Abstract
Objective: In view of the existence of multifarious pathologic subtypes of spindle cell lipoma (SCL), which is easily misdiagnosed as other benign and malignant soft tissue tumors, we performed this study and aimed to better define the category of SCL. Methods: We collected and analyzed 40 cases of SCL with complete clinical and pathologic information from January 2010 to December 2018. Clinical and histopathologic analyses of SCL were performed, as well as immunohistochemical staining and fluorescence in situ hybridization (FISH) using probes for RB1 and MDM2, and the related literature was reviewed. Results: In 40 cases, the male to female ratio was 3.4:1, and the mean age was 54 years old. SCL of our study included six pathologic subtypes: classic (25/40), fibrous (4/40), myxoid (4/40), low-fat (3/40), pseudoangiomatous (2/40), and fat-rich (2/40) changes. Microscopically, SCL showed distinctive morphology, with uniform spindle cells and a variably adipocytic component. The spindle cells were bland in morphology, without prominent atypia or pleomorphism, set in a myxoid or fibrous matrix. Immunohistochemically, CD34 and vimentin were positive in spindle cells, and spindle cells of 6 cases also expressed S-100 protein. FISH analysis of 10 cases revealed that heterozygous deletion of RB1 was in six samples with chromosome 13 aberrations and MDM2 gene amplification was not detected in any cases. Surgical resection is considered as the primary treatment for SCL, as there was no any recurrence or metastasis in our cases after 2-105 months of follow-up. Conclusions: SCL is a rare benign lipoma, and the proportion of spindle cells and adipocytic component varies, which may form various pathologic changes. The diagnosis needs to be combined with clinicopathologic features, immunophenotypes, and genetics. It has to be differentiated from mammary-type myofibroblastoma, cellular angiolipoma, solitary fibrous tumor, and myxoid liposarcoma.
Keywords: Spindle cell lipoma (SCL), S-100, RB1, MDM2, differential diagnosis
Introduction
Spindle cell lipoma (SCL) is a rare benign subcutaneous adipose tissue tumor that was first reported by Enzinger in 1975 and often occurs in the neck and back of middle-aged men [1,2]. Tumor tissue mainly includes mild-shaped spindle cells, a large amount of collagen fibers and part of adipose tissue. In view of the existence of several pathologic subtypes of SCL, and the morphologic overlap in a variety of benign and malignant soft tissue tumors, its clinical misdiagnosis often occurs. Therefore, we collected and analyzed 40 cases of SCL with complete clinical and pathologica information in the hospital and an outside hospital. Then, H&E staining, immunohistochemical staining and FISH analysis were performed, and we reviewed the relevant literature, identifying SCLs with a variety of benign and malignant tumors. This subject was intended to improve the level of understanding of the disease, and provide new evidence and strategies for diagnosis, treatment, and prognosis.
Materials and methods
Patients and samples
With Institutional Board Review approval, the Department of Pathology of the Affiliated Hospital of Jining Medical University and the Second People’s Hospital of Jining City and consultation archives from January 2010 to December 2018 were searched for cases of SCL and analyzed for their clinical and histopathologic features. All available hematoxylin and eosin-stained sections were reviewed and confirmed by two pathologists with expertise in bone and soft tissue pathology. Moreover, the clinical and pathologic data of the patients were collected, and the relevant case literature was reviewed for retrospective review.
Immunohistochemistry
The immunohistochemical analysis was conducted on paraffin-embedded sections using the EnVision two-step method. Primary antibodies used in the study were displayed as follows: CD34 (Kit-0004, QBEnd/10), Vimentin (MAB-0735, MX034), S-100 (Kit-0007, 4C4.9), and Ki-67 (MAB-0672, MX006), Ready-to-use; Maixin Bio, Fujian, China. Appropriate positive and negative controls were performed coincidentally for all the markers tested. The brown staining of the spindle-shaped tumor cells was regarded as positive immunohistochemical staining. The positive rate of the cells under the microscope was observed and calculated. No positive cells were judged as negative “-”, and the proportion of positive cells was less than 25% as weak positive “+”, 25% or more regarded as positive “++”, and 50% or more regarded as strong positive “+++”. Normal vascular endothelium and mature adipose tissue cells were not counted in the count range.
FISH analysis
The following two probes were used for FISH analysis: RB1 deletion probe, purchased from Kanglu (Kanglu, Wuhan, China), was used to detect deletion of the RB1 gene locus in 13q14.2. It consists of a red probe spanning RB1. MDM2 (12q15) Probe was purchased from Anbiping (Anbiping, Guangzhou, China). This probe (red signal) is often used for detection of MDM2 gene amplification, containing a 12q15 specific probe as a control, labeled in green.
