Abstract
B7H4 is a member of the B7 family, which is expressed on antigen-presenting cells (APCs) and which negatively regulates the immune response of T cells through the inhibition of their proliferation, cytokine production, and cell cycle progression. Acyl-CoA thioesterase 4 (ACOT4) is an isoform of the ACOTs family that catalyzes the hydrolysis of fatty acyl-CoA to CoA-SH and free fatty acids. An abnormal metabolism of lipids and fatty acids is observed during tumor progression. In our study, a tissue microarray was constructed from 288 cases of gastric adenocarcinoma (GC). ACOT4 expression in cancer-associated fibroblasts (CAFs) and B7H4 expression in cancer tissues were analyzed by immunohistochemistry. The correlations among B7H4 in GC cells, ACOT4 in CAFs, and survival were analyzed. The results showed that the expression rate of B7H4 in tumor cells and ACOT4 in CAFs in 288 tissues was 71.9% (207/288) and 26.4% (76/288), respectively, and a Kaplan-Meier survival analysis showed that a low expression of ACOT4 in fibroblasts was positively correlated with poor survival. However, in a subgroup showing a high ACOT4 expression, the overall survival rate was associated with a high expression of B7H4 and correlated with poor prognosis in GC. In conclusion, ACOT4 expression in CAFs could be an independent prognostic factor for GC patients, and the co-expression with B7H4 in cancer tissues was significantly correlated with GC patients’ prognosis. This evidence can represent a comprehensive prediction and a targeted therapy for gastric cancer patients. Tumor immunotherapy targeting might be affected by tumor microenvironment metabolism.
Keywords: Gastric adenocarcinoma, ACOT4, CAFs, B7H4, survival
Introduction
Gastric cancer is one of the most prevalent malignant tumors, displaying both a high incidence and mortality worldwide [1]. Although some efforts have been made in the early diagnosis and treatment of gastric cancer, the overall survival is still disappointing. Indeed, the 5-year overall survival rate is only 20-25% due to postoperative recurrence and inefficient chemotherapy [2]. Therefore, a deeper knowledge of the molecular mechanism of gastric cancer progression for the discovery of other aspects still unknown is highly necessary to improve the treatment and prognosis of gastric cancer [3].
B7H4 (also called B7S1, B7x, or VTCN1), belonging to the B7 family of costimulatory proteins, is mainly expressed in antigenpresenting cells (APCs) and is a negative regulator of T cell proliferation, cell-cycle progression and cytokine production [4]. B7H4 is implicated in various types of human tumors including gastric cancer, playing an important role in tumor progression and is associated with a poor prognosis [5-8]. At present, immunotherapy using programmed cell death-1/programmed cell death ligand-1 (PD-1/PD-L1) inhibitors have been approved for the treatment of more than nine forms of cancer, including gastric cancer [9], since the PD-1 surface protein on CD4+ and CD8+ T cells is overexpressed in gastric cancer patients after gastric cancer surgery [10]. Meyer et al. [11] demonstrated that B7H4 receptor expression in CD8+ tumor infiltrating lymphocytes was positively correlated with PD-1 expression. Therefore, in this work the expression of the B7H4 gene in gastric adenocarcinoma (GC) patients and its relationship with prognosis were explored. Acyl-CoA thioesterase (ACOT) catalyzes the hydrolysis of acyl-CoA to the corresponding non-esterified (free) fatty acid and coenzyme A (CoA-SH). These enzymes play a very important role in lipid metabolism by maintaining cellular levels and proper ratios of free and activated fatty acids and CoA-SH [12]. ACOT4 is located in the peroxisome [13] and is a member of the gene family of type I acyl CoA thiolase [14], a peroxidase that hydrolyzes a variety of coenzyme esters. ACOT4 mainly hydrolyzes succinyl CoA, which is a peroxisome proliferator-activated receptor gene [15]. Recently, Wang et al. [16] demonstrated an aberrant ACOT1 overexpression in gastric cancer tissues, which was significantly correlated with the poor prognosis of gastric cancer patients. More and more studies on tumor immunological effects have been conducted, and tumor metabolism has received more attention. However, the correlation between tumor metabolism and tumor immunity has not been studied yet.
