Abstract
The abnormal expression of microRNAs (miRNAs) is critical for the development of human cancers. However, the functions of many miRNAs remain to be elucidated. miR-373 was reported to involve the tumorigenesis of multiple cancers, but its role in nasopharyngeal carcinoma (NPC) is not clear. Quantitative real-time PCR was performed to analyze miR-373 expression in NPC cell lines. The connection between membrane associated ring-CH-type finger 5 (MARCH5) and miR-373 was analyzed using a luciferase activity reporter assay and western blot. A cell counting kit-8 assay, a colony formation assay, and a wound-healing assay were performed to investigate the biological functions of miR-373 and MARCH5. We showed miR-373 expression is downregulated, and MARCH5 expression is upregulated, in NPC cells. MARCH5 was validated as a direct target of miR-373. miR-373 regulates NPC cell proliferation, colony formation, and cell migration by regulating MARCH5. In conclusion, our study showed that miR-373 has a tumor suppressive role in NPC by targeting MARCH5. This may provide novel therapeutic targets for NPC.
Keywords: miR-373, MARCH5, nasopharyngeal carcinoma, growth, migration
Introduction
Nasopharyngeal carcinoma (NPC) is a relative uncommon cancer type and accounted for approximately 0.6% of all human cancers in 2012 [1]. Risk factors for the initiation of NC include Epstein-Barr virus infection and cigarette smoking [2]. The mechanisms related to NPC tumorigenesis remain largely unknown. Therefore, a better understanding of mechanisms concerning NPC progression could help us to identify novel NPC targets.
microRNAs (miRNAs) were reported to have dual roles, a tumor suppressor role and an oncogenic role, in human cancers [3]. miRNAs are endogenous non-coding RNAs at the length of 18-25 nucleotides that regulate gene expression by 3’-untranslated region (3’-UTR) binding [4]. The numbers of identified miRNAs are increasing; however, only a fraction of them have been functionally studied [4]. Emerging evidence has revealed that miRNAs play crucial roles in human cancer, including NPC [5]. Hence, it is imperative to investigate the abnormally expressed miRNAs to identify novel therapy targets in NPC.
miR-373 was previously reported to function as either an oncogene or a tumor suppressor gene in human cancers [6-9]. For example, low miR-373 was identified in pancreatic cancer and correlated with the poor overall survival of cancer patients [6]. Forcing the expression of miR-373 inhibits glioma cell migration and invasion by regulating CD44 and TGFBR2 [7]. These findings suggested a possible tumor suppressive role of miR-373. On the other hand, miR-373 was also reported to be an oncogenic miRNA in cancers, including lung cancer and renal cell carcinoma [8,9]. Knocking down the expression of miR-373 in lung cancer was reported to enable the enhancement of radiosensitivity and also to decrease cell migration and invasion ability by targeting TIMP2 [8]. Moreover, miR-373 was shown to promote renal cell carcinoma progression both in vitro and in vivo [9].
Membrane associated ring-CH-type finger 5 (MARCH5) is an E3 ubiquitin-protein ligase located at the outer mitochondrial membrane [10]. Recently, MARCH5 was shown to promote breast cancer metastasis by inducing cell cycle arrest and the epithelial-mesenchymal transition [11]. Meanwhile, it was reported that MARCH5 was capable of being regulated by miR-30a in breast cancer [11].
In this work, the expression of miR-373 and MARCH5 in NPC cell lines and in normal cell lines was analyzed by quantitative real-time PCR and western blot, respectively. The effects of miR-373 and MARCH5 on NPC cell growth, colony formation, and migration were analyzed with a cell counting kit-8 (CCK-8) assay, a colony formation assay, and a wound-healing assay. The connection between miR-373 and MARCH5 was validated using a luciferase activity reporter assay and western blot.
