Abstract
MicroRNA-145-5p downregulation has been shown to play important roles in the oncogenesis and progression of many cancer types including glioblastoma (GBM). However, the potential role of serum miR-145-5p in the diagnosis and prognosis of glioblastoma (GBM) remains poorly known. This study was designed to explore the clinical significance of serum miR-145-5p in patients with GBM. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was carried out to measure the serum levels of miR-145-5p in 117 GBM patients, 52 grade I/II glioma patients and 50 healthy volunteers. The associations between serum miR-145-5p level and the clinical variables as well as prognosis were analyzed. The bioinformatic analysis of the downstream targets of miR-145-5p was also performed. Compared to grade I/II glioma patients and healthy controls, serum miR-145-5p levels were significantly decreased in GBM patients. In addition, the receiver operating characteristic (ROC) analysis demonstrated that serum miR-145-5p might be a reliable diagnostic marker of GBM with an AUC of 0.895, combing with 84.6% sensitivity and 78.0% specificity. Low serum miR-145-5p level had significant correlation with aggressive clinicopathological parameters. Moreover, the Kaplan-Meier curve revealed that patients in the high serum miR-145-5p group survived significantly longer than those in the low serum miR-145-5p group. Multivariate analysis confirmed that serum miR-145-5p expression was an independent prognostic indicator for overall survival. The bioinformatic analysis revealed that many downstream genes and pathways that miR-145-5p regulated were closely associated with the initiation and development of cancer. Taken together, decreased serum miR-145-5p is a promising diagnostic and prognostic biomarker for GBM.
Keywords: miR-145-5p, prognosis, serum, glioblastoma
Introduction
Glioblastoma (GBM) is an extremely aggressive form of glioma, which is the most frequent type of primary brain tumor in human adults [1,2]. Though great efforts have been made to improve the clinical outcome of GBM by adopting multimodal therapy over the past decades, patients with GBM still experience poor prognosis with a median survival of only 12-14 months [3,4]. One of the main reasons is the lack of specific and reliable biomarkers in clinical practice. Therefore, identifying easily accessible and effective biomarkers for this disease are urgently needed to improve the diagnosis and the prognosis of GBM patients.
MicroRNAs (miRNAs) are single-stranded, noncoding regulatory RNA molecules that modulate the expression of multiple target genes at the post-transcriptional level by binding to their 3’-untranslated regions (3’ UTR) [5,6]. Growing evidence has shown miRNAs play important roles in diverse cellular biological processes including, but not limited to, cell proliferation, differentiation, migration and apoptosis [7,8]. miRNAs are abnormally expressed in different human cancers and might function as oncogenes or tumor suppressors [9]. Up to now, several circulating miRNAs have been found to be dysregulated in the serum or plasma of GBM patients. For instance, serum miR-137 level was significantly decreased in patients with GBM and was negatively associated with aggressive clinical parameters [10]. Similarly, Wang et al revealed reduced serum miR-485-3p predicted an unfavorable prognosis and was an independent prognostic factor for GBM [11].
miR-145-5p had been shown to play a tumor suppressive role in many cancer types, such as GBM [12], non-small cell lung cancer [13,14], gastric cancer [15,16], prostate cancer [17,18], melanoma [19], esophageal squamous cell carcinoma [20], renal cell carcinoma [21]. However, the clinical significance of serum miR-145-5p in GBM had not yet been investigated. In this study, serum miR-145-5p levels in GBM patients were detected, then the associations between serum miR-145-5p and clinical variables and as well as prognosis were subsequently analyzed.
Materials and methods
Study population
A total of 117 cases with GBM, comprising 85 men and 32 women, were enrolled in this study. Moreover, 52 subjects diagnosed with a low-grade glioma (I/II) and 50 healthy volunteers were recruited as the control groups, respectively. Of all the GBM patients, 86 patients received total resection while 31 patients received partial resection. Ninety-nine cases (84.6%) died during the follow-up period, with a median overall survival (OS) duration of 15.84 months. Details of clinical characteristics of the patients were presented in Table 1. OS was measured from the day of diagnosis until death from any cause or last follow up. The study was approved by Ethics Committee of the Second Hospital of Lanzhou University, and written informed consent was collected from all patients.