Cancer-associated fibroblasts (CAFs) [17], as the main components of tumor stroma, play a key role in tumor progression. Previous studies [18] showed that a tumor is not just a bunch of tumor cells, but an ecosystem composed of cells, infiltrating immune cells, stromal cells, and other related molecules. In 2009, Pavlides [19] discovered that cancer cells mainly induce an anti-Warburg effect in the nearby CAFs. A recent study showed that CAFs stromal cells, which dominate the tumor microenvironment, provide energy-rich metabolites to fuel the mitochondrial respiration and anabolic metabolism of cancer cells [20,21]. Increased evidence suggested that CAFs play a key role in promoting tumor cell invasion and metastasis by overexpressing a variety of factors [22-24]. However, how CAFs affect tumor cell progression is still unclear. Therefore, in this study, we are the first demonstrating that ACOT4 is expressed in CAFs in 26.4% (76/288) of GC patients’ tissues, and we characterize the association between its expression and the expression of B7H4, and finally, we evaluate the correlation of the associated results with survival.
Materials and methods
Patients and tissue samples
A total of 302 GC patients were enrolled in this study from June 2006 to November 2011 according to the diagnosis and treatment records of gastric carcinoma patients at the Department of Pathology, Affiliated Hospital of Jiangnan University, China. However, only 288 patients arrived through the end of the follow up on October 2017. The clinicopathological characteristics of the 288 GC patients are shown in Table 1 and include patient gender, age, tumor size, histological grade, primary tumor, nodal metastasis, pathologic stage, vascular invasion, neural invasion, and lymphatic invasion. Paraffin-embedded specimens were provided by the Department of Pathology of the Affiliated Hospital of Jiangnan University. The clinicopathological parameters were evaluated using chi-square and fisher exact tests.
Table 1.
The correlation between ACOT1 expression in CAFs, B7-H4 expression in tumor cells and the clinical pathological variables in 288 gastric adenocarcinoma patients
| Clinical parameters | ACOT4 | P value | B7H4 | P value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Positive (N = 76) | Negative (N = 212) | Positive (N = 207) | Negative (N = 81) | |||
| Gender | 0.883 | 0.123 | ||||
| Female | 18 (23.7%) | 52 (24.5%) | 46 (22.2%) | 24 (29.6%) | ||
| Male | 58 (76.3%) | 160 (75.5%) | 161 (77.8%) | 57 (70.4%) | ||
| Age | 0.612 | 0.336 | ||||
| < 60 | 29 (38.2%) | 74 (34.9%) | 72 (34.8%) | 31 (38.3%) | ||
| ≥ 60 | 47 (61.8%) | 138 (65.1%) | 135 (65.2%) | 50 (61.7%) | ||
| Tumor size | 0.561 | 0.429 | ||||
| < 3 cm | 29 (38.2%) | 89 (42.0%) | 86 (41.5%) | 32 (39.5%) | ||
| ≥ 3 cm | 47 (61.8%) | 123 (58.0%) | 121 (58.5%) | 49 (60.5%) | ||
| Histological grade | 0.249 | 0.023* | ||||
| Well/moderately | 19 (25.0%) | 68 (32.1%) | 55 (26.6%) | 32 (39.5%) | ||
| Poorly | 57 (75.0%) | 144 (67.9%) | 152 (73.4%) | 49 (60.5%) | ||
| Vascular invasion | 0.634 | 0.362 | ||||
| Absent | 56 (73.7%) | 162 (76.4%) | 155 (74.9%) | 63 (77.8%) | ||
| Present | 20 (26.3%) | 50 (23.6%) | 52 (25.1%) | 18 (22.2%) | ||
| Neural invasion | 0.913 | 0.391 | ||||
| Absent | 45 (59.2%) | 124 (58.5%) | 123 (59.4%) | 46 (56.8%) | ||
| Present | 31 (40.8%) | 88 (41.5%) | 84 (40.6%) | 35 (43.2%) | ||
| Lymph node metastasis | 0.867 | 0.452 | ||||
| N0 | 35 (46.1%) | 100 (47.2%) | 98 (47.3%) | 37 (45.7%) | ||
| N1/N2/N3 | 41 (53.9%) | 112 (52.8%) | 109 (52.7%) | 44 (54.3%) | ||
| Distant metastasis | 0.911 | 0.550 | ||||
| Absent | 73 (96.1%) | 203 (95.8%) | 198 (95.7%) | 78 (96.3%) | ||
| Present | 3 (3.9%) | 9 (4.2%) | 9 (4.3%) | 3 (3.7%) | ||
| TNM stage | 0.505 | 0.303 | ||||
| I-II | 68 (89.5%) | 195 (92.0%) | 111 (53.6%) | 40 (49.4%) | ||
| III-IV | 8 (10.5%) | 17 (8.0%) | 96 (46.4%) | 41 (50.6%) | ||
| T stage | 0.194 | 0.403 | ||||
| T1-2 | 35 (46.1%) | 116 (54.7%) | 190 (91.8%) | 73 (90.1%) | ||
| T3-4 | 41 (53.9%) | 96 (45.3%) | 17 (8.2%) | 8 (9.9%) | ||
NOTE: Values are n (%).