Materials and methods
Cell lines
NPC cell lines (CNE-1 and CNE-2) were incubated in RPMI-1640 (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen). Human nasopharyngeal epithelial cell line NP69 was incubated in a keratinocyte/serum-free medium accompanied by bovine pituitary extract (Invitrogen). These cell lines were incubated in a 37°C humidified incubator containing 5% of CO2. All cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Knockdown and forced expression of target genes
A miR-373 mimic and the corresponding negative control (NC-mimic) were obtained from GenePharma (Shanghai, China) and were used to manipulate the expression of miR-373. pcDNA3.1 containing the open reading frame of MARCH5 (pMARCH5) and pcDNA3.1 were purchased from GenScript (Nanjing, China). Cell transfection was conducted using Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocols.
Quantitative real-time PCR (RT-qPCR) analysis
Total RNA was isolated from cell lines with Trizol reagent (Invitrogen). Complementary DNA was synthesized with a RevertAid RT Reverse Transcription kit (Thermo Fisher Scientific, Inc.) and then subjected to RT-qPCR analysis at ABI 7500 PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR Green Mix (Takara, Dalian, China). The primer sequences used were listed as follows: miR-373 forward, 5’-ATTTTGGTTAATACGGTGAAATTTC-3’ and reverse, 5’-CTATCGCCCAAACTAAAATACGAT-3’; U6 snRNA forward, 5’-CTCGCTTCGGCAGCACA-3’ and reverse, 5’-ACGCTTCACGAATTTGCGT-3’. The relative expression level was analyzed with 2-ΔΔCq method using U6 snRNA as an internal control.
Western blot analysis
RIPA lysis buffer purchased from Beyotime (Haimen, Jiangsu, China) was used for total protein extraction. The same amount of the protein sample was isolated using 10% SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Beyotime) after being quantified with a BCA kit (Beyotime). Subsequently, the membranes were blocked with 5% fat-free milk and incubated with specific primary antibodies (anti-MARCH5: ab77585, anti-GAPDH: 181602; Abcam, Cambridge, MA, USA) at 4°C overnight. Finally, bands were developed with BeyoECL (Beyotime) after they were incubated with a secondary antibody (ab6721, Abcam) at 37°C for 4 h.
CCK-8 assays
The cells were seeded in a 96-well plate at the density of 1 × 104 cells/well. Furthermore, 10 μl CCK-8 solution (Beyotime) was added to each well after incubation at 0, 24, 48, and 72 h. After further incubation for 4 h, the absorbance at 450 nm was measured using a microplate reader.
Colony formation assay
1,000 cells were seeded in 6-well plates and incubated at the above-mentioned conditions for 2 weeks. The colonies were fixed with ethanol, stained with crystal violet, and counted under a microscope.
Wound healing assay
The cells were seeded into 6-well plates and incubated until they reached 90% confluence. After that, a wound at the cell surface was generated using a plastic pipette tip. The cells were then washed with PBS and photographed at 0 and 48 h under a microscope.
Bioinformatic analysis and luciferase activity reporter assay
TargetScan and miRDB were conducted to analyze the targets of miR-373. The wild-type 3’-UTR of MARCH5 was cloned into pmiR-RB-REPORT (RiboBio, Guangzhou, China) and named as wt-MARCH5. The mutant type of MARCH5 3’-UTR (mt-MARCH5) was established using a Site-direct mutagenesis kit (Takara). The cells were cotransfected with luciferase reporter vectors and miRNAs using Lipofectamine 2000. After 48 h of transfection, relative luciferase activity was assessed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Statistical analysis
The statistical analysis was performed using GraphPad 6.0 (Graphpad, San Diego, CA, USA). Student’s t-test was used for comparisons between two groups. ANOVA and Tukey’s post-hoc test were used for comparisons among three or more groups. The results were presented as the mean ± SD, and P < 0.05 was considered statistically significant.
Results
miR-373 expression was downregulated, but MARCH5 was upregulated in NPC
We measured miR-373 expression in NPC cell lines and normal epithelial cell lines using RT-qPCR. Compared with the NP69 cell line, the miR-373 expression in the CNE-1 and CNE-2 cell lines was significantly reduced (Figure 1A). Moreover, we showed that the MARCH5 expression was significantly elevated in the NPC cell lines compared with the normal cell lines (Figure 1B).
