Abstract
Background: Timely endothelial repair after intervention-associated vascular injury is critical to prevent restenosis and thrombosis. Compared to living cell therapies, exosomes may be a better alternative. Thus, we aimed to compare the role of exosomes derived from human umbilical vein endothelial cells (HUVECs) versus those derived from endothelial progenitor cells (EPCs) in vascular endothelial repair. Material/methods: Exosomes secreted from HUVECs and EPCs were isolated and characterized respectively. In vitro, the effects of the two types of exosomes on migration and proliferation of endothelial cells were studied. In vivo, rats were systemically treated with the two exosome groups respectively after carotid artery endothelial injury induced by a balloon. The efficacy in promoting re-endothelialization was measured by Evans blue dye and histological examination. Results: Both types of exosomes, sized from 30 nm to 100 nm, had sphere-shaped or cupped morphology, and expressed CD63, CD9, and CD81; but the total yield of exosomes (particles/mL) based on number of cells, was 4.2 times higher from EPCs than HUVECs. Compared with control treatment, both exosome treated groups manifested significant enhancement of migration and proliferation in vitro and vascular recovery at an early stage in vivo. The two exosome-treated groups, however, did not statistically significantly differ. Conclusion: In conclusion, our results indicated that exosomes derived from HUVECs and EPCs had similar morphology, size distributions and characteristics, but those derived from EPCs were more abundant with comparable biologic activity. Therefore, EPCs may be a robust source of exosomes to promote vascular repair.
Keywords: Exosomes, endothelial cells, re-endothelialization, HUVECs, endothelial progenitor cells
Introduction
Endovascular therapies are widely used techniques to treat patients with cardiovascular disease [1-3]. However, such procedures are associated with an approximately 30% risk of lumen restenosis during the first 3 months after intervention, even with drug-eluting balloons [4]. Intervention-related vascular endothelial cell damage is a key factor in the initiation and progression of restenosis [5]. Vascular endothelial injury may bring about potential changes such as inflammation, thrombosis, and smooth muscle cell proliferation, which cause neointimal hyperplasia, adverse arterial remodeling, and restenosis [5,6]. Thus, timely promotion of endothelial cell (ECs) repair is crucial to prevent progression of restenosis.
Recent studies have revealed that paracrine-mediated migration of ECs from the adjacent healthy endothelium may be important in endothelial regeneration after damage [5,7,8]. Exosomes, an important component of cellular paracrine secretion, are eliciting interest for biotherapeutics in endothelial cell repair and angiogenesis [5,9-11]. Exosomes originate from multivesicular bodies (MVBs), with small bilipid membranes and 40-150 nanometers size [12]. They play critical roles in mediating intercellular communication by shuttling mRNAs and proteins and altering gene expression and cellular behavior of the recipient cells. Compared with classic cell-based therapies, administration of exosomes reduces the risk of thrombosis, unwanted immune responses, or tumorigenesis [13,14].
Identification of ideal cell types for exosome isolation is important in developing exosome-based therapy for vascular endothelial cell injury. Recent studies show that exosomes secreted from human coronary artery endothelial cells (HCAECs) and human microvascular endothelial cell line (HMEC-1) containing miRNAs could be internalized by recipient ECs to regulate gene expression and stimulate ECs proliferation and migration [5,9]. However, HCAECs and HMEC-1 are hard to obtain, since harvesting of coronary artery and microvascular tissue may be invasive [15]. In addition, unwanted cell types often contaminate ECs obtained from fat [16].
In contrast, EPCs can be obtained in a non-invasive way [15]. After harvest of peripheral blood and cord blood, mononuclear cells have been isolated and induced to EC-like cells ex vivo [15,17]. Moreover, EPCs are better at cell proliferation and sensitivity to angiogenic factors compared to adult mature vascular endothelial cells [18].
To date, there is no direct comparison of exosomes derived from ECs and EPCs in terms of their features, as well as their therapeutic effects. In this study, we set out to compare exosomes derived from HUVECs and EPCs in vivo and in vitro to identify a robust source of exosomes to promote vascular repair.
Materials and methods
Exosome isolation
Exosomes from HUVECs and EPCs were collected as described [9]. When grown up to approximately 70% to 80% confluence, HUVECs and EPCs (Lonza) were cultured in serum-free EBM-2 medium (Lonza) for 24-40 hours. The conditioned media of both cell lines were collected and the number of both cell lines was counted. Collected culture medium was first centrifuged at 2,000 × g (Beckman Coulter) for 20 minutes to remove cellular debris, then collected by differential centrifugation (Hitachi), 10,000 × g for 30 minutes, and 100,000 × g for 120 minutes. The pelleted exosomes were resuspended with 15 ml PBS and filtered with a 0.22 μm filter (Millipore), then centrifuged at 4,000 × g to 200 μL.
