Abstract
Background
Previously we have shown that volatile anesthetic isoflurane attenuated neutrophil recruitment and phagocytosis in mouse sepsis and skin inflammation models. The objectives of this study were to test ex vivo function of neutrophils in patients who underwent cardiac catheterization under volatile anesthesia versus intravenous anesthesia (IA), and also to assess the effect of anesthesia on surgical site infections (SSIs) using mouse model to understand the clinical relevance of anesthesia-induced immunomodulation.
Methods
Whole blood from patients who underwent cardiac catheterization procedures either by volatile anesthesia or IA was collected and subjected to phagocytosis assay and a lipopolysaccharide-induced tumor necrosis factor-α assay. Mouse SSI with Staphylococcus aureus USA300 was created, and the effect of isoflurane and propofol exposure (short or long exposure) on bacterial loads was tested.
Results
Neutrophil phagocytosis was significantly attenuated after the induction of volatile anesthesia in patients, but not by IA. Monocyte phagocytosis was not affected by the anesthesia regimen. Bacterial loads following SSIs were significantly higher in mice receiving long, but not short, isoflurane exposure. Propofol exposure did not affect bacterial loads.
Discussion
Neutrophil phagocytosis can be affected by the type of anesthesia, and preclinical model of SSIs showed potential clinical relevance. The effects of anesthesia regimen on SSIs in patients needs to be studied extensively in the future.
Keywords: Neutrophil, Phagocytosis, Volatile anesthetics, Intravenous anesthetics, Surgical site infections
Introduction
Phagocytes are cells that ingest harmful particles and bacteria to protect the host and include an array of innate immune cells such as neutrophils, monocytes, macrophages, and dendritic cells. These professional phagocytes express surface receptors that assist clearance of a wide range of microbial pathogens and their products, including toll-like receptors (TLRs), Fc receptors, and C3b receptors.1,2 Phagocytes are also important sources of pro- and anti-inflammatory cytokines, which help to regulate the host immune response. Thus, phagocytes are considered to be the front-line defense cells.3 Because perioperative infections, such as surgical site infections (SSIs) result in significant morbidity, mortality, and financial burdens,4–7 it is of great interest to understand the factors that potentially affect the behavior of these professional phagocytes in the perioperative setting, thereby mitigating the risk of infection.
The effect of anesthetics on the immune system was described more than a century ago when Graham reported that ether anesthesia significantly inhibited leukocyte phagocytosis of streptococci.8 This observational study did not lead to subsequent investigations by others for a long time, but recently there is a renewed interest in understanding the effect of anesthetics on immune cells.9–11 Ether is no longer used in clinical practice, but its derivatives isoflurane and sevoflurane are the main volatile anesthetics (VAs) in clinical use. We previously showed that isoflurane exposure attenuated the recruitment and phagocytic capacity of neutrophils in mouse experimental abdominal sepsis and skin inflammation models.12,13 Neutrophils are initial responders in surgical procedures during which anesthesia is provided, and their adequate function is critical to control SSIs.14 However, studies that directly examine the effect of different anesthetics on phagocytes, including neutrophils, in surgical patients are limited. Therefore, the objective of this study was to test if VAs would affect phagocyte function in patients. Because anesthesia for younger pediatric patients are often induced and maintained by VAs, we studied ex vivo phagocyte function in these patients under VA-based anesthesia. We also included an intravenous anesthesia (IA) group as a comparator. An additional study objective was to assess the clinical relevance of anesthesia-induced immunomodulation by using a clinically relevant mouse SSI model.
Materials and methods
Study design and sample collection
In this study, we compared the effect of volatile anesthesia and IA on neutrophil function in patients who underwent routine cardiac catheterization procedures between November 2014 and January 2017. The study was approved by the Institutional Review Board at Boston Children’s Hospital, and written informed consent was obtained from all patients. The study was registered in ClinicalTrials.gov () and carried out in accordance with Declaration of Helsinki. We included patients greater than or equal to 1 y of age for the study because the maturation of phagocytes may take up to 1 y of age.15,16 We excluded patients with noncardiac comorbidities such as known underlying hematological disorders, known oncological disorders, or cyanotic heart disease. In addition, we excluded patients who did not require preoperative laboratory testing or preoperative intravenous catheter insertion.
