Abstract
The purpose of this report is to determine the clinicopathological and immuno-phenotypical characteristics of myoid hamartoma of the breast (MHB). The clinical data, histological morphology, and immunohistochemical results of 2 patients diagnosed with MHB were analyzed, and 15 cases of MHB reported in China were reviewed. Both patients were female, aged 28 and 35 years old, and their lesions were located in the upper outer quadrant of the left breast and the right breast respectively. The lesions measured 3 cm × 3 cm × 2.5 cm and 6.5 cm × 6 cm × 4.5 cm. A gross examination indicated a grayish solid block with clear boundaries. A microscopic examination showed different proportions of ducts and acini, beam myoid cells, adipose tissue, and fibrous stroma. The myoid cell bundles of both specimens were positive for vimentin, SMA, h-caldesmon and desmin, but negative for ER, PR, PCK, s-100, Calponin, and P63. Both cases were confirmed as MHB based on their clinical, histological and immuno-phenotypical characteristics. Our findings provide further insights into the pathological basis of MHB, which can help avoid both misdiagnosis and missed diagnosis.
Keywords: Mammary gland, myoid hamartoma, clinicopathological features, immune phenotype
Introduction
Breast hamartoma (BH) is a benign tumor involving the ducts, lobules, and the fibrous and fatty tissues in varying proportions, accounting for approximately 0.04%~1.15% of all benign breast tumors [1]. Myoid hamartoma of the breast (MHB) is a very rare variant of BH and primarily consists of smooth muscles and stromal structures. Owing to its benign and atypical nature, MHB can be easily missed or misdiagnosed during routine clinical examination. We analyzed the clinicopathological and immuno-phenotypical characteristics of two MHB specimens in order to gain more insights into its effective and accurate diagnosis.
Materials and methods
Specimens
Paraffin-embedded tissue samples from 2 MHB patients were obtained from the Department of Pathology, the Affiliated Hospital of Southwest Medical University between January 2017 and November 2018. Both patients were residents of Sichuan, Luzhou. The clinicopathological information of the patients, including their ages, tumor sizes, tumor locations, gender, operation methods and prognoses, were also obtained. The experimental protocol was pre-approved by the Medical Ethics Committee of Southwest Medical University (no. 20130051).
Immunohistochemistry
The paraffin embedded tissues were cut into 3 μm thick sections and placed on poly-L-Lysine coated slides. After drying overnight at 60°C, the sections were de-paraffinized, quenched with hydrogen peroxide, boiled in Tris-EDTA (pH 8.0-9.0) for 3 min to unmask antigens, and rinsed with tap water. The sections were then incubated with the primary antibodies against SMA, h-caldesmon, desmin, vimentin, ER, PR, PCK, s-100, calponin or P63 at 37°C for 30 min, rinsed thrice with tris-buffered saline (TBS), and incubated with a secondary antibody (DAKO Rabbit/Mouse, Cheng du China) at room temperature for 30 min. After washing with TBS, color was developed using DAB, and the sections were counterstained with hematoxylin. ER, PR, and P63 localized to the nuclei, SMA, h-caldesmon, Calponin, desmin, and vimentin to the cytoplasm, and PCK to the membrane. A sample was considered positive for any of the above markers if ≥ 1% of the tumor cells stained positive.
Results
Patients and clinical history
Specimen 1 was from a 28-year-old female patient, and specimen 2 from a 35-year-old female patient. The patients had sought clinical intervention for a “painless breast mass”. A physical examination showed actively growing round or oval masses without tenderness in the left quadrant of the left breast in patient 1 and in the right breast in patient 2. Breast ultrasonography was performed in both patients and showed well-defined mass shadows and uneven internal echoes (Figure 1A). The diagnosis was “fibroadenoma”, and both patients underwent simple mastectomies. The patients recovered well and have not shown any signs of recurrence or the presence of other breast lesions so far.
Figure 1.
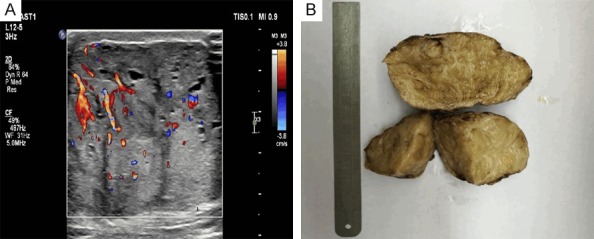
Gross observation. A. Ultrasonograph showing a clear boundary and uneven internal echo of the MHB mass. B. Tumor tissue masses seen with the naked eye.
