Abstract
Background/Objectives
Normative Mini‐mental state examination (MMSE) reference values in elderly are scarce. Therefore, the aim is to present normative MMSE values for 85–93 year olds.
Design
A longitudinal age cohort study.
Setting
A population study of the residents in the municipality of Linköping, Sweden.
Participants
Residents (n = 650) born in 1922 during the course of 2007. In total, 374 individuals participated and were tested with MMSE at age 85, 280 of these were willing and able to also participate at age 86, 107 at age 90 and 51 at age 93.
Measurements
MMSE, from 0–30, with lower scores denoting more impaired cognition.
Results
Median MMSE values for the total population over the ages 85, 86, 90 and 93 years was 28 for all ages investigated. The 25th percentile values were 26, 26, 26 and 27, respectively. For a “brain healthy” sub‐group median values were 28, 29, 28, and 28. The 25th percentile values were 27, 28, 26 and 27, respectively. Comparisons for age‐effects showed no differences when all individuals for each age group were compared. When only the individuals reaching 93 years of age (n = 50) were analyzed, there was a significant lowering of MMSE in that age group.
Conclusion
The literature is variable and in clinical practice a low (24) MMSE cut off is often used for possible cognitive impairment in old age. The present data indicate that MMSE 26 is a reasonable cut off for possible cognitive decline in older persons up to the age of 93. J Am Geriatr Soc 67:534–538, 2019.
Keywords: mental status, dementia test, aged 80 and over
The Mini‐Mental State Examination (MMSE)1 is the most widely used instrument for pragmatic cognitive testing in healthcare. It is recommended in several guidelines for use as the primary cognitive test to screen for cognitive decline.2 Because most cases of cognitive impairment due to disease, for example, those caused by dementia, occur at a higher age, the importance of normative MMSE reference values for the corresponding age is obvious. In Sweden, more than 80% of prevalent dementia cases are 80 years or older. Normative studies (age, education) covering large populations that do not exclude patients with possible cognitive deficits reflect lower performance levels due to both “normality” and various conditions and disease states affecting brain function. MMSE is not discriminative in terms of the etiology of the cognitive deficit.1 Therefore, the lower percentiles of the MMSE in a population include various conditions in need of further investigation for appropriate medical management. In a 2016 Cochrane meta‐analysis, the MMSE was evaluated for the screening for dementia in clinically unevaluated people aged 65 and older in community and primary care populations.3 The review demonstrated diagnostic accuracy (for dementia) at various cut points. But there was insufficient evidence to recommend a specific score on the MMSE to confidently rule out or rule in dementia in patients 65 years and older.4
Some previous age‐ and education‐normative studies examining age cohorts for whom the educational level was considerably lower than today's, have suggested cutoff values of MMSE 21 for persons aged over 85.5 Even though cutoff values discriminating between pathologic/healthy cognitive states are explicit, many countries have used MMSE 24 as the cutoff pathologic value (eg, Creavin et al3). Even so, most guidelines state there is not an absolute value for pathologic MMSE, but that the evaluation result should be used as one piece in a larger set of data,2 and normative values are still of importance for the evaluation and interpretation of MMSE data.
We found a variety of results from normative studies on total populations or on so‐called brain‐healthy individuals. Only a few had a substantial number of observations over the age of 80. In a longitudinal (but not normative) study of older persons, the mean MMSE was 24.1 at age 85 and 20.2 at age 88 for an age cohort born in 1901‐1902.6 In an additional longitudinal (but not normative) study of very old age of the same age cohorts, mean MMSE values for 97, 99, and 100 years of age were around 17.7 In a study of 85‐year‐olds born in 1923‐1924, the MMSE mean values were significantly (approximately 1.5 points) higher than for those born 22 years earlier in 1901, suggesting improvements over time between age cohorts.8
We used the longitudinal Elderly in Linköping Screening Assessment9 that studied an age cohort of 85‐year‐olds (born in 1922) and then followed the same individuals for 8 years until the age of 93. We present normative MMSE values and suggest that the 25th percentile values be used for the possible discrimination of pathologic conditions.