Table 1.
Serum miR-145-5p expression and clinicopathologic parameters of 117 GBM patients
| Clinical variables | No. of cases | Low miR-145-5p | High miR-145-5p | P |
|---|---|---|---|---|
| Age | 0.0878 | |||
| <50 | 39 | 17 | 22 | |
| ≥50 | 78 | 47 | 31 | |
| Sex | 0.8364 | |||
| Men | 85 | 46 | 39 | |
| Women | 32 | 18 | 14 | |
| KPS | 0.0183 | |||
| <70 | 24 | 8 | 16 | |
| ≥70 | 93 | 56 | 37 | |
| IDH1 mutation | 0.0126 | |||
| Wild type | 78 | 49 | 29 | |
| Mutated | 39 | 15 | 24 | |
| Extent of resection | 0.0030 | |||
| Total | 86 | 40 | 46 | |
| Partial | 31 | 24 | 7 | |
| Radioresistance | 0.0387 | |||
| Yes | 38 | 26 | 12 | |
| No | 79 | 38 | 41 |
Blood collection and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Blood (7 ml) was withdrawn from all participants and collected in EDTA-K2 tubes. Blood samples were separated by centrifugation at 3000 g for 10 min, and then centrifuged at 12000 rpm for 5 min. The supernatant was transferred to RNase/DNase free tubes and stored at -80°C until use. Total RNA was isolated from the blood samples using a mirVana PARIS isolation kit (Ambion, Austin, Texas, USA), 25 fmol/ml of synthesized cel-miR-39 (Qiagen, Hilden, Germany) was spiked in after adding the denaturing solution to the blood samples before RNA extraction. The quantity and concentration of RNA were spectrophotometrically assessed by measuring absorbance at A260/280. Quantitative RT-PCR was run on a LightCycler 480 II System (Roche Diagnostics GmbH, Mannheim, Germany) using SYBR Green PCR Mix (Takara, Dalian, China). Triplicates were performed for all qRT-PCR reactions. The relative serum miR-145-5p levels were calculated by the comparative 2-ΔΔCt method using cel-miR-39 level for normalization.
Statistical analysis
All statistical analyses were implemented using MedCalc V15.2 (MedCalc Software, Ostend, Belgium). Statistical comparison between groups was performed with Kruskal-Wallis test. Chi-square analysis was conducted to compare the distinction of categorical variables. Receiver-operating characteristic (ROC) curves were derived and area under the curve (AUC) analysis was performed to assess the diagnostic feasibility of serum miR-145-5p. Multivariate logistic analysis for the correlation with the risk of OS was tested for serum miR-145-5p expression and several clinical parameters. Survival curves were constructed using the Kaplan-Meier method, and differences were estimated using the log-rank test. For the bioinformatic analysis, the targeted gene of miR-145-5p was obtained miRWalk2.0. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) database. STRING online database and Cytoscape software (Version 3.7.0, Institute of Systems Biology, Seattle, WA, USA) were used for the protein-protein interaction (PPI) analysis. Differences were considered statistically significant at the level of P<0.05.
Results
Downregulation of serum miR-145-5p in GBM patients
We first detected the levels of serum miR-145-5p in all the participants using qRT-PCR. As shown in Figure 1, serum miR-145-5p levels in GBM patients were greatly lower than those in patients with grade I/II gliomas and the control group (P=0.008). Furthermore, the levels of miR-145-5p were significantly higher in healthy controls compared to patients with grade I/II glioma (P=0.022).
Figure 1.
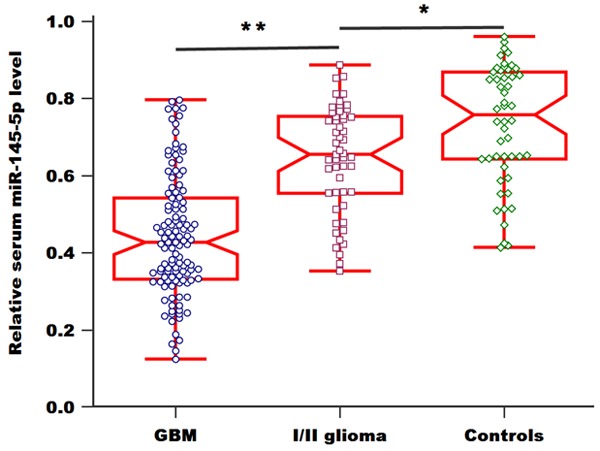
Relative levels of serum miR-145-5p in GBM patients, grade I/II glioma patients and healthy controls.