Results
Clinical features
There were 40 cases of SCL, 31 males and 9 females, aged 14-81 years. The mean age and median age were 54 and 55 years, respectively. According to Table 1, tumors with a wide anatomic distribution, mainly occurred in the neck (11 cases, 27.5%), shoulder (4 case, 10.0%), back (4 cases, 10.0%), inguinal region (3 cases, 7.5%), forehead (2 cases, 5.0%), occipital region (2 case, 5.0%), tongue (2 case, 5.0%), left lower jaw (1 case, 2.5%), left ring finger (1 case, 2.5%), left anterior chest wall (1 case, 2.5%), mediastinum (1 case, 2.5%), retroperitoneum (1 case, 2.5%), sacrococcygeal region (1 case, 2.5%), Right perineum (1 case, 2.5%), left scrotum (1 case, 2.5%), left thigh (1 case, 2.5%), knee join (1 case, 2.5%), left lower extremity (1 case, 2.5%) and foot (1 case, 2.5%). Clinically, many patients had gradually increased subcutaneous painless masses. The masses were predominantly oval and mobile, with a long history. Among them, 30 cases (75.0%) had a history of more than one year (1 to 20 years).
Table 1.
Clinical data of 40 cases of spindle cell lipoma
| Case no. | Sex | Age (y) | Medical history | Site | Size (cm) | Follow-up (m) |
|---|---|---|---|---|---|---|
| 1 | F | 49 | Left lower extremity mass for 1 year | Left lower extremity | 8.5 × 7 × 5 | 2 |
| 2 | F | 47 | Neck mass for 6 years | Posterior neck | 8 × 5 × 3 | 3 |
| 3 | M | 49 | Tongue mass for more than 4 years | Tongue abdomen | 2.5 × 2.2 × 1.5 | 5 |
| 4 | M | 51 | Left neck mass for more than 10 days | Left neck | 3.5 × 2.5 × 1.5 | 5 |
| 5 | M | 50 | Neck mass for 6 years | Right posterior neck | 6.5 × 4 × 2.7 | 6 |
| 6 | M | 70 | Post-neck mass for more than 1 year | Posterior neck | 3 × 2.5 × 2 | 8 |
| 7 | M | 66 | Post-neck mass for more than 10 years | Posterior neck | 12 × 7 × 5 | 10 |
| 8 | M | 53 | Back mass for 1 week | Right back | 2.2 × 2 × 1.5 | 16 |
| 9 | M | 58 | Neck mass for several years | Right neck | 2 × 1.5 × 1.5 | 18 |
| 10 | M | 14 | Left ring finger mass for more than 2 years | Left ring finger | 1.2 × 1 × 0.6 | 18 |
| 11 | M | 67 | Neck mass for more than 20 yeas | Neck | 6 × 4 × 2.5 | 18 |
| 12 | M | 64 | Right occipital scalp mass for 2 years | Right occipital scalp | 3 × 2.5 × 2 | 19 |
| 13 | M | 35 | Right perineal mass for more than 4 years | Right shoulder | 6.5 × 4.5 × 2.5 | 21 |
| 14 | F | 64 | Right perineal mass for more than 4 years | Right perineum | 3.5 × 2 × 1.5 | 21 |
| 15 | M | 66 | Right back mass more than 10 years | Right back | 2.8 × 2 × 1 | 25 |
| 16 | F | 69 | Left inguinal mass for 2 weeks | Left inguinal area | 3.8 × 2.5 × 1.5 | 30 |
| 17 | M | 61 | Back mass for more than 1 year | Back | 13 × 9 × 6 | 37 |
| 18 | M | 63 | Neck mass for 10 years | Neck | 2 × 1.5 × 1 | 40 |
| 19 | M | 60 | Forehead scalp mass for 20 years | Right forehead | 6.3 × 5.5 × 2 | 46 |
| 20 | M | 65 | Back mass for 5 years | Right back | 8 × 4 × 4 | 50 |
| 21 | F | 52 | Forehead mass for 3 years | Forehead | 3 × 3 × 1.2 | 51 |
| 22 | M | 47 | Occipital mass for 4 years | Occipital region | 1.5 × 1 × 1 | 51 |
| 23 | F | 25 | Left lower jaw mass 2 years | Left lower jaw | 3 × 2 × 1 | 53 |
| 24 | F | 52 | PE found abdominal mass for 1 day | Right retroperitoneum | 18 × 16 × 10 | 53 |
| 25 | M | 42 | Left thigh mass for 3 years | Left thigh | 17 × 8 × 5 | 54 |
| 26 | M | 40 | Left inguinal mass for more than 8 years | Left inguinal area | 9 × 8 × 4 | 55 |
| 27 | M | 45 | Shoulder mass for more than 2 years | Shoulder | 5 × 3 × 2 | 60 |
| 28 | M | 81 | Neck mass for 10 years | Neck | 8.5 × 6.5 × 5 | 63 |
| 29 | F | 61 | Shoulder mass for more than 5 years | Right Shoulder | 7.5 × 6.5 × 2.5 | 64 |
| 30 | M | 48 | Right knee joint mass for 2 months | Right knee | 9 × 6 × 3 | 64 |
| 31 | M | 38 | Left scrotal mass for more than half a year | Left scrotum | 7.5 × 4 × 3 | 65 |
| 32 | M | 67 | Left anterior chest wall mass for 2 years | Left anterior chest wall | 7 × 5 × 3 | 66 |
| 33 | M | 22 | Sacrococcygeal region mass for 4 years | Sacrococcygeal region | 4.5 × 1.5 × 1 | 74 |
| 34 | M | 68 | Left shoulder mass for 20 years | Left shoulder | 7 × 7 × 6 | 78 |
| 35 | M | 63 | Neck mass for 1 year | Neck | 2 × 2 × 1 | 78 |
| 36 | M | 42 | Left foot mass for 2 years | Left foot sole | 4 × 3 × 2 | 79 |
| 37 | M | 56 | Left inguinal mass for 5 months | Left inguinal region | 5 × 3 × 2 | 84 |
| 38 | M | 60 | Neck mass for 8 years | Neck | 2.5 × 2 × 1 | 90 |
| 39 | M | 35 | PE found mediastinum mass for 6 days | Mediastinum | 15 × 14 × 10 | 91 |
| 40 | F | 81 | Right tongue mass for half a year | Anterior part of right tongue | 1.1 × 1 × 0.6 | 105 |
y: year, m: months, PE: Physical examination.
