Abstract
A 70-year-old woman was referred to our hospital due to symptoms of dry eyes, dry mouth, and epigastric pain. Computed tomography showed distal pancreatic swelling, liver edge dullness and surface irregularities. Serum anti-nuclear antibody titers, immunoglobulin G and IgG4 levels were elevated. Autoimmune pancreatitis (AIP) was diagnosed based on endoscopic findings and a histopathological examination. Her AIP improved after starting prednisolone treatment. A liver biopsy revealed interface hepatitis with lymphoplasmacyte and IgG4-positive plasma cell infiltration. In addition, non-alcoholic steatohepatitis (NASH) was diagnosed based on the presence of parenchymal steatosis, ballooning hepatocytes, and pericellular fibrosis. We experienced a unique liver disease case showing IgG4-related liver disease overlapping with NASH.
Keywords: autoimmune hepatitis, autoimmune pancreatitis, IgG4-related disease, non-alcoholic steatohepatitis, overlap
Introduction
Autoimmune pancreatitis (AIP) is a systemic inflammatory disease characterized by the infiltration of immunoglobulin (Ig)G4-positive plasma cells (1, 2), and it can be complicated by other synchronous or metachronous forms of IgG4-related disease (IgG4-RD) in the hepatobiliary region (3-5). Recently, cases of IgG4-RD involving inflammation, mainly in the liver parenchyma and portal region, as well as IgG4-positive plasma cell infiltration have been reported, and IgG4-related autoimmune hepatitis (IgG4-AIH) is representative of these conditions. However, the number of reported cases of IgG4-AIH is small, so this entity is still being investigated.
In the present case, IgG4-AIH was suspected because the patient had a revised International Autoimmune Hepatitis Group score of 16 points and high serum IgG4 levels, and IgG4-positive plasma cell infiltration was detected in the portal region. Furthermore, based on the patient's histological findings, it was considered that non-alcoholic steatohepatitis (NASH) had caused liver cirrhosis.
No cases in which IgG4-RD overlapped with NASH-related liver cirrhosis have been reported. We herein report an interesting case and include a consideration of the literature.
Case Report
In February 2016, a 70-year-old woman became aware that she had dry eyes and a dry mouth. In addition, she had been suffering from epigastric pain since May 2015. She visited a nearby clinic and underwent an examination to investigate the cause of her abdominal symptoms. Gastrointestinal endoscopy showed esophageal varices (Li, F1, Cb, RC-) but no abnormal findings in the gastroduodenal mucosa. Abdominal computed tomography (CT) revealed swelling of the distal pancreas, liver edge dullness, and liver surface irregularities. Therefore, the patient was referred to our gastrointestinal medicine outpatient facility for a further examination and treatment.
She had a history of hypertension, arrhythmia, and spinal canal stenosis, which had been treated with telmisartan, nifedipine CR, verapamil, and pregabalin. She was not consuming any dietary supplements. She had no history of blood transfusions, no remarkable family history, and no history of autoimmune disease and did not consume significant amounts of alcohol or smoke.