Subsequently, ROC analysis revealed that serum miR-145-5p could distinguish GBM patients from grade I/II glioma patients. The AUC of serum miR-145-5p was 0.825, and the sensitivity and specificity were 76.1% and 76.9%, respectively (Figure 2A). Moreover, the ROC curve data showed an increased AUC value of 0.895, with a sensitivity of 84.6% and a specificity of 78.0%, indicating serum miR-145-5p could effectively discriminate GBM patients from healthy controls (Figure 2B).
Figure 2.
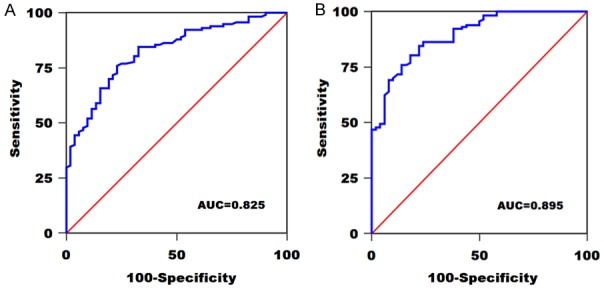
A. ROC curve of serum miR-145-5p in distinguishing GBM patients and grade I/II glioma patients. B. ROC curve of serum miR-145-5p in distinguishing GBM patients and healthy controls.
Relationship between serum miR-145-5p and clinicopathologic features of GBM patients
To analyze the role of serum miR-145-5p in the progression of GBM, its correlation with clinicopathologic data of GBM patients was evaluated. The clinical variables included age, sex, Karnofsky performance status (KPS), isocitrate dehydrogenase 1 (IDH1) mutation, extent of resection and raidoresistance. We divided all cases into low (below the median, n=64) and high (above the median, n=53) serum miR-145-5p expression groups based on the median value of serum miR-145-5p levels. The results demonstrated that reduced serum miR-145-5p expression was closely associated with KPS (P=0.0183), IDH1 mutation (P=0.0126), extent of resection (P=0.0030) and raidoresistance (P=0.0387). Conversely, no significant relationship was observed between serum miR-145-5p expression and age, sex (both P>0.05) (Table 1).
Prognostic value of serum miR-145-5p in GBM patients
Kaplan-Meier survival analysis was performed to evaluate the relationship between serum miR-145-5p level and OS of GBM patients. GBM patients in the high serum miR-145-5p group had prolonged OS in comparison with those in low serum miR-145-5p group (Figure 3A, P<0.0001). For the patients with total resection, patients in low serum miR-145-5p group had worse OS (Figure 3B, P<0.0001). Likewise, for the patients with partial resection, those with higher serum miR-145-5p expression had significantly longer OS than those with the lower serum miR-145-5p expression (Figure 3C, P=0.0008).
Figure 3.
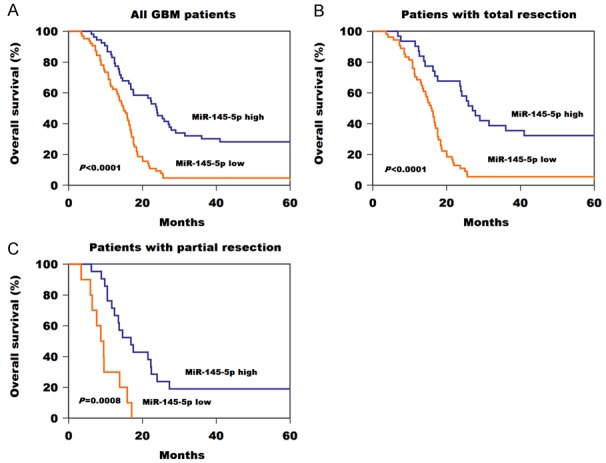
A. Kaplan-Meier survival curves for GBM patients according to serum miR-145-5p level. B. Kaplan-Meier survival curves for GBM patients with total resection according to serum miR-145-5p level. C. Kaplan-Meier survival curves for GBM patients with partial resection according to serum miR-145-5p level.
