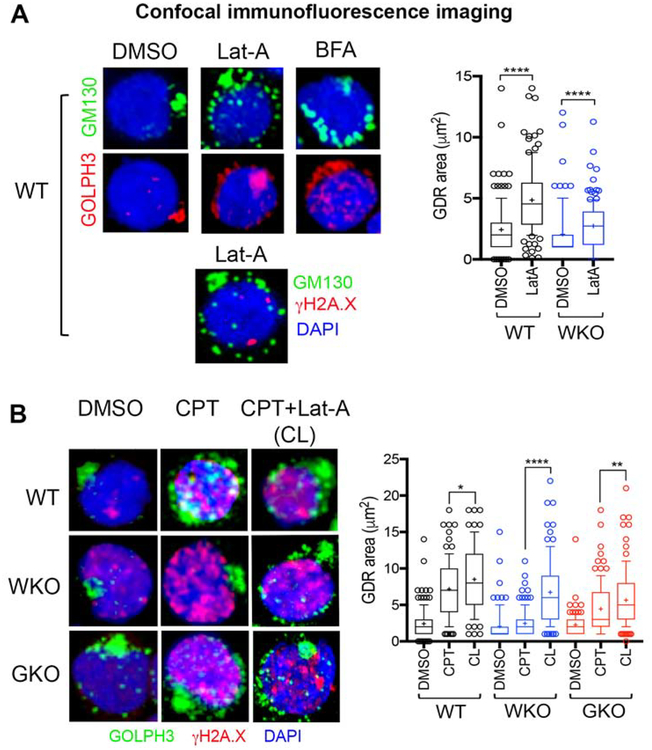
Pharmacological depolymerization of F-actin does not prevent DNA-damage-induced GDR in human Th lymphocytes
Because F-actin was shown to be part of the GDR signaling pathway, we asked how depolymerization of cellular F-actin by Latrunculin A (Lat-A) influences DNA-damage-mediated GDR in human Th cells. First, we show that F-actin depolymerization in normal (WASp-sufficient) Th cells does not cause DNA damage (γH2A.X foci) on its own, and yet induces spontaneous dispersal of both Golgi and GOLPH3 (Figure 5A). This suggests a role for F-actin cytoskeleton in maintaining the physiological Golgi ribbon morphology in human Th cells. As such, decreasing or increasing cellular F-actin content was previously shown to both disperse and condense Golgi.27, 38–40 These contrasting findings suggest that the final effect of modulating cellular F-actin content on basal Golgi morphology is context and cell-type dependent. Notably, we show that F-actin depolymerization in normal Th cells subjected to CPT-induced DNA-damage (CPT + Lat-A sample, aka CL) allows the development of GDR, the magnitude of which is comparable to that in Lat-A untreated, CPT-treated Th cells (CPT sample) (Figure 5B). This suggests that F-actin depolymerization does not alter the ability of endogenous WASp and/or GOLPH3 to modulate Golgi morphology after DNA damage in human Th cells. Surprisingly, the defective GDR seen in the CPT-treated WASp-deficient Th cells (WKO) is at least partially restored upon F-actin depolymerization (Figure 5B), and in the CPT-treated GOLPH3-deficient Th cells (GKO), F-actin depolymerization increases the degree of GDR relative to CPT alone control. These findings suggest that DNA damage-induced GDR can proceed in the setting of reduced cellular F-actin content in normal, WASp-deficient, or GOLPH3-deficient human Th cells.
Figure 5. F-actin depolymerization does not impede DNA damage induced Golgi dispersal response in WASp-sufficient Th cells.
(A) Confocal immunofluorescence images of the human Th cells (WT, ND1 donor) multi-labelled with the indicated reagents after treating Th cells with Latrunculin A (Lat-A, F-actin depolymerizing agent), Brefeldin A (BFA, inhibitor of protein transport/secretion that induces spontaneous Golgi dispersal), or DMSO control. Note, Lat-A per se does not induce DNA damage (γH2A.X foci) and yet disperses Golgi. Box-and-whisker plots depict the magnitude of GDR under Lat-A or DMSO treatment in WT and WKO Th cells. Other descriptions of the images, plots, and p-values as per Figure 2A. (B) Confocal immunofluorescence images of human Th cells, WT, WKO, GKO from ND1 donor multi-labelled with the indicated reagents after treating Th cells with CPT alone or CPT followed by Lat-A (CL) or DMSO control. Box-and-whisker plots (on right) depict the magnitude of GDR under indicated conditions. *p<0.05, **p<0.001, ****p<0.0001 Mann-Whitney nonparametric.