Figure 1.
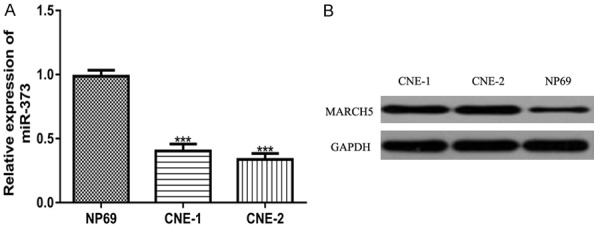
miR-373 expression was downregulated, while MARCH5 expression was upregulated, in the NPC cell lines compared with the normal cell line. (A) miR-373 expression and (B) MARCH5 expression in NPC cell lines and the normal cell line. miR-373: microRNA-373; MARCH5: membrane associated ring-CH-type finger 5; NPC: nasopharyngeal carcinoma.
The overexpression of miR-373 inhibits NPC cell proliferation and migration
To investigate the biological role of miR-373, we transfected the synthetic miRNAs into NPC cell lines and found that the miR-373 mimic increased the levels of miR-373 in NPC cell lines (Figure 2A). A CCK-8 assay and colony formation were conducted to investigate the effects of miR-373 on cell proliferation. The results showed that miR-373 overexpression inhibits NPC cell proliferation and colony formation (Figure 2B and 2C). Moreover, a wound-healing assay revealed the that the miR-373 mimic transfection significantly inhibited cell migration ability compared to the NC-mimic (Figure 2D).
Figure 2.
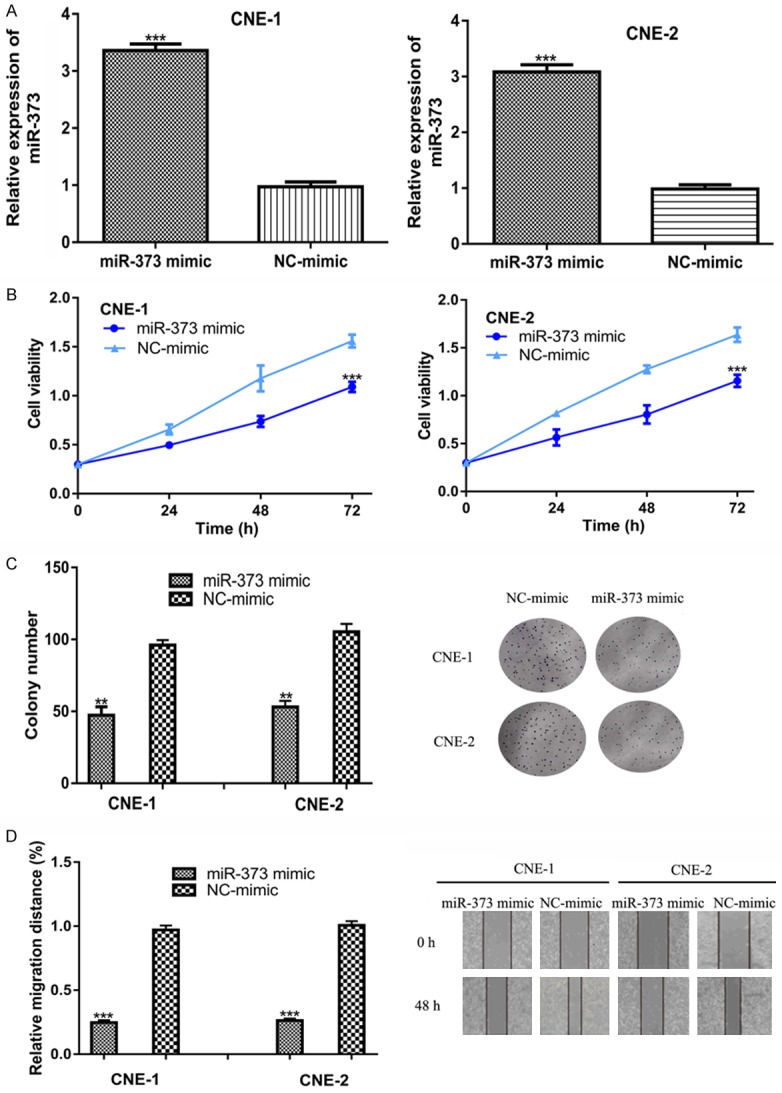
The overexpression of miR-373 inhibits NPC cell growth and migration. (A) miR-373 expression, (B) Cell proliferation, (C) Colony formation, and (D) Cell migration in NPC cells transfected with miR-373 mimic and NC-mimic. miR-373: microRNA-373; NPC: nasopharyngeal carcinoma.