P < 0.05 by the χ2 test.
Construction of tumor tissue microarray (TMA) and CAFs TMA
The hematoxylin and eosin-stained sections of formalin fixed, paraffin-embedded tumor tissue blocks were screened to validate cancerous tissue, adjacent fibroblast tissue, and adjacent normal tissue. The corresponding spots on the tissue block were marked for a correct tissue core punch. TMA was assembled using a manual TMA spotting (Quick-Ray; UNITMA Co, Ltd, Seoul, Korea). Two representative tumor cores with a diameter of 1.5 mm were obtained from each GC tissue block. Two tissue cores per case were arranged to increase the concordance rate between the immunohistochemistry (IHC) results of the TMA and the integral sections. Two normal gastric tissue cores from the same block were included in each TMA core and were arranged according to the corresponding tumor core below. The same method was used on the issue of CAFs. Hematoxylin and eosin staining was performed on each block to confirm tumor tissue integrity and cell morphology. Cases with inadequate carcinoma tissue or cases that did not contain adenocarcinoma tissue in the cores were not included.
IHC staining
Formalin-fixed, paraffin-embedded tissues were cut into 4-μm thick sections, deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. An antigen retrieval procedure was applied in boiling water using a sodium citrate solution for 20 minutes. Endogenous peroxidase was blocked by incubating the sections with hydrogen peroxide for 5 minutes. Next, sections were incubated with primary polyclonal antibodies overnight at 4°C against ACOT4 (1:150, ab133948; Abcam, Hong Kong, China), and B7H4 (1:100, 12080-1-AP; proteinch, Wuhan, China). Sections were washed, incubated with an amplification agent and polymerase (reagent A; GTVisionTM III Kit supply, Shanghai, China), and then stained with 3,3’-diaminobenzidine (reagent B and C; GTVisionTM III Kit supply). Nuclei were counterstained with hematoxylin. The negative control was treated with phosphate buffered saline instead of the primary antibody.
IHC staining evaluation
B7H4 expression was mainly found in the cytoplasm of carcinoma cells. Gastric cancer ACOT4 expression was mainly observed in the cytoplasm of CAFs. Each case was scored according to the percentage of positively stained cells in the entire section (0, no positive staining or ≤ 5%; 1, 6%-25% positive staining; 2, 26%-50% positive staining; 3, 51%-75% positive staining; and 4, 76%-100% positive staining). The scoring intensity was determined as previously described: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The score was obtained by multiplying the stain mode score by the intensity score. Each case had 2 cores that were observed in 2 different fields, with one score viewed under high magnification (×400), and the final score was calculated by the average of the four scores. This was a double-blinded study, and the two pathologists who scored the staining did not know the clinical parameters. In case of serious differences, the final score was determined by reassessing the staining using a multi-head microscope. The expressions of B7H4 in the tumor tissue and ACOT4 in the CAFs tissue were evaluated using chi-square and Fisher’s exact tests.
Statistical analysis
The statistical analysis was performed using SPSS 19.0 software (IBM, Chicago, IL USA). The clinicopathological parameters and the expression of B7H4 in the tumor tissue and ACOT4 in the CAFs tissue were evaluated using chi-square and Fisher’s exact test. The Pearson chi-square test or Fisher’s exact test methods were used to analyze the relationship between the B7H4 and ACOT4 expressions. The proportional hazard model was calculated by multivariate survival analysis using Cox regression analysis, and the overall survival was calculated using the Kaplan-Meier curve (log-rank test). P < 0.05 was considered statistically significant.