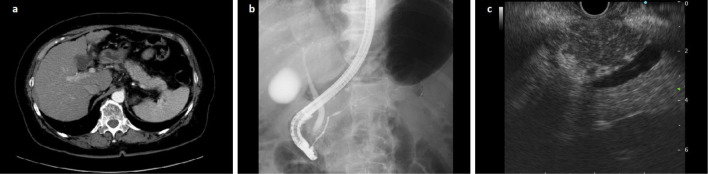
Upon admission, a physical examination showed spontaneous epigastric pain and obesity (body mass index: 31.2). Swelling of the salivary and lacrimal glands was not seen. Regarding ophthalmology, corneal disorders were not seen, but the Schirmer test demonstrated that the patient's tear volume was reduced [3 mm (severe dry eye: <5 mm) wetting of the paper after 5 minutes]. The results of her laboratory tests are shown in Table 1. Her total white blood cell count was within the normal limits, although eosinophilia was detected. Her platelet count was 12.1×104/μL (reference range: 13-36×104), and her glycated hemoglobin (HbA1c) level was 6.4% (reference range: 4.6-6.2%). Her prothrombin time and aspartate aminotransferase, alanine aminotransferase, total bilirubin, and pancreatic amylase levels were within the normal limits. A test for the hepatitis B virus core antibody produced a positive result; however, tests for hepatitis B virus DNA and hepatitis B virus core-related antigen both produced negative results. No hepatitis C virus RNA was detected in the patient's serum by polymerase chain reaction. The patient's anti-nuclear antibody (ANA) titer was 1:80, and the homogeneous and speckled ANA patterns were seen. The patient's rheumatoid factor level was elevated to 39 U/mL (reference range: ≤15). Tests for the anti-liver/kidney microsome type 1 antibody and anti-mitochondrial M2 antibody produced negative results. The patient's serum IgG level was elevated to 1,837 mg/dL (reference range: 870-1,700 mg/dL), and her serum IgG4 level was 558 mg/dL (reference range: 4.5-117 mg/dL). The patient's type IV collagen・7s domain level was mildly elevated to 6.2 ng/mL (reference range: ≤6 ng/mL), and her hyaluronic acid level was 69.2 ng/mL (reference range: ≤50 ng/mL). The human leukocyte antigen (HLA) test detected DR8 and DR15. Contrast-enhanced CT (Fig. 1a) showed findings that were indicative of liver cirrhosis; i.e. the margins of the liver were blunted, the liver surface was irregular, and diffuse distal pancreatic swelling and a capsule-like hepatic rim were seen. Endoscopic retrograde cholangiopancreatography (Fig. 1b) showed diffuse irregular narrowing of the distal main pancreatic duct. Endoscopic ultrasound (EUS) (Fig. 1c) showed that parts of the parenchyma of the distal pancreas were hypoechoic and lobulated, whereas other parts were swollen and exhibited a hyperechoic patchy/mesh-like appearance. An EUS-fine-needle aspiration biopsy specimen showed marked lymphoplasmacytic cell and IgG4-positive plasma cell infiltration [≥10 cells per high-power field (HPF)] in the pancreas (Fig. 2a, b). No marked storiform fibrosis or obliterative phlebitis was observed.
Table 1.
Laboratory Data on Admission.
| Peripheral blood | Blood chemistry | Blood coagulation factors | ||||||
| WBC | 6,010 | /μL | TP | 6.8 | g/dL | PT | 90.0 | % |
| Neutro | 49.8 | % | Alb | 3.6 | g/dL | APTT | 35.5 | sec |
| Eos | 17.3 | % | T-Bil | 1.0 | mg/dL | Fib | 266 | mg/dL |
| Baso | 0.8 | % | AST | 29 | U/L | FDP | 1.8 | µg/mL |
| Mono | 5.5 | % | ALT | 27 | U/L | |||
| Lymph | 26.6 | % | LDH | 169 | U/L | Serological tests | ||
| RBC | 410×104 | /μL | ALP | 284 | U/L | IgA | 331 | mg/dL |
| Hb | 12.1 | g/dL | γ-GTP | 43 | U/L | IgM | 104 | mg/dL |
| Plt | 12.1×104 | /μL | Amyl | 75 | U/L | IgG | 1,837 | mg/dL |
| P-Amyl | 29 | U/L | IgG4 | 558 | mg/dL | |||
| Virus markers | T-Chol | 152 | mg/dL | RF | 39 | U/mL | ||
| HBs-Ag | (−) | TG | 81 | mg/dL | ANA | ×80 | (homo, speckled) | |
| HBs-Ab | (−) | UN | 9.6 | mg/dL | Anti-SSA antibody | (−) | ||
| HBe-Ag | (−) | Cr | 0.60 | mg/dL | Anti-SSB antibody | (−) | ||
| HBe-Ab | (−) | Na | 141 | mEq/L | AMA-M2 | (−) | ||
| HBc-Ab | (+) | K | 4.2 | mEq/L | Anti-LKM1 antibody | (−) | ||
| HBV-DNA | (−) | Glu | 95 | mg/dL | Hyaluronic acid | 69.2 | ng/mL | |