From electronic medical records, we obtained the age, weight, primary diagnosis, procedure, comorbidities, list of regular medications, preoperative complete blood count with differential (if available), American Society of Anesthesiologist physical status, and medications administered intraoperatively. The function of phagocytes for each patient was assessed at two different time points. The initial time point was at the preoperative blood draw or for some patients at the preoperative intravenous line insertion. The secondary time point was 1 h after the induction of anesthesia. As parameters of phagocyte function, we measured phagocytosis and lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production. In addition, the activation level of phagocytes at the time of blood collection was probed by testing CD18 and m24 expression levels. The method for each assay is described in the following. For the VA group, patients underwent mask induction with sevoflurane. Once induced, anesthesia was maintained with sevoflurane (end-tidal concentration of 1.0%–2.5%) or isoflurane (end-tidal concentration of 0.3%–1.1%). For the IA group, an intravenous catheter was placed preoperatively. Anesthesia was induced and maintained with propofol infusion. One hour after anesthesia, blood was collected in heparin-containing tubes. At the time of intraoperative blood collection, none of patients had received catheter intervention by cardiologists yet. Assays were initiated within 30 min of sample collection.
Phagocytosis assay
Phagocytosis assay was performed using Phagotest (Glycotope Biotechnology; Berlin, Germany) per the company protocol with minor modification. Phagotest is a widely used phagocytosis assay for clinical samples.17–19 Briefly, 50 μL of heparinized whole blood was incubated with 2.5 μL of opsonized and fluorescein isothiocyanate (FITC)-labelled Escherichia coli (E coli) at 37°C for 5 min. Negative controls were kept on ice. After quenching, samples were centrifuged and washed. After blood erythrocytes were lysed with FACS lysis solution (BD Biosciences; Billerica, MA), samples were subjected to flow cytometry analysis. Granulocytes and monocyte population were gated by forward and side scatter analysis. Phagocytosis percentage was defined as [the number of FITC positive granulocytes (or monocytes)/the number of granulocytes (or monocytes)] × 100 (%).
Ex vivo TNF-α production assay
The capacity of the subjects’ phagocytes to respond to a challenge was also tested by using an ex vivo TNF-α production assay as previously described.20 Monocytes are the primary producers of TNF-α. Briefly, 50 μL of whole blood collected in a heparinized tube was incubated with 500 pg/mL of LPS (O111:B4, purified by phenol extraction; SigmaeAldrich, St. Louis, MO) and incubated for 4 h at 37°C. After centrifugation, the plasma was subjected to TNF-α measurements using a human TNF-α ELISA kit (R&D systems; Minneapolis, MN) per the company protocol.
CD18 and m24 expression on neutrophils
After Fc blocking, whole blood was incubated with anti-human CD18-phycoerythrin antibody (Biolegend; San Diego, CA) and anti-human m24-FITC antibody (kindly provided by Professor Nancy Hogg, London Research Institute). After blood erythrocytes were lysed with FACS lysis solution, samples were subjected to flow cytometry analysis. Granulocytes and monocyte populations were gated from forward and side scatter analysis.
Mice
Wild-type mice on the C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions with 12-h light and dark cycles. Male mice at 8–10 wk of age were used for the experiments.
Mouse USA300 wound infection model and bacterial loads
All the experimental procedures complied with institutional and ARRIVE guidelines regarding the use of animals in research,21 and were approved by our institution’s animal care and use committee. The deep wound SSI model was previously reported.22,23 Briefly, mice were anesthetized with ketamine/xylazine anesthesia. The hair over the thigh muscle was shaved and disinfected. Then a 1-cm incision was made with a scalpel directly into the thigh muscle to the depth of the femur. One silk suture was placed in the muscle. S aureus (USA300, 103 CFU/3 mL, USA300 was kindly provided by Dr Jean C. Lee, Brigham and Women’s Hospital) was added into the incision under the suture. Once the incision and the skin were closed, mice were either exposed to isoflurane (0.8%) or propofol (10 mg/kg/h) for 2 or 6 h in randomized fashion. Postoperative pain was treated with subcutaneous buprenorphine. The mice were euthanized 72 h after the S aureus inoculation, and bacterial loads in the wounds were assessed by plating tissue homogenates on blood agar plates for quantitative culture.
Statistical analysis
Statistical analysis was performed with PRISM5 software (La Jolla, CA) using the Mann–Whitney test or the Kruskal–Wallis test. Data were shown as median and interquartile range, and P < 0.05 was considered statistically significant.