Exosome identification
Exosome size and concentration were measured by NTA (Nanosight NS300, Malvern, UK). Sample that were 1000-fold diluted were pipetted with a 1 mL syringe and slowly pushed into the sample chamber. The average of the three movie recordings captured with 20 × magnification, 30 frames per second, was taken to measure the size and concentration of the exosomes. The morphologies of both exosomes were observed by transmission electron microscopy (TEM) (Tecnai G2, Spirit Biotwin, USA) as previously described [19]. Western blot was used to analyze the characteristic surface markers of exosomes including CD63, CD9, and CD81. Briefly, exosome samples (total protein of 30 μg/lane) were separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis, then transferred to polyvinylidene fluoride membranes (Millipore, USA). The membranes were blocked with 5% BSA in TBST (TBS with 0.2% Tween-20) for 2 h, following by incubation with primary antibodies against CD63, CD9, and CD81 (Abcam, UK) at 4°C overnight. Then they were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. The immunoreactive protein bands were visualized using an ECL detection kit (Thermo Fisher Scientific) and imaged with the ChemiDoc XRS Plus Luminescent Image Analyser (Bio-Rad).
Measurement of uptake of both exosomes by HUVECs
To observe exosome uptake by HUVECs, we stained the exosomes from HUVECs and EPCs with Vybrant DiO dye (Molecular Probes) following the manufacturer’s protocol, then added them (1 × 1011 particles/mL) to the medium of HUVECs and incubated for 2 hours at 37°C [19]. The cells were then washed with PBS and fixed in 4% paraformaldehyde for 20 minutes, then stained with DAPI for 5 minutes at room temperature. After washing, cells were observed using a laser confocal fluorescence microscope (Leica Microsystems, Wetzlar, Germany) [10].
Cell proliferation assay
HUVECs at an initial density of 1.5 × 103 cells/well were seeded into 96-well plates and cultured in serum-free EGM media with 1 × 1011 particles/mL of exosomes from HUVECs, EPCs, or PBS, respectively. At days 0, 1, 2, and 3, 20 μl MTT reagent (0.5 mg/ml) was added to each well media. Then cells were cultured for another 4 h at 37°C, and 150 μL dimethyl sulfoxide was added to each well. The absorbance representing the proliferation of HUVECs, was measured at 490 nm.
Migration assay
HUVEC migration was evaluated by scratching the confluent HUVEC cell layer (6 × 104 cells/well) in a 12-well plate with a 200 μL pipette tip. The detached cells were washed with PBS, and cultured in 500 mL of serum-free medium with exosomes (1 × 1011 particles/mL) from HUVECs or EPCs, or without exosomes for 24 hours. Cell migration was assessed by light microscopy at magnification × 100 (Olympus Corporation, Tokyo, Japan) and Image-Pro Plus software.
Rat model of balloon-induced vascular injury and treatment
All procedures were approved by the Animal Research Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Female SD rats (200-250 g) were intraperitoneally injected with 50 mg/kg pentobarbital for anesthesia and 100 U/kg heparin sodium for heparinization. After exposing the left common carotid artery, the left internal carotid artery, and external carotid artery, we ligated the distal external carotid artery and temporarily occluded the common carotid artery and internal carotid artery using artery clips. For endothelial injury, a 2F Fogarty arterial embolectomy balloon catheter (Edwards Lifesciences) was inserted through the internal carotid artery to the common carotid artery. The balloon was slowly distended with saline and was pulled back with rotation three times. The total length of denudation was about 5-6 mm from the bifurcation of the carotid arteries. Following the wire removal, the external carotid artery near the bifurcation was ligated permanently, and the artery clamps in the internal carotid artery and common carotid artery were taken away to restore antegrade blood flow. After injury for 2 h and 24 h, the two experimental groups were injected with 2 × 1012 exosome particles from HUVECs or EPCs dissolved in 200 μL of PBS respectively, and the control group was injected with an equal volume of PBS through the tail vein.