| HBcrAg | ≤2.9 | HbA1c (N) | 6.4 | % | Type IV collagen·7S | 6.2 | ng/mL | |
| HCV-RNA | (−) | CRP | 0.04 | mg/dL | HLA | DR8, DR15 | ||
γ-GTP: γ -glutamyltransferase, Alb: albumin, ALP: alkaline phosphatase, ALT: alanine aminotransferase, AMA-M2: anti-mitochondrial M2 antibody, Amyl: amylase, ANA: antinuclear antibody, Anti-LKM1: anti-liver kidney microsome 1 antibody, Anti-SSA: anti-Sjögren’s syndrome-related antigen A, Anti-SSB: anti-Sjögren’s syndrome-related antigen B, APTT: activated partial thromboplastin time, AST: aspartate aminotransferase, Baso: basophils, Cr: creatinine, CRP: C-reactive protein, Eos: eosinophils, FDP: fibrin/fibrinogen degradation products, Fib: fibrinogen, Glu: glucose, Hb: hemoglobin, HBA1c (N): glycated hemoglobin, HBc-Ab: hepatitis B core antibody, HBcrAg: hepatitis B virus core-related antigen, HBe-Ab: hepatitis B envelope antibody, HBe-Ag: hepatitis B envelope antigen, HBs-Ab: hepatitis B surface antibody, HBs-Ag: hepatitis B surface antigen, HBV-DNA: hepatitis B virus DNA, HCV-RNA: hepatitis C virus RNA, HLA: human leukocyte antigen, IgA: immunoglobulin A, IgG: immunoglobulin G, IgG4: immunoglobulin G4, IgM: immunoglobulin M, K: potassium, Lymph: lymphocytes, LDH: lactate dehydrogenase, Mono: monocytes, Na: sodium, Neutro: neutrophils, P-Amyl: pancreatic amylase, Plt: platelet count, PT: prothrombin time, RBC: red blood cell count, RF: rheumatoid factor, T-Bil: total bilirubin, T-Chol: total cholesterol, TG: triglycerides, TP: total protein, UN: urea nitrogen, WBC: white blood cell count
Figure 1.
Imaging findings obtained using contrast-enhanced computed tomography (CECT), endoscopic retrograde cholangiopancreatography (ERCP), and endoscopic ultrasound (EUS). (a) CECT showed findings that were indicative of liver cirrhosis, e.g. the margins of the liver were blunted, the liver surface was irregular, and diffuse distal pancreatic swelling and a capsule-like rim were seen. (b) ERCP showed diffuse irregular narrowing of the distal main pancreatic duct and no abnormalities in the extrahepatic bile ducts. (c) EUS showed that some parts of the parenchyma of the distal pancreas were hypoechoic and lobulated, whereas other parts were swollen and exhibited a hyperechoic patchy/mesh-like appearance.
Figure 2.
Histopathological findings of the pancreas (a, b) and liver biopsies (c-h). (a, b) Marked lymphoplasmacytic cell infiltration and marked immunoglobulin G4 (IgG4) -positive plasma cell infiltration was seen in the pancreatic parenchyma. (a) (IgG staining, magnification: ×400), (b) (IgG4 staining, magnification: ×400). (c, d) Severe interface hepatitis combined with lymphoplasmacytic cell infiltration. (c) [Hematoxylin and Eosin (H&E) staining, magnification: ×100], (d) Lymphoplasmacytic cell (arrows) (H&E staining, magnification: ×400). (e) Detection of IgG4-positive plasma cells. Forty IgG4-positive plasma cells/high-power field were detected in the portal region (IgG4 staining, magnification: ×400). (f) Mild liver parenchymal inflammation and steatosis (H&E staining, magnification: ×100). (g) Ballooning hepatocytes (arrows) (H&E staining, magnification: ×400). (h) Pericellular fibrosis and bridging fibrosis (Masson’s trichrome staining, magnification: ×200)
A liver biopsy revealed severe interface hepatitis together with lymphoplasmacytic cell infiltration (Fig. 2c, d), 40 IgG4-positive plasma cells/HPF in the portal region (Fig. 2e). Furthermore, mild liver parenchymal inflammation and steatosis (covering about 10% of the liver) (Fig. 2f), ballooning hepatocytes (Fig. 2g), pericellular fibrosis, and bridging fibrosis (Fig. 2h) were seen, whereas no intrahepatic destruction; concentric periductal fibrosis (“onion skin”), which is suggestive of primary sclerosing cholangitis (PSC); or storiform fibrosis or obliterative phlebitis, which are characteristics of IgG4-RD, was noted. No multinucleated giant cells were observed.