Results
Patient characteristics
The clinicopathological characteristics of the 288 GC patients are shown in Table 1. The study cohort included 218 males (75.7%) and 70 females (24.3%). The age at initial diagnosis was classified as < 60 years (35.8%) or ≥ 60 years (64.2%), and the tumor size was classified as < 3 cm (41.0%) and ≥ 3 cm (59%). Peripheral neuropathy, vascular invasion and lymphatic metastasis (N1-N3) accounted for 41.3%, 24.3%, and 53.1% of the total cases, respectively. According to the pathological classification, 263 (91.3%) cases were stage I-II disease and 25 (8.7%) cases were stage III-IV disease.
Expression of ACOT4 and B7H4 in GC patients
GC histomorphology was assessed by hematoxylin and eosin staining (Figure 1A). Similar to previous studies, ACOT4 positive expression was mainly found in the cytoplasm of CAFs (Figure 1B). B7H4 was highly expressed in GC tissues and the staining indicated its localization in the cytoplasm (Figure 1C). The positive rate of ACOT4 and B7H4 in 288 tissues was 26.4% (76/288) and 71.9% (207/288) respectively, by immunohistochemical staining.
Figure 1.
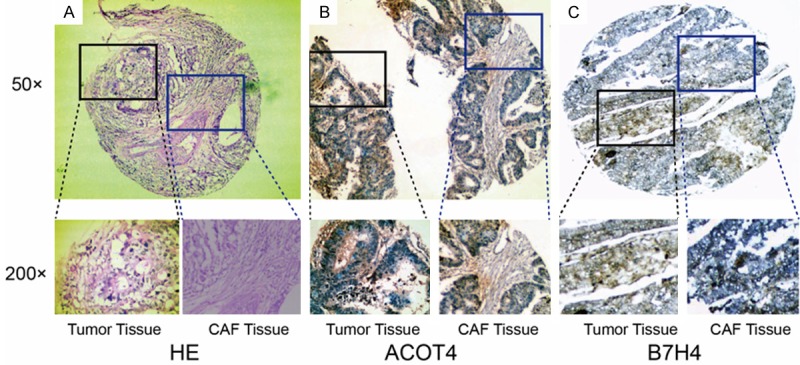
Representative results of hematoxylin and eosin staining, ACOT4 and B7H4 levels in gastric cancer patients. A. Show the hematoxylin and eosin (HE) staining of gastric adenocarcinoma tissues and in CAFs. B. Are representative images showing the expression of ACOT4 in gastric carcinoma tissue and in CAFs, respectively. C. Show the expression of B7H4 in gastric carcinoma tissue and in CAFs. The photomicrographs were taken at 50× and 200× magnification.
Univariate and multivariate analysis of survival
The ACOT4 expression in the CAFs of GC patients was independent from several factors including age, gender, tumor stage, and metastasis (Table 1). The positive rate of B7H4 expression in cancer cells was associated with histological grade (P = 0.023), but not with gender, age, tumor size, vascular invasion, neural invasion, lymph node metastasis, histological grade, distant metastasis, clinical stage, or primary tumor stage (Table 2).
Table 2.
Correlation analysis of ACOT4 and B7H4 expression levels
| B7H4 expression | |||
|---|---|---|---|
|
|
|||
| Negative | Positive | ||
| ACOT4 expression | Negative | 52 | 160 |
| Positive | 29 | 47 | |
| p value | 0.018* | ||
P < 0.05 by the χ2 test.
The effect of the combined expression of B7H4 and ACOT4 in GC patients’ prognosis
Among the gastric cancer patients, the shortest survival time was 25 days, while the longest was 2672 days, and the median survival time was 768.07 days. The Kaplan-Meier survival curve showed a positive correlation between ACOT4 expression in the CAFs and survival (log-rank P = 0.024). The correlation between B7H4 expression in the cancer tissues and overall survival was not statistically significant (log-rank P = 0.903). A Kaplan-Meier survival curve of the co-expression of ACOT4 and B7H4 showed that the overall survival of the ACOT4(-)/B7H4(-) patients was the highest, and the overall survival of ACOT4(+)/B7H4(-) was the lowest (Figure 2).
Figure 2.