Evans blue dye analysis of rat carotid artery re-endothelialization
On day 7, the rats were anesthetized according to the method described above. After intravenous injection of the Evans blue dye (3%), dye was allowed to circulate for 10 minutes. Then 2 mL of 0.9% NaCl was administered to wash away the unbound Evans blue dye. The left common carotid artery was harvested after rats were euthanized by anesthesia. The area stained blue represents nonendothelialization, while the area not stained represents reendothelialization. Images were observed using a stereo microscope (Leica M205 FA). Re-endothelialization is expressed as the ratio of the remaining area to the total area.
Histologic analysis
On day 7, the left common carotid artery of both the exosome-treated groups and the control group were harvested. Then the samples were fixed in 10% formalin for 10 minutes, followed by dehydration and embedding in paraffin. Five transverse sections of 5 μm thickness of each sample were collected, and stained with hematoxylin and eosin according to standard protocols. The area of intima and media, and the ratio of intimal and medial area (I/M) were observed and measured with a Nikon E200 microscope and Image-Pro Plus 6 software.
Statistical analysis
SPSS 18 was used for statistical analysis. All experiments were carried out with at least three replicates per group, and in vitro experiments were repeated three times. Data are expressed as means ± standard deviation (SD). Differences were compared by one-way analysis of variance (ANOVA). P < 0.05 was considered significant.
Results
Characterization of exosomes
Characterization of exosomes derived from HUVECs and EPCs was determined by NTA, TEM, and western blot. NTA measurement showed that the size of both exosome groups ranged from approximately 30 nm to 100 nm (Figure 1A). For comparison of exosome yields from HUVECs and EPCs, total cell counts were adjusted. The results of NTA revealed that the particles/mL of exosomes from EPCs was 4.2 times higher than those from HUVECs, independent of exosome size (95% CI 1.58 × 1011, 8.22 × 1012, P < 0.001). TEM showed that both exosomes exhibited a sphere-shaped or cupped morphology (Figure 1B). Western blotting indicated the expression of exosomal surface markers including CD63, CD9, and CD81 in both groups (Figure 1C).
Figure 1.
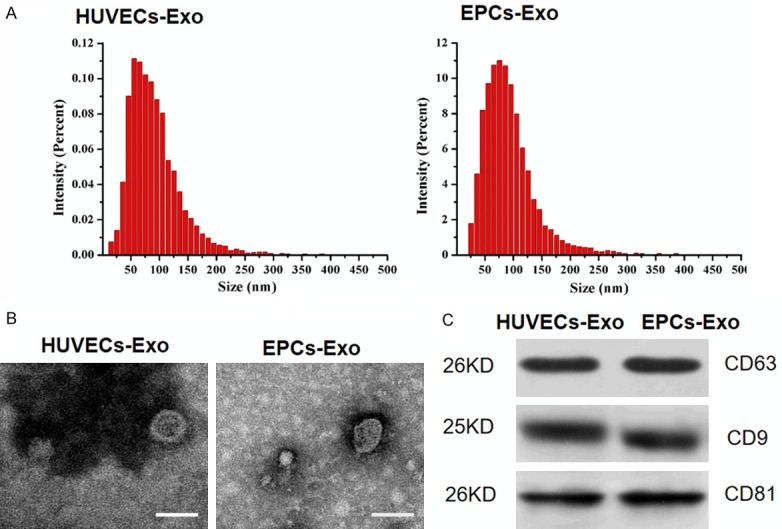
Characterization of HUVEC-derived exosomes (HUVECs-Exo) and EPC-derived exosomes (EPCs-Exo). A. Particle size distribution of HUVECs-Exo and EPCs-Exo was measured by NTA. B. Morphology of HUVECs-Exo and EPCs-Exo was observed with transmission electron microscopy. Scale bar: 50 nm. C. Western blot analysis of exosomal surface markers CD63, CD9, and CD81 in HUVECs-Exo and EPCs-Exo.
Endothelial cell internalization of exosomes derived from HUVECs and EPCs
To determine whether exosomes from HUVECs and EPCs could be internalized by ECs, HUVECs (as an example) were cultured with the DiO-labeled exosomes derived from HUVECs and EPCs for 2 hours. Fluorescence microscopy showed that the labeled exosomes were transferred to the perinuclear region of HUVECs, demonstrating that the EPCs and HUVECs exosomes were incorporated into HUVECs (Figure 2).
Figure 2.