Pathological examination
Visual inspection
The MHB mass in both cases was ovoid with clear boundaries. The lesions measured 3 cm × 3 cm × 2.5 cm in patient 1 and 6.5 cm × 6 cm × 4.5 cm in patient 2. Sample 1 had no obvious capsule, but sample 2 had an incomplete envelope attached to its surface. The tumor tissues appeared gray to grayish yellow, solid, with a medium or tough texture. A large portion of both masses was lobulated, with visible grayish yellow braided stripes (Figure 1B).
Microscopic examination
The specimens were composed of different proportions of mammary duct, acinus, myoid cell bundle, adipose tissue and fibrous stroma. The myoid cells were distributed in a diffuse, fascicular or braided pattern, and interspersed with ducts and lobular acini. They were mixed with interstitial fibers in specimen 1 and with the fibrous stroma in specimen 2, forming a solid structure and interacting with the surrounding ductal myoepithelium, and interstitial mucoid degeneration can be seen in some areas (Figure 2A). The myoid cells were well differentiated, with abundant erythematous cytoplasms, spindle shaped nuclei, and a lack of atypia and mitotic figures (Figure 2B). The proportion of MHB muscle-like tissues in both specimens was around 20%~50%. In addition, sclerosing adenopathy and ductal dilation were seen in specimen 1 (Figure 2C) and apocrine glandular metaplasia and fibroadenomas in specimen 2 (Figure 2D).
Figure 2.
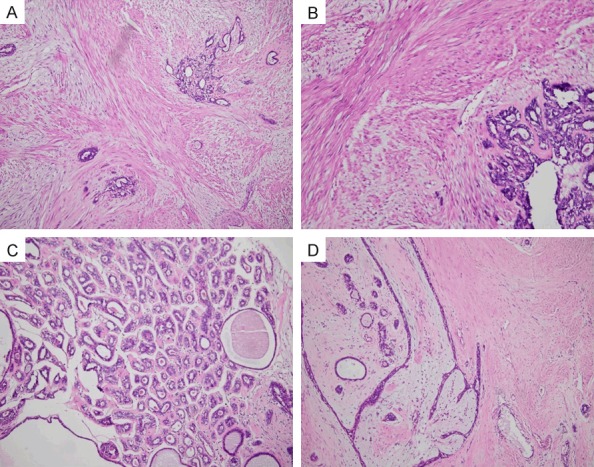
Histopathological examination with H&E staining. A. Diffuse mixture of sarcoplasmic bundles with interstitial myxoid degeneration, 100 ×. B. Myoid cells with abundant erythrocytoplasms, fusiform nuclei, no atypia, and no mitosis, 200 ×. C. Sclerosing adenopathy and ductal dilation in the surrounding breast tissue in sample 1, 100 ×. D. Fibroadenomas in the surrounding breast tissue in sample 2, 100 ×.
Immuno-phenotyping
The intramuscular cell bundles of both specimens were positive for vimentin, SMA (Figure 3A), h-caldesmon and desmin (Figure 3B). In addition, the surrounding mammary ducts and acinar epithelial cells were positive for ER, PR, PCK, and CK7, and the ducts and acinar epithelial cells were positive for s-100, calponin and P63. Both specimens were negative for PCK, CK7, s-100, and P63.
Figure 3.
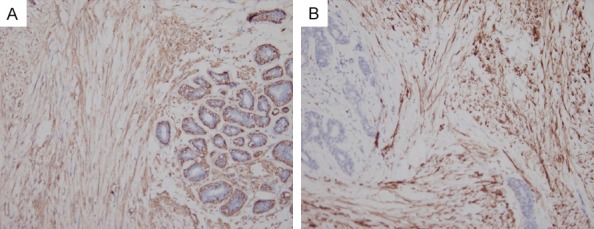
Immuno-histochemical analysis. A. Intramuscular cell bundles showing a positive expression of SMA, 200 ×. B. Intramuscular cell bundles positive for desmin, 200 ×.
Pathological diagnosis
Breast myoid hamartomas.
Discussion
Breast Hamartomas (BH) are rare benign tumors composed of varying proportions of ducts, lobules, fibers and lipids, accounting for only 0.04%~1.15% of benign breast tumors observed in females [1]. MHB, an even rarer subtype of BH characterized by smooth muscle-like structures in the stroma, was first reported by Davies et al. [2] in 1973. Most patients present with an asymptomatic breast mass, which is often misdiagnosed by ultrasound or X-ray examination. At present, only 15 cases have been reported in China, and 35 cases have been reported elsewhere.