Histologic features
Pathological examination: In 40 cases of SCL, the largest tumor was 18 cm × 16 cm × 10 cm, located in the Right retroperitoneum, and the smallest was 1.1 cm × 1 cm × 0.6 cm, located in the tongue. 31 cases were intact and 9 cases had no complete envelope, but with well-circumscribed margins. The cut surface was grayish, or grayish yellow, and often nodular. Due to the different ratio of collagen fibers and fat cells, the texture can appear from soft (Figure 1A) to tough (Figure 1B). The focal area showed a jelly-like appearance and no obvious bleeding and necrosis.
Figure 1.
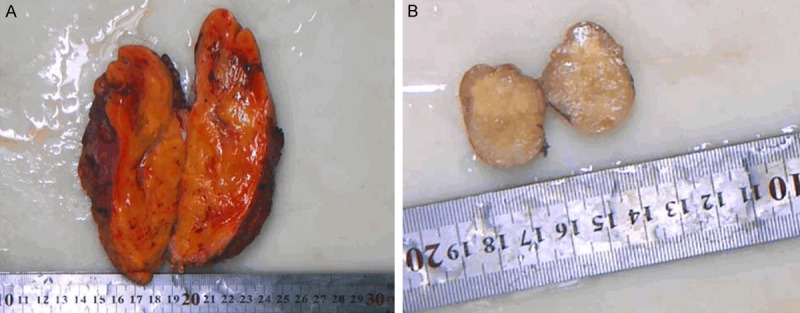
Gross findings of SCL. The texture can appear from soft to tough, because of the different ratio of collagen fibers and fat cells (A, B).
Microscopically, the changes of tumors in 40 cases were almost the same. Most of the tumors were composed of spindle cells, rope-like collagen fibers and mature adipocytes, surrounded by a clear fibrous capsule (Figure 2A, 2B). In addition, scattered mast cells were frequently observed. Spindle cells were bland, without obvious atypia, pleomorphism or nuclear mitoses, and arranged in bundles between the collagen fibers. In addition to the classic pathologic features, some cases also exhibit non-classical subtypes. Case 5, 9, 22 and 38 showed a small amount of spindle cells scattered in a large number of collagen fiber bundles, called fibroblastic subtype (Figure 2C). Case 3, 15, 16 and 33 showed prominent myxoid degeneration of the stroma, and the spindle-shaped nucleus was compact and deeply stained, which was called myxoid subtype (Figure 2D). Cases 18 (previously published as a case report) and 20 included some vascular-like space or branching dilated fissures formed by flattened spindle cells, without visible red blood cells, similar to hemangioma-like changes, called pseudoangiomatous subtype (Figure 2E). Case 7 and 14 showed a lipomatous tumor with scattered spindle cells and minor fibrous components, called fat-rich type (Figure 2F), while case 10 and 17 showed a large number of spindle cells, with a small proportion of fat components (less than 5%), and case 6 was basically no adipose tissue, called low-fat subtype (Figure 2G, 2H). However, as shown in Tables 2 and 3, both classic and non-classical forms have the basic pathological morphology, and there is no significant correlation between this morphology and anatomical location distribution.
Figure 2.