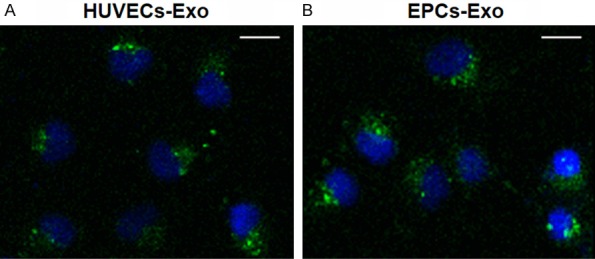
Fluorescence microscopy analysis of Vybrant DiO-labeled HUVECs-Exo and EPCs-Exo internalization by HUVECs. The green-labeled exosomes are visible in the perinuclear region of HUVEC proliferation. *P < 0.05. Scale bar: 50 μm.
Exosomes derived from HUVECs and EPCs promote endothelial target cell proliferation and migration in vitro
The effect of exosomes secreted from HUVECs and EPCs on proliferation of HUVECs was examined by MTT analysis. Exosomes from both groups could significantly promote the proliferation of HUVECs compared to controls (P < 0.05), but there was no significant difference between the two exosome treatment groups (P > 0.05) (Figure 3B). The role of exosomes derived from HUVECs and EPCs in ECs migration was determined by scratch wound assay. Both exosome treatment groups markedly enhanced the motility and migration of HUVECs compared to controls (55.46 ± 8.7%, 65.41 ± 4.1% versus 30.26 ± 4.8%; P < 0.001, P = 0.002) (Figure 3A), but there was no significant difference between the two exosome-treated groups (P = 0.096) (Figure 3A).
Figure 3.
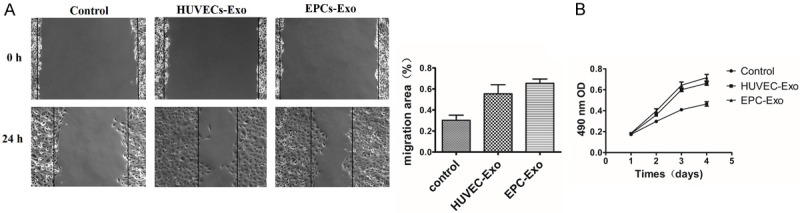
A. A scratch wound assay demonstrated that HUVECs treated with HUVECs-Exo and EPCs-Exo had increased migration compared to the control group. B. MTT analysis of exosomes derived from HUVECs and EPCs in promoting HUVEC proliferation. *P < 0.05.
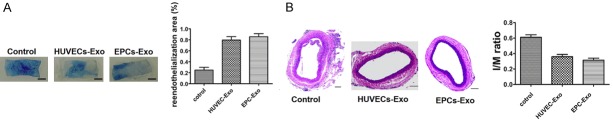
EPCs exosomes promote re-endothelialization after vascular injury
The result of endothelial repair in injured arteries on postoperative day 7 was assessed by Evans blue staining (Figure 4A). The re-endothelialized area appeared white, whereas nonendothelialized lesions were stained blue. The re-endothelialization index was expressed by the ratio of white area to total area. Both exosome treatments significantly accelerated re-endothelialization of the injured arteries compared with the PBS control (79.57 ± 6.2%, 85.61 ± 5.6% versus 24.87 ± 5.1%; n = 4; P < 0.001), but there was no significant difference between the two exosome treatment groups (P = 0.239). Quantitative analysis of intimal area/medial area ratios (I/M) in the lumen of injured vessels revealed that neointimal hyperplasia was significantly reduced by treatment with both exosomes compared with the control (0.31 ± 0.03, 0.36 ± 0.02 versus 0.61 ± 0.03; n = 4; P < 0.001), but there was no significant difference between the two exosome treatment groups (P = 0.108) (Figure 4B).
Figure 4.
In vivo effect of exosomes on carotid artery injury. A. Evans blue dye was used to calculate the re-endothelialization ratio. Nonendothelialized lesions are represented by blue staining and the re-endothelialized area displays as white. The index of re-endothelialization was calculated as the white area/total area ratio. Data are displayed as mean ± SD, P < 0.05, Scale bar: 1 mm. B. Hematoxylin-eosin staining of carotid artery sections. The intimal to medial area ratio (I/M) was used to evaluate neointimal hyperplasia and is shown as the mean ± SD, P < 0.05, Scale bar: 100 μm.
Discussion
In this study, we performed the first comparison of the characteristics of exosomes derived from HUVECs and EPCs and their effect on repair of endothelial damage. Our data showed that exosomes derived from both HUVECs and EPCs were similar in morphology, size distribution, and presence of surface markers, while the production of exosomes derived from EPCs was 4.2 times higher than those of HUVECs under the same culture conditions. Moreover, the ability of both exosomes derived from HUVECs and EPCs in expediting re-endothelialization at the early stage after endothelial injury in the rat carotid artery and promoting proliferation and migration of endothelial cells in vitro showed no significant difference. Our results indicated that abundant exosome-derived EPCs had comparable biologic activity. Therefore, EPCs may be a robust source of exosomes in promoting vascular repair.
Recently, the characterization of human EPC-derived exosomes has been described [10,11,19,20] and the first study of HUVECs-derived exosomes was published in 2017 [21]. It has been reported that exosome content and characteristics may be influenced by different cell culture conditions and isolation methods [22-24]. Therefore, in this study, the classic and widely employed methods were selected for both cell lines. We found that total exosome yield from EPCs according to the numbers of cells adjusted was higher than that of HUVECs. This can be attributed to EPCs exhibiting an inordinately higher self-renewal and expansion ratecompared to HUVECs [18].
In this study, exosomes derived from HUVECs and EPCs both improved EC repair after vascular damage in the rat carotid artery in vitro and EC proliferation and migration in vitro. van Balkom et al. [9] reported that endothelial cell-derived exosomes stimulated migration and proliferation of neighboring target cells through miR-214, which repressed the expression of ataxia telangiectasia mutation, and hence prevented senescence and promoted vascularization. Li et al. [10] demonstrated that EPC-derived exosomes promoted vascular endothelial cell repair by increasing expression of angiogenesis-related molecules. Our result is consistent with previous studies [5,9-11]. The underlying mechanism of exosome uptake in murine endothelium was recently investigated. Murine ECs took up intravenously injected exosomes of human origin in a developmental endothelial locus-1 dependent manner [5,25]. The local uptake of exosomes by non-professional phagocytic cells (e.g., ECs) might play a key role in altering the biologic behavior of adjacent cells, such as inhibition of senescence, stimulation of proliferation, and migration of initiating countermeasures against perceived damage [5,9].
Contrary to expectations, with the same number of exosomes of the two groups employed in vivo and in vitro, the results showed no significant difference either in promoting vascular injury or in EC proliferation and migration. We speculate that components such as microRNAs or proteins in exosomes may play a crucial role. Future studies should focus on the components present in exosomes from HUVECs and EPCs that take part in vascular damage repair, and the underlying mechanism.
This study had several limitations. First, the exact mechanisms through which exosomes secreted by HUVECs and EPCs promote endothelial cell recovery remain unclear. Second, the effects of differing concentrations of both exosome sources on in vivo endothelial cell recovery at different timepoints need further study. Third, the effects of both exosome sources on smooth muscle cells (SMCs) have not been investigated. All of these represent important and intriguing avenues for future investigation.
Conclusions
In sum, our results indicate that exosomes originating from both ECs and EPCs may promote vascular repair in rat carotid artery injury. However, total exosome yield (particles/mL) was higher from EPCs than HUVECs. Further studies are needed to investigate the exact mechanisms by which both exosome sources promote vascular repair, in order to develop robust exosome-based therapy for vascular diseases.
Acknowledgements
This study was supported by grant from the National Natural Science Foundation of China (81671791).
Disclosure of conflict of interest
None.
References
- 1.Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Perez-Vizcayno MJ, Byrne RA, Kastrati A, Meier B, Salanti G, Juni P, Windecker S. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015;386:655–664. doi: 10.1016/S0140-6736(15)60657-2. [DOI] [PubMed] [Google Scholar]
- 2.Malyar NM, Reinecke H, Freisinger E. Restenosis after endovascular revascularization in peripheral artery disease. Vasa. 2015;44:257–270. doi: 10.1024/0301-1526/a000440. [DOI] [PubMed] [Google Scholar]
- 3.Pizzolato R, Hirsch JA, Romero JM. Imaging challenges of carotid artery in-stent restenosis. J Neurointerv Surg. 2014;6:32–41. doi: 10.1136/neurintsurg-2012-010618. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt A, Piorkowski M, Werner M, Ulrich M, Bausback Y, Braunlich S, Ick H, Schuster J, Botsios S, Kruse HJ, Varcoe RL, Scheinert D. First experience with drug-eluting balloons in infrapopliteal arteries: restenosis rate and clinical outcome. J Am Coll Cardiol. 2011;58:1105–1109. doi: 10.1016/j.jacc.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D, Vosen S, Franklin BS, Fleischmann BK, Nickenig G, Werner N. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 6.Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular inflammation and repair: implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc Interv. 2011;4:1057–1066. doi: 10.1016/j.jcin.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation. 2010;121:898–905. doi: 10.1161/CIRCULATIONAHA.109.885459. [DOI] [PubMed] [Google Scholar]