Multivariate Cox regression analysis was used to determine the influence of serum miR-145-5p levels and clinical parameters (KPS, IDH1 mutation, extent of resection) on patient survival. KPS (risk ratio =3.84; 95% CI, 1.25-6.58; P=0.017), IDH1 mutation (risk ratio =4.15; 95% CI, 1.46-6.97; P=0.012), extent of resection (risk ratio =5.21; 95% CI, 1.81-8.94; P=0.003) and serum miR-145-5p expression (risk ratio =4.72; 95% CI, 1.67-8.05; P=0.008) were independent prognostic factors for OS (Table 2).
Table 2.
Multivariate analysis of the prognostic values of factors on OS in GBM
| Characteristics | Risk Ratio | 95% CI | P | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| KPS | 3.84 | 1.25 | 6.58 | 0.017 |
| IDH1 mutation | 4.15 | 1.46 | 6.97 | 0.012 |
| Extent of resection | 5.21 | 1.81 | 8.94 | 0.003 |
| Serum miR-145-5p | 4.72 | 1.67 | 8.05 | 0.008 |
CI, Confidence Interval.
Bioinformatic analysis
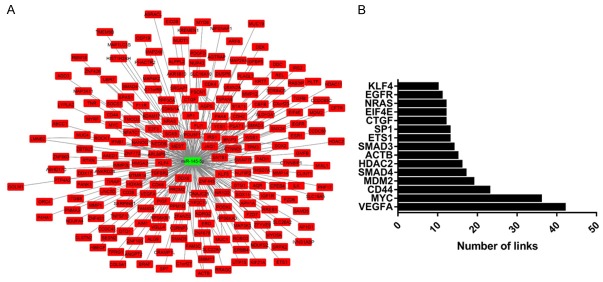
Our results revealed that GO: 0045944~positive regulation of transcription from RNA polymerase II promoter, GO: 0008284~positive regulation of cell proliferation, GO: 0045893~positive regulation of transcription, DNA-templated, GO: 0006366~transcription from RNA polymerase II promoter, GO: 0001666~response to hypoxia, GO: 0035019~somatic stem cell population maintenance, GO: 0007165~signal transduction, GO: 0010628~positive regulation of gene expression, GO: 0030335~positive regulation of cell migration and GO: 0048870~cell motility were the top ten enriched biological processes (Figure 4A). GO: 0005829~cytosol, GO: 0000790~nuclear chromatin, GO: 0005667~transcription factor complex, GO: 0005654~nucleoplasm, GO: 0005737~cytoplasm, GO: 0001726~ruffle, GO: 0071141~SMAD protein complex, GO: 0005913~cell-cell adherens junction, GO: 0005634~nucleus and GO: 0070062~extracellular exosome were the top ten enriched cellular component (Figure 4B). GO: 0003700~transcription factor activity, sequencespecific DNA binding, GO: 0005515~protein binding, GO: 0043565~sequence-specific DNA binding, GO: 0004672~protein kinase activity, GO: 0019899~enzyme binding, GO: 0098641~cadherin binding involved in cell-cell adhesion, GO: 0046934~phosphatidylinositol-4,5-bisphosphate 3-kinase activity, GO: 0004713~protein tyrosine kinase activity, GO: 0031435~mitogen-activated protein kinase kinase kinase binding and GO: 0005178~integrin binding were the top ten enriched molecular function (Figure 4C). For the KEGG pathway analysis, bladder cancer, proteoglycans in cancer, pathways in cancer, pancreatic cancer, FoxO signaling pathway, ErbB signaling pathway, signaling pathways regulating pluripotency of stem cells, PI3K-Akt signaling pathway, MicroRNAs in cancer and TGF-beta signaling pathway were the top enriched pathways (Figure 4D). In addition, Figure 5A revealed the downstream genes of miR-145-5p. Downregulation of miR-145-5p (green color) might result in upregulation of many targeted genes (red color). PPI analysis revealed the central nodes of the gene network that miR-145-5p regulated (Figure 5B).