Discussion
Our study identifies a new function for WASp that links nuclear DNA-damage signaling to cytoplasmic organelle (Golgi) morphology, and proposes impaired GDR as a new cell-biological defect that contributes, at least in part, to the development of dysfunctional Th and B cell phenotypes in WAS patients. Previously, we showed an essential role of nuclear-WASp in preventing the occurrence of DNA DSBs in human Th cells.13 These findings together propose a three-step model for cell dysfunction in WAS lymphocytes. Step-1: WASp-deficiency triggers DNA damage (i.e., high DSB induction).13 Step-2: WASp-deficiency impairs efficient repair of the damaged DNA due to defective homology-directed repair (i.e., low DSB repair efficiency).16 Step-3: Failure of GDR in the face of high DNA damage load. This deleterious triad will lead to decreased lymphocyte proliferation, increased cell death, and/or senescence, thus contributing to the development of lymphocyte dysfunction in WAS. Accordingly, WASp is an essential factor in multiple phenomena, including the GDR, that ensures restoration of genome stability and cell viability following DNA strand breaks. Of the various peripheral blood cells, Th and B cells have been reported to be more radiosensitive than CTLs, Tregs, dendritic cells, or natural killer cells.41 In this regards, impaired cytoprotection and organelle (Golgi) dysfunction in WAS Th and B cells, in the context of ionizing radiation (X Ray) and radiomimetic chemotherapy agent (camptothecin), proposes that a subset of WAS patients may manifest a “radiosensitive” form of primary immune deficiency disorder, a susceptibility that is currently not recognized. WAS patients are exposed to diagnostic radiation from imaging, and require myeloablative/cytoreductive chemotherapy as preparation for curative stem cell transplantation and/or for treating WAS-linked lymphomas. Our findings that a radiomimetic chemotherapy drug (camptothecin) also inflicts organelle damage in WAS lymphocytes will compel future testing of how other chemotherapeutic agents affect WAS lymphocyte survival and function. Future studies linking the GDR defect to patient mutations and clinical phenotypes in WAS will inform practice-changing approaches in a subset of patients whose lymphocytes are deemed radiosensitive.
From a mechanistic standpoint, our study provides multiple new insights into the DNA damage-induced GDR in human lymphocytes and the role of WASp in this phenomenon. First, WASp is located also at the Golgi, where it co-localizes with a core component of the GDR signaling pathway, GOLPH3. Second, loss of WASp disrupts the nuclear translocation of GOLPH3 and its colocalization with nuclear DNA-PKcs, an event proposed to phosphorylate GOLPH3, the latter driving the MYO18A/F-actin processive motor for the GDR.21 Because reagents that can unequivocally monitor phosphorylated GOLPH3 (T143/T148) are currently unavailable, our data cannot conclude whether the decreased GOLPH3/DNA-PK signal co-localization observed in WASp-deficient Th cells results in decreased GOLPH3 phosphorylation and the consequent impairment in GOLPH3:MYO18A complexation. Moreover, because we did not capture the physical binding of WASp and GOLPH3 under “native” coimmunoprecipitation conditions, we favor a model wherein, WASp influences GOLPH3 activity indirectly, via a second message (downstream factor), the identification of which will require insightful follow-up