A lip biopsy revealed slight fibrosis and lymphocytic infiltration (containing approximately 50 lymphocytes per 4 mm2) focally around the small salivary gland. It was suspected that the patient might have been complicated with Sjögren's syndrome.
The patient's clinical and pancreatic histological findings met the 2011 clinical diagnostic criteria for AIP (segmental AIP). AIH was considered pathologically based on the existence of interface hepatitis combined with lymphoplasmacytic cell infiltration, and the patient's revised International Autoimmune Hepatitis Group score was 16 points (AIH, definite). IgG4 immunohistochemical staining revealed 40 IgG4-positive plasma cells/HPF in the portal region. In addition, NASH was diagnosed based on the detection of parenchymal steatosis, ballooning hepatocytes, and pericellular fibrosis. Therefore, we considered that the patient was suffering from AIP combined with IgG4-RD overlapping NASH.
Steroid therapy involving a daily dose of 40 mg of prednisolone for AIP was started. The patient's serum levels of IgG and IgG4 immediately decreased and soon approached the normal limits. In addition, an imaging study indicated that the distal pancreatic swelling had improved. The prednisolone treatment was eventually discontinued after being tapered, and the patient's condition has not recurred.
Discussion
IgG4-RD is a systemic disease, while AIP is the most common of its organ-specific manifestations (pancreatic disease is seen in 60% of patients with IgG4-RD) (2). Furthermore, AIP is often complicated with hepatobiliary conditions, including PSC, inflammatory pseudotumors, and chronic active hepatitis (3-5). IgG4-related sclerosing cholangitis (IgG4-SC) is a representative IgG4-RD and most frequently occurs in combination with AIP. In the current case, no marked liver dysfunction was seen in blood tests. However, a histological examination of a liver biopsy sample was carried out, due to the possibility of AIH (i.e., the patient was ANA-positive and exhibited elevated serum IgG levels). The liver biopsy specimen showed interface hepatitis combined with lymphoplasmacytic cell infiltration, and the patient was confirmed to have AIH based on her revised International Autoimmune Hepatitis Group score (16 points; a score of ≥16 points indicates AIH, definite). Although the marked inflammatory cell infiltration and hepatocyte collapse seen in the portal region and liver parenchyma were suggestive of AIH, AIP often exhibits a portal inflammation pattern or lobular hepatitis characterized by parenchymal inflammation and focal necrosis (6).
Recently, cases of IgG4-RD without storiform fibrosis and obliterative phlebitis, which are characteristics of IgG4-RD, have been reported, and IgG4-AIH and IgG4 hepatopathy have been suggested to be representative of these conditions (4-12). Umemura et al. (5) were the first to propose the existence of IgG4-AIH, and they subsequently reported two further cases of the condition (5, 7, 8). Ishizu et al. (9) reported a case of IgG4-AIH involving metachronous type 1 AIP, and Suto et al. (11) and Araki et al. (12) each reported one case of IgG4-AIH without other synchronous or metachronous forms of IgG4-RD. Therefore, to our knowledge, six cases of IgG4-AIH, including our own, have been reported in Japan.
Among these cases, the male-to-female ratio was 2:4, and the patients' median age was 59.5 years old (range: 42-73 years old). HLA-DR4, which exhibits a strong correlation with AIH, was found in one case. Synchronous or metachronous IgG4-RD complications were encountered in three cases (two cases involved type 1 AIP, and one case involved IgG4-SC). Prednisolone was administered as a treatment in all of these cases (Table 2).
Table 2.