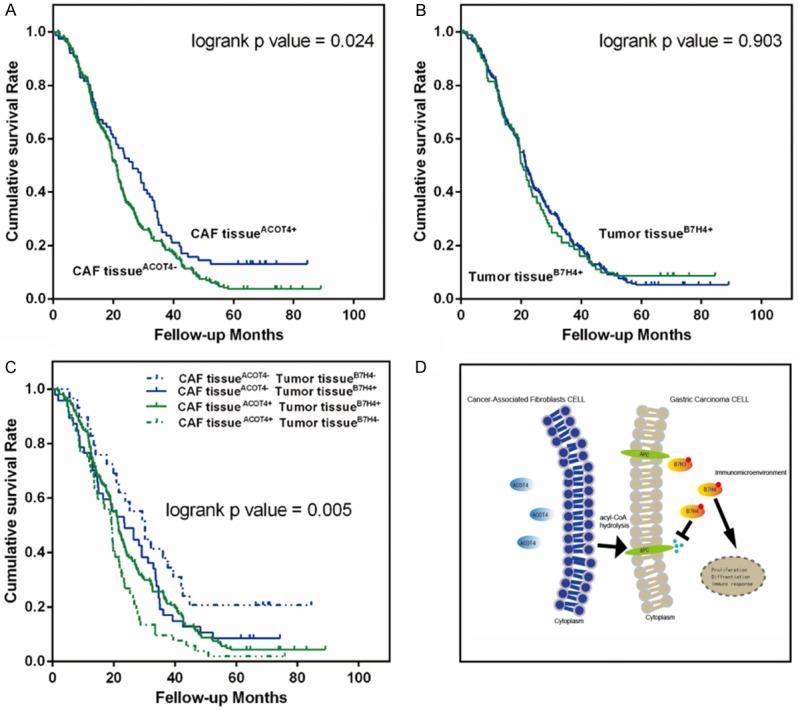
Kaplan-Meier curves showing the impact of CAFs ACOT4 expression and carcinoma B7-H4 expression on overall survival in 288 gastric cancer patients. A. Kaplan-Meier survival method showed the survival curves of patients with ACOT4 negative expressions with a significantly poor prognosis compared with the ACOT4 positive expressions in the CAFs, (log-rank P = 0.024). B. The correlation between B7H4 expression in cancer tissues and total survival was not statistically significant (log-rank P = 0.903). C. The relationship between survival and the combined expressions of ACOT4 and B7-H4 (P = 0.005). D. The potential mechanism involved. +, positive; -, negative.
Correlation between B7H4 and ACOT4 protein expression
As shown in Table 3, a correlation analysis was used to determine whether the B7H4 expression in GC was associated with ACOT4 expression in CAFs. A total of 47 (22.7%) GC patients showed positive ACOT4 and B7H4 co-expression, while in 52 (64.2%) patients, ACOT4 expression in CAFs and B7H4 in GC tissue were expressed in the opposite way. These findings indicated a significant correlation between the expression of B7H4 in GC tissue and ACOT4 expression in CAFs (P = 0.018).
Table 3.
Univariate and multivariate analyses of clinicopathological variables for overall survival rate in 288 gastric adenocarcinoma patients
| Clinical parameters | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | HR (95% CI) | |||
| Sex (female vs male) | 1.027 (0.777-1.358) | 0.851 | 1.056 (0.791-1.411) | 0.710 |
| Age (≥ 60 y vs < 60 y) | 1.039 (0.810-1.333) | 0.764 | 1.031 (0.793-1.341) | 0.818 |
| Tumor size (≥ 3 cm vs < 3 cm) | 0.910 (0.714-1.159) | 0.446 | 0.913 (0.710-1.175) | 0.480 |
| Histologic grade (poor vs well to moderately) | 0.880 (0.679-1.140) | 0.334 | 0.900 (0.691-1.172) | 0.432 |
| Vascular invasion (present vs absent) | 1.238 (0.939-1.634) | 0.131 | 1.137 (0.845-1.531) | 0.397 |
| Neural invasion (present vs absent) | 0.998 (0.783-1.272) | 0.987 | 0.983 (0.762-1.270) | 0.898 |
| T stage (T1-2/T3-4) | 0.950 (0.748-1.206) | 0.673 | 0.955 (0.741-1.230) | 0.720 |
| Lymph node metastasis (present vs absent) | 1.116 (0.878-1.418) | 0.371 | 1.086 (0.835-1.413) | 0.540 |
| Distant metastasis (present vs absent) | 0.789 (0.419-1.485) | 0.463 | 0.759 (0.395-1.456) | 0.407 |
| TNM stage (III-IV vs I-II) | 0.785 (0.507-1.215) | 0.277 | 0.828 (0.529-1.296) | 0.408 |
| ACOT4 (positive/negative) | 0.722 (0.546-0.954) | 0.022* | 0.748 (0.563-0.994) | 0.045* |
| B7H4 (positive/negative) | 0.984 (0.753-1.285) | 0.903 | 0.892 (0.673-1.182) | 0.427 |
P < 0.05.