studies. By the same token, because the WASp:GOLPH3 co-association was captured after formaldehyde fixing, we cannot completely exclude the possibility of a transient and/or low-affinity co-association between GOLPH3 and WASp, and if so, additional studies will be needed to pin-point the subcellular location (cytosol, golgi, nucleus) where such GOLPH3:WASp co-association occurs. At the minimum, our findings insert WASp into GDR signaling pathway, and further suggest that when WASp and GOLPH3 are co-expressed in Th cells, GOLPH3 effect on GDR likely funnels through WASp. As such, loss of GOLPH3 alone is insufficient to impair GDR in WASp-sufficient human Th cells, but depleting WASp in the GOLPH3-deficient Th cells (i.e. double GKO/WKO mutant), profoundly impairs GDR. These findings, while challenging the absolute requirement of GOLPH3 in the GDR,21 places WASp at a critical juncture within the GDR pathway. However, given the previous demonstration of GOLPH3’s role in GDR, our study raises the possibility for at least 2 pathways supporting GDR in different cell lineages, i.e., WASp-dependent pathway for the hematolymphoid cell lineages, in which WASp is highly-expressed, and GOLPH3-dependent pathway for non-hematolymphoid cell lineages including malignant cells, in which WASp is minimally/or not expressed. To substantiate this dualistic-pathway model for GDR, we will need to identify other Golgi-resident proteins that partner with WASp to modulate DNA damage-induced GDR in the context of GOLPH3 deficiency. Moreover, testing the intactness of GDR after depleting N-WASp (a WASp-family member that is ubiquitously expressed in all tissues) in nonhematolymphoid/malignant cells is required to determine whether N-WASp has a function in the GDR, and whether GOLPH3 and N-WASp are epistatic or additive in these cell lineages.
The ribbon structure of Golgi is critical for modulating cell proliferation and survival, and hence various human disorders including neurodegeneration and cancer associate with disruption of the Golgi ribbon structure.36, 42, 43 While these disorders have been reported to associate with pathological Golgi fragmentation/dispersal, our study links pathological Golgi condensation, both in steady-state and during DNA-damage as a contributor to the immunodeficient phenotype in WAS. The clinical relevance of DNA damage-mediated GDR to cancer biology is presented by the observation that both GOLPH3 and WAS gene mutations are found in different cancers, and that the GDR defect occurs in both GOLPH3-deficient and WASp-deficient cells. However, there is a fundamental difference between WASp- and GOLPH3-pathways. Over-expression of GOLPH3 is linked with poor prognosis and the development of chemotherapy-resistance in a subset of cancers, which has formed the basis to propose a role for GOLPH3-mediated cell protective GDR in the development of the “chemo-resistance” phenotype in GOLPH3-overexpressing cancer cells.43 In contrast, under/or loss of expression of WASp is linked with hematolymphoid cancers in humans.44–46 In the irradiation-induced murine model of cancer, compared to p53+/−WASp+/+ mice, p53+/−WASp−/− mice develops lymphoma.47 Accordingly, GOLPH3 functions as an oncoprotein, whereas WASp functions as a tumor-suppressor protein, at least in a subset of cancers. Thus, the effect of GOLPH3 or WASp dysfunction on DNA damage-induced GDR is likely modulated by the cell lineage and/or cancer subtype.