MARCH5 was a direct target of miR-373
Using an online prediction tools analysis, we found MARCH5 contains a binding site for miR-373 (Figure 3A). A luciferase reporter assay revealed that the miR-373 mimic inhibited luciferase activity in the cells transfected with wt-MARCH5, but it did not affect the luciferase activity of the cells transfected with mt-MARCH5 (Figure 3B). Western blot further validated that MARCH5 expression was inhibited by the miR-373 mimic transfection in the NPC cell lines (Figure 3C).
Figure 3.
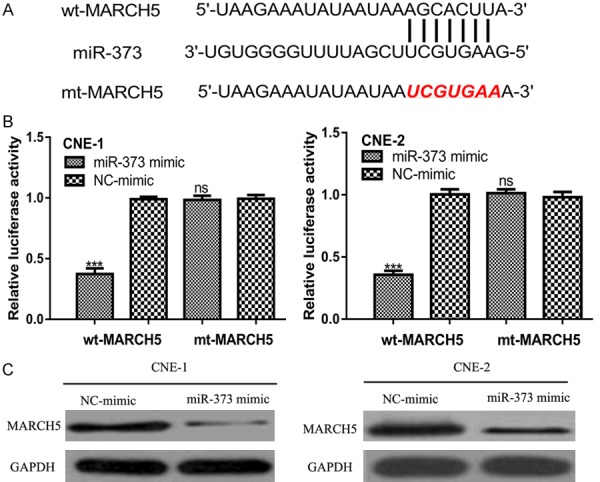
MARCH5 was a direct target of miR-373. A. Predicted binding site of miR-373 and 3’-UTR of MARCH5. B. MARCH5 3’-UTR luciferase reporter assays in NPC cells. C. MARCH5 expression in NPC cells transfected with synthetic miRNAs. miR-373: microRNA-373; MARCH5: membrane associated ring-CH-type finger 5; NPC: nasopharyngeal carcinoma; UTR: untranslated region; wt: wild-type; mt: mutant.
Overexpression of MARCH5 promotes NPC cell proliferation and migration
To analyze role of MARCH5 in miR-373-induced NPC cell behavior inhibition, pMARCH5 was transfected into NPC cells. We found pMARCH5 transfection increased MARCH5 expression in NPC cell lines, and partially reversed the suppression effects of the miR-373 mimic on MARCH5 expression (Figure 4A). Figure 4B-D displayed that overexpression of MARCH5 promotes NPC cell proliferation and migration. Furthermore, we showed that pMARCH5 transfection partially abolished the inhibitory effects of the miR-373 mimic on NPC cell behaviors (Figure 4B-D).
Figure 4.