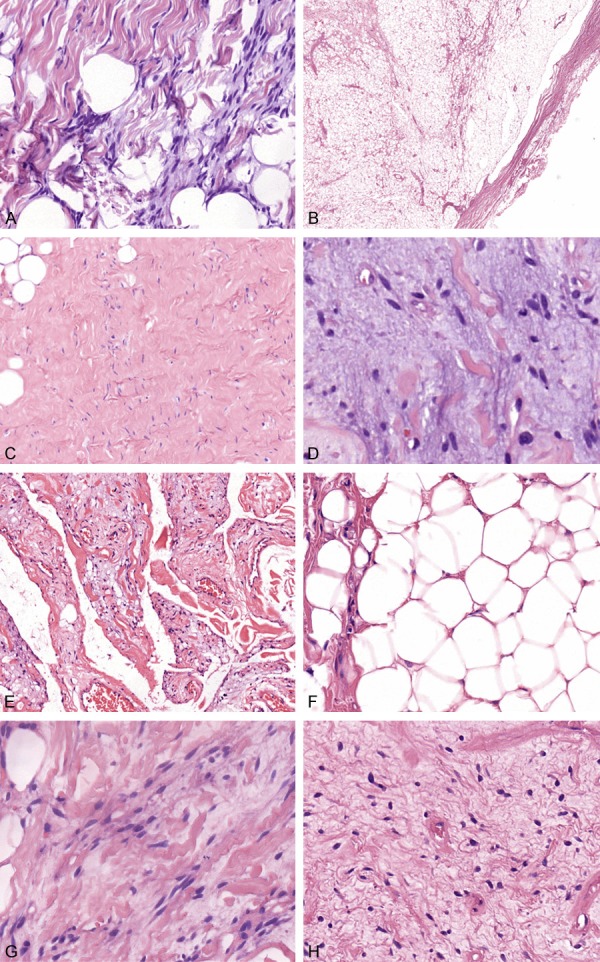
Morphology of SCL. SCL mainly composed of spindle cells, rope-like collagen fibers and mature adipocytes (A, H&E × 200). Most of the tumors were surrounded by clear fibrous capsule (B, H&E × 10). Case 5 showed that a small amount of spindle cells are set in a predominantly collagenous stoma, called fibrous subtype (C, H&E × 100). Case 15 showed obvious myxoid stromal changes, with compact and deeply stained spindle-shaped nucleus, which was called myxoid subtype (D, H&E × 400). Case 18 contained numerous branching dilated fissures formed by flattened spindle cells, without visible red blood cells, resembling vascular channels, called pseudoangiomatous subtype (E, H&E × 10). Case 14 showed the apparent adipose tissue, only with scattered spindle cells and rope-like collagen fibers, called fat-rich type (F, H&E × 200), while case 17 showed a large number of spindle cells, with a small proportion of fat components (less than 5%) (G, H&E × 400), and case 6 was free of adipose tissue, called low-fat subtype (H, H&E × 200).
Table 2.
Pathologic features of various subtypes of spindle cell lipoma
| Case | Subtypes | Pathological features | Number (%) |
|---|---|---|---|
| 1 | Classic | Spindle cell, rope-like collagen, adipose tissue | 25 (62.5%) |
| 2 | Fibrous | Massive collagen fiber | 4 (10.0%) |
| 3 | Myxoid | Myxoid stroma | 4 (10.0%) |
| 4 | Low-fat | Little or almost no adipose tissue | 3 (7.5%) |
| 5 | Pseudoangiomatous | Pseudovascular fissure | 2 (5.0%) |
| 6 | Fat-rich | Large amount of adipose tissue | 2( 5.0%) |
Table 3.
Summary of histologic features of spindle cell lipoma by anatomic location
| Total = 40 | Number of Cases |
|---|---|
| Neck | 11 |
| Classic | 5 |
| Fibrous | 3 |
| Pseudoangiomatous | 1 |
| Low-fat | 1 |
| Fat-rich | 1 |
| Shoulder | 4 |
| Classic | 4 |
| Back | 4 |
| Classic | 1 |
| Myxoid | 1 |
| Pseudoangiomatous | 1 |
| Low-fat | 1 |
| Inguinal region | 3 |
| Classic | 2 |
| Myxoid | 1 |
| Forehead | 2 |
| Classic | 2 |
| Occipital region | 2 |
| Classic | 2 |
| Tongue | 2 |
| Classic | 1 |
| Myxoid | 1 |
| Left ring finger | 1 |
| Low-fat | 1 |
| Sacrococcygeal region | 1 |
| Myxoid | 1 |
| Right perineum | 1 |
| Fat-rich | 1 |
| Other anatomic locations | 10 |
| Classic | 1 |
Non-classic: Fibrous, Myxoid, Pseudoangiomatous, Low-fat and Fat-rich. Other anatomic locations: Left lower jaw, Left anterior chest wall, Mediastinum, Right retroperitoneum, Left scrotum, Left thigh, Knee joint, Left lower extremity, Left foot sole.
Immunohistochemical findings
Immunohistochemical staining revealed that CD34 (Figure 3A) and vimentin (Figure 3B) were strongly positive in 40 cases of SCL. And Ki67 decorated less than 3% lesional cells in all cases. In addition, there were some spindle to dendritic cells expressing S-100 in 6 cases, of which case 18 and 26 was positive for S-100 (Figure 3C), while other cases were partly positive for S-100 (Figure 3D), with 20% of positive cells, respectively. Specific immunohistochemical staining characteristics and case statistics are shown in Table 4.