Results
Demographics of patients and preoperative phagocyte counts
Demographics of patients enrolled in the study are shown in Table. Patients in the VA group were younger than those in the IA group. Because inhalational induction with VA is typically offered to younger children to mitigate fear for preoperative intravenous catheter insertion, this result was in line with our expectation. Preoperative neutrophil, granulocyte, and monocyte counts were not significantly different between the VA and IA groups (Supplemental Fig. 1A–C).
Table –
The characteristics of patients.
| VA | IA | |
|---|---|---|
| Patient number | 25 (male 16, female 9) | 15 (male 8, female 7) |
| Age (y) | 7.0 (3.5, 8.5) | 13.0 (11.00, 20.00) |
| Weight (kg) | 24.5 (15.6, 31.5) | 48.3 (40.0, 58.0) |
Age and weight are shown as median (25th, 75th interquartile range).
The VA group showed reduced phagocytosis by granulocytes, but not by monocytes
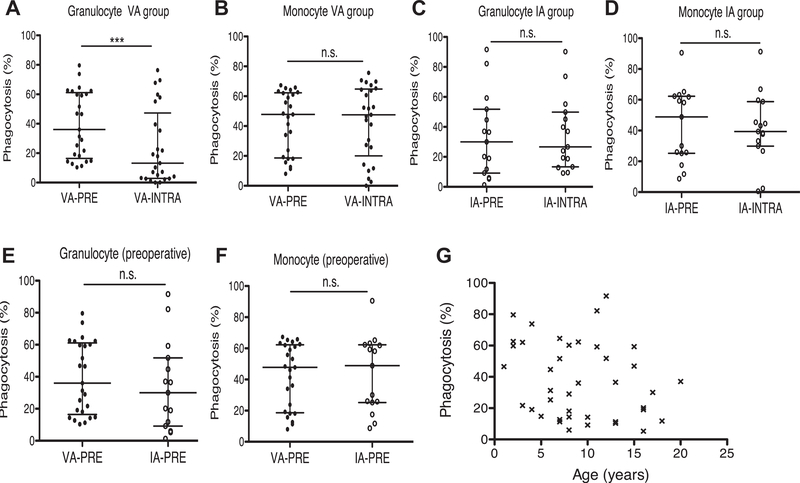
We compared preoperative and intraoperative phagocytosis by granulocytes and monocytes. VA exposure was associated with significant reductions in phagocytosis by granulocytes (Fig. 1A), but not by monocytes (Fig. 1B). We observed no significant differences in phagocytosis by granulocytes or monocytes with IA exposure (Fig. 1C and D). We compared the preoperative granulocyte and monocyte phagocytosis between VA and IA group and did not see any difference between the two groups (Fig. 1E and F). This is consistent with our finding that there was no correlation between age and phagocytosis in study patient population (Fig. 1G).
Fig. 1 –
Granulocyte and monocyte phagocytosis in VA and IA groups. Granulocyte and monocyte phagocytosis was compared in VA and IA groups. (A) Preoperative and intraoperative granulocyte phagocytosis in VA group. (B) Preoperative and intraoperative monocyte phagocytosis in VA group. (C) Preoperative and intraoperative granulocyte phagocytosis in IA group. (D) Preoperative and intraoperative monocyte phagocytosis in IA group. (E) Preoperative granulocyte phagocytosis between VA and IA groups. (F) Preoperative monocyte phagocytosis of VA and IA groups. (G) Relationship between age and granulocyte phagocytosis. Data were shown as median ± interquartile range. Statistical analysis was performed using Wilcoxon matched-pairs signed rank test (A-D), Mann–Whitney test (E, F) and regression analysis (G). *** denotes P < 0.001. IA-PRE = preoperative IA group; IA-INTRA = intraoperative IA group; n.s. = not significant; VA-PRE = preoperative VA group; VA-INTRA = intraoperative VA group.