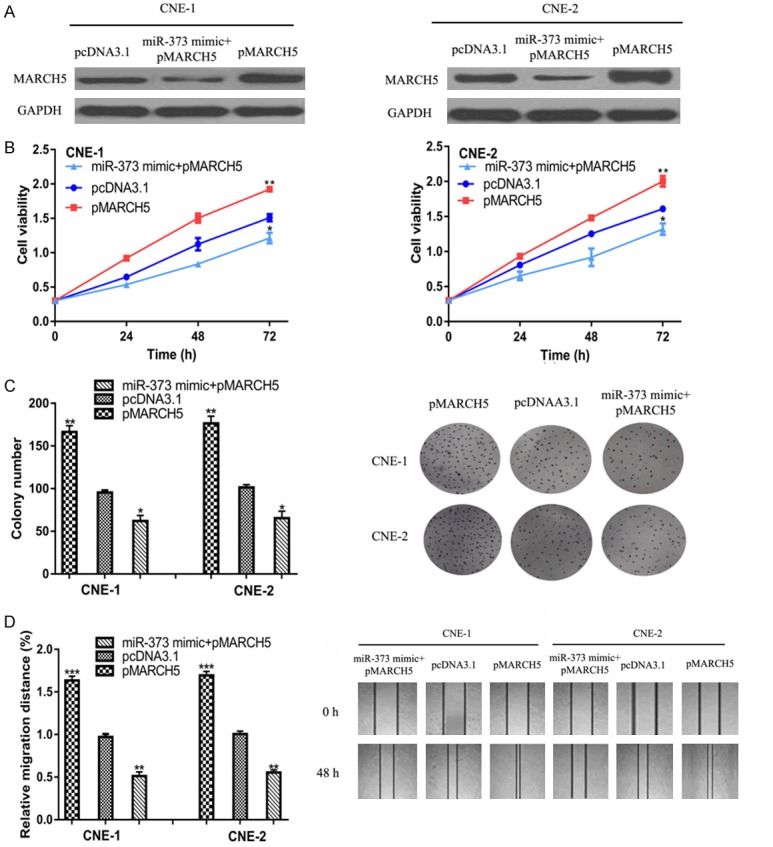
MARCH5 promotes NPC cell growth and migration. (A) miR-373 expression, (B) Cell proliferation, (C) Colony formation, and (D) Cell migration in NPC cells transfected with pMARCH5, pcDNA3.1, or pMARCH5 and miR-373 mimic. miR-373: microRNA-373; MARCH5: membrane associated ring-CH-type finger 5; NPC: nasopharyngeal carcinoma.
Discussion
The GENCODE projects showed that only 3% of the genes in the human genome are for protein coding, while approximately 75% of the genes in the human genome are transcribed into non-coding RNAs, including miRNAs, long non-coding RNAs, etc. [12]. The roles of non-coding RNAs in NPC tumorigenesis have been increasingly appreciated [13-16], but most of them are still not functionally characterized. Here, we showed miR-373 expression was reduced in NPC cell lines compared with the normal cell line. In the meantime, we found MARCH5 expression was elevated in NPC cell lines compared with the normal cell line. We also validated that the overexpression of miR-373 inhibits NPC cell growth and migration. Mechanistically, we demonstrated miR-373 directly interacted with MARCH5 through luciferase activity reporter and western blot analyses. The overexpression of MARCH5 promotes NPC cell growth and migration. Importantly, miR-373 exerts the tumor suppressive role on NPC partially due to its regulating the expression of MARCH5. These results collectively suggest that miR-373 has a tumor suppressive function and plays a crucial role in NPC.
It has been widely recognized that one miRNA can regulate multiple genes in the human genome [4]. Previous studies have identified several targets for miR-373, including CD44, TGFBR2, and TIMP2 [7,8]. Hence, one of our most important findings was that MARCH5 participated in the tumor suppressive role of miR-373 on NPC. The function of MARCH5 in human cancers remains largely unknown. Hu et al. demonstrated that MARCH5 was able to promote ovarian cancer cell autophagy, migration, and invasion by regulating the TGFB1-SMAD2/3 pathway, suggesting an oncogenic role of MARCH5 [17]. Here, we also revealed that MARCH5 was overexpressed in NPC and its overexpression could promote the malignancy behaviors of NPC cells, indicating an oncogenic role of MARCH5 in NPC.
To sum up, our results reveal the tumor suppressive role of miR-373 on NPC. The overexpression of miR-373 inhibited NPC cell growth and migration by downregulating MARCH5. We suggest that miR-373 and MARCH5 are possible targets for NPC treatment. However, further in vitro and in vivo studies are urgently needed to confirm the conclusions of this work.