Discussion
ACOT4 in CAFs of cancer cells
IHC showed ACOT4 protein expression in the cytoplasm, and this result was consistent with the findings of Hunt et al. [25]. The ACOT4 positive rate in the CAFs among the 288 tissues was 26.4% (76/288). This expression was independent from the genders, ages, tumor stages, and tumor sizes of the gastric cancer patients. ACOT4 is a peroxisome protein expressed in the cytoplasm [26]. There are two known possible pathways for the production of succinyl CoA, such as the breakdown of amino acids and the oxidation of dicarboxylic acids. ACOT4 is expressed in the kidney, liver and proximal intestine, it regulates the oxidation of dicarboxylic acid in peroxisomes, and may hydrolyze succinyl-CoA under certain conditions. Wang et al. [16] demonstrated an aberrant ACOT1 overexpression in gastric cancer tissues, which was significantly correlated with patients’ poor prognosis. ACOT1 [27] catalyzes a reaction that produces free fatty acids, reducing the flux of fatty acids to downstream metabolic pathways. ACOT7 [28] ACOT11, and ACOT13 [29] are also associated with a poor overall survival rate in lung adenocarcinoma patients. Hunt [26] identified and characterized a mouse ACOT gene cluster made up of six genes that apparently arose from gene duplications encoding ACOTs localized in the cytosol (ACOT1), mitochondria (ACOT2), and peroxisomes (ACOT3-6), and the data strongly suggest that the human ACOT4 gene acquired the functions of three mouse genes by a functional convergent evolution, providing an explanation for the unexpected complexity of the ACOT4 gene. Therefore, we assumed that the promotion of fatty acid metabolism is closely related to the regulation of tumor apoptosis or proliferation, depending on the circumstances.
Relationship between B7H4 and prognosis
PD-L1 is overexpressed in tumor cells and its blockage has a significant effect on human tumors. However, most patients did not respond to this therapy, suggesting that there may be other mechanisms of T cell failure. In our result, the positive rate of B7H4 in 288 tissues was 71.9% (207/288), it was highly expressed in GC cells, and its expression was significantly correlated with histological grade. B7H4 expression in gastric cancer tissues with low histological grades was higher than it was in the high/moderate histological grades, suggesting that B7H4 might be involved in the development of GC. Nevertheless, unlike most of the previous results [30-32], our analysis showed that B7H4 was not an independent prognostic factor. The reason might be that most of the patients with gastric cancer enrolled in this study were early stage patients (91.3% cases in stages I-II). B7H4 expression is mostly negatively correlated with cancer patient outcomes [30-33]. Whether and how B7H4 regulates tumor-specific CD8+ T cell responses, in particular, CD8+ T cell exhaustion in the context of tumors remains largely unknown. According to Li et al. [34], B7H4 can potentially become a new immunotherapy checkpoint in cancer treatment.
The relationship between tumor metabolism and immunity
In this study, we were the first to demonstrate that ACOT4 was expressed in gastric cancer-related CAFs and that ACOT4 was positively correlated with survival. It was interesting to see that the B7H4 positive expression rate was not significantly correlated with the survival of gastric cancer patients, since ACOT4 is known as a protective gene. In low B7H4 expression patients, the prognosis of low ACOT4 expression patients was significantly better than that of the high ACOT4 expression patients; in patients with positive B7H4, no difference in survival was observed between patients with negative ACOT4 and those with positive ACOT4. Multiple studies showed that cancer cells increased fatty acid synthesis, providing lipids for membrane components, beta-oxidation, and lipid modification of lipoproteins [35,36]. Fatty acid can serve as the main energy source for many carcinoma types [37-39]. Our results suggest that there might be an intersection between tumor metabolism and tumor immunity, and the correlation between the two proteins was statistically significant. However, further studies are needed to confirm our discovery.