- 8.Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93:223–231. doi: 10.1093/cvr/cvr278. [DOI] [PubMed] [Google Scholar]
- 9.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel DM, Stoorvogel W, Wurdinger T, Verhaar MC. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. S3991–3915. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Chen C, Wei L, Li Q, Niu X, Xu Y, Wang Y, Zhao J. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy. 2016;18:253–262. doi: 10.1016/j.jcyt.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Kong J, Wang F, Zhang J, Cui Y, Pan L, Zhang W, Wen J, Liu P. Exosomes of endothelial progenitor cells inhibit neointima formation after carotid artery injury. J Surg Res. 2018;232:398–407. doi: 10.1016/j.jss.2018.06.066. [DOI] [PubMed] [Google Scholar]
- 12.De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5:608. doi: 10.3389/fimmu.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger D, Vinas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD. Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol. 2015;185:2309–2323. doi: 10.1016/j.ajpath.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Huang C, Song B, Xiao Y, Fang M, Feng J, Wang P. CD4+CD25+ regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model. Cell Immunol. 2013;285:62–68. doi: 10.1016/j.cellimm.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Pinto DJ, Erndt-Marino JD, Becerra-Bayona SM, Guiza-Arguello VR, Samavedi S, Malmut S, Reichert WM, Russell B, Hook M, Hahn MS. Evaluation of late outgrowth endothelial progenitor cell and umbilical vein endothelial cell responses to thromboresistant collagen-mimetic hydrogels. J Biomed Mater Res A. 2017;105:1712–1724. doi: 10.1002/jbm.a.36045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arts CH, Hedeman Joosten PP, Blankensteijn JD, Staal FJ, Ng PY, Heijnen-Snyder GJ, Sixma JJ, Verhagen HJ, de Groot PG, Eikelboom BC. Contaminants from the transplant contribute to intimal hyperplasia associated with microvascular endothelial cell seeding. Eur J Vasc Endovasc Surg. 2002;23:29–38. doi: 10.1053/ejvs.2001.1532. [DOI] [PubMed] [Google Scholar]
- 17.Tura O, Skinner EM, Barclay GR, Samuel K, Gallagher RC, Brittan M, Hadoke PW, Newby DE, Turner ML, Mills NL. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. Stem Cells. 2013;31:338–348. doi: 10.1002/stem.1280. [DOI] [PubMed] [Google Scholar]
- 18.Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, Tolla-Le Port C, Leboeuf M, Charbord P, Bikfalvi A, Uzan G. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;103:2577–2584. doi: 10.1182/blood-2003-08-2770. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Chen C, Hu B, Niu X, Liu X, Zhang G, Zhang C, Li Q, Wang Y. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci. 2016;12:1472–1487. doi: 10.7150/ijbs.15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, Fan H. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther. 2018;26:1375–1384. doi: 10.1016/j.ymthe.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao B, Chai Y, Lv S, Ye M, Wu M, Xie L, Fan Y, Zhu X, Gao Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int J Mol Med. 2017;40:1201–1209. doi: 10.3892/ijmm.2017.3106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Royo F, Diwan I, Tackett MR, Zuniga P, Sanchez-Mosquera P, Loizaga-Iriarte A, Ugalde-Olano A, Lacasa I, Perez A, Unda M, Carracedo A, Falcon-Perez JM. Comparative miRNA analysis of urine extracellular vesicles isolated through five different methods. Cancers (Basel) 2016;8 doi: 10.3390/cancers8120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truong G, Guanzon D, Kinhal V, Elfeky O, Lai A, Longo S, Nuzhat Z, Palma C, Scholz-Romero K, Menon R, Mol BW, Rice GE, Salomon C. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells - Liquid biopsies for monitoring complications of pregnancy. PLoS One. 2017;12:e0174514. doi: 10.1371/journal.pone.0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tracy SA, Ahmed A, Tigges JC, Ericsson M, Pal AK, Zurakowski D, Fauza DO. A comparison of clinically relevant sources of mesenchymal stem cell-derived exosomes: bone marrow and amniotic fluid. J Pediatr Surg. 2019;54:86–90. doi: 10.1016/j.jpedsurg.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Dasgupta SK, Le A, Chavakis T, Rumbaut RE, Thiagarajan P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation. 2012;125:1664–1672. doi: 10.1161/CIRCULATIONAHA.111.068833. [DOI] [PubMed] [Google Scholar]