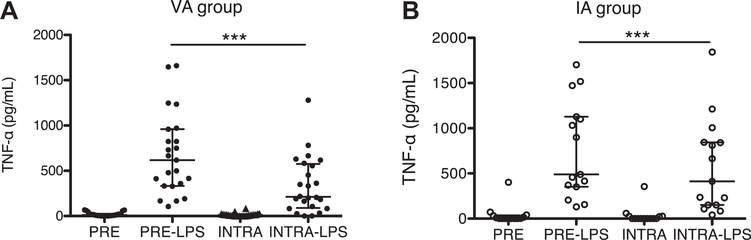
TNF-α response to LPS in patient blood before and after anesthesia
We used activation of the TLR pathway by LPS as a readout of phagocyte responsiveness. TNF-α is a major proinflammatory mediator and is predominately produced by monocytes after LPS challenge.20 LPS-mediated TNF-α production was significantly attenuated in the both the VA group (Fig. 2A) and the IA group (Fig. 2B) after anesthesia.
Fig. 2 –
TNF-α production in whole blood stimulated by LPS. Whole blood was stimulated with LPS for 4 h at 37°C, then plasma TNF-α was measured in VA group (A) or IA group (B). Data were shown as median ± interquartile range. Statistical analysis was performed using Kruskal–Wallis test with Dunn’s post hoc analysis. *** denotes P < 0.001. INTRA = intraoperative blood without LPS stimulation; INTRA + LPS = intraoperative blood stimulated by LPS; PRE = preoperative blood without LPS stimulation; PRE + LPS = preoperative blood stimulated with LPS.
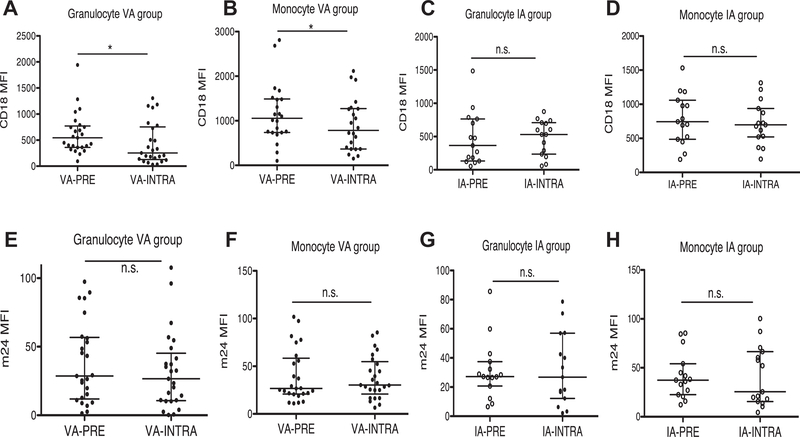
Preoperative and intraoperative CD18 and m24 expression levels
Phagocytes express a number of cell adhesion molecules on their cell surface. CD18 pairs with one of four different CD11 molecules (CD11a, CD11b, CD11c, or CD11d) to form leukocyte adhesion molecules called β2 integrins.24 The surface expression level of β2 integrins increases on neutrophil and monocyte activation.25,26 Furthermore, they change conformation on neutrophil activation to facilitate binding to their ligands. Antibody m24 recognizes an epitope exposed on CD18 on activation.27 CD18 expression was reduced on both granulocytes and monocytes after exposure to VA (Fig. 3A and B) but was unchanged in the IA group (Fig. 3C and D). There was no significant alteration in m24 expression in either the VA or IA groups after anesthesia (Fig. 3E and H).
Fig. 3 –
CD18 and m24 expression level on granulocytes and monocytes in VA and IA groups. CD18 and m24 expression levels were probed by PE labeled anti-CD18 antibody and FITC labeled anti-m24 antibody, respectively. (A) CD18 expression on granulocytes in VA group, (B) CD18 expression on monocytes in VA group, (C) CD18 expression on granulocytes in IA group, (D) CD18 expression on monocytes in IA group, (E) m24 expression on granulocytes in VA group, (F) m24 expression on monocytes in VA group, (G) m24 expression on granulocytes in IA group, and (H) m24 expression on monocytes in IA group. Data were shown as median ± interquartile range. Statistical analysis was performed using Wilcoxon matched-pairs signed rank test. * denotes P < 0.05. IA-PRE = preoperative IA group; IA-INTRA = intraoperative IA group; n.s. = not significant; PE = phycoerythrin; VA-PRE = preoperative VA group; VA-INTRA = intraoperative VA group.