The altered metabolic landscape of the tumor microenvironment restrains the infiltration of immune cells and other antitumor immunity functions through the production of immuno-suppressive metabolites. Metabolic dysregulation in cancer cells may affect the expression of cell surface markers, which interferes with immune surveillance. An in vitro study also showed that both co-inhibitory (PD-L1 and B7H4) and co-stimulatory (CD86) molecules are up-regulated when fibroblasts are cultured [40]. We hypothesized that ACOT4 might inhibit the infiltration of immune cells and other anti-tumor immunity functions through the combination of fatty acid metabolites with APCs, thereby mutating the immune response of B7H4. The partial antibody experiment previously performed on B7H4 also demonstrated the expression instability of B7H4, which is easy to be identified and abnormally expressed [41].
Immune checkpoint therapies revolutionized the standard treatment in some cancer patients, but cancer in many other patients is resistant to immunotherapy. Regarding immunotherapy in gastric cancer, Keytruda (PD-1 antibody), as a second-line drug, failed to improve the overall survival and non-progressive survival of patients with PD-L1 positive gastric cancer compared with standard chemotherapy, according to the research of Keynote 061 [42]. According to ROBIN [43], specific metabolic pathways involved in immunotherapy resistance include PI3K-Akt-mTOR, hypoxia-inducible factor (HIF), adenosine, JAK/STAT, and Wnt/Beta-catenin. It is known that there is a metabolic symbiosis among cancer cells and mesenchymal cells (especially CAFs) [44]. Cancer cells demonstrate a metabolic heterogeneity, and immunotherapy has become a promising approach in a selective anticancer therapy. In the future, combination strategies with the addition of antistromal drugs should be considered. As a matter of fact, end-stage disease patients with cachexia may be more affected by lipid metabolism [45]. Thus, more patients with advanced tumors should be included in further studies.
Conclusion
Our study revealed that ACOT4 and B7H4 were both expressed in GC. ACOT4 overexpression in CAFs was associated with survival rate among GC patients. In addition, a strong correlation between ACOT4 in CAFs and B7H4 in GC tissue was found. These findings suggested that a pre-individualized therapy targeting both ACOT4 and B7H4 might serve as a promising treatment modality in GC patients. Thus, our results provided new ideas for studying the correlation between immune and tumor microenvironment metabolism in GC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81372375 to D. Hua) and by a grant from Fundamental Research Funds for the Central Universities funded by the Ministry of Education of China (no. JUSRP51710A to Y. Cheng). This study was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Jiangnan university.
This study was funded by the Ministry of Education of China (JUSRP51710A). The recruitment of subjects was based on the principle of voluntary and informed consent, and the rights and privacy of the subjects will be protected to the greatest extent possible.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Razzak M. Targeted therapies: hope for antiangiogenic therapy in advanced gastric cancer. Nat Rev Clin Oncol. 2013;10:548. doi: 10.1038/nrclinonc.2013.156. [DOI] [PubMed] [Google Scholar]
- 3.Solomon BL, Garrido-Laguna I. Upper gastrointestinal malignancies in 2017: current perspectives and future approaches. Future Oncol. 2018;14:947–962. doi: 10.2217/fon-2017-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podojil JR, Miller SD. Potential targeting of B7-H4 for the treatment of cancer. Immunol Rev. 2017;276:40–51. doi: 10.1111/imr.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JB, Stashwick C, Powell DJ Jr. B7-H4 as a potential target for immunotherapy for gynecologic cancers: a closer look. Gynecol Oncol. 2014;134:181–9. doi: 10.1016/j.ygyno.2014.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maskey N, Li K, Hu M, Xu Z, Peng C, Yu F, Cao H, Chen J, Li Y, Yang G. Impact of neoadjuvant chemotherapy on lymphocytes and co-inhibitory B7-H4 molecule in gastric cancer: low B7-H4 expression associates with favorable prognosis. Tumour Biol. 2014;35:11837–43. doi: 10.1007/s13277-014-2410-2. [DOI] [PubMed] [Google Scholar]