Because DNA DSB damage can elicit multiple cell-survival programs, including the GDR, an unanswered question in the field of DDR-mediated GDR is whether GDR per se is necessary and sufficient for cell survival. Our MTS assays do show retarded Th cell proliferation in all 3 mutant Th cell lines (WKO, GKO, WGKO) relative to their isogenic WT control, with the proliferation defect most pronounced in this order: WGKO>WKO >GKO. While these data link impaired GDR to cell-survival, our study cannot definitively establish whether the GDR defect is a cause (driver defect) or a consequence (secondary effect) of the underlying Th cell immune dysfunction from WASp-deficiency. As such, increased DNA damage in WASp-deficient cells is expected to activate CDK1/Cyclin B1-complex and CDC25c, which in turn will trigger G2/M arrest and cellular apoptosis (as seen in WGKO T cells). Therefore, perturbation of this pathway, we speculate, might be responsible for the observed differences between the single GKO deficient T cells versus double GKO+WKO (WGKO) T cells. Other possibility is that WASp could influence G2/M state by disrupting the mitotic spindle structures, causing mitotic arrest. This speculation is based on the previously demonstrated role of both N-WASp and F-actin in this process.48
Because Golgi harbors a diverse range of signaling proteins,32 another key question in the field is to define the functional role of GDR in the DDR process in general, and for WAS specifically, to define how impaired GDR contributes to the development of autoimmunity and/or autoinflammatory cellular and clinical phenotypes. Because defective GDR will impair Golgi-to-endoplasmic reticulum (ER) transport, a defect previously shown to cause another primary immune-deficiency/autoimmunity disorder, “Copa syndrome”,49 we speculate that the observed Golgi defect may directly contribute to the autoimmunity phenotype in a subset of WAS patients. As such, loss of WASp could potentially affect cell survival/function also via non-GDR mechanisms, such as by impaired homology-directed DNA repair mechanism,16 resulting in the accumulation of DNA DSB load that can alter multiple signaling/transcriptional networks including the metabolic status of the cell. Accordingly, while our studies and those of others clearly link WASp-deficiency to the defects in the DDR signaling, it remains a challenge to estimate the relative contributions of the GDR defect versus DNA damage defect to the development of WAS Th and B cell phenotypes. At the minimum, our study broadens the field of play for WASp by uncovering an essential role in the cell-protective phenomenon linked to IR-induced DNA damage, and proposes a novel etiologic mechanism for Th and B cell immune-deficiency and immune-dysregulation in human WAS.
Supplementary Material
Key Messages:
WASp is essential for the irradiation-mediated, DNA damage-induced Golgi-dispersal response (GDR) in human Th and B cells.
WASp-deficient Th and B cells are radiosensitive.
Acknowledgements
This work was supported by the NIH, National Institute of Allergy and Infectious Diseases (NIAID) grant R21AI138051 (to Y.M.V.). Part of the studies were funded by the Research Bridge Award from the Carver College of Medicine and the Endowment from the Mary Joy and Jerre Stead Foundation. A subset of data was obtained at the Flow Cytometry Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa. We thank the University of Iowa Dance Marathon organization (UIDM) for supporting the research laboratory space where this work was carried out.
Abbreviations:
- WAS
Wiskott-Aldrich syndrome
- WASp
Wiskott-Aldrich syndrome protein
- N-WASp
neural WASp
- WHAMM
WASP Homolog-Associated Protein With Actin, Membranes And Microtubules
- Wave
WASP Family Member 1
- ROCK1
Rho Associated Coiled-Coil Containing Protein Kinase 1
- MTOC
Microtubule organizing center
- RNA Pol II
RNA polymerase II
- LAMP-1
Lysosomal-associated membrane protein 1
- IR
ionizing radiation
- CPT
Camptothecin
- DSB
double strand break
- HDR
homology-directed repair
- DDR
DNA damage repair and response
- GDR
Golgi-dispersal response
- GOLPH3
Golgi Phosphoprotein 3
- GOLPH3L
Golgi Phosphoprotein 3 like
- DNA-PKcs
DNA-Dependent Protein Kinase Catalytic Subunit
- MYO18A