Figure 3.
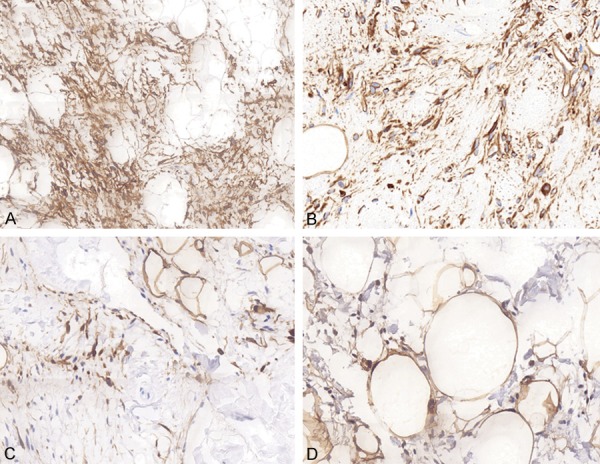
Immunohistochemical findings of SCL. Immunohistochemical staining revealed that CD34 (A, IHC × 100) and vimentin (B, IHC × 200) were strongly positive in 40 cases of SCL. Non-fat spindle cells expressing S-100 protein with a cytoplasmic and nuclear pattern in 6 cases, of which case 18 was strongly positive for S-100 (C, IHC × 200), while case 20 was partly positive for S-100 (D, IHC × 200).
Table 4.
Immunohistochemical findings of 40 cases of spindle cell lipoma
| NO. | Subtypes | CD34 +++ | Vimentin +++ | S-100 - | S-100 + | S-100 ++ | Ki-67 < 3% |
|---|---|---|---|---|---|---|---|
| 1 | Classic | 25/25 | 25/25 | 21/25 | 3/25 | 1/25 | 25/25 |
| 2 | Non-classic | 15/15 | 15/15 | 13/15 | 1/15 | 1/15 | 15/15 |
FISH findings
Six of 10 cases showed a heterozygous deletion of the RB1 gene in > 45% of the cells by FISH, according to our threshold value for reporting a positive result (Figure 4A). However, no evidence of the MDM2 gene regional gain or loss was identified by FISH in all 10 cases tested (Figure 4B).
Figure 4.
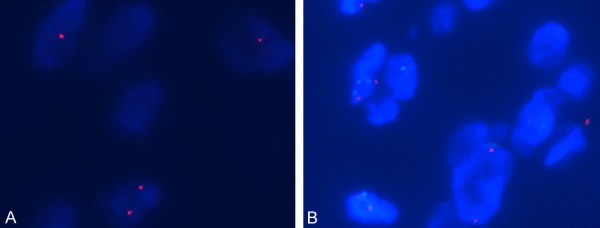
FISH analysis of SCL. FISH with the RB1 deletion probe for case 1: a nucleus with heterozygous deletion of the RB1 gene (one red signal) (A). FISH with the MDM2 (12q15) gene amplification in ten cases were all negative (red single/green single < 2) (B).
Discussion
SCL is a rare benign adipose tissue tumor commonly found in middle-aged and older men. Most cases are located in the neck, back, and shoulders [1,2]. In addition, a few cases can be present in the scalp, eyelids, forehead, face, mouth, the lower jaw, kidney, vulva, perianal and upper and lower extremities [3-13], as our case 36 occurred in the left sole of foot. We also report herein two very rare cases with RB1 gene deletion, one located in the left ring finger and another in left scrotum. Clinically, it is manifested as a single painless nodule under the skin, and some cases can occur under the dermis, muscle and submucosa. The mass can be active, often accompanied by a long history, and a few familial cases can be multiple nodules, which are common in male patients.
The general shape often shows nodules and lobes, with the clear boundary. The diameter ranges from 1 cm to 18 cm and the texture is soft to tough. The cut surface can be yellow, yellowish white and grayish white due to the different ratio of fat to fiber. Occasionally, the surface can be gelatinous. The morphology of the tumors is clear, and most of them contained envelopes. In all of our cases, 31 cases had complete capsules, which is also very supportive. Microscopically, SCL shows distinctive morphology, with spindle-shaped cells and variable proportions of adipocytic component. In this background, a few mast cells are scattered, which may be accompanied by a myxoid or collagenous matrix. As a consequence, there are many variant subtypes of SCL, such as fibrous, myxoid, fat-rich, low-fat and pseudoangiomatous type [3-15]. Most of the spindle cells in our cases were bland in morphology, short in spindle shape, and have no atypia and mitotic figures, sometimes showing a fence-like appearance. In some cases, scattered clusters of multinucleated giant cells can be seen, which is easy to misdiagnose. In a few of cases, the tumors were accompanied by irregular fissure-like structures and villiform connective tissue projections, called “pseudoangiomatous type” [7,10]. In addition, in a small number of cases, it is difficult to see fat components, called low-fat type [4,7,15], which is easily misdiagnosed as other soft tissue tumors. The histology of our cases basically has the structural components of classical spindle cell lipoma: bland spindle cells, coarse collagen, and mature adipocytes. In addition to the above classic features, cases 18 and 20 had a special structure, that is, some dilated and fissure-like structures formed by flattened spindle cells, which are referred to as a pseudoangiomatous spindle cell lipoma. Cases 5 and 38 showed that most areas were a bunch of rope-like collagen fiber bundles, accompanied by a few spindle cells and scattered mast cells, called fibrous type. Additionally, due to the difference in the ratio of spindle cells to adipose tissue in some cases, the following two pathologic subtypes were produced. Namely, cases 7 and 14 contained a large amount of adipose tissue, during which there were some consistently spindle cells and a small amount of fibrous collagen, named as fat-rich type. In case 17, a large number of spindle cells were only accompanied by a small proportion of fat components (less than 5%), and case 6 was substantially free of adipose tissue, which was called a low-fat or fat-free subtype. In view of the above-mentioned multifarious histomorphologic features of SCL, it is sometimes diagnosed as other benign and malignant tumors such as solitary fibrous tumor and shuttle-shaped cell liposarcoma. Therefore, combined immunohistochemical staining and molecular pathological examination can further confirm the diagnosis.