METHODS
The Elderly in Linköping Screening Assessment is a longitudinal population study that has followed a cohort (born in 1922) initially assessed at the age of 85 in 2007 (TI).9 The individuals reside in Linköping municipality, Sweden, a town with about 145 000 inhabitants, situated in the southeast of Sweden and known for its university and high‐technology industry.
Follow‐ups were completed after 1 year at age 86 (T2), 5 years at age 90 (T3), and 8 years at age 93 (T4). T1 and T2 consisted of three phases: a postal questionnaire, a home visit by an occupational therapist, and a visit to the memory clinic in Linköping. The protocol at T3 and T4 was shortened to include only a home visit, during which the MMSE and self‐report questionnaires were administered. The MMSE examination was performed in the home of the patient in most cases. The research reported in this article, including permission to obtain data from all registers held by the County Council of Östergötland, complies with the ethical rules for human experimentation stated in the Declaration of Helsinki. The study was approved by the Research Ethics Committee of Linköping University, Sweden (2006: 141‐06; 2012: 332‐31; 2014: 455‐31). Written informed consent was collected from all participants at each phase of the study.
All 650 residents in the municipality of Linköping born in 1922 were invited to participate during the course of 2007 (T1). In total, 374 individuals were tested with MMSE at T1, 280 at T2, and 107 of these were willing and also able to participate at T3 and 51 at T4 (Figure 1). Medical records were scrutinized systematically. For the analysis of the brain‐healthy group, persons with known diseases that can cause cognitive decline were excluded. The conditions concerned were neurologic (eg, stroke, epilepsy, multiple sclerosis, normal pressure hydrocephalus, Parkinson disease), cognitive (eg, dementia, objective mild cognitive impairment), somatic (eg, chronic obstructive pulmonary disease with hypoxia, insulin‐dependent diabetes, metastasized cancer), and psychiatric disease (eg, psychosis, depression), severe head trauma, and drug abuse.
Figure 1.
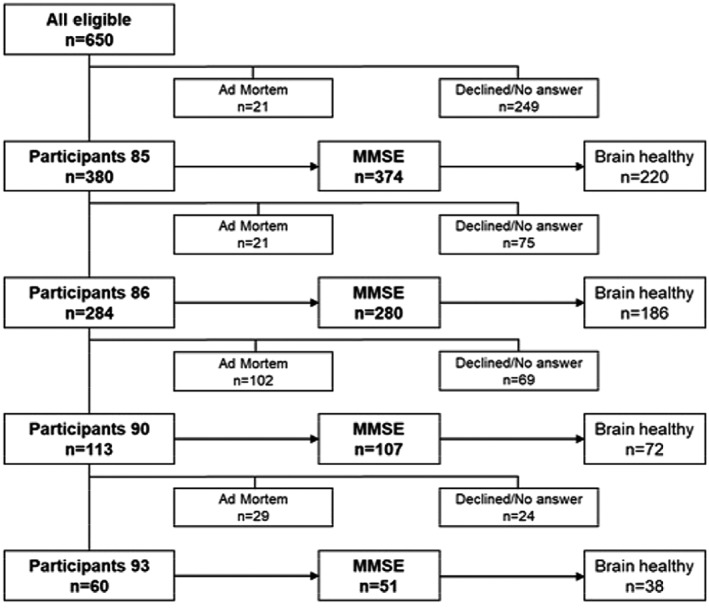
Flowchart of the participants in the study.
Cognitive functioning was assessed using the MMSE.1 It consists of 12 items to assess orientation to time and place, attention, memory, language, and visual construction. It yields a single total score ranging from 0 to 30, with lower scores denoting more impaired cognition. The tests were performed by a trained nurse or a trained occupational therapist. Educational levels was grouped into three categories because most individuals in this age cohort belong to these categories, that is, 6 to 9 years (elementary school), 10 to 12 years (high school), or more than 12 years (higher education).
Statistics
Normative values are presented as percentiles for each age group. The MMSE scores originate from the same population on four separate occasions between ages 85 and 93. Analyses were performed using SPSS v.24. Age effects on the repeated MMSE measure were analyzed using mixed models including all the tested individuals for each time point, respectively. We also separately analyzed age effects only on the same cohort of individuals reaching the age of 93 years (n = 50), that is, the same 50 individuals studied for each time point. The mixed‐models analyses used a diagonal repeated covariance structure for the data. Differences in MMSE between men and women, and between the total group and the brain‐healthy group were tested using the Mann‐Whitney U test. Differences in MMSE between educational levels were tested using the Kruskal‐Wallis analysis of variance.
RESULTS
The normative values represented longitudinal data for the whole cohort following through from 85 years of age for 8 years until they reached 93 years of age (Table 1). The lower quartile MMSE was 26 for ages 85 to 90 and 27 for age 93.
Table 1.
MMSE Normative Data at Ages 85, 86, 90, and 93
| Age, y | 85 | 86 | 90 | 93 | |
|---|---|---|---|---|---|
| N | 374 | 280 | 107 | 51 | |
| Median | 28 | 28 | 28 | 28 | |
| Mean | 27.0 | 26.9 | 26.6 | 27.4 | |
| Standard deviation | 3.4 | 4.3 | 4.4 | 2.3 | |
| Range | 24 | 26 | 27 | 10 | |
| Minimum | 6 | 4 | 3 | 20 | |
| Maximum | 30 | 30 | 30 | 30 | |
| Percentiles | 5 | 21 | 17 | 18 | 22 |
| 10 | 23 | 23 | 22 | 23 | |
| 25 | 26 | 26 | 26 | 27 | |
| 50 | 28 | 28 | 28 | 28 | |
| 75 | 29 | 29 | 29 | 29 | |
| 90 | 30 | 30 | 30 | 30 | |
| 95 | 30 | 30 | 30 | 30 | |
When only the group defined as brain healthy was evaluated, the mean score for all age groups was, as expected, significantly higher than for the excluded individuals for all age groups (values not shown; p < 001, Mann‐Whitney U test). The mean MMSE for the brain‐healthy group was around 28 over all age groups. The lower quartile was 27, 28, 26, and 27 for each age group, respectively (Table 2).
Table 2.
MMSE Normative Data for the Brain healthy at Ages 85, 86, 90, and 93
| Age, y | 85 | 86 | 90 | 93 | |
|---|---|---|---|---|---|
| N | 220 | 186 | 72 | 38 | |
| Median | 28 | 29 | 28 | 28 | |
| Mean | 27.8 | 28.1 | 27.6 | 28.0 | |
| Standard deviation | 2.4 | 2.5 | 2.4 | 1.8 | |
| Range | 19 | 20 | 15 | 9 | |
| Minimum | 12 | 10 | 16 | 22 | |
| Maximum | 30 | 30 | 30 | 30 | |
| Percentiles | 5 | 23 | 24 | 23 | 22 |
| 10 | 26 | 26 | 24 | 26 | |
| 25 | 27 | 28 | 26 | 27 | |
| 50 | 28 | 29 | 28 | 28 | |
| 75 | 29 | 29 | 29 | 29 | |
| 90 | 30 | 30 | 30 | 30 | |
| 95 | 30 | 30 | 30 | 30 | |
There were no differences in MMSE between men and women.
Normative values were also analyzed in relation to educational level (Supplementary Table S1). For the lowest educational level (1‐9 y), the median MMSE was 28 and lower quartile 26. For the intermediate level (high school, 10‐12 y), the median MMSE was 28 and the lower quartile was 26 to 27. And for the highest (university) educational level (>12 y of education), the median was 28 to 29 and the lower quartile was 27 to 28. There was a significant difference in MMSE between educational groups for the 85‐ and 86‐year‐old groups only (Supplementary Table S1).
The effect of age on MMSE was studied both in the total sample for each time point and also separately in the limited cohort that reached 93 years of age (n = 50). There was no significant age effect on MMSE when the whole population for each time point was studied. But for the selected group that reached 93 years, there was a significant negative age effect (Figure 2).
Figure 2.
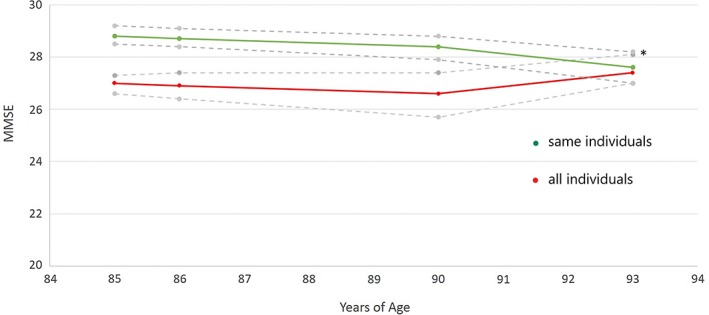
Upper (green): MMSE vs age for the same individuals (n = 50) who reached 93 years of age and delivered data at all four time points, mean (95% confidence interval [CI]), p = .003, mixed models. * Pairwise post hoc comparisons showed that MMSE at 93 years of age was lower than at the three earlier ages (p < .037). Lower (red): MMSE vs age for all individuals between 85 and 93 years of age, mean (95% CI), p = .419, mixed models. For numbers, see Table 1.
DISCUSSION
The normative values of the present study are one of the more recent (age cohort 1922) and larger samples at the age of 85 and older. With a median MMSE of 28 and lower quartile of 26 from 85 to 93 years of age, it appears that the MMSE in clinical practice can use the same norms across these age groups. The exclusion of persons with diagnosed conditions known to affect cognition (eg, dementia, stroke) did not change the picture, leaving the brain‐healthy group with similar reference values (lower quartile 26). Because MMSE of 27 or higher indicates the absence of cognitive decline for younger age groups in several clinical studies, we conclude that a lower cutoff (lower quartile) for cognitive decline can be set to MMSE 26 for most age groups including the elderly aged 85 to 93. It is true that this lower quartile will include healthy subjects, but it will also have a high probability of including dementia cases (eg, Aevarsson and Skoog6).This contrasts with the commonly used MMSE cutoff of 243 that increases the risk of false‐negative cases.
Longitudinal population studies require considerable effort by scientists to be successful. One shortcoming of the present study is the number of nonparticipants that may have limited our ability to draw conclusions about the whole population originally selected. Taking into account the number of deaths that took place before the investigations started, the present study had a response rate of 59%. This is in the same range as some other similar studies (eg, Skoog et al.7 and Stephan et al.10). There are earlier studies with higher response rates (70%‐80%),11, 12 but given an adequate (high) direct response rate, additionally increased response may not reduce the risk of selection bias.13 We analyzed the nonparticipants in our study and found that nonparticipants and participants had similar healthcare utilization, but the nonparticipants were more often accommodated in nursing homes in comparison with participants.14 Thus given the fact that dementia is common in nursing homes, there is a risk that the investigated population is underrepresented regarding persons with cognitive deficits like dementia and more closely reflects a population that is able to visit healthcare facilities. However, the 25th percentile of MMSE 25 in an earlier Canadian study with a relative oversampling of older persons living in institutions suggests that the differences in accommodation have a low impact on the suggested cutoff.12
We found similar reference values for the brain healthy in one study covering ages over 85, in which Heeren et al reported median scores of 28 and lowest quartile values of 25 to 26 for elderly people aged 85 to 99.11 A previous, more limited, study reported a lowest quartile cutoff of 26 for those aged 80 and older.15 Many age‐normative studies set a limit for upper age or involve a limited number of cases. In a 2017 Japanese population study on older adults, 96 individuals were aged 85 years or older,16 the 10th percentile was 18 for those aged 85 and older (n = 73), and 13 for those 90 years of age and over (n = 23). These 10th percentile values contrast with the present values of around MMSE 22 to 23. In an Irish longitudinal study of aging, the oldest age cohort was 85 years of age, and the 25th percentile MMSE level was 23 to 27 from low to high educational level. In their study, however, individuals with severe cognitive impairment as well as those living in long‐term care institutions were excluded.17
In a normative Mexican study, in which those with severe cognitive impairment were excluded, 370 individuals aged 80 years and older were examined, with 25th percentile MMSE values of 14 to 19, depending on educational level. This clearly differs from most other studies.18 In a Shanghai longitudinal aging study, 92 individuals aged 85 and over were examined, but no normative values in percentiles were given.19 In a much earlier Canadian study, in which 853 individuals aged 85 and over were studied, the mean MMSE was 25.9, and the 25th percentiles were 23 to 26, depending on education.12 For the group with a similar educational level to most individuals in our study (6‐9 years), the 25th percentile was 25. In a study by Skoog et al, in which two age cohorts of 85‐year‐olds were compared, 22% of those born in 1923‐1924 had MMSE 24 or lower.7 Taken together, in the studies described here from different countries and continents,7, 11, 12 it appears that the 25th percentile remains around MMSE 25 to 26.
In summary, even though normative values for MMSE at high age are variable, the present study, together with most other data, suggests that the 25th percentile MMSE values of 25 to 26 are given by most studies involving 85‐year‐olds.
Interestingly, our longitudinal data revealed relative stability over the 8 years studied, similar to the cross‐sectional data of 85‐ to 99‐year‐olds in Heeren et al.11 However, when analyzing the same individuals over time, that is, the individuals reaching 93 years of age, there was a small but significant age‐related decline. This can be interpreted as meaning that this possible age effect is hidden when the cohorts are not identical. Like most other studies, we found no differences in MMSE between men and women.11, 16, 20 Similarly to the Dutch study, a low educational level did not affect the MMSE results,11 even though other studies have described such differences (eg, Aevarsson and Skoog6).
We conclude that an MMSE cutoff (corresponding to the 25th percentile) of 26 is an evidence‐based, pragmatic cutoff for further medical evaluation of 85‐year‐olds and older. This conclusion, based on our data, is supported by other normative studies with a valid number of samples of individuals aged 85 years and older with more than 6 years of education.11, 12 Because similar data appear in studies in both Europe and Canada, we suggest that the 26 MMSE cutoff (rather than 24) can be used in countries with similar social contexts. We regard an MMSE cutoff of 26 to be a safety measure, indicating that the individual subject to the examination should be recommended for further medical evaluation. In this respect, this new higher cutoff (for further investigation) instead of the current lower value (MMSE 24) would result in a higher sensitivity (proportion of true dementia cases) and a lower specificity (proportion of true negative cases), which reduces the risk of missing individuals with dementia.
The MMSE is not a diagnostic test but merely a screening test based on clinical suspicion of cognitive decline, irrespective of its cause. In Scandinavia and elsewhere, a non–evidence‐based use of cutoff 24 was used in clinical practice, for example, in primary care.3 The present study corroborates a few older studies and a 2016 Czech study,21 but with more homogeneous educational levels similar to those of most individuals aged 80 and older in European and North American countries today, suggesting an MMSE cutoff of 26 for individuals aged 85 to 93 years.
Supporting information
Supplementary Table S1. MMSE normative data age 85, 86, 90, and 93 in relation to educational levels.
ACKNOWLEDGMENTS
Financial Disclosure
This study was performed by the authors while employed by Linköping University and/or the County Council of Östergötland. Support staff (nurse, occupational therapist) were funded by a research grant from Linköping University.
Conflict of Interest
None.
Author Contributions
Study design: Wressle and Marcusson. Methods: All authors. Data collection: Marcusson, Wressle, and Fällman. Analysis and interpretation of the data: Kvitting, Fällman, and Marcusson. Preparation of the manuscript: Kvitting, Fällman, and Marcusson. All authors commented on all phases of the manuscript preparation and data analysis.
Sponsor's Role: The funders had no input or influence on the study.
REFERENCES
- 1. Folstein MF, Folstein SE, Mchugh PR. “Mini‐mental state.” A practical method for grading cognitive state of patients for clinician. J Psychiatr Res 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 2.National Institue for Health and Care Excellence (NICE). Dementia: Supporting people with dementia and their carers in health and social care. Clinical guideline CG42;2006.
- 3. Creavin ST, Wisniewsk S, Noel‐Storr AH, et al. Mini‐Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev 2016;13:CD011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalish VB, Lerner B. Mini‐Mental State Examination for the detection of dementia in older patients. Am Fam Physician 2016;2016:880‐881. [Google Scholar]
- 5. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population‐based norms for the Mini‐Mental State Examination by age and educational level. JAMA 1993;269:2386‐2391. [PubMed] [Google Scholar]
- 6. Aevarsson O, Skoog I. A longitudinal population study of the Mini‐Mental State Examination in the very old: Relation to dementia and education. Dementia Geriatr Cognit Disord 2000;11:166‐175. [DOI] [PubMed] [Google Scholar]
- 7. Skoog J, Backman K, Ribbe M, et al. A longitudinal study of the Mini‐Mental State Examination in late nonagenarians and its relationship with dementia, mortality, and education. J Am Geriatr Soc 2017;65:1296‐1300. [DOI] [PubMed] [Google Scholar]
- 8. Skoog I, Borjesson‐Hanson A, Kern S, et al. Decreasing prevalence of dementia in 85‐year olds examined 22 years apart: The influence of education and stroke. Sci Rep 2017;7:6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagga K, Dong HJ, Marcusson J, Skoglund SO, Wressle E. Health‐related factors associated with hospitalization for old people: Comparisons of elderly aged 85 in a population cohort study. Arch Gerontol Geriatr 2012;54:391‐397. [DOI] [PubMed] [Google Scholar]
- 10. Stephan BCM, Muniz‐Terrera MG, Granic A, et al. Longitudinal changes in global and domain specific cognitive function in the very‐old: Findings from the Newcastle 85+ study. Int J Geriatr Psychiatry 2018;33:298‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heeren TJ, Lagaay AM, von Beek WC, Rooymans HG, Hijmans W. Reference values for the Mini‐Mental State Examination (MMSE) in octo‐ and nonagenarians. J Am Geriatr Soc 1990;38:1093‐1096. [DOI] [PubMed] [Google Scholar]
- 12. Bravo G, Hebert R. Age‐ and education‐specific reference values for the Mini‐Mental and modified Mini‐Mental State Examinations derived from a non‐demented elderly population. Int J Geriatr Psychiatry 1997;12:1008‐1018. [DOI] [PubMed] [Google Scholar]
- 13. der Wiel AB, van Exel A, de Craen AJ, et al. A high response is not essential to prevent selection bias: Results from the Leiden 85‐plus study. J Clin Epidemiol 2002;55:1119‐1125. [DOI] [PubMed] [Google Scholar]
- 14. Dong HJ, Wressle E, Marcusson J. Unaltered image of health maintenance: An observation of non‐participants in a Swedish cohort study of 85 to 86 year olds. J Frailty Aging 2015;4:93‐99. [DOI] [PubMed] [Google Scholar]
- 15. Bleecker ML, Bolla‐Wilson K, Kawas C, Agnew J. Age‐specific norms for the Mini‐Mental State Exam. Neurology 1988;38:1565‐1568. [DOI] [PubMed] [Google Scholar]
- 16. Sakuma N, Ura C, Miyamae F, et al. Distribution of Mini‐Mental State Examination scores among urban community‐dwelling older adults in Japan. Int J Geriatr Psychiatry 2017;32:718‐725. [DOI] [PubMed] [Google Scholar]
- 17. Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, Savva GM. Normative values of cognitive and physical function in older adults: Findings from the Irish longitudinal study on ageing. J Am Geriatr Soc 2013;61:S279‐S290. [DOI] [PubMed] [Google Scholar]
- 18. Mokri H, Avila‐Funes JA, Meillon C, Gutierrez Robledo LM, Amiev H. Normative data for the Mini‐Mental State Examination, the free and cued selective reminding test and the Isaacs set test for an older adult Mexican population: The Coyoacan cohort study. Clin Neuropsychol 2013;27:1004‐1018. [DOI] [PubMed] [Google Scholar]
- 19. Xiao S, Lewis M, Mellor D, et al. The China longitudinal ageing study: Overview of the demographic, psychosocial and cognitive data of the Shanghai sample. J Ment Health 2016;25:131‐136. [DOI] [PubMed] [Google Scholar]
- 20. Han C, Jo SA, Jo I, Kim E, Park MH, Kang Y. An adaptation of the Korean Mini‐Mental State Examination (K‐MMSE) in elderly Koreans: Demographic influence and population‐based norms (the AGE study). Arch Gerontol Geriatr 2008;47:302‐310. [DOI] [PubMed] [Google Scholar]
- 21. Bartos A, Raisova M. The Mini‐Mental State Examination: Czech norms and cutoffs for mild dementia and mild cognitive impairment due to Alzheimer's disease. Dementia Geriatr Cognit Disord 2016;42:50‐57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. MMSE normative data age 85, 86, 90, and 93 in relation to educational levels.