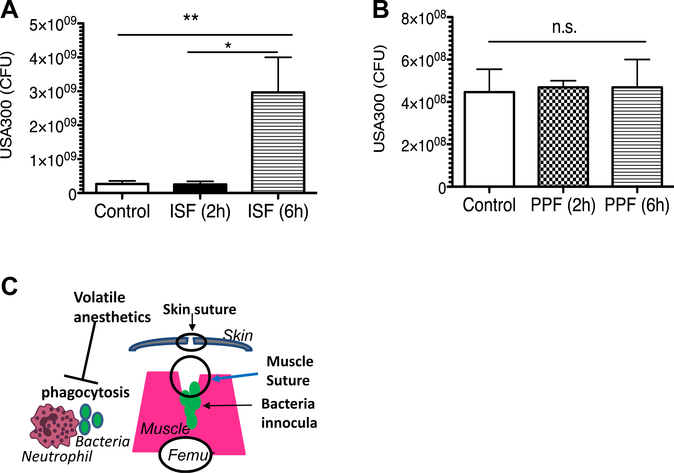
Isoflurane exposure was associated with higher bacterial loads in experimental wounds than propofol
SSIs are the most common nosocomial infection in the United States. S aureus is the most common microbial species involved in SSIs,28 and methicillin-resistant S aureus (MRSA) strains predominate in nosocomial infections.29,30 Our data indicate that mice exposed to isoflurane for 6 h had significantly higher MRSA USA300 bacterial loads in the wound tissue than mice given no isoflurane (control) or exposed to isoflurane for only 2 h (Fig. 4A). Mice given propofol for 2 or 6 h had bacterial loads in the tissues similar to those given no propofol (Fig. 4B).
Fig. 4 –
The effect of anesthetics on bacterial loads in surgical wound infection model. Surgical wounds were infected with MRSA USA300. A group of mice were exposed to isoflurane (0.8 %) for 2 or 6 h (A) or propofol (10 mg/kg/h) for 2 or 6 h (B). Bacterial loads of infected muscles were tested 3 d after surgical wound infection. Data represent median ± interquartile range of 8 mice. Statistical analysis was performed Kruskal–Wallis test with Dunns post hoc analysis. * and ** denote P < 0.05 and P < 0.01, respectively. ISF = isoflurane; n.s. = not significant; PPF = propofol. (C) Scheme of surgical wound infections and the effect of volatile anesthetics on bacteria phagocytosis by neutrophils. (Color version of figure is available online.)
Discussion/conclusions
In this study, we found that patients who received VA were associated with reduced phagocytosis by granulocytes, in line with previous animal experiments reported by our group and others.12,13,31 Interestingly, we did not see any difference in phagocytosis by monocytes. In addition, we did not observe any difference in phagocytosis in the IA group. The responsiveness of phagocytes in patient blood was probed by TNF-α production induced by LPS, and this response was attenuated in both the VA and IA groups.
Several investigators have examined the degree of phagocytosis using intraoperative samples. Heine et al.19 examined the effect of isoflurane and propofol general anesthesia on patients (median age 38 y old) who underwent elective super-selective embolization due to arteriovenous malformation. After 4 h of anesthesia, the mean granulocyte phagocytosis of E coli was 93.2% and 94.3% in the propofol and isoflurane arms, respectively. Procedural stress response could have been elicited by the 4-h time point, which could affect the baseline activation profile of neutrophils. In addition, the isoflurane arm was initially induced with propofol, suggesting that there was a crossover of anesthetic medication. In our study, the VA arm was induced and maintained without propofol, and blood was collected at 1 h after anesthesia induction, when we did not see any phenotypic difference in phagocytes probed by m24 expression. Kotani et al.32 evaluated the phagocytosis of alveolar macrophages obtained by bronchoalveolar lavage during orthopedic surgery under isoflurane or propofol anesthesia. In both groups, alveolar macrophage phagocytosis was attenuated in a time-dependent manner. Isoflurane anesthesia attenuated phagocytosis slightly more than propofol anesthesia. One of the interesting findings in our study was that the VA group showed lower phagocytosis by granulocytes during the intraoperative period, but not by monocytes. We probed the activation level of granulocytes and monocytes using an m24 antibody. We did not observe any differences between preoperative and intraoperative activation levels at the two time points. Both neutrophils (majority of granulocyte constituents) and monocytes are myeloid cells.33 Phagocytosis is largely divided into opsonic phagocytosis via Fc receptor family and complement receptors, nonopsonic phagocytosis via macrophage receptor with collagenous structure (MARCO) and nonspecific phagocytosis via TLRs.34 Although neutrophils and monocytes share various receptors and show functional similarity, there are some differences. While monocytes express FcγRI (CD64) constitutively, neutrophils express this only in response to inflammatory stimuli.1 Also monocytes express MARCO.35 Thus monocytes may have more phagocytosis machinery than neutrophils, which might have contributed to the difference between granulocytes and monocytes in our results in the VA group. Identifying how isoflurane attenuated phagocytosis of granulocytes would provide an insight to this answer.
Monocytes are categorized by their surface expression of CD14 and CD16 into classical (CD14+/CD16−), intermediate (CD14+/CD16+), and nonclassical (CD14dim/CD16+) monocytes.36–38 Classical monocytes are primarily phagocytic, consisting of ~90% of monocytes. Intermediate and nonclassical monocytes constitute 2%~3% and 7%~8% of monocytes, respectively, and they primarily produce cytokines. In our ex vivo study, VAs and propofol significantly attenuated TNF-α production in response to LPS. LPS stimulates TLR4 and activates nuclear factor-κB (NFκB) for TNF-α production. Sevoflurane inhibits NFκB activation.39 Similarly, isoflurane exposure attenuates TLR4 signaling.40 These results are in line with our findings. Propofol also attenuates TLR4-mediated NFκB signaling and suppresses TNF-α production by LPS-stimulated macrophages.41 These results are in line with the report that VAs and propofol have anti-inflammatory properties.42
Previously Koo et al.43 retrospectively compared the incidence of SSI after colorectal surgery anesthetized by VAs versus propofol in a propensity-matched groups. They found that the VA group had a higher incidence of SSIs. Also, the prospective study by von Dossow et al.44 showed that isoflurane-based anesthesia was associated with an increased incidence of postoperative infection over propofol-based anesthesia in a small number of alcoholic patients undergoing abdominal surgery. In this study, we did not evaluate the clinical outcome after cardiac catheterization. Because the heterogeneity of the patients’ underlying diseases and operative courses could affect infection in clinical studies, and the incidence of SSIs is ~3%,45 we would need a larger number of patients for assessment. Instead, we attested to the clinical relevance of anesthesia in a preclinical SSI infection model. We showed that the longer-exposure VA arm was associated with higher bacterial loads than the propofol group, suggesting that selection of anesthetics could affect the course of infection. Short exposures did not affect differences in bacterial loads. Although the half-life of VAs is very short, we expect that the neutrophil function would be hindered for the duration of exposure. Because S aureus can grow exponentially with a relative short doubling time, the duration for the longer exposure might have been long enough to make a difference in S aureus bacterial loads in the wounds by the end of exposure in comparison with control arm. In line with this notion, it has been reported that SSI can establish within a few hours.46 Approximately 400,000 SSIs occur each year with the aggregate annual cost of $3.5 to $10.1 billion.6 Thus our findings are potentially significant, and further analysis of the underlying mechanism is warranted.
One limitation of this study is that patients received additional medication other than VAs and propofol. Although we primarily tried to evaluate the effect of VAs on phagocytes, a bolus dose of fentanyl (1–2 mcg/kg) and also muscle relaxants were used in most cases. Fentanyl has not shown any effect on phagocytosis in vitro.47 The effect of muscle relaxants on phagocytosis has not been reported, and both the VA and propofol arms received these medications. Thus it is very unlikely that they would be responsible for phenotypic differences between the VA and IA groups. We have chosen patients who underwent cardiac catheterization procedures because these procedures do not pose a significant stress response, and it is easy to obtain blood samples from indwelling catheters during the procedure. Among cardiac patients, we chose acyanotic heart diseases because hypoxia can affect neutrophil phagocytosis.48 However, Parikh et al.49 reported that granulocyte phagocytosis was reduced in children with cyanotic and acyanotic congenital heart disease. And we do not know the impact of underlying illness requiring catheterization on innate immune function that we studied here. Thus, we may need to test nonheart disease patients in the future to further validate our findings in other populations. In addition, we tested only MRSA in mouse wound infection models. Thus, the effect of anesthetics on different microbes needs to be tested as well. Our mouse model does not accommodate surgical stress, which may also affect neutrophil function. With these limitations, our findings indicate that the selection of anesthetic drugs may pose impact on surgical outcomes, particularly after long duration of anesthesia. Certainly the benefit of VAs has also been described,50 and determination of clinical benefits need to be further examined.
In conclusion, we found that use of VAs was associated with reduction in granulocyte phagocytosis in patients undergoing cardiac catheterization, whereas propofol anesthesia did not. In vivo murine experiments showed that VA exposure worsened bacterial loads in wounds compared with propofol anesthesia, suggesting potential clinical significance of our findings in patients. Further analysis to elucidate the underlying mechanism is needed, as well as outcome assessment in patients in the future.
Supplementary Material
Acknowledgment
The authors thank Dr Jean C. Lee (Brigham and Women’s Hospital, Boston) for critical comments and advice.
Financial support: This work was in part supported by CHMC Anesthesia Foundation (K.Y.) and R01GM118277 (K.Y.)
Footnotes
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jss.2018.07.075.
R E F E R E N C E S
- 1.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch M Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995;5:85–87. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 4.Allegranzi B, Bischoff P, de Jonge S, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e276–e287. [DOI] [PubMed] [Google Scholar]
- 5.Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e288–e303. [DOI] [PubMed] [Google Scholar]
- 6.Shepard J, Ward W, Milstone A, et al. Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg. 2013;148:907–914. [DOI] [PubMed] [Google Scholar]
- 7.Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals. Public Health Rep. 2002;122:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham E The influence of ether and esther anesthesia on bacteriolysis, agglutination, and phagocytosis. J Infect Dis. 1911;8:147–175. [Google Scholar]
- 9.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–277. [DOI] [PubMed] [Google Scholar]
- 10.Stollings LM, Jia LJ, Tang P, Dou H, Lu B, Xu Y. Immune modulation by volatile anesthetics. Anesthesiology. 2016;125:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuki K, Eckenhoff RG. Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth Analg. 2016;123:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbo C, Yuki K, Demers M, Wagner DD, Shimaoka M. Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model. J Anesth. 2013;27:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutsogiannaki S, Schaefers MM, Okuno T, et al. From the cover: prolonged exposure to volatile anesthetic isoflurane worsens the outcome of polymicrobial abdominal sepsis. Toxicol Sci. 2017;156:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano H, Kinoshita M, Fujino K, et al. Insulin treatment directly restores neutrophil phagocytosis and bactericidal activity in diabetic mice and thereby improves surgical site Staphylococcus aureus infection. Infect Immun. 2012;80:4409–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storm SW, Mariscalco MM, Tosi MF. Postnatal maturation of total cell content and up-regulated surface expression of Mac-1 (CD11b/CD18) in polymorphonuclear leukocytes of human infants. J Leukoc Biol. 2008;84:477–479. [DOI] [PubMed] [Google Scholar]
- 16.Yasui K, Masuda M, Tsuno T, et al. An increase in polymorphonuclear leucocyte chemotaxis accompanied by a change in the membrane fluidity with age during childhood. Clin Exp Immunol. 1990;81:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filias A, Theodorou GL, Mouzopoulou S, Varvarigou AA, Mantagos S, Karakantza M. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr. 2011;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi A, Lord J. Adiponectin inhibits neutrophil phagocytosis of Escherichia coli by inhibition of PKB and ERK 1/2 MAPK signalling and Mac-1 activation. PLoS One. 2013;8:e69108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heine J, Jaeger K, Osthaus A, et al. Anaesthesia with propofol decreases FMLP-induced neutrophil respiratory burst but not phagocytosis compared with isoflurane. Br J Anaesth. 2000;85:424–430. [DOI] [PubMed] [Google Scholar]
- 20.Hall MW, Geyer SM, Guo CY, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med. 2013;41:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds P, Wall P, van Griensven M, McConnell K, Lang C, Buchman T. Shock supports the use of animal research reporting guidelines. Shock. 2012;38:1–3. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Lee JC. Murine models of bacteremia and surgical wound infection for the evaluation of Staphylococcus aureus vaccine candidates. Methods Mol Biol. 2016;1403:409–418. [DOI] [PubMed] [Google Scholar]
- 23.McLoughlin RM, Solinga RM, Rich J, et al. CD4þ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc Natl Acad Sci U S A. 2006;103:10408–10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov. 2003;2:703–716. [DOI] [PubMed] [Google Scholar]