Myosin XVIIIA
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- PBMCs
Peripheral blood mononuclear cells
- EBV-BLCL
Epstein-Barr virus immortalized B lymphoblastoid cell line
- HTLV-1
Human T-cell lymphotropic virus type 1
- WT
wild-type
- WKO/WKD
WAS gene knock-out/knock-down
- GKO
GOLPH3 gene knock-out
- WGKO
WAS and GOLPH3 double knock-out
Footnotes
Conflict of interest: All authors declare no conflict of interest with the current study
References
- 1.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 1994;78:635–44. [DOI] [PubMed] [Google Scholar]
- 2.Villa A, Notarangelo L, Macchi P, Mantuano E, Cavagni G, Brugnoni D, et al. X-linked thrombocytopenia and Wiskott-Aldrich syndrome are allelic diseases with mutations in the WASP gene. Nat Genet 1995;9:414–7. [DOI] [PubMed] [Google Scholar]
- 3.Zhou L, Li W, Zhang X, Liu D, Ding Y, Dai R, Zhao X. Abnormal distribution of distinct lymphocyte subsets in children with Wiskott-Aldrich syndrome. Hum Immunol. 2017. September;78(9):565–573. [DOI] [PubMed] [Google Scholar]
- 4.Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, et al. Clinical course of patients with WASP gene mutations. Blood 2004;103:456–64. [DOI] [PubMed] [Google Scholar]
- 5.Albert MH, Bittner TC, Nonoyama S, Notarangelo LD, Burns S, Imai K, et al. X-linked thrombocytopenia (XLT) due to WAS mutations: clinical characteristics, long-term outcome, and treatment options. Blood 2010;115:3231–8. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y, Mazza C, Christie JR, Giliani S, Fiorini M, Mella P, et al. Mutations of the Wiskott-Aldrich Syndrome Protein (WASP): hotspots, effect on transcription, and translation and phenotype/genotype correlation. Blood 2004;104:4010–9. [DOI] [PubMed] [Google Scholar]
- 7.Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, McCormick F, et al. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell 1996;84:723–34. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MD, Sadhukhan S, Kottangada P, Ramgopal A, Sarkar K, D’Silva S, et al. Nuclear role of WASp in the pathogenesis of dysregulated TH1 immunity in human Wiskott-Aldrich syndrome. Sci Transl Med 2010;2:37ra44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadhukhan S, Sarkar K, Taylor M, Candotti F, Vyas YM. Nuclear role of WASp in gene transcription is uncoupled from its ARP2/3-dependent cytoplasmic role in actin polymerization. J Immunol 2014;193:150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar K, Sadhukhan S, Han SS, and Vyas YM. Disruption of hSWI/SNF complexes in T cells by WAS mutations distinguishes X-linked thrombocytopenia from Wiskott-Aldrich syndrome. Blood 2014;124:3409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar K, Sadhukhan S, Han SS, Vyas YM. SUMOylation-disrupting WAS mutation converts WASp from a transcriptional activator to a repressor of NF -kappaB response genes in T cells. Blood 2015;126:1670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teitell MA. Alternative control: what’s WASp doing in the nucleus? Sci Transl Med 2010;2:37ps31. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar K, Han SS, Wen KK, Ochs HD, Dupré L, Seidman MM, Vyas YM. R-loops cause genomic instability in T helper lymphocytes from patients with Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2018. July;142(1):219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano-Pozo M, Santos-Pereira JM, Rondón AG, Barroso S, Andújar E, Pérez-Alegre M, García-Muse T, Aguilera A. R loops are linked to histone H3 S10 phosphorylation and chromatin condensation. Mol Cell. 2013. November 21;52(4):583–90 [DOI] [PubMed] [Google Scholar]
- 15.García-Pichardo D, Cañas JC, García-Rubio ML, Gómez-González B, Rondón AG, Aguilera A. Histone Mutants Separate R Loop Formation from Genome Instability Induction. Mol Cell. 2017. June 1;66(5):597–609.e5. [DOI] [PubMed] [Google Scholar]
- 16.Schrank BR, Aparicio T, Li Y, Chang W, Chait BT, Gundersen GG, Gottesman ME, Gautier J. Nuclear ARP2/3 drives DNA break clustering for homology-directed repair. Nature. 2018. July;559(7712):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rengan R, Ochs HD, Sweet LI, Keil ML, Gunning WT, Lachant NA, Boxer LA, Omann GM. Actin cytoskeletal function is spared, but apoptosis is increased, in WAS patient hematopoietic cells. Blood. 2000. February 15;95(4):1283–92. [PubMed] [Google Scholar]