MHB mostly occurs in women before and after menopause [3] and affects a single breast in the majority of the cases. Only one case of bilateral MHB has been reported in a male patient [4]. The course of the illness can run up to 5 years. Both MHB cases reported in this paper were females, aged 28 and 35 years old, and were unilateral. We also reviewed the 15 MHB cases reported in China. All patients were female, aged 19 to 60 years, and the maximum tumor diameter ranged from 1.4 to 4 cm. The specific clinical characteristics are summarized in Table 1.
Table 1.
Clinical features of MHB cases recorded in China
| Number of cases | Age (years) | Tumor Location | Maximum Mass Diameter (cm) | Operation Method | Prognosis |
|---|---|---|---|---|---|
| 5 | 29~44 Mean-39 | Not to mention | 1.4~2.0 An average of 1.7 | Simple mass resection | Local recurrence occurred in 1 case 10 months after surgery; 1 case respectively in the postoperative recurrence of 36, 41 months twice. |
| 4 | 19~26 Mean-23.5 | All left breasts | 2.0~4.0 | One case with intraductal carcinoma was treated with expanded mass resection; The remaining 3 cases underwent simple mass resection | Follow-up of 1~9 months, no recurrence. |
| 1 | 45 | Left breast | 4.0 | Simple mass resection | No |
| 5 | 22~60 Mean-37 | 2 cases of left breast 3 cases of right breast | 1.5~3.5 An average of 2.6 | Simple mass resection | Followed up for 3~55 months, an average of 20 months, no recurrence. |
Pathological examination
Visual inspection
MHB masses are usually located in the upper outer quadrant of the breast, and are well-defined solid masses covered with partial or complete fibrous capsule [5]. The shape can be round, oval or lobulated, with gray-white and yellowish sections and textures ranging from soft to hard. A fibroma weave pattern and cystic cavities of different sizes filled with brown-yellow liquid and no yellow spot calcification have been observed in some cases [6-8].
Microscopic examination
The mass is composed of a mixture of breast ducts and leaflets, fibers and fat in different proportions. Because of the presence of sclerosing adenosis, ductal dilatation, apocrine metaplasia, common ductal epithelial hyperplasia, and lobular atrophy in breast lobules and ducts in MHB, there is no specific morphology of MHB histology [9-11], such as histologic non-specific forms, so overall MHB presents diverse changes. It is characterized by smooth muscle-like areas of varying proportions, with solid lamellar, fascicular, and braided arrangements in the interstitium [12]. The cytoplasms of the myoid cells are eosinophilic or lightly stained, and the nuclei are elongated and spindle-shaped with two blunt ends and fine chromatin. No obvious cell heteromorphism is observed. In a few reported cases, the myoid cells showed epithelioid differentiation and formed an infiltrating lobular carcinoma [13]. In our specimens, the myoid cells showed a solid flaky, parallel or fascicular arrangement, a mild morphology, with no atypia or mitosis. In addition, we observed breast tissues in the tumor, along with sclerosing adenopathy and ductal dilation in case 1 and columnar epithelial hyperplasia in case 2. The pathological features of the 15 reviewed cases are summarized in Table 2.
Table 2.
Pathological features of MHB cases recorded in China
| Pathological Feature | Number Of Cases |
|---|---|
| Visual inspection | |
| Gray, grayish-yellow hard masses with well-defined borders | 12 (80%) |
| Gray, grayish-yellow hard non plastic tissue with well-defined borders | 1 (6%) |
| Gray, well-defined, medium-hard lobulated tissue | 1 (6%) |
| Mermatone biopsy | 1 (6%) |
| There is a membrane or part of the membrane | 3 (20%) |
| No membrane | 12 (80%) |
| Microscopic inspection | |
| Bundles of myoid cells are solid, braided, and fascicular in arrangement | 8 (53%) |
| Bundles of myoid cells are diffusely distributed, intermixed with a fibrous fat background | 6 (40%) |
| There is a transitional transition between the bundle of myoid cells and the periductal myoepithelium | 3 (20%) |
| There is no transitional transition between the bundle of myoid cells and the periductal myoepithelium | 3 (20%) |
| Other lesions | |
| Catheter dilation or atrophy | 4 (26%) |
| Adenopathy | 2 (13%) |
| Hyperhidrosis | 4 (26%) |
| Columnar epithelial hyperplasia | 1 (6%) |
| Usual ductal epithelial hyperplasia | 3 (20%) |
| Atypical intraductal hyperplasia | 1 (6%) |
| Intraductal papilloma | 1 (6%) |
| Ductal carcinoma in situ | 1 (Low level, 6%) |