Figure 4.
GO and KEGG analysis of the downstream genes of miR-145-5p.
Figure 5.
The downstream targeted genes regulated by miR-145-5p (A); The central nodes of PPI network (B).
Discussion
In our study, we demonstrated that serum miR-145-5p levels were decreased in GBM patients compared to patients with grade I/II gliomas as well as healthy controls. ROC curve analysis showed that serum miR-145-5p could well identify GBM patients from grade I/II glioma patients and healthy controls with a relative high AUC value. Moreover, downregulation of serum miR-145-5p was strongly correlated with KPS, IDH1 mutation, extent of resection and radioresistance. Also, patients in high serum miR-145-5p group had longer OS than those in low serum miR-145-5p group. Furthermore, serum miR-145-5p was identified as an independent prognostic indicator for GBM patients. The bioinformatic analysis revealed that many downstream genes and pathways that miR-145-5p regulated were closely associated with the initiation and development of cancer. These results suggested that low serum miR-145-5p might serve as a promising biomarker for the diagnosis and prognosis of GBM. Consistent with our results, Zhao et al showed reduced serum miR-145-5p levels were strongly correlated with shorter 2-year overall survival and disease-free survival, as well as a poor GBM prognosis [12].
The findings of this study also were in line with those from previous studies on different types of cancer. For instance, miR-145-5p expression was markedly decreased in non-small cell lung cancer (NSCLC) tissues compared with that in normal tissues, and downregulation of miR-145-5p was associated with lymph node metastasis [13]. Similarly, Chang et al showed miR-145-5p overexpression greatly inhibited epithelial-mesenchymal transition (EMT) by targeting mitogen-activated protein kinase kinase kinase 1 (MAP3K1), MAP3K1 upregulation reversed the anti-tumorigenic effect in NSCLC cells [14]. In gastric cancer (GC), miR-145-5p levels were significantly decreased in primary GC tissues. miR-145-5p overexpression markedly decreased GC cell invasion and EMT via targeting zinc-finger E-box binding homeobox 2 (ZEB2) and N-cadherin. Moreover, low miR-145-5p expression was found to be strongly correlated with worse clinical variables and shortened survival time [15,16]. Also, Ozem and colleagues demonstrated that upregulation of miR-145-5p dramatically inhibited cell proliferation, suppressed cell migration and induced cell apoptosis of prostate cancer (PCa), and SOX2 was identified as its downstream target [17]. Xu et al revealed miR-145-5p expression was reduced both in PCa tissues and cells, restoration of miR-145-5p restrained the carcinogenesis by silencing Fascin-1 expression [18]. In melanoma, Liu et al reported enhanced miR-145-5p expression caused the inhibition of cell proliferation, migration, and stimulation of cell apoptosis in vitro, as well as the repression of tumorigenesis in xenograft models [19]. In esophageal squamous cell carcinoma (ESCC), in vitro evidence showed miR-145-5p overexpression significantly inhibited tumorigenic effects of cancer cells and decreased the expressions of cell cycle genes by downregulating the Sp1/NF-κB signaling pathway [20]. Moreover, Liep et al revealed cooverexpression of miR-145-5p and miR-141-3p greatly reduced cell migration and invasion of renal cell carcinoma [21].
However, our study still had several limitations. Firstly, the sample size was relatively small. Further research with a larger cohort of patients is needed to validate our findings. Secondly, this miRNA was only measured in blood samples and not in comparison with the expression in tissue specimens. Finally, the molecular mechanism underlying the role of miR-145-5p in GBM needs further exploration.
In summary, we found that serum miR-145-5p levels were reduced in GBM patients and associated with aggressive clinicopathological variables. More importantly, low serum miR-145-5p could well distinguish GBM from healthy volunteers and predict shorter survival time. Based on these findings, serum miR-145-5p might serve as a noninvasive biomarker for early detection and prognostic prediction of GBM.