According to the previous literature, CD34 and Vimentin were positively expressed in SCL, and our immunohistochemistry results also confirmed these reports. However, we unexpectedly found that six of the 40 SCLs expressed S-100 protein, which was less common in previous reports [2,16,17]. The S-100 protein is a soluble acidic protein isolated from bovine brain. It is mainly found in nerve tissue, pituitary, adrenal medulla and some mesenchymal cells, and some tumors, such as lymphoid hematopoietic tumors, malignant melanoma and liposarcoma. As reported, immunohistochemical staining of S-100 protein is located in the cytoplasm or nucleus. In our SCLs, the pseudoangiomatous types and other four classic cases expressed S-100 with nuclear and cytoplasmic location, and some regions with dendritic appearance were strongly positive (+++). It is possible that these spindle cells are specific immunophenotypes exhibited at some stage of adipocyte development. According to the data, it is known that S-100 is mostly negative in the isolated fibrous tumor, but positively expressed in neurofibromatosis. This may play a certain auxiliary diagnosis role, especially when the histomorphology changes are difficult to identify. Spindle tumor cells expressing S-100 in SCL also expressed CD34, which appeared as rod-shaped, immature mesenchymal cells. Therefore, these spindle cells may be derived from immature mesenchymal cells with various differentiation capabilities. It is true that heterozygous loss of the RB1 gene in SCL, and the specific expression of STAT6 in solitary fibrous tumors may further assist in the differential diagnosis.
In recent years, cell and molecular genetics have confirmed that lipomas are tumorous lesions, and some subtypes of it have been proven to have some characteristic chromosomal abnormalities. For example, the chromosome phenotype of chondrolipoma is t(11;16)(q13;p12-13), accompanied by abnormal C11 of 95-MKL2 gene. Forcucci and Panagopoulos et al. [7,10] showed that there were 13q14 partial chromosomes (including RB1 gene and FOXO1 gene) and 16q22 chromosome deletion in SCL by fluorescence in situ hybridization analysis. In addition, the C13orf1 gene in the corresponding region of 13q of SCL was found to be significantly lower than of classical lipoma by PCR from the study of Bartuma. Uehara et al. found that the RB1 and FOXO1 genes encoded on chromosome 13 in SCL were lower than those in solitary fibrous tumors with normal chromosome 13. It was also noted that the expression of oxidative stress related protein, 8-hydroxy-2-deoxyguanosine (8-OHDG) and 4-hydroxy-2-nonenal (4-HNE), was increased, which proved that the p38 MAPK pathway was activated in SCL [18]. As previously reported, our research also showed that SCL has structural abnormality of 13q resulting in deletion of RB1. Of course, many scholars have found that the cell-rich angiolipoma and mammary myofibroblastoma similar to SCL in morphology also have deletions of chromosomes 13 and 16 [18-20], suggesting SCL and cell-rich angiolipoma and mammary myofibroblastic tumors may have similar molecular pathologic mechanisms. This may be a morphologic difference caused by different anatomical locations. The above reports also provide more evidence for further revealing the pathogenesis of the disease and clarifying the clinical diagnosis of SCL.