- 18.Rawlings SL, Crooks GM, Bockstoce D, Barsky LW, Parkman R, Weinberg KI. Spontaneous apoptosis in lymphocytes from patients with Wiskott-Aldrich syndrome: correlation of accelerated cell death and attenuated bcl-2 expression. Blood. 1999. December 1;94(11):3872–82. [PubMed] [Google Scholar]
- 19.Westerberg LS, Meelu P, Baptista M, Eston MA, Adamovich DA, Cotta-de-Almeida V, et al. Activating WASP mutations associated with X-linked neutropenia result in enhanced actin polymerization, altered cytoskeletal responses, and genomic instability in lymphocytes. J Exp Med 2010;207:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moulding DA, Blundell MP, Spiller DG, White MR, Cory GO, Calle Y, et al. Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J Exp Med 2007;204:2213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farber-Katz SE, Dippold HC, Buschman MD, Peterman MC, Xing M, Noakes CJ, Tat J, Ng MM, Rahajeng J, Cowan DM, Fuchs GJ, Zhou H, Field SJ. DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3. Cell. 2014. January 30;156(3):413–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alekhina O, Burstein E, Billadeau DD. Cellular functions of WASP family proteins at a glance. J Cell Sci. 2017. July 15;130(14):2235–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matas OB, Martínez-Menárguez JA, Egea G. Association of Cdc42/N-WASP/Arp2/3 signaling pathway with Golgi membranes. Traffic. 2004. November;5(11):838–46. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya K, Swaminathan K, Peche VS, Clemen CS, Knyphausen P, Lammers M, Noegel AA, Rastetter RH. Novel Coronin7 interactions with Cdc42 and N-WASP regulate actin organization and Golgi morphology. Sci Rep. 2016. May 4;6:25411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egorov M, Polishchuk R. Identification of CDC42 Effectors Operating in FGD1-Dependent Trafficking at the Golgi. Front Cell Dev Biol. 2019. February 4;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campellone KG, Webb NJ, Znameroski EA, Welch MD. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell. 2008. July 11;134(1):148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondylis V, van Nispen tot Pannerden HE, Herpers B, Friggi-Grelin F, Rabouille C. The golgi comprises a paired stack that is separated at G2 by modulation of the actin cytoskeleton through Abi and Scar/WAVE. Dev Cell. 2007. June;12(6):901–15. [DOI] [PubMed] [Google Scholar]
- 28.Colón-Franco JM, Gomez TS, Billadeau DD. Dynamic remodeling of the actin cytoskeleton by FMNL1γ is required for structural maintenance of the Golgi complex. J Cell Sci. 2011. September 15;124(Pt 18):3118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei JH, Seemann J. Unraveling the Golgi ribbon. Traffic. 2010. November;11(11):1391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouasti S, Matarrese P, Paddon R, Khosravi-Far R, Sorice M, Tinari A, Malorni W, Degli Esposti M. Death receptor ligation triggers membrane scrambling between Golgi and mitochondria. Cell Death Differ. 2007. March;14(3):453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chia J, Goh G, Racine V, Ng S, Kumar P, Bard F. RNAi screening reveals a large signaling network controlling the Golgi apparatus in human cells. Mol Syst Biol. 2012;8:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makhoul C, Gosavi P, Gleeson PA. The Golgi architecture and cell sensing. Biochem Soc Trans. 2018. October 19;46(5):1063–1072. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Mei M, Li Q, Roboti P, Pang Q, Ying Z, Gao F, Lowe M, Bao S. Loss of the golgin GM130 causes Golgi disruption, Purkinje neuron loss, and ataxia in mice. Proc Natl Acad Sci U S A. 2017. January 10;114(2):346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dippold HC, Ng MM, Farber-Katz SE, Lee SK, Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu S, Fuchs GJ, Meerloo T, Farquhar MG, Zhou H, Field SJ. GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009. October 16;139(2):337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.How PC, Shields D. Tethering function of the caspase cleavage fragment of Golgi protein p115 promotes apoptosis via a p53-dependent pathway. J Biol Chem. 2011. March 11;286(10):8565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei JH, Seemann J. Golgi ribbon disassembly during mitosis, differentiation and disease progression. Curr Opin Cell Biol. 2017. August;47:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott KL, Kabbarah O, Liang MC, Ivanova E, Anagnostou V, Wu J, Dhakal S, Wu M, Chen S, Feinberg T, Huang J, Saci A, Widlund HR, Fisher DE, Xiao Y, Rimm DL, Protopopov A, Wong KK, Chin L. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009. June 25;459(7250):1085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang D, Zhang X, Huang S, Yuan H, Li J, Wang Y. Mena-GRASP65 interaction couples actin polymerization to Golgi ribbon linking. Mol Biol Cell. 2016. January 1;27(1):137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egea G, Lázaro-Diéguez F, Vilella M. Actin dynamics at the Golgi complex in mammalian cells. Curr Opin Cell Biol. 2006. April;18(2):168–78. [DOI] [PubMed] [Google Scholar]
- 40.Colón-Franco JM, Gomez TS, Billadeau DD. Dynamic remodeling of the actin cytoskeleton by FMNL1γ is required for structural maintenance of the Golgi complex. J Cell Sci. 2011. September 15;124(Pt 18):3118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heylmann DR. del F, Kindler T, Kaina B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014. August;1846(1):121–9. doi: 10.1016/j.bbcan.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Bexiga MG, Simpson JC. Human diseases associated with form and function of the Golgi complex. Int J Mol Sci. 2013. September 10;14(9):18670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buschman MD, Rahajeng J, Field SJ. GOLPH3 links the Golgi, DNA damage, and cancer. Cancer Res. 2015. February 15;75(4):624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menotti M, Ambrogio C, Cheong TC, Pighi C, Mota I, Cassel SH, Compagno M, Wang Q, Dall’Olio R, Minero VG, Poggio T, Sharma GG, Patrucco E, Mastini C, Choudhari R, Pich A, Zamo A, Piva R, Giliani S, Mologni L, Collings CK, Kadoch C, Gambacorti-Passerini C, Notarangelo LD, Anton IM, Voena C, Chiarle R. Wiskott-Aldrich syndrome protein (WASP) is a tumor suppressor in T cell lymphoma. Nat Med. 2019. January;25(1):130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimi A, Kamachi Y, Imai K, Watanabe N, Nakadate H, Kanazawa T, Ozono S, Kobayashi R, Yoshida M, Kobayashi C, Hama A, Muramatsu H, Sasahara Y, Jakob M, Morio T, Ehl S, Manabe A, Niemeyer C, Kojima S. Wiskott-Aldrich syndrome presenting with a clinical picture mimicking juvenile myelomonocytic leukaemia. Pediatr Blood Cancer. 2013. May;60(5):836–41. [DOI] [PubMed] [Google Scholar]
- 46.Stieglitz E, Taylor-Weiner AN, Chang TY, Gelston LC, Wang YD, Mazor T, Esquivel E, Yu A, Seepo S, Olsen S, Rosenberg M, Archambeault SL, Abusin G, Beckman K, Brown PA, Briones M, Carcamo B, Cooper T, Dahl GV, Emanuel PD, Fluchel MN, Goyal RK, Hayashi RJ, Hitzler J, Hugge C, Liu YL, Messinger YH, Mahoney DH Jr, Monteleone P, Nemecek ER, Roehrs PA, Schore RJ, Stine KC, Takemoto CM, Toretsky JA, Costello JF, Olshen AB, Stewart C, Li Y, Ma J, Gerbing RB, Alonzo TA, Getz G, Gruber T, Golub T, Stegmaier K, Loh ML. The genomic landscape of juvenile myelomonocytic leukemia. Nat Genet. 2015. November;47(11):1326–1333. doi: 10.1038/ng.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keszei M, Kritikou JS, Sandfort D, He M, Oliveira MMS, Wurzer H, Kuiper RV, Westerberg LS. Wiskott-Aldrich syndrome gene mutations modulate cancer susceptibility in the p53± murine model. Oncoimmunology. 2018. July 30;7(9):e1468954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H, Yang F, Dong P, Liao S, Liu WR, Zhao G, Qin B, Dou Z, Liu Z, Liu W, Zang J, Lippincott-Schwartz J, Liu X, Yao X. NDP52 tunes cortical actin interaction with astral microtubules for accurate spindle orientation. Cell Res. 2019. August;29(8):666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vece TJ, Watkin LB, Nicholas S, Canter D, Braun MC, Guillerman RP, Eldin KW, Bertolet G, McKinley S, de Guzman M, Forbes L, Chinn I, Orange JS. Copa Syndrome: a Novel Autosomal Dominant Immune Dysregulatory Disease. J Clin Immunol. 2016. May;36(4):377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.