Acknowledgements
This study was supported by the funding from the Second Hospital of Lanzhou University and Lanzhou Modern Vocational College.
Disclosure of conflict of interest
None.
References
- 1.Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82. doi: 10.1016/j.pharmthera.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67:279–283. doi: 10.1001/archneurol.2010.5. [DOI] [PubMed] [Google Scholar]
- 4.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G German Glioma Network. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11:145–156. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Stahlhut C, Slack FJ. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Med. 2013;5:111. doi: 10.1186/gm516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farazi TA, Spitzer JI, Morozov P, Tuschl T. MiRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li HY, Li YM, Li Y, Shi XW, Chen H. Circulating microRNA-137 is a potential biomarker for human glioblastoma. Eur Rev Med Pharmacol Sci. 2016;20:3599–3604. [PubMed] [Google Scholar]
- 11.Wang ZQ, Zhang MY, Deng ML, Weng NQ, Wang HY, Wu SX. Low serum level of miR-485-3p predicts poor survival in patients with glioblastoma. PLoS One. 2017;12:e0184969. doi: 10.1371/journal.pone.0184969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Shen J, Hodges TR, Song R, Fuller GN, Heimberger AB. Serum microRNA profiling in patients with glioblastoma: a survival analysis. Mol Cancer. 2017;16:59. doi: 10.1186/s12943-017-0628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gan TQ, Xie ZC, Tang RX, Zhang TT, Li DY, Li ZY, Chen G. Clinical value of miR-145-5p in NSCLC and potential molecular mechanism exploration: a retrospective study based on GEO, qRT-PCR, and TCGA data. Tumour Biol. 2017;39:1010428317691683. doi: 10.1177/1010428317691683. [DOI] [PubMed] [Google Scholar]
- 14.Chang Y, Yan W, Sun C, Liu Q, Wang J, Wang M. MiR-145-5p inhibits epithelial mesenchymal transition via the JNK signaling pathway by targeting MAP3K1 in non-small cell lung cancer cells. Oncol Lett. 2017;14:6923–6928. doi: 10.3892/ol.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang SB, He XJ, Xia YJ, Hu WJ, Luo JG, Zhang J, Tao HQ. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. Onco Targets Ther. 2016;9:2305–2315. doi: 10.2147/OTT.S101853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Zhang Y, Wen X, Hu XL, Cheng LZ, Yu JY, Wei ZB. Downregulation of miR-145-5p correlates with poor prognosis in gastric cancer. Eur Rev Med Pharmacol Sci. 2016;20:3026–3030. [PubMed] [Google Scholar]
- 17.Ozen M, Karatas OF, Gulluoglu S, Bayrak OF, Sevli S, Guzel E, Ekici ID, Caskurlu T, Solak M, Creighton CJ, Ittmann M. Overexpression of miR-145-5p inhibits proliferation of prostate cancer cells and reduces SOX2 expression. Cancer Invest. 2015;33:251–258. doi: 10.3109/07357907.2015.1025407. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Chang J, Du X, Hou J. Long non-coding RNA PCAT-1 contributes to tumorigenesis by regulating FSCN1 via miR-145-5p in prostate cancer. Biomed Pharmacother. 2017;95:1112–1118. doi: 10.1016/j.biopha.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Gao G, Yan D, Chen X, Yao X, Guo S, Li G, Zhao Y. Effects of miR-145-5p through NRAS on the cell proliferation, apoptosis, migration, and invasion in melanoma by inhibiting MAPK and PI3K/AKT pathways. Cancer Med. 2017;6:819–833. doi: 10.1002/cam4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J, Shi ZZ. MiR-145-5p suppresses tumor cell migration, invasion and epithelial to mesenchymal transition by regulating the Sp1/NF-κB signaling pathway in esophageal squamous cell carcinoma. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18091833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liep J, Kilic E, Meyer HA, Busch J, Jung K, Rabien A. Cooperative effect of miR-141-3p and miR-145-5p in the regulation of targets in clear cell renal cell carcinoma. PLoS One. 2016;11:e0157801. doi: 10.1371/journal.pone.0157801. [DOI] [PMC free article] [PubMed] [Google Scholar]