Because of the existence of myriad pathologic subtypes of spindle cell lipoma, it is necessary to identify various related soft tissue tumors. First, a differential diagnosis of fibrous types of SCL is the elastofibroma. Elastofibroma often occurs in the scapula of women, consisting of a large number of collagen fibers and shaped elastic fibers, accompanied by mature adipose tissue, but with positive elastic fiber staining. Another important differential diagnosis is solitary fibrous tumors. Compared with low-fat types of SCL, it usually occurs in deep soft tissues of middle-aged people, with the most common in retroperitoneum and thigh. Microscopically, it can reveal mature adipocytes and a prominent vasculature, but the tumor cells are fusiform or ovoid, patternless, cytoplasmic reddish, diffusely expressing CD99 and STAT6 [21]. In addition, angiomyolipoma occurs in the liver and kidney, and is more common in women. It shows a bundle of spindle-shaped cells and deformed thick-walled blood vessels, no irregular branched pseudovascular structure different from PASCL. Tumor cells express SMA, HMB45 and Melan-A, and do not express CD34 and bcl-2. Cellular angiolipoma, also devoid of branched pseudovascular structures, is rarely reported at present, primarily composed of a large number of small blood vessels and abundant fusiform mesenchymal cells and a small amount of adipose tissue. The regions of small blood vessels diffusely express CD31, CD34, FVIIIRAg and α-SMA [22]. As well as myxoid types of SCL, myxoid liposarcoma also consists of a mucoid-like matrix rich in mucopolysaccharide, and the tumor cells can be positive for S-100 and vimentin. However, it mainly occurs in the limbs, trunk and other parts of young people. The tumor consists of round, short fusiform primitive mesenchymal cells, imprinted fat cells of different sizes, and branched capillary networks [23,24]. There is also a mammary myofibroblastoma with a 13q14 chromosome deletion, which is also composed of bland short spindle tumor cells and hyaline degeneration collagen, but the spindle cells diffusely express desmin, and it often occurs in the mammary gland, scrotum, vulva, and inguinal area.
The SCLs reported in the literature were all benign and can be completely removed locally. In this study, 40 patients recovered well after surgery, without tumor recurrence and other discomfort after 2-105 months of follow-up.
Although spindle cell lipoma is a rare, benign adipose tissue tumor with variable morphology, the non-classic subtypes showed no distinctive clinical or immunohistochemical features, compared with classic type. The pathologic morphology almost all includes mild spindle cells, rope-like collagen fibers, and a certain proportion of adipose tissue. Meanwhile, we have comprehensively reported and discussed several pathological subtypes of SCL, and further explored their differential diagnosis in combination with immunophenotype and molecular detection. It was noted that SCL is similar to many benign and malignant soft tissue tumors in pathologic morphology. Therefore, pathologists and clinicians should be conscious of multiple variant subtypes of SCL when encountering spindle cell-associated soft tissue tumors to avoid misdiagnosis.
Acknowledgements
This work was supported by the Supporting Fund for Teachers’ research of Jining Medical University (Grant No. JYFC2018FKJ015) and Miao Pu Project Foundation of Affiliated Hospital of Jining Medical University (Grant No. MP-2017-009). We thank the patients for agreeing to participate and supply their detailed medical histories.
Disclosure of conflict of interest
None.
References
- 1.Enzinger FM, Harvey DA. Spindle cell lipoma. Cancer. 1975;36:1852–1859. doi: 10.1002/1097-0142(197511)36:5<1852::aid-cncr2820360542>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Ko JS, Daniels B, Emanuel PO, Elson P, Khachaturov V, McKenney JK, Goldblum JR, Billings SD. Spindle cell lipomas in women: a report of 53 cases. Am J Surg Pathol. 2017;41:1267–1274. doi: 10.1097/PAS.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 3.Bhat A, Vijaya C, Rao SB. Pseudoangiomatous variant of spindle cell lipoma: report of a rare case. Indian J Pathol Microbiol. 2016;59:376–378. doi: 10.4103/0377-4929.188142. [DOI] [PubMed] [Google Scholar]
- 4.Billings SD, Folpe AL. Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variants. Am J Dermatopathol. 2007;29:437–442. doi: 10.1097/DAD.0b013e31813735df. [DOI] [PubMed] [Google Scholar]
- 5.Cheah A, Billings S, Goldblum J, Hornick J, Uddin N, Rubin B. Spindle cell/pleomorphic lipomas of the face: an under-recognized diagnosis. Histopathology. 2015;66:430–437. doi: 10.1111/his.12548. [DOI] [PubMed] [Google Scholar]