- 7.Abadi YM, Jeon H, Ohaegbulam KC, Scandiuzzi L, Ghosh K, Hofmeyer KA, Lee JS, Ray A, Gravekamp C, Zang X. Host B7x promotes pulmonary metastasis of breast cancer. J Immunol. 2013;190:3806–14. doi: 10.4049/jimmunol.1202439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crispen PL, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED. Predicting disease progression after nephrectomy for localized renal cell carcinoma: the utility of prognostic models and molecular biomarkers. Cancer. 2010;113:450–460. doi: 10.1002/cncr.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takaya S, Saito H, Ikeguchi M. Upregulation of immune checkpoint molecules, PD-1 and LAG-3, on CD4+ and CD8+ T cellsafter gastric cancer surgery. Yonago Acta Medica. 2015;58:39–44. [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer MA, Denardo DG. Better together: B7S1 checkpoint blockade synergizes with anti-PD1. Immunity. 2018;48:621–623. doi: 10.1016/j.immuni.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Brocker C, Carpenter C, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human acyl-CoA thioesterase gene (ACOT) family. Hum Genomics. 2010;4:411–420. doi: 10.1186/1479-7364-4-6-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tillander V, Alexson SEH, Cohen DE. Deactivating fatty acids: Acyl-CoA thioesterase-mediated control of lipid metabolism. Trends Endocrinol Metab. 2017;28:473–484. doi: 10.1016/j.tem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt MC, Lindquist PJ, Nousiainen S, Huttunen M, Orii K, Svensson TL, Aoyama T, Hashimoto T, Diczfalusy U, Alexson SE. Acyl-CoA thioesterases belong to a novel gene family of peroxisome proliferator-regulated enzymes involved in lipid metabolism. Cell Biochem Biophys. 2000;32 Spring:317–24. doi: 10.1385/cbb:32:1-3:317. [DOI] [PubMed] [Google Scholar]
- 15.Westin MA, Hunt MC, Alexson SE. The identification of a succinyl-CoA thioesterase suggests a novel pathway for succinate production in peroxisomes. J Biol Chem. 2005;280:38125–32. doi: 10.1074/jbc.M508479200. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Wu J, Qiu Z, Ge X, Liu X, Zhang C, Xu W, Wang F, Hua D, Qi X, Mao Y. ACOT1 expression is associated with poor prognosis in gastric adenocarcinoma. Hum Pathol. 2018;77:35–44. doi: 10.1016/j.humpath.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, He Y, Chen H. Autophagic tumor stroma: mechanisms and roles in tumor growth and progression. Int J Cancer. 2013;132:1–8. doi: 10.1002/ijc.27664. [DOI] [PubMed] [Google Scholar]
- 18.Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30:1002–19. doi: 10.1101/gad.279737.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 20.Shan T, Chen S, Chen X, Lin WR, Li W, Ma J, Wu T, Cui X, Ji H, Li Y, Kang Y. Cancer-associated fibroblasts enhance pancreatic cancer cell invasion by remodeling the metabolic conversion mechanism. Oncol Rep. 2017;37:1971–1979. doi: 10.3892/or.2017.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, Daumer KM, Zhou J, Wang C, Katiyar S, Xu H, Bosco E, Quong AA, Aronow B, Witkiewicz AK, Minetti C, Frank PG, Jimenez SA, Knudsen ES, Pestell RG, Lisanti MP. Caveolin-1-/- null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts. Am J Pathol. 2009;174:746–61. doi: 10.2353/ajpath.2009.080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi: 10.1186/s13046-015-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK, Vander Heiden MG, Migneco G, Chiavarina B, Frank PG, Capozza F, Flomenberg N, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle. 2010;9:1960–1971. doi: 10.4161/cc.9.10.11601. [DOI] [PubMed] [Google Scholar]
- 25.Hunt MC, Siponen MI, Alexson SE. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim Biophys Acta. 2012;1822:1397–410. doi: 10.1016/j.bbadis.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Hunt MC, Rautanen A, Westin MA, Svensson LT, Alexson SE. Analysis of the mouse and human acyl-CoA thioesterase (ACOT) gene clusters shows that convergent, functional evolution results in a reduced number of human peroxisomal ACOTs. FASEB J. 2006;20:1855–64. doi: 10.1096/fj.06-6042com. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Yasuoka A, Shimizu M, Saito Y, Kumakura K, Asakura T, Nagai T. Transcriptomic responses of the liver and adipose tissues to altered carbohydrate-fat ratio in diet: an isoenergