Abstract
This study was conducted to investigate the hypotensive effect of egg white protein (EWP) hydrolysate (EWH) in spontaneously hypertensive rats (SHRs). The hydrolysis of EWP was effectively performed with a combination of 0.5% bromelain and 1% papain at 50°C for 60 min. The resulting hydrolysate did not elicit an allergic reaction as confirmed by human mast cell activation test. The systolic and diastolic blood pressures of the SHRs fed the EWH diet were observed to be significantly or numerically lower than those of the other groups during the experimental period of 28 d. EWH treatment significantly (p<0.05) upregulated the nitric oxide levels in hCMEC/D3 cells and the plasma of the SHRs compared to those in the control. Moreover, EWH ingestion significantly (p<0.01) reduced the plasma angiotensin II level of the SHRs compared with that in the control. In conclusion, beyond its basic nutritional value, EWH prevents and manages hypertension, and thus can be an invaluable resource for functional food development.
Keywords: egg white protein hydrolysate, spontaneously hypertensive rats, hypotensive effect, nitric oxide, angiotensin II
Introduction
Hypertension is defined as blood pressure (BP) chronically higher than 140/90 mmHg (Chockalingam, 2008). It has been identified as a risk factor for cardiovascular diseases (CVDs) and related complications, including atherosclerosis and stroke (Danaei et al., 2013). The underlying mechanisms of hypertension involve numerous pathways and factors, including the renin-angiotensin system (RAS), oxidative stress, inflammation, and impaired nitric oxide (NO) generation (Jahandideh et al., 2016). In RAS, angiotensin-converting enzyme (ACE) converts angiotensin (Ang) I into Ang II, which increases BP. ACE inhibitors block this conversion and cause the blood vessels to relax, decreasing the blood volume, whereby they exert a BP-lowering effect and improve endothelial function (Wu et al., 2017).
Another mechanism affecting BP involves endothelial nitric oxide synthase (eNOS) (Heiss et al., 2015). This enzyme is vital for the maintenance of endothelial homeostasis. It generates NO, which stimulates guanylate cyclase to form cyclic guanosine monophosphate. This cyclic nucleotide causes proliferation of vascular smooth muscle cells and prevents platelet adhesion and inflammation (Behrendt and Ganz, 2002). A substantial amount of evidence indicates that NO has a modulatory effect on BP, and impaired NO bioavailability is correlated with endothelial dysfunction, promoting atherosclerosis in both hypertension and CVDs (Endemann and Schiffrin, 2004; Hermann et al., 2006). Furthermore, NO has been demonstrated to downregulate plasma Ang II level in spontaneously hypertensive rats (SHRs), thereby inhibiting Ang II-dependent vasoconstriction (Rajapakse et al., 2016).
Development of functional foods using food-derived proteins and peptides has become a major research interest for the use of natural resources in the prevention and management of hypertension due to the side-effects of the commonly used hypotensive pharmaceuticals (Khanna et al., 2008; Viera, 2012). The ACE-inhibitory potentials of various food-derived peptides have been extensively determined (FitzGerald et al., 2004; Gobbetti et al., 2004; Miguel et al., 2005). Moreover, it has been appreciated that food-derived peptides can be therapeutically used for the modulation of NO levels to reduce BP (Aluko, 2015).
Egg white is an inexpensive and rich source of high-quality proteins. Egg white protein (EWP) hydrolysate (EWH) obtained with various proteases has been found to exhibit several bioactivities, such as ACE inhibition, vasodilation, and antioxidant activity, whereby it suppress CVDs (Davalos at al., 2004; Grootaert et al., 2017; Jahandideh et al., 2016; Miguel et al., 2007). Therefore, this study aimed to prepare the EWH with hypoallergenic property using food-grade proteases and to investigate the hypotensive effect of EWH in SHRs for the development of functional foods as BP modulators.
Materials and Methods
Sample preparation
Egg white powder was purchased from SANOVO technology group (Odense, Denmark). To prepare the EWH (EggNOpepTM), a mix of bromelain (0.5%, w/v) and papain (1%, w/v) was added into the egg white solution (10%, w/v), and the resulting mixture was incubated at 50°C for 60 min. Subsequently, the mixture was heated at 95°C for 10 min to inactivate the enzymes and then lyophilized. The degree of hydrolysis of the EWH was confirmed by SDS-PAGE according to the method of Laemmli (1970).
Measurement of allergenic properties
The allergenic properties of EWP and EWH were investigated using the method described by Jung et al. (2017). A human mast cell line (HMC-1 cells) was grown in Dulbecco's modified Eagle's medium (Merck, Darmstadt, Germany) containing 10% fetal bovine serum (Cellgro, USA) and 1% penicillin (Gibco, USA) at 37°C in a humidified atmosphere with 5% CO2. The cells were cultured in a 24-well plate (105 cells/well) overnight, and then incubated for an additional 24 h with 10 μg/mL of compound 48/80 (Merck) as a positive control, 100 μg/mL of EWP or 100 μg/mL of EWH. Subsequently, the tumor necrosis factor-α (TNF-α) and histamine levels in the culture supernatants were quantified using the human TNF-α ELISA Ready-SET-Go (eBioscience, San Diego, CA, USA) and histamine ELISA kits (IBL International, Hamburg, Germany), respectively as per the manufacturers' instructions.
Measurement of NO levels
The blood-brain barrier hCMEC/D3 cell line (MD Millipore, MA, USA) was cultured with the EndoGRO LS complete culture media kit (Merck) as recommended by the manufacturer. The cells were incubated in a 24-well plate at 37°C in a humidified atmosphere with 5% CO2 and then treated with EWP or EWH at 1% for 24 h. Subsequently, the NO levels in the culture supernatants were quantitated using the total NO and nitrate/nitrite parameter assay kit (R&D Systems, Minneapolis, MN, USA). The plasma NO levels of SHRs were determined with the same kit. Briefly, 100 μL of sample was reacted with 100 μL of the reaction diluent, and Griess reagents I and II following the kit protocol. Absorbance was measured at 540 nm using a microplate reader (Emax; Molecular Devices, San Jose, CA, USA).
Animal study
This study was approved by the Animal Ethics Committee of Sahmyook University (SYU-IACUC-2018-008). Male SHRs that were 8 weeks old and weighed 264±21 g, were obtained from Charles River Laboratories Inc. (Japan). The SHRs were housed in a room maintained at 22±2°C with a 12 h light/dark cycle. All the SHRs had free access to water, and food was provided ad libitum in powdered form. After acclimatization for 1 wk, the SHRs were randomly divided into three groups (n=5), which were fed for 28 d either the basal diet (AIN-93A) or one of the two test diets containing either EWP or EWH at 1% (w/w).
Post-mortem procedures
At the end of the experiment, the SHRs were sacrificed by asphyxiation using CO2 gas. The blood was collected by cardiac puncture, and a portion was immediately transferred into a BD vacutainer blood collection tube containing EDTA (Plymouth, UK). The blood plasma was isolated by centrifugation at 10,000×g at 4°C for 20 min and then stored at –80°C until further analysis.
Measurement of BP
BP was measured every third day during the experimental period (at 0, 3, 6, 9, 12, 15, 18, 21, 24, and 27 d) by non-invasive tail-cuff plethysmography. The systolic BP (SBP) and diastolic BP (DBP) were measured using the BP analysis system (NIBP-2000, Visitech Systems, Apex, NC, USA). All the measurements were recorded by the same person in a quiet room to reduce stress-induced variations. The final values were calculated from ten successive measurements.
Analysis of plasma Ang II levels
The plasma Ang II levels of the SHRs fed the test diets were measured with the Ang II ELISA kit (LifeSpan BioSciences, Seattle, WA, USA) according to the manufacturer's guidelines.
Statistical analysis
Results are expressed as means±SEM. Data were analyzed using ANOVA and X2 test using the SAS software (GLM procedures, version 9.1, SAS Institute, Cary, NC, USA). Means were compared using Duncan's multiple range test. The two-tailed p-value less than 0.05 was considered significant.
Results and Discussion
Preparation and allergenic properties of EWH
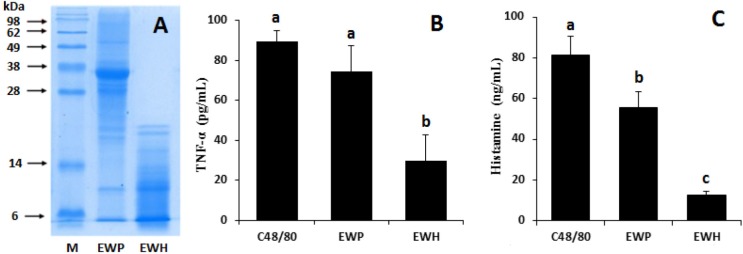
EWP was effectively hydrolyzed by incubation with the combination of 0.5% bromelain and 1% papain at 50°C for 60 min, as assessed by the reduced levels of the major protein bands observed in SDS-PAGE (Fig. 1A). The HMC-1 cells were incubated with EWH or EWP to investigate whether these samples elicited an allergic reaction. Toward this end, the TNF-α and histamine levels in the culture supernatant were measured. These compounds are known to be released by mast cells as an inflammatory response to allergens. The ovalbumin in EWP is reported to result in allergic response by inducing TNF-α and histamine release (Chen et al., 2019).
Fig. 1. SDS-PAGE analysis of EWP and EWH (A), and measurement of TNF-α (pg/mL) and histamine (ng/mL) levels in the culture supernatant of human mast cells (HMC-1) treated with EWP or EWH (B, C).
Values are expressed as mean±SEM (n=5). a,b Means with superscripts without a common letter differ, p<0.01. M, protein molecular weight marker; C48/80, compound 48/80; EWP, egg white protein; EWH, egg white protein hydrolysate.
Treatment of compound 48/80 (positive control) stimulated HMC-1 cells to release both compounds, while EWH significantly (p<0.01) suppressed this inflammatory response compared to the control. Moreover, the TNF-α and histamine levels were significantly (p<0.01) lower in the culture treated with EWH than in EWP-treated culture (Fig. 1B and 1C).
Although EWP is considered one of the common food allergens, EWP-derived peptides have been documented to have antioxidant and ACE inhibitory bioactivities (Duan et al., 2014; Jahandideh et al., 2014). Our results showed that the enzymatic hydrolysis of EWP effectively reduced allergic response. The complex peptide population produced by the hydrolysis of EWP may have contributed to the hypotensive effect observed in this study.
Measurement of BP in SHRs
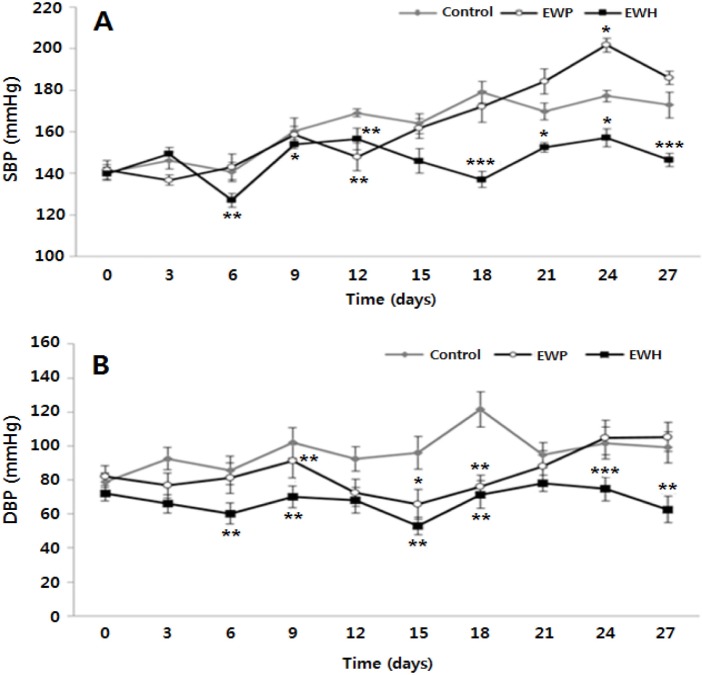
The SHR strain is a suitable rodent model for hypertension. These rats show impaired vascular function and higher levels of Ang II, oxidative stress, and inflammation compared to wild type rats (Ikarashi et al., 2018). The SBP and DBP baselines in all the experimental groups were approximately 140 mmHg and 80 mmHg, respectively, indicating the presence of hypertension, and these values are similar to those in humans with essential hypertension (Okamoto and Aoki, 1963).
To evaluate whether EWH exerted a hypotensive effect, SBPs and DBPs of the SHRs fed the test diets were measured every 3 d for 4 wk. The SBP of EWH group was significantly lower than those of the other groups after 6 d of the treatment except for 15 d (Fig. 2A). At the end of the study, the SBP of EWH group was similar (146±3.27 mmHg) to the baseline value at the beginning, whereas the SBPs of the control and EWP groups were 172.9±6.22 mmHg and 186.0±3.26 mmHg, respectively. Moreover, the DBP of EWH group was maintained at a constant rate of 62.9±7.95 mmHg during the experiment period and was significantly lower than the DBPs of the other groups except for 12 and 21 d (Fig. 2B).
Fig. 2. Comparison of the blood pressure of the spontaneously hypertensive rats fed the test diets for 28 d.
Values are expressed as mean±SEM (n=5). (A) Systolic blood pressure, (B) diastolic blood pressure. * p<0.05, ** p<0.01, *** p<0.001 vs. the control group. Control, untreated; EWP, egg white protein; EWH, egg white protein hydrolysate.
A number of studies have investigated complementary approaches that are based on dietary resources to attenuate hypertension. Especially, the enzymatic hydrolysates of various food proteins, such as those of rice bran, egg white, and milk have been proven to be rich sources of bioactive peptides that exert beneficial effects on hypertension, lipid profile, inflammation, and oxidative stress in vitro and in vivo (Boonla et al., 2015; Davalos et al., 2004; Ganguly et al., 2019; Manso et al., 2008; Miguel et al., 2005; Phelan and Kerins, 2011).
Measurement of NO and Ang II levels
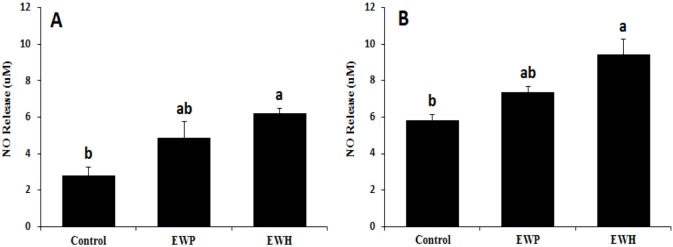
The blood-brain barrier hCMEC/D3 cells were incubated with EWH or EWP. The NO level in the culture supernatant increased more than 2-fold upon EWH treatment as compared to that in the control group (Fig. 3A). Similarly, the NO levels in the blood of the SHRs fed the EWH diet were significantly (p<0.05) higher than in the control group (Fig. 3B).
Fig. 3. Nitric oxide levels in the culture supernatant of hCMEC/D3 cells (A) and blood plasma of the spontaneously hypertensive rats fed the test diets for 28 d (B).
Values are expressed as mean±SEM (n=5). a,b Means with superscripts without a common letter differ, p<0.05. Control, untreated; EWP, egg white protein; EWH, egg white protein hydrolysate.
In general, BP is modulated through various mechanisms, such as adjustment of ACE activity, vascular function, and oxidative status. Upregulation of the NO level is another mechanism underlying vascular relaxation. It has been suggested that NO in the brain regulates BP by affecting sympathetic nerve activity (Rajapakse et al., 2016). NO in specific regions of the central nervous system has been shown to play a significant role in cardiovascular regulation. Among these regions, the rostral ventrolateral medulla is responsible for basal and reflex-action control of sympathetic nerve activity and has been shown to be related to CVDs, such as hypertension (Kishi et al., 2001). Our results suggest that EWH could contribute to these vasodilatory mechanisms.
Additionally, the plasma Ang II levels were assessed in the SHRs fed the test diets. As shown in Fig. 4, orally administered EWH significantly (p<0.01) reduced the plasma Ang II level compared with that in the control group. However, the effect of EWH did not significantly differ than that of EWP.
Fig. 4. Angiotensin II levels in the plasma of the spontaneously hypertensive rats fed the test diets for 28 d.
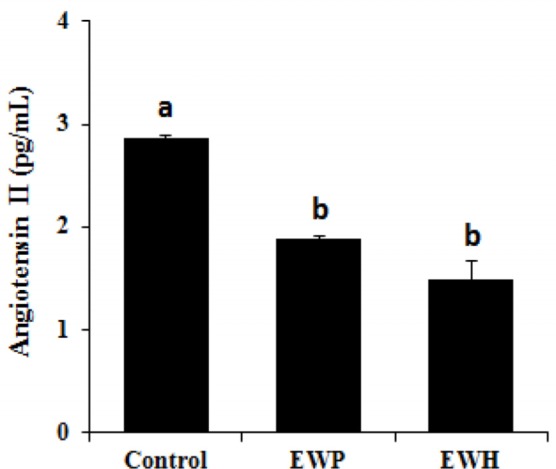
Values are expressed as mean±SEM (n=5). a,b Means with superscripts without a common letter differ, p<0.01. Control, untreated; EWP, egg white protein; EWH, egg white protein hydrolysate.
We also observed that EWH had an inhibitory effect on ACE in vitro (data not shown), and thus it is possible that EWH downregulates circulating Ang II level in SHRs. ACE inhibitors are essentially used as a therapeutic approach in the regulation of high BP. Downregulation of Ang II suppresses the causative mechanisms of high BP through the RAS pathways (Bader and Ganten, 2008; Hermann et al., 2006). Aluko (2015) has reported that enzymatic hydrolysis of inactive food proteins releases bioactive peptides that enhance the eNOS pathway, resulting in upregulation of NO within vascular walls, and interrupt the interaction between Ang II and its receptors.
In conclusion, bioactive peptides obtained by hydrolysis of food-proteins such as EWP can prevent and manage hypertension beyond their basic nutritional values. The results of this study confirm the hypotensive and hypoallergenic properties of EWH, which can be a valuable resource for functional food development. However, further research is required to identify the specific peptides associated with the modulation of BP.
Acknowledgments
This paper was supported by the Fund of the Sahmyook University in 2017.
Conflict of Interest
The authors declare no potential conflict of interest.
Author Contributions
Conceptualization: Han KS, Yun SS. Data curation: Lee DE. Formal analysis: Jung TH. Methodology: Lee DE, Jo YN. Investigation: Lee DE, Jung TH, Jo YN. Writing – original draft: Lee DE, Han KS. Writing – review & editing: Lee DE, Jung TH, Jo YN, Yun SS, Han KS.
Ethics Approval
This study was approved by the Animal Ethics Committee of Sahmyook University (SYU-IACUC-2018-008).
References
- Aluko RE. Antihypertensive peptides from food proteins. Annu Rev Food Sci Technol. 2015;6:235–262. doi: 10.1146/annurev-food-022814-015520. [DOI] [PubMed] [Google Scholar]
- Bader M, Ganten D. Update on tissue renin-angiotensin systems. J Mol Med. 2008;86:615–621. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90:40L–48L. doi: 10.1016/S0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- Boonla O, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Pannangpetch P, Thawornchinsombut S. Peptides-derived from thai rice bran improves endothelial function in 2K-1C renovascular hypertensive rats. Nutrients. 2015;7:5783–5799. doi: 10.3390/nu7075252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Chen G, Shu S, Xu Y, Ma X. Metabolomics analysis of baicalin on ovalbumin-sensitized allergic rhinitis rats. R Soc Open Sci. 2019;6:181081. doi: 10.1098/rsos.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockalingam A. World hypertension day and global awareness. Can J Cardiol. 2008;24:441–444. doi: 10.1016/S0828-282X(08)70617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Singh GM, Paciorek CJ, Lin JK, Cowan MJ, Finucane MM, Farzadfar F, Stevens GA, Riley LM, Lu Y, Rao M, Ezzati M. The global cardiovascular risk transition: Associations of four metabolic risk factors with national income, urbanization, and western diet in 1980 and 2008. Circulation. 2013;127:1493–1502. doi: 10.1161/CIRCULATIONAHA.113.001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos A, Miguel M, Bartolome B, Lopez-Fandino R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J Food Prot. 2004;67:1939–1944. doi: 10.4315/0362-028X-67.9.1939. [DOI] [PubMed] [Google Scholar]
- Duan X, Wu F, Li M, Yang N, Wu C, Jin Y, Yang Y, Jin Z, Xu X. Naturally occurring angiotensin I-converting enzyme inhibitory peptide from a fertilized egg and its inhibitory mechanism. J Agric Food Chem. 2014;62:5500–5506. doi: 10.1021/jf501368a. [DOI] [PubMed] [Google Scholar]
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- FitzGerald RJ, Murray BA, Walsh DJ. Hypotensive peptides from milk proteins. J Nutr. 2004;134:980S–988S. doi: 10.1093/jn/134.4.980S. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Sharma K, Majumder K. Food-derived bioactive peptides and their role in ameliorating hypertension and associated cardiovascular diseases. Adv Food Nutr Res. 2019;89:165–207. doi: 10.1016/bs.afnr.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Gobbetti M, Minervini F, Rizzello CG. Angiotensin I-converting-enzyme-inhibitory and antimicrobial bioactive peptides. Int J Dairy Technol. 2004;57:173–188. doi: 10.1111/j.1471-0307.2004.00139.x. [DOI] [Google Scholar]
- Grootaert C, Matthijs B, Voorspoels S, Possemiers S, Smagghe G, Van Camp J. Egg-derived bioactive peptides with ACE-inhibitory properties: A literature update. Food Funct. 2017;8:3847–3855. doi: 10.1039/C7FO00839B. [DOI] [PubMed] [Google Scholar]
- Heiss C, Rodriguez-Mateos A, Kelm M. Central role of eNOS in the maintenance of endothelial homeostasis. Antioxid Redox Signal. 2015;22:1230–1242. doi: 10.1089/ars.2014.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens. 2006;8:17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikarashi N, Toda T, Hatakeyama Y, Kusunoki Y, Kon R, Mizukami N, Kaneko M, Ogawa S, Sugiyama K. Anti-hypertensive effects of acacia polyphenol in spontaneously hypertensive rats. Int J Mol Sci. 2018;19:700. doi: 10.3390/ijms19030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahandideh F, Chakrabarti S, Majumder K, Li Q, Panahi S, Morton JS, Davidge ST, Wu J. Egg white protein hydrolysate reduces blood pressure, improves vascular relaxation and modifies aortic angiotensin II receptors expression in spontaneously hypertensive rats. J Funct Foods. 2016;27:667–673. doi: 10.1016/j.jff.2016.10.019. [DOI] [Google Scholar]
- Jahandideh F, Majumder K, Chakrabarti S, Morton JS, Panahi S, Kaufman S, Davidge ST, Wu J. Beneficial effects of simulated gastro-intestinal digests of fried egg and its fractions on blood pressure, plasma lipids and oxidative stress in spontaneously hypertensive rats. PLOS ONE. 2014;9:e115006. doi: 10.1371/journal.pone.0115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TH, Hwang HJ, Yun SS, Lee WJ, Kim JW, Ahn JY, Jeon WM, Han KS. Hypoallergenic and physicochemical properties of the A2 β-casein fraction of goat milk. Korean J Food Sci Anim Resour. 2017;37:940–947. doi: 10.5851/kosfa.2017.37.6.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Lefkowitz L, White WB. Evaluation of recent fixed-dose combination therapies in the management of hypertension. Curr Opin Nephrol Hypertens. 2008;17:477–483. doi: 10.1097/MNH.0b013e3283069d72. [DOI] [PubMed] [Google Scholar]
- Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901. doi: 10.1161/hyp.38.4.896. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manso MA, Miguel M, Even J, Hernandex R, Aleixandre A, Lopez-Fandino R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem. 2008;109:361–367. doi: 10.1016/j.foodchem.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Miguel M, Alvarez Y, Lopez-Fandino R, Alonso MJ, Salaices M. Vasodilator effects of peptides derived from egg white proteins. Regul Pept. 2007;140:131–135. doi: 10.1016/j.regpep.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Miguel M, Lopez-Fandino R, Ramos M, Aleixandre A. Short-term effect of egg-white hydrolysate products on the arterial blood pressure of hypertensive rats. Br J Nutr. 2005;94:731–737. doi: 10.1079/BJN20051570. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Phelan M, Kerins D. The potential role of milk-derived peptides in cardiovascular disease. Food Funct. 2011;2:153–167. doi: 10.1039/c1fo10017c. [DOI] [PubMed] [Google Scholar]
- Rajapakse NW, Head GA, Kaye DM. Say NO to obesity-related hypertension: Role of the l-Arginine-Nitric oxide pathway. Hypertension. 2016;67:813–819. doi: 10.1161/HYPERTENSIONAHA.116.06778. [DOI] [PubMed] [Google Scholar]
- Viera AJ. Resistant hypertension. J Am Board Fam Med. 2012;25:487–495. doi: 10.3122/jabfm.2012.04.110275. [DOI] [PubMed] [Google Scholar]
- Wu J, Liao W, Udenigwe CC. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci Technol. 2017;69:214–219. doi: 10.1016/j.tifs.2017.07.011. [DOI] [Google Scholar]