- 6.Cooper TJ, Lincoln T, James DT, Borgna S. Intraosseous spindle cell lipoma of the mandible: case report. Br J Oral Maxillofac Surg. 2017;55:839–840. doi: 10.1016/j.bjoms.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Forcucci JA, Sugianto JZ, Wolff DJ, Maize JC Sr, Ralston JS. “Low-fat” pseudoangiomatous spindle cell lipoma: a rare variant with loss of 13q14 region. Am J Dermatopathol. 2015;37:920–923. doi: 10.1097/DAD.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khatib Y, Khade AL, Shah VB, Khare MS. Cytohistological features of spindle cell lipoma-a case report with differential diagnosis. J Clin Diagn Res. 2017;11:ED10–ED11. doi: 10.7860/JCDR/2017/23292.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau SK, Bishop JA, Thompson LD. Spindle cell lipoma of the tongue: a clinicopathologic study of 8 cases and review of the literature. Head Neck Pathol. 2015;9:253–259. doi: 10.1007/s12105-014-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panagopoulos I, Gorunova L, Lobmaier I, Andersen HK, Bjerkehagen B, Heim S. Cytogenetic analysis of a pseudoangiomatous pleomorphic/spindle cell lipoma. Anticancer Res. 2017;37:2219–2223. doi: 10.21873/anticanres.11557. [DOI] [PubMed] [Google Scholar]
- 11.Ud Din N, Zhang P, Sukov WR, Sattler CA, Jenkins SM, Doyle LA, Folpe AL, Fritchie KJ. Spindle cell lipomas arising at atypical locations. Am J Clin Pathol. 2016;146:487–495. doi: 10.1093/ajcp/aqw137. [DOI] [PubMed] [Google Scholar]
- 12.Val-Bernal JF, Hermana S, Gomez-Roman JJ. Incidental, low-fat variant of spindle cell lipoma: a novel tumour of the small intestine. Pol J Pathol. 2018;69:82–86. doi: 10.5114/pjp.2018.75341. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z, Selvarajan S, Tiong AL, Lim TH, Khor LY. Spindle cell lipoma in an end-stage renal allograft: case report. Transplant Proc. 2016;48:3145–3148. doi: 10.1016/j.transproceed.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Cascajo C, Borghi S, Weyers W. Fibrous spindle cell lipoma: report of a new variant. Am J Dermatopathol. 2001;23:112–115. doi: 10.1097/00000372-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Dos Santos JL, Ocamoto EA, Almeida LY, Teixeira LR, Ribeiro-Silva A, Leon JE. Low-fat plexiform spindle cell lipoma with prominent myxoid stroma: an unusual oral presentation and immunohistochemical analysis. J Craniofac Surg. 2017;28:e158–e160. doi: 10.1097/SCS.0000000000003348. [DOI] [PubMed] [Google Scholar]
- 16.Mentzel T, Rutten A, Hantschke M, Hornick JL, Brenn T. S-100 protein expressing spindle cells in spindle cell lipoma: a diagnostic pitfall. Virchows Arch. 2016;469:435–438. doi: 10.1007/s00428-016-1996-8. [DOI] [PubMed] [Google Scholar]
- 17.Val-Bernal JF, Cagigal ML, Mazorra R. Low-fat, plexiform spindle cell lipoma of the lip expressing S100 protein: a neural tumor simulator. Rom J Morphol Embryol. 2018;59:385–390. [PubMed] [Google Scholar]
- 18.Uehara K, Ikehara F, Shibuya R, Nakazato I, Oshiro M, Kiyuna M, Tanabe Y, Toyoda Z, Kurima K, Kina S, Hisaoka M, Kinjo T. Molecular signature of tumors with monoallelic 13q14 deletion: a case series of spindle cell lipoma and genetically-related tumors demonstrating a link between FOXO1 status and p38 MAPK pathway. Pathol Oncol Res. 2018;24:861–869. doi: 10.1007/s12253-017-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howitt BE, Fletcher CD. Mammary-type myofibroblastoma: clinicopathologic characterization in a series of 143 cases. Am J Surg Pathol. 2016;40:361–367. doi: 10.1097/PAS.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 20.Marino-Enriquez A, Nascimento AF, Ligon AH, Liang C, Fletcher CD. Atypical spindle cell lipomatous tumor: clinicopathologic characterization of 232 cases demonstrating a morphologic spectrum. Am J Surg Pathol. 2017;41:234–244. doi: 10.1097/PAS.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 21.Tan SY, Szymanski LJ, Galliani C, Parham D, Zambrano E. Solitary fibrous tumors in pediatric patients: a rare and potentially overdiagnosed neoplasm, confirmed by stat6 immunohistochemistry. Pediatr Dev Pathol. 2018;21:389–400. doi: 10.1177/1093526617745431. [DOI] [PubMed] [Google Scholar]
- 22.Panagopoulos I, Gorunova L, Andersen K, Lobmaier I, Bjerkehagen B, Heim S. Consistent involvement of chromosome 13 in angiolipoma. Cancer Genomics Proteomics. 2018;15:61–65. doi: 10.21873/cgp.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahadir B, Behzatoglu K, Hacihasanoglu E, Koca SB, Sigirci BB, Tokat F. Atypical spindle cell/pleomorphic lipomatous tumor: a clinicopathologic, immunohistochemical, and molecular study of 20 cases. Pathol Int. 2018;68:550–556. doi: 10.1111/pin.12719. [DOI] [PubMed] [Google Scholar]
- 24.Muratori F, Bettini L, Frenos F, Mondanelli N, Greto D, Livi L, Franchi A, Roselli G, Scorianz M, Capanna R, Campanacci D. Myxoid liposarcoma: prognostic factors and metastatic pattern in a series of 148 patients treated at a single institution. Int J Surg Oncol. 2018;2018:8928706. doi: 10.1155/2018/8928706. [DOI] [PMC free article] [PubMed] [Google Scholar]