| False angiomatoid interstitial hyperplasia | 1 (6%) |
| Interstitial mucus change | 3 (20%) |
| Fibroadenoma formation | 1 (6%) |
| Immuno-phenotype of myoid cells | |
| MSA positive | 9 (60%) |
| SMA positive | 15 (100%) |
| Desmin positive | 11 (73%) |
| h-Caldesmon positive | 10 (66%) |
| P63 positive | 1 (6%) |
| S-100 positive | 1 (6%) |
Immunohistochemistry and tissue origin
Most MHB cells strongly express the smooth muscle cell (SMC) markers vimentin, SMA and MSA, in addition to desmin, calponin and h-caldesmon [15]. SMA is also expressed in myofibroblasts and myoepithelial cells. Therefore, the muscle-like cells in MHB likely originate from the SMCs. A few cases have also reported myoid cells expressing s-100, p63, and CD34 [16]. Furthermore, in some cases, the muscle-like cells and myoepithelial cells of the surrounding mammary ducts migrate to each other. Since sclerosis-induced adenopathy is common in the surrounding mammary tissue, the smooth muscle components in MHB may originate from SMCs or myoepithelial cells of the nipple and blood vessels [17]. We also observed mature cartilage tissues in the MHB interstitium as documented in some previous reports, which indicates that the smooth muscle components in MHB may also originate from the undifferentiated interstitium of the mammary gland [6]. In specimen 1, a mutual migration of myoid cells and the surrounding mammary duct myoepithelial cells was observed, but the cells were negative for s-100, calponin and P63. Taken together, the origin of MHB myoid cells is highly ambiguous and needs to be verified by studying more cases.
Differential diagnosis
MHB is difficult to distinguish from other benign breast lesions by visible inspection and has a high risk of biopsy. Therefore, its diagnosis depends on the histo-morphological and immuno-phenotypical examination of the masses. MHB can be misdiagnosed as one of the following benign lesions: (1) Fibroadenoma-characteristic periductal or intraductal growth pattern, rare normal mammary lobules in the interstitium, and absence of muscle-like components and adipose tissue [5], (2) Spindle cell lesions of the breast including leiomyoma, fibroblastoma, schwannoma and fibromatosis-characterized by a dominance of muscle-like components; leiomyoma, myofibroblastoma, and schwannoma lack breast ducts, lobules and adipose tissue components, while fibromatosis presents invasive growth, loose edema or myxoid lesions in the background, with few cell components and the absence of h-caldesmon and desmin [9], (3) Male mammary gland hyperplasia-the absence of a mucus-edematous halo around the glandular duct, interstitial lymphocyte infiltration, and micro-papillary ductal epithelial hyperplasia [8] and (4) Mammary gland disease-the absence of capsule, muscle-like components and adipose tissue.
Treatment and prognosis
Although MHB is a benign lesion, the possibility of malignant transformation or recurrence cannot be excluded. Therefore, a complete resection of the tumor is recommended. Among the 15 cases of MHB that we reviewed, there was only 1 case of postoperative local recurrence, and 1 case with recurrence at 36 and 41 months post-operation. Daya et al. [18] reported 25 cases of MHB, of which 2 cases showed postoperative recurrence at 7 and 18 months, while Tse et al. [19] reported 1 case with a 10-month postoperative recurrence. The recurrent tumor lacks a clear boundary and shows increased fission and cell atypia. Furthermore, it is unclear whether the recurrence of MHB is also related to the epithelial components in the lesion. If there are signs of ductal carcinoma, the resection area should be expanded to ensure a negative resection margin.
Lin Yu et al. [5] hypothesized that an undefined tumor boundary was indicative of mitosis in the interstitium and malignant lesions in the surrounding ducts and lobules, which correlates with an increased risk of recurrence. Few reports have been published on MHB so far, with short follow-up durations. Therefore, more cases need to be reviewed and followed for longer durations in order to determine the recurrence rate and long-term prognosis after a simple mastectomy with greater accuracy. The 2 cases of MHB reported in this paper were followed for 3~12 months after the mastectomy, and recurrence was not observed.
Acknowledgements
This study was partly supported by operating research grants from Luzhou People’s Government-Sichuan Medical University Science and Technology Projects, no. 2015LZCYD-S02.
Disclosure of conflict of interest
None.
References
- 1.Krings G, McIntire P, Shin SJ. Myofibroblastic, fibroblastic and myoid lesions of the breast. Semin Diagn Pathol. 2017;34:427–437. doi: 10.1053/j.semdp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Davies JD, Riddell RH. Muscular hamartomas of the breast. J Pathol. 1973;111:209–11. doi: 10.1002/path.1711110309. [DOI] [PubMed] [Google Scholar]
- 3.Felipe Lima J, Sirkin W, Hanna WM. Myoid hamartoma of the breast with symplastic changes. Breast J. 2016;22:583–4. doi: 10.1111/tbj.12642. [DOI] [PubMed] [Google Scholar]
- 4.Nangia A, Patiri K, Pujani M, Sehgal S. Bilateral myoid hamartoma of breast: an exceptionally rare lesion. Breast Dis. 2013;34:77–80. doi: 10.3233/BD-130355. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y, Shen XX, Yang WT. Clinicopathological analysis and literature review of myohamartoma of mammary gland. J Clin Exp Pathol. 2010;26 275-279+285. [Google Scholar]
- 6.Su CC, Chen CJ, Kuo SJ, Chen DR. Myoid hamartoma of the breast with focal chondromyoxid metaplasia and pseudoangiomatous stromal hyperplasia: a case report. Oncol Lett. 2015;9:1787–1789. doi: 10.3892/ol.2015.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Pan GQ, Ma Y. Clinicopathologic analysis of four cases of mammary musculoid hamartoma. Zhonghua Bing Li Xue Za Zhi. 2016;45:715–716. doi: 10.3760/cma.j.issn.0529-5807.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Yang YJ, Wei B, Bu H. Mammary myoid hamartomas. J Clin Exp Pathol. 2014;30:550–552. [Google Scholar]
- 9.Tian BL, Gao AF, Yang XH. Clinicopathologic analysis of mammary myoid hamartoma. Journal of Practical Oncology. 2011;26:625–627. [Google Scholar]
- 10.Filho OG, Gordan AN, Mello Rde A, Neto CS, Heinke T. Myoid hamartomas of the breast: report of 3 cases and review of the literature. Int J Surg Pathol. 2004;12:151–3. doi: 10.1177/106689690401200211. [DOI] [PubMed] [Google Scholar]
- 11.Amir RA, Sheikh SS. Breast hamartoma: a report of 14 cases of an under-recognized and under-reported entity. Int J Surg Case Rep. 2016;22:1–4. doi: 10.1016/j.ijscr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sevim Y, Kocaay AF, Eker T, Celasin H, Karabork A, Erden E, Genc V. Breast hamartoma: a clinicopathologic analysis of 27 cases and a literature review. Clinics (Sao Paulo) 2014;69:515–23. doi: 10.6061/clinics/2014(08)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäfer FK, Biernath-Wuepping J, Eckmann-Scholz C, Order BM, Mathiak M, Hilpert F, Strauss A, Jonat W, Schäfer PJ. Rare benign entities of the breast-myoid hamartoma and capillary hemangioma. Geburtshilfe Frauenheilkd. 2012;72:412–418. doi: 10.1055/s-0031-1298571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasit JG, Parikh B, Trivedi P, Shah M. Myoid (muscular) hamartoma of the breast with chondroid metaplasia. Indian J Pathol Microbiol. 2012;55:121–2. doi: 10.4103/0377-4929.94883. [DOI] [PubMed] [Google Scholar]
- 15.Mizuta N, Sakaguchi K, Mizuta M, Imai A, Nakatsukasa K, Morita M, Soshi M, Goto M, Yasukawa S, Konishi E, Taguchi T. Myoid hamartoma of the breast that proved difficult to diagnose: a case report. World J Surg Oncol. 2012;10:12. doi: 10.1186/1477-7819-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu L, Yang W, Xu X, Gu Y, Wang C, Lu H, Sheng W, Shi D. Myoid harmatoma of the breast: clinicopathologic analysis of a rare tumor indicating occasional ecurrence potential. Breast J. 2011;17:322–4. doi: 10.1111/j.1524-4741.2011.01085.x. [DOI] [PubMed] [Google Scholar]
- 17.Stafyla V, Kotsifopoulos N, Grigoriadis K, Bakoyiannis CN, Peros G, Sakorafas GH. Myoid hamartoma of the breast: a case report and review of the literature. Breast J. 2007;13:85–7. doi: 10.1111/j.1524-4741.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 18.Daya D, Trus T, D’Souza TJ, Minuk T, Yemen B. Hamartoma of the breast, an underrecognized breast lesion. A clinicopathologic and radiographic study of 25 cases. Am J Clin Pathol. 1995;103:685–9. doi: 10.1093/ajcp/103.6.685. [DOI] [PubMed] [Google Scholar]
- 19.Tse GM, Law BK, Ma TK, Chan AB, Pang LM, Chu WC, Cheung HS. Hamartoma of the breast: a clinicopathological review. J Clin Pathol. 2002;55:951–4. doi: 10.1136/jcp.55.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]