- 25.Jones SL, Knaus UG, Bokoch GM, Brown EJ. Two signaling mechanisms for activation of alphaM beta2 avidity in polymorphonuclear neutrophils. J Biol Chem. 1998;273:10556–10566. [DOI] [PubMed] [Google Scholar]
- 26.Thaler B, Hohensinner PJ, Krychtiuk KA, et al. Differential in vivo activation of monocyte subsets during low-grade inflammation through experimental endotoxemia in humans. Sci Rep. 2016;6:30162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schurpf T, Springer TA. Regulation of integrin affinity on cell surfaces. EMBO J. 2011;30:4712–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. [DOI] [PubMed] [Google Scholar]
- 29.Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis. 2006;42:389–391. [DOI] [PubMed] [Google Scholar]
- 30.National Nosocomial Infections Surveillance, S. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. [DOI] [PubMed] [Google Scholar]
- 31.Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS One. 2008;3:e1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotani N, Hashimoto H, Sessler DI, et al. Intraoperative modulation of alveolar macrophage function during isoflurane and propofol anesthesia. Anesthesiology. 1998;89:1125–1132. [DOI] [PubMed] [Google Scholar]
- 33.Silva MT, Correia-Neves M. Neutrophils and macrophages: the main partners of phagocyte cell systems. Front Immunol. 2012;3:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobzik L, Swirski FK. MARCOing monocytes for elimination. Sci Transl Med. 2014;6:219fs214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep. 2015;5:13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stansfield BK, Ingram DA. Clinical significance of monocyte heterogeneity. Clin Transl Med. 2015;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun XJ, Li XQ, Wang XL, Tan WF, Wang JK. Sevoflurane inhibits nuclear factor-kappaB activation in lipopolysaccharide-induced acute inflammatory lung injury via toll-like receptor 4 signaling. PLoS One. 2015;10:e0122752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun M, Deng B, Zhao X, et al. Isoflurane preconditioning provides neuroprotection against stroke by regulating the expression of the TLR4 signalling pathway to alleviate microglial activation. Sci Rep. 2015;5:11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu GJ, Chen TL, Chang CC, Chen RM. Propofol suppresses tumor necrosis factor-alpha biosynthesis in lipopolysaccharide-stimulated macrophages possibly through downregulation of nuclear factor-kappa B-mediated toll-like receptor 4 gene expression. Chem Biol Interact. 2009;180:465–471. [DOI] [PubMed] [Google Scholar]
- 42.Cruz FF, Rocco PR, Pelosi P. Anti-inflammatory properties of anesthetic agents. Crit Care. 2017;21:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koo BW, Sim JB, Shin HJ, et al. Surgical site infection after colorectal surgery according to the main anesthetic agent: a retrospective comparison between volatile anesthetics and propofol. Korean J Anesthesiol. 2016;69:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Von Dossow V, Baur S, Sander M, et al. Propofol increased the interleukin-6 to interleukin-10 ratio more than isoflurane after surgery in long-term alcoholic patients. J Int Med Res. 2007;35:395–405. [DOI] [PubMed] [Google Scholar]
- 45.Barie PS. Surgical site infections: epidemiology and prevention. Surg Infect (Larchmt). 2002;3:S9–S21. [DOI] [PubMed] [Google Scholar]
- 46.Miles AA, Miles EM, Burke J. The value and duration of defence reactions of the skin to the primary lodgement of bacteria. Br J Exp Pathol. 1957;38:79–96. [PMC free article] [PubMed] [Google Scholar]
- 47.Krumholz W, Endrass J, Knecht J, Hempelmann G. The effects of midazolam, droperidol, fentanyl, and alfentanil on phagocytosis and killing of bacteria by polymorphonuclear leukocytes in vitro. Acta Anaesthesiol Scand. 1995;39:624–627. [DOI] [PubMed] [Google Scholar]
- 48.Fritzenwanger M, Jung C, Goebel B, Lauten A, Figulla HR. Impact of short-term systemic hypoxia on phagocytosis, cytokine production, and transcription factor activation in peripheral blood cells. Mediators Inflamm. 2011;2011:429501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parikh S, Bharucha B, Kamdar S, Kshirsagar N. Polymorphonuclear leukocyte functions in children with cyanotic and acyanotic congenital heart disease. Indian Pediatr. 1993;30:883–890. [PubMed] [Google Scholar]
- 50.Uhlig C, Bluth T, Schwarz K, et al. Effects of volatile anesthetics on mortality and postoperative pulmonary and other complications in patients undergoing surgery: a systematic review and meta-analysis. Anesthesiology. 2016;124:1230–1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.