Disclosure of conflict of interest
None.
References
- 1.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 2.Lv JW, Chen YP, Zhou GQ, Tang LL, Mao YP, Li WF, Guo R, Lin AH, Ma J, Sun Y. Cigarette smoking complements the prognostic value of baseline plasma Epstein-Barr virus deoxyribonucleic acid in patients with nasopharyngeal carcinoma undergoing intensity-modulated radiation therapy: a large-scale retrospective cohort study. Oncotarget. 2016;7:16806–16817. doi: 10.18632/oncotarget.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of MicroRNAs in cancer. Cancer Res. 2016;76:3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S, Gregory RI. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua Y, Chen H, Wang L, Wang F, Wang P, Ning Z, Li Y, Liu L, Chen Z, Meng Z. Low serum miR-373 predicts poor prognosis in patients with pancreatic cancer. Cancer Biomark. 2017;20:95–100. doi: 10.3233/CBM-170231. [DOI] [PubMed] [Google Scholar]
- 7.Wei F, Wang Q, Su Q, Huang H, Luan J, Xu X, Wang J. miR-373 inhibits glioma cell U251 migration and invasion by down-regulating CD44 and TGFBR2. Cell Mol Neurobiol. 2016;36:1389–1397. doi: 10.1007/s10571-016-0338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y, Jiang Y, Sang M, Xu C. Down-regulation of miR-373 increases the radiosensitivity of lung cancer cells by targeting TIMP2. Int J Biochem Cell Biol. 2018;99:203–210. doi: 10.1016/j.biocel.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zhang D, Wang J. MicroRNA-373 promotes tumorigenesis of renal cell carcinoma in vitro and in vivo. Mol Med Rep. 2017;16:7048–7055. doi: 10.3892/mmr.2017.7443. [DOI] [PubMed] [Google Scholar]
- 10.Nagashima S, Tokuyama T, Yonashiro R, Inatome R, Yanagi S. Roles of mitochondrial ubiquitin ligase MITOL/MARCH5 in mitochondrial dynamics and diseases. J Biochem. 2014;155:273–279. doi: 10.1093/jb/mvu016. [DOI] [PubMed] [Google Scholar]
- 11.Tang H, Peng S, Dong Y, Yang X, Yang P, Yang L, Yang B, Bao G. MARCH5 overexpression contributes to tumor growth and metastasis and associates with poor survival in breast cancer. Cancer Manag Res. 2018;11:201–215. doi: 10.2147/CMAR.S190694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigó R, Hubbard TJ. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji Y, Wang M, Li X, Cui F. The long noncoding RNA NEAT1 targets miR-34a-5p and drives nasopharyngeal carcinoma progression via Wnt/β-Catenin signaling. Yonsei Med J. 2019;60:336–345. doi: 10.3349/ymj.2019.60.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Zhou J, Liu J, Wu G, Yu Y, Zhu H, Liu J. LncRNA ANCR promotes proliferation and radiation resistance of nasopharyngeal carcinoma by inhibiting PTEN expression. Onco Targets Ther. 2018;11:8399–8408. doi: 10.2147/OTT.S182573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang TS, Zheng YJ, Wang J, Zhao JY, Yang DK, Liu ZS. MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2. J Exp Clin Cancer Res. 2019;38:97. doi: 10.1186/s13046-019-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Zheng YQ, Bai YF, Yang S, Cui YR, Wang YP, Hu WL. MircoRNA-629 promotes proliferation, invasion and migration of nasopharyngeal carcinoma through targeting PDCD4. Eur Rev Med Pharmacol Sci. 2019;23:207–216. doi: 10.26355/eurrev_201901_16766. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Meng Y, Zhang Z, Yan Q, Jiang X, Lv Z, Hu L. MARCH5 RNA promotes autophagy, migration, and invasion of ovarian cancer cells. Autophagy. 2017;13:333–344. doi: 10.1080/15548627.2016.1256520. [DOI] [PMC free article] [PubMed] [Google Scholar]