List and Clinical Features of Reported IgG4-AIH Cases.
| No. | References | Age | Sex | ANA | Serum IgG | Serum IgG4 | IgG4 plasma cells/HPF or IgG4/IgG cell ratio | Revised International Autoimmune Hepatitis Group score | HLA-DR | Synchronous or metachronous IgG4-RD | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (5) | 54 | F | ×80 | 2,403 | 557 | ≥30 IgG4+ plasma cells/HPF | 18 (definite) | DRB1(*1302 and *1501) | IgG4-SC | 40 mg PSL/day |
| 2 | (7) | 42 | M | ×20,480 | 5,622 | 642 | ≥30 IgG4+ plasma cells/HPF | definite | DRB1(*0405) | (no disease) | 60 mg PSL/day |
| 3 | (9) | 73 | M | ×2,560 | 2,850 | 284 | 30-40 IgG4+ plasma cells/HPF | 18 (definite) | DRB1(*1302 and *1502) | type 1 AIP | 40 mg PSL/day |
| 4 | (11) | 48 | F | ×40 | 1,538 | 669 | 25.8 IgG4+ plasma cells/HPF | 21 (definite) | DR1, DR15 | (no disease) | 30 mg PSL/day + 600 mg UDCA |
| 5 | (12) | 65 | F | ×10,240 | 3,262 | 535 | IgG4/IgG cell ratio: 85% | 20 (definite) | DR8, DR15 | (no disease) | 50 mg PSL/day |
| 6 | This case | 70 | F | ×80 | 1,837 | 558 | 40 IgG4+ plasma cells/HPF | 16 (definite) | DR8, DR15 | type 1 AIP | 40 mg PSL/day |
AIH: autoimmune hepatitis, AIP: autoimmune pancreatitis, ANA: anti-nuclear antibody, F: female, HLA: human leukocyte antigen, HPF: high-power field, IgG: immunoglobulin G, IgG4: immunoglobulin G4, IgG4-RD: immunoglobulin G4-related disease, M: male, PSL: prednisolone, SC: sclerosing cholangitis, UDCA: ursodeoxycholic acid
Umemura et al. (8) proposed the diagnostic criteria for IgG4-related AIH as follows: 1) having definite AIH according to the International Autoimmune Hepatitis Group (IAIHG) scoring system, 2) serum IgG4 concentration ≥135 mg/dL, and 3) immunostaining of IgG4 showing ≥10 IgG4-positive plasma cells/HPF in the portal tract. Nakanuma et al. (10) recommended the following criteria: 1) serum IgG4 concentration ≥135 mg/dL, 2) immunostaining of IgG4 showing ≥10 IgG4-positive plasma cells/HPF in liver tissue, 3) chronic hepatitis with zonal and bridging necrosis or broad collapse, and 4) other metachronous or synchronous IgG4-related disease(s). Although Chung et al. (13) and Aydemir et al. (14) also reported cases of IgG4-AIH, they did not use a high serum IgG4 concentration, one of the major diagnostic items for IgG4-RD, as a diagnostic criterion; therefore, the conditions they reported might have been different from the IgG4-AIH described by Umemura et al. and Nakanuma et al. The current case met the diagnostic criteria of Umemura et al. and would have been classified as possible IgG4-associated AIH according to the diagnostic criteria of Nakanuma et al.
Umemura et al. also reported that IgG4 hepatopathy causes liver damage in 60-70% of AIP cases (6). In the present case, a hepatopathological examination showed as many as 40 IgG4-positive plasma cells/HPF in the portal region and an IgG4-/IgG-positive cell ratio of >40%; however, the possibility of IgG4 hepatopathy should be considered because zonal and bridging necrosis, which are characteristic findings of the IgG4-AIH proposed by Nakanuma et al., were not seen. Furthermore, liver cirrhosis due to NASH [grade 1, stage 4 according to the classification of Brunt et al. (15)] was suspected because mild liver parenchymal inflammation, steatosis, ballooning hepatocytes, Mallory bodies, pericellular fibrosis, and advanced fibrosis (pseudo-lobule formation and bridging fibrosis) were noted. It was reported that about half of NASH patients are ANA-positive (16, 17), and Yatsuji et al. (18) reported that 97% of Japanese female non-alcoholic fatty liver disease (NAFLD) patients exhibited revised International Autoimmune Hepatitis Group scores of ≥10 points (indicating AIH, probable or definite). It is difficult to distinguish NASH from AIH, as the revised International Autoimmune Hepatitis Group scoring system does not include criteria for excluding NASH. Furthermore, a few cases of NAFLD that overlapped with AIH have been reported (19-22). Clinicians should keep overlapping NASH and AIH in mind in cases involving ANA positivity, an AIH score of ≥10 points, and/or difficultly in pathological differentiation. In cases in which active hepatitis is caused by NASH, the administration of prednisolone might exacerbate the liver damage; therefore, histopathological examinations of liver biopsy samples are important for determining the optimal treatment strategy.
The current case involved an obese patient, and liver cirrhosis due to NASH was suspected because of pericellular fibrosis, disseminated ballooning hepatocytes, and the presence of very few central veins, which suggest previous lobule-centered inflammation. However, the degree of liver inflammation was severe based on the presence of IgG4-positive plasma cell infiltration. Therefore, we considered that the observed inflammation was due to IgG4 hepatopathy or IgG4-AIH overlapping with AIP, on a background of NASH cirrhosis. We continued to treat the patient with prednisolone for her AIP. Long-term and high-dose glucocorticoid therapy carry a risk of exacerbating NASH; however, no liver damage suggesting the exacerbation of NASH occurred during the prednisolone treatment.
Although there have been several reports of NASH complicated by autoimmune diseases (23-25), there have not been any reported cases of NASH complicated by IgG4-related disease, autoimmune pancreatitis, or IgG4-related liver disease. The pathogenesis of NASH-induced autoimmune diseases has not been elucidated in detail and will be the topic of future discussion. However, the involvement of IL-17 is assumed as an element common to both NASH and autoimmune diseases. In recent years, it has become known that IL-17 produced by Th17 cells, a kind of Th cell, induces autoimmune diseases such as psoriasis, rheumatoid arthritis, Crohn's disease, and many others (26). In particular, Th17 cells are frequently found in the intestinal lamina propria, and it has recently been clarified that the induction of Th17 cells involves the presence of enterobacteria, such as segment bacteria. Primarily, Th17 cells enhance the gastrointestinal mucosal barrier function by producing IL-22, which is an antimicrobial peptide inducer, in addition to IL-17, which promotes the migration of leukocytes. However, it has been reported that the overactivation of Th17 cells is closely related to the pathogenesis of autoimmune diseases. In addition, the involvement of Th17 cells and IL-17 has been reported in several animal models of NAFLD progressing to NASH (27-31). Tedesco et al. (32) reported the development of liver fibrosis and inflammation requires hepatic activation of γδ TCR+ cells and production of IL-17 mediated by exposure to Lactobacillus gasseri that is translocated to the liver due to increased intestinal permeability in Mdr2−/− mice. It is thought that dysbiosis, which is one of the pathogeneses of NASH, causes the proliferation and activation of Th17 cells and induces the overproduction of IL-17, thereby leading to autoimmune diseases.
This case was difficult to distinguish from AIH because of the existence of NASH in the background. This is the first reported case of NASH overlapping with IgG4-RD, and it will be necessary to accumulate and consider further cases in the future.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Dr. Koshi Matsumoto from Ebina General Hospital for helpful discussions and comments on the manuscript.
References
- 1. Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344: 732-738, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 94: e680, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zen Y, Harada K, Sasaki M, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol 28: 1193-1203, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Zen Y, Fujii T, Sato Y, et al. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol 20: 884-894, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Umemura T, Zen Y, Hamano H, et al. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut 56: 1471-1472, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Umemura T, Zen Y, Hamano H, et al. Immunoglobulin G4-hepatopathy: association of immunoglobulin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology 46: 463-471, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Umemura T, Zen Y, Nakanuma Y, et al. Another cause of autoimmune hepatitis. Hepatology 52: 389-390, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Umemura T, Zen Y, Hamano H, et al. Clinical significance of immunoglobulin G4-associated autoimmune hepatitis. J Gastroenterol 46 (Suppl 1): 48-55, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Ishizu Y, Ishigami M, Kuzuya T, et al. Immunoglobulin G4-associated autoimmune hepatitis later complicated by autoimmune pancreatitis: a case report. Hepatol Res 46: 601-606, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Nakanuma Y, Ishizu Y, Zen Y, et al. Histopathology of IgG4-related autoimmune hepatitis and IgG4- related hepatopathy in IgG4-related disease. Semin Liver Dis 36: 229-241, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Suto D, Murata K, Otake T, et al. A case of IgG4-related autoimmune hepatitis incidentally found by liver injury. Kanzo 59: 277-283, 2018. [Google Scholar]
- 12. Araki T, Arinaga-Hino T, Koga H, et al. Marked accumulation of fluorodeoxyglucose and inflammatory cells expressing glucose transporter-3 in immunoglobulin G4-related autoimmune hepatitis. Hepatol Res 48: 937-944, 2018. [DOI] [PubMed] [Google Scholar]
- 13. Chung H, Watanabe T, Kudo M, et al. Identification and characterization of IgG4-associated autoimmune hepatitis. Liver Int 30: 222-231, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Aydemir Y, Akcoren Z, Demir H, et al. Clinical and histopathological features of immunoglobulin G4-associated autoimmune hepatitis in children. J Gastroenterol Hepatol 34: 742-746, 2019. [DOI] [PubMed] [Google Scholar]
- 15. Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467-2474, 1999. [DOI] [PubMed] [Google Scholar]
- 16. Niwa H, Sasaki M, Haratake J, et al. Clinicopathological significance of antinuclear antibodies in non-alcoholic steatohepatitis. Hepatol Res 37: 923-931, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Tsuneyama K, Baba H, Kikuchi K, et al. Autoimmune features in metabolic liver disease: a single-center experience and review of the literature. Clin Rev Allergy Immunol 45: 143-148, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Yatsuji S, Hashimoto E, Kaneda H, et al. Diagnosing autoimmune hepatitis in nonalcoholic fatty liver disease: is the International Autoimmune Hepatitis Group scoring system useful? J Gastroenterol 40: 1130-1138, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Adams LA, Lindor KD, Angulo P. The prevalence of autoantibodies and autoimmune hepatitis in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 99: 1316-1320, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Loria P, Lonardo A, Leonardi F, et al. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Dig Dis Sci 48: 2173-2181, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Fukuda S, Komori A, Itoh M, et al. Histological remission during corticosteroid therapy of overlapping nonalcoholic steatohepatitis and autoimmune hepatitis: case report and literature review. Case Rep Gastroenterol 5: 553-557, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komura T, Ohta H, Seike T, et al. The efficacy of corticosteroid therapy in a patient with non-alcoholic steatohepatitis overlapping autoimmune hepatitis. Intern Med 57: 807-812, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kashiwagi Y, Hatsushika T, Tsutsumi N, et al. Gastrointestinal and liver lesions in primary childhood Sjögren syndrome. Clin Rheumatol 36: 1433-1435, 2017. [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Wang N, Chen Y, et al. The association of non-alcoholic fatty liver disease with thyroid peroxidase and thyroglobulin antibody: a new insight from SPECT-China study. Autoimmunity 51: 238-244, 2018. [DOI] [PubMed] [Google Scholar]
- 25. Ogdie A, Grewal SK, Noe MH, et al. Risk of incident liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis: a population-based study. J Invest Dermatol 138: 760-767, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tabarkiewicz J, Pogoda K, Karczmarczyk A, et al. The role of IL-17 and Th17 lymphocytes in autoimmune diseases. Arch Immunol Ther Exp (Warsz) 63: 435-449, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng F, Wang K, Aoyama T, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143: 765-766.e1-3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y, She W, Wang F, et al. 3, 3'-Diindolylmethane alleviates steatosis and the progression of NASH partly through shifting the imbalance of Treg/Th17 cells to Treg dominance. Int Immunopharmacol 23: 489-498, 2014. [DOI] [PubMed] [Google Scholar]
- 29. Rolla S, Alchera E, Imarisio C, et al. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci (Lond) 130: 193-203, 2016. [DOI] [PubMed] [Google Scholar]
- 30. Giles DA, Moreno-Fernandez ME, Stankiewicz TE, et al. Regulation of inflammation by IL-17A and IL-17F modulates non-alcoholic fatty liver disease pathogenesis. PLoS One 11: e0149783, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang Y, Bian Z, Zhao L, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol 166: 281-290, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tedesco D, Thapa M, Chin CY, et al. Alterations in intestinal microbiota lead to production of interleukin 17 by intrahepatic gd T-cell receptor-positive cells and pathogenesis of cholestatic liver disease. Gastroenterology 154: 2178-2193, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]