Abstract
Objects: To investigate the expression and clinical significance of H-caldesmon which is considered a myogenic marker in GIST. Methods: The clinical information of 105 patients diagnosed with GIST was obtained from Yantai Yuhuangding Hospital and Rambam Health Care Campus. Morphology, the results of immunohistochemical staining and available molecular detection were reviewed. The expression of H-caldesmon was detected for each specimen by immunohistochemical staining. Comparative analysis was carried out between H-caldesmon expression and clinicopathologic parameters. Results: H-caldesmon was expressed in all patients with GIST including tumors outside the gastrointestinal tract and with CD117-negative expression. Although the pattern of expression was different, the positive rate in our study group was 100%. There was no statistically difference between H-caldesmon expression and parameters such as gender, age, location, morphology, risk, immunologic markers, and molecular mutation. Conclusions: H-caldesmon is expressed positively in GIST and might not be a specific marker for smooth muscle and associated tumors. GIST outside the gastrointestinal tract or with CD117-negative expression should not be misdiagnosed assmooth muscle tumor because of the positive expression of H-caldesmon in the differential diagnosis. Comprehensive analysis combined with other immunological markers and molecular detection is needed.
Keywords: H-caldesmon, GIST, differential diagnosis
Introduction
GIST is one of the common mesenchymal tumors and the morbidity accounts for 3% of all neoplasms in the gastrointestinal tract [1]. This type of tumor used to be considered as leiomyoma or leiomyosarcoma until the origin from interstitial Cajal cells was revealed by later studies. The tumor cells are usually positive for CD117, Dog-1, and CD34 [2,3]. However, we recently encountered some cases of GIST expressing H-caldesmon very well, which prompted this study investigating its potential clinical significance.
Materials and methods
Materials
The clinical information of 105 patients diagnosed as GIST were collected randomly including five cases of outside the gastrointestinal tract and two cases with CD117 negative expression from Department of Pathology of Yantai Yuhuangding Hospital and Department of Pathology, Rambam Health Care Campus from January 2015 to December 2016. Clinical information of all patients was obtained from the corresponding Department of Pathology database. The cases with the diagnosis of needle biopsy or lacking risk assessment were excluded. All procedures performed in our study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Pathological features assessment
Morphology, immunohistochemical markers and available molecular results of all the cases in our group were re-evaluated by two senior pathologists.
Immunohistochemical staining of H-caldesmon
Immunohistochemical staining of H-caldesmon (EnVision two-step method) was done on an immunohistochemical automatic staining instrument (Ventana Benchmark XT, Roche). Each slice was stained with leiomyoma as the positive control, while negative controls replaced the first antibody with PBS. Information on H-caldesmon antibody was: Clone: EP19; Concentration: 1:100; Source: Beijing Zhongshan Jinqiao Biological Technology Co., Ltd. The positive signal was localized in the cytoplasm and a positive standard was designated as more than 10% of the tumor cells showing a positive reaction [4].
Statistical analysis
Statistical software was used in analyzing significance between expression of H-caldesmon and clinicopathological parameters in GIST.
Results
Clinical data of 105 patients diagnosed with GIST
Among 105 cases of GIST, 48 were male and 57 were female. The patients were aged from 28 to 83 with a median age of 58. 32 cases were at high risk (32/105, 30.5%), 23 cases at medium risk (23/105, 21.9%), 27 cases at low risk (27/105, 25.7%) and 23 cases at very low risk (23/105, 21.9%). The location was: 75 cases in the stomach (75/105, 71.4%), 19 cases in the small intestine (19/105, 18.1%), 6 cases in the large intestine (6/105, 5.7%) and 5 cases outside the gastrointestinal tract (5/105, 4.8%).
Morphology feature of GIST
According to the morphology of tumor cells, GIST could be divided into spindle cell type and epithelial cell type. Tumor cells in spindle cell type were long or short spindles arranged as a bundle or vortex (Figure 1A). Tumor cells in the epithelial cell type had abundant eosinophilic or transparent cytoplasm, round or oval nuclei, and clear borders (Figure 1B). Signet ring cells could also be found (Figure 1C). In some cases, tumor cells were composed of spindle and epithelial cells.
Figure 1.
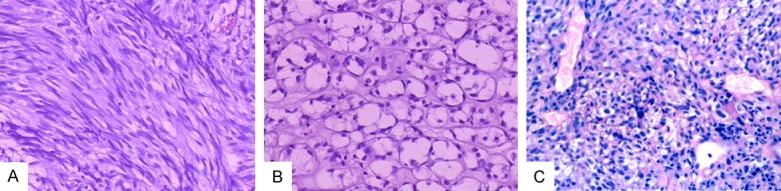
Morphology of GIST. Spindle cell type (A) (H&E, 20 ×); Epithelial cell type (B) (H&E, 20 ×). The tumor showed signet ring cells (C) (H&E, 10 ×).
Evaluation of immunohistochemical staining for CD117, CD34, Dog-1, SMA, Desmin, S-100
Respectively, the positive rates of biomarkers in the 105 cases were CD117 (103/105, 98.10%), CD34 (103/105, 98.10%), Dog-1 (104/105, 99.05%), SMA (10/105, 9.52%), desmin (4/105, 3.81%) and S-100 (focally positive, 5/105, 4.76%).
Evaluation of genetic sequencing of c-kit and PDGFRa
The mutation results of 9, 11, 12, 13, 14, 17, and 18 exons in c-kit gene and 12, 14, and 18 exons in PDGFRa gene were obtained by genetic sequencing from 21 patients out of 105 patients. Among 21 cases, c-kit gene mutation was positive in 18 cases (14 cases with 11 exon mutation, 3 cases with a 9 exon mutation and 1 case with an 18 exon mutation), PDGFRa gene mutation was positive in 2 cases (1 case with 12 exon mutation and 1 case with 18 exon mutation), and mutation was not detected in one case. The mutations in two cases with CD117-negative expression were all found in PDGFRa genes just like the above discrimination: one case with mutation site in exon 12 (Figure 2A) and another in exon 18 (Figure 2B) respectively. There was no c-kit mutation found in these two cases.
Figure 2.
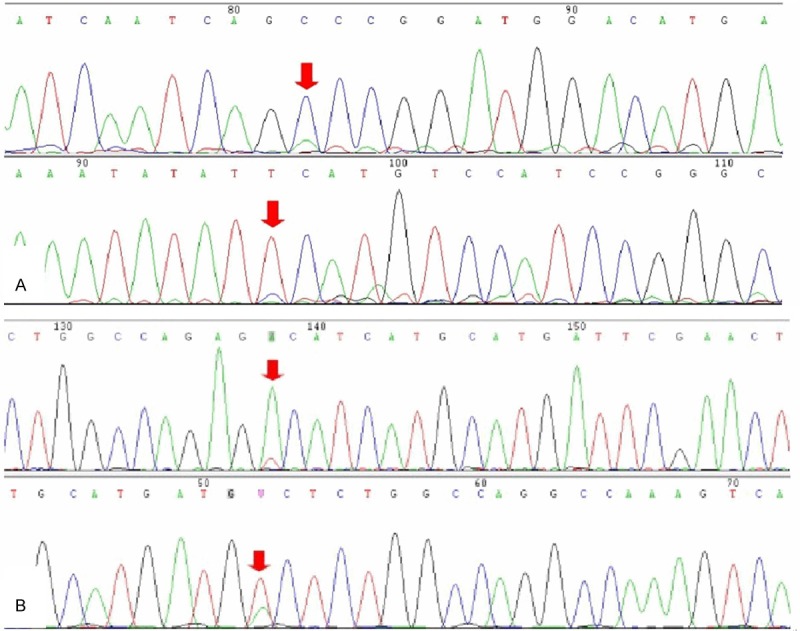
Genetic sequencing showed the mutations in PDGFRa gene. One case mutation site located in 12 exon (A) and another in 18 exon (B).
Expression of H-caldesmon in GIST
H-caldesmon was expressed in all cases (105/105, 100%). The number of tumor cells positively expressing H-caldesmon in each case exceeded 50%. The positive signal was mainly located in the cytoplasm (Figure 3A) and in one case positive signal localization was at the membrane of the tumor cells (Figure 3B). Positive expression patterns varied from mottled (Figure 3C) to diffuse and strong. H-caldesmon was also positive in five cases outside the gastrointestinal tract (Figure 4) and two cases that were CD117 negative, which were diagnosed as GIST and confirmed by genetic sequencing (Figure 5).
Figure 3.
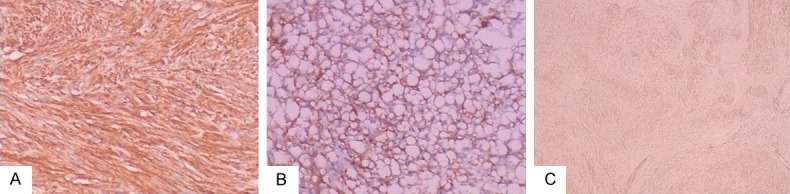
Immunohistochemical staining of H-caldesmon. Positive signal was located in the cytoplasm (A) (H&E, 10 ×) or membrane (B) (H&E, 10 ×) of the tumor cells. Positive expression patterns varied from mottled (C) (H&E, 4 ×) to diffuse and strong.
Figure 4.
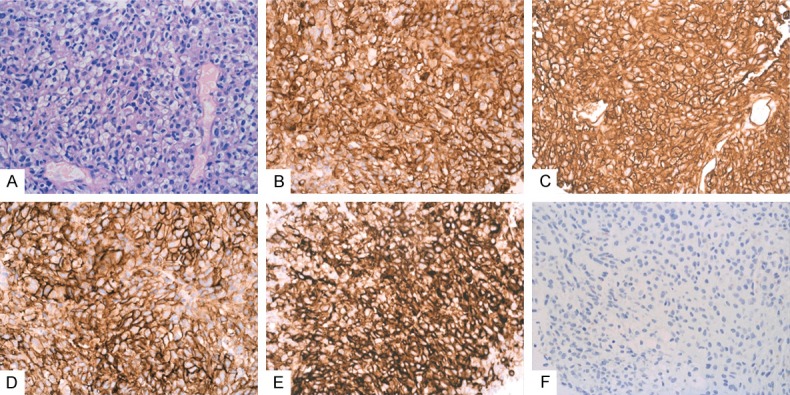
H-caldesmon is expressed positively in a case outside the gastrointestinal tract. The tumor cells were epithelioid and vacuoles were seen in the cytoplasm (A) (H&E, 10 ×). CD117 (B) (Envision, 10 ×), CD34 (C) (Envision, 10 × magnification), Dog-1 (D) (Envision, 10 × magnification) and H-caldesmon (E) (Envision, 10 × magnification) were expressed positively while Desmin (F) (Envision, 10 ×) was negative.
Figure 5.
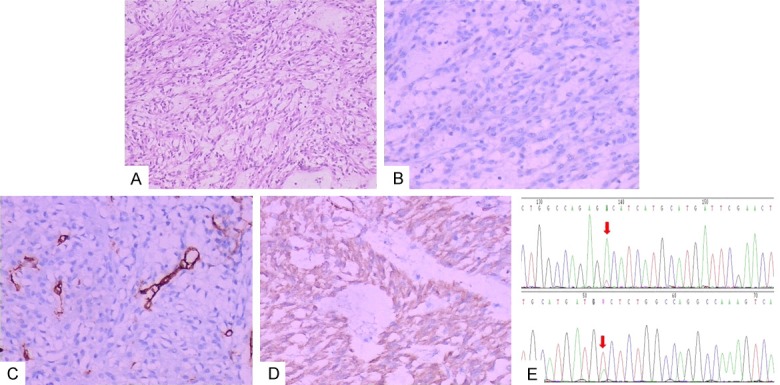
H-caldesmon was expressed positively in the case with CD117-negative expression. The tumor cells were spindled (A) (H&E staining, 10 × magnification) and CD117 (B) (Envision, 20 × magnification), CD34 (C) (Envision, 20 × magnification) had negative expression, while H-caldesmon (D) (Envision, 20 × magnification) was expressed positively. The 18 exon in PDGFR-a gene was mutated (E).
Result of statistical analysis
Because the tumors in our group were all positive for H-caldesmon, there was no statistical difference between H-caldesmon expression and GIST clinicopathologic parameters.
Discussion
Caldesmon is the cytoskeletal-associated protein in the smooth muscle cells, including two subtypes: H-caldesmon and L-caldesmon [5]. H-caldesmon is considered to be the protein binding to actin and tropomyosin, regulating smooth muscle contraction function and being mainly expressed in smooth muscle cells and smooth muscle tumors [6,7]. Therefore H-caldesmon was considered a specific marker of smooth muscle and its associated tumors. We found that there were also related studies about the expression of H-caldesmon in GIST by literature review [4,8-10]. In those papers, the positive expression rate of H-caldesmon ranged from 50% to 80%, which was different from our results. The difference might be related to the source of immunohistochemical antibody or positive evaluation criteria. Although we designated more than 10% of the stained tumor cells as the positive standard, our results showed that the positive expression of H-caldesmon was more than 50% in every specimen. In any case, H-caldesmon was expressed highly in GIST. Most importantly, these papers accepted without question that myogenic differentiation was present whenever positive expression of H-caldesmon was seen in GIST, whereas we doubted this. First, all of the results showed that H-caldesmon was expressed in GIST with a high ratio. It is uncertain whether all these GIST possessed myogenic differentiation. Second, desmin is a recognized myogenic marker and the positive expression of desmin might beproof of myogenic differentiation. In all the cases in our group, the results of desmin expression were only about 5% which was consistent with previous studies [9,10]. This value was too different from the expression of H-caldesmon. Martinez-Ciarpaglini et al presented 13 cases of atypical fibroxanthoma with H-caldesmon positive expression but desmin negative expression [11]. Therefore H-caldesmon expression seemed to be inappropriate for myogenic differentiation. Third, by literature review, we found that H-caldesmon was also expressed in malignant pleural mesothelioma (68/70, 97.14%), adult granuloma (20/22, 90.91%), and fibrothecoma (31/31, 100%) of the ovary [12,13]. Could the expression of H-caldesmon in those tumors be interpreted as myogenic differentiation? Based on the above analysis, we hypothesized that smooth muscle and its related tumors could express H-caldesmon, but H-caldesmon expression was notevidence of myogenic differentiation. Therefore H-caldesmon might not be a specific marker for smooth muscle and its corresponding tumors. In our study, we found that the positive expression of H-caldesmon in one case was localized in the membrane tumor cells rather than in the cytoplasm where cytoskeletal proteins usually exist. Whether H-caldesmon has other functions needs further study.
GIST is prevalent in the elderly and the stomach was the main site. Spindle cells and epithelial cells are main morphologic types. CD34 and Dog-1 are highly sensitive antibodies for GIST just like CD117. The 11 exon mutation in c-kit gene was common and PDGFRα mutation was usually found in CD117-negative GIST. All of these findings were consistent with those in previous clinical studies [8,10,14-16].
Due to the high expression of H-caldesmon in GIST, there was no statistically difference between H-caldesmon expression and GIST clinicopathologic parameters such as gender, age, location, morphology, risk, immunological markers and molecular mutation. However, one point worth noting was that H-caldesmon is also expressed in some special GISTs, for instance GIST with CD117-negative expression and GISTs outside the gastrointestinal tract. During the diagnosis and differential diagnosis of soft tissue neoplasms, tumors could not be misdiagnosed as the smooth muscle tumor due to the positive expression of H-caldesmon. GIST with CD117 negative expression and GIST outside the gastrointestinal tract should be given more attention by pathologists especially. Combined CD117, CD34, Dog-1, and molecular detection was necessary for the correct diagnosis.
Conclusions
H-caldesmon is also expressed in some non-myogenic tumors and might not be a specific marker for smooth muscle and tumors of its origin. In the differential diagnosis, the pitfall of misdiagnosis as smooth muscle tumor due to expression of H-caldesmon should be avoided. It is necessary to combine other antibodies and molecular detection to prevent misdiagnosis.
Acknowledgements
This project was supported in part by the Natural Science Foundation of Shandong Province, China (Grant No. ZR2015HQ013).
Written informed consents were obtained from patients and/or their family members who were consent to participate in our study for publication of this article.
Disclosure of conflict of interest
None.
Abbreviations
- GIST
gastrointestinal stromal tumor
- H-caldesmon
high molecular weight caldesmon
- L-caldesmon
low molecular weight caldesmon
- PDGFRa
platelet derived growth factor receptor alpha
References
- 1.Miettinen M, Lasota J. GISTs: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 2.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for GISTs that is more specific than CD34. Mod Pathol. 1998;11:728–34. [PubMed] [Google Scholar]
- 3.Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA, Demetri GD, Fletcher CD, Fletcher JA. KIT activation is a ubiquitous feature of GISTs. Cancer Res. 2001;61:8118–21. [PubMed] [Google Scholar]
- 4.Fujimoto Y, Nakanishi Y, Yoshimura K, Shimoda T. Clinicopathologic study of primary malignant GIST of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer. 2003;6:39–48. doi: 10.1007/s101200300005. [DOI] [PubMed] [Google Scholar]
- 5.Ueki N, Sobue K, Kanda K, Hada T, Higashino K. Expression of high and low molecular weight caldesmons during phenotypic modulation of smooth muscle cells. Proc Natl Acad Sci U S A. 1987;84:9049–53. doi: 10.1073/pnas.84.24.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobieszek A. Helical model of smooth muscle myosin filament and the ribbons made of caldesmon: history revisited. Eur Biophys J. 2016;45:861–67. doi: 10.1007/s00249-016-1175-5. [DOI] [PubMed] [Google Scholar]
- 7.Sharma RK, Parameswaran S. Calmodulin-binding proteins: a journey of 40 years. Cell Calcium. 2018;75:89–100. doi: 10.1016/j.ceca.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Tazawa K, Tsukada K, Makuuchi H, Tsutsumi Y. An immunohistochemical and clinicopathological study of GISTs. Pathol Int. 1999;49:786–98. doi: 10.1046/j.1440-1827.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S. GIST: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol. 2002;33:669–76. doi: 10.1053/hupa.2002.124116. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Lasota J. GISTs: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Ciarpaglini C, Agustí J, Alvarez E, Hueso L, Terrádez L, Monteagudo C. h-caldesmon immunoreactivity in atypical fibroxanthoma: implications for the differential diagnosis. Pathology. 2018;50:358–61. doi: 10.1016/j.pathol.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Comin CE, Dini S, Novelli L, Santi R, Asirelli G, Messerini L. h-Caldesmon, a useful positive marker in the diagnosis of pleural malignant mesothelioma, epithelioid type. Am J Surg Pathol. 2006;30:463–9. doi: 10.1097/00000478-200604000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Yu G, Qu G. High molecular weight caldesmon expression in ovarian adult granulosa cell tumour and fibrothecoma. Histopathology. 2018;72:359–61. doi: 10.1111/his.13365. [DOI] [PubMed] [Google Scholar]
- 14.Liu QY, He RF, Xu ZG, Fu FF, Zhou SL, Lei QQ, Liang S, Xue HZ. Clinicopathological and genetic features of mutant gastrointestinal stromal tumors with rare lymph node metastasis and literature review. Int J Clin Exp Pathol. 2017;10:5189–96. [Google Scholar]
- 15.Weldon CB, Madenci AL, Boikos SA, Janeway KA, George S, von Mehren M, Pappo AS, Schiffman JD, Wright J, Trent JC, Pacak K, Stratakis CA, Helman LJ, La Quaglia MP. Surgical management of wild-type GISTs: a report from the national institutes of health pediatric and wildtype GIST clinic. J. Clin. Oncol. 2017;35:523–8. doi: 10.1200/JCO.2016.68.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi S, Gasparotto D, Miceli R, Toffolatti L, Gallina G, Scaramel E, Marzotto A, Boscato E, Messerini L, Bearzi I, Mazzoleni G, Capella C, Arrigoni G, Sonzogni A, Sidoni A, Mariani L, Amore P, Gronchi A, Casali PG, Maestro R, Dei Tos AP. KIT, PDGFRA, and BRAF mutational spectrum impacts on the natural history of imatinib-naive localized GIST: a population-based study. Am J Surg Pathol. 2015;39:922–30. doi: 10.1097/PAS.0000000000000418. [DOI] [PubMed] [Google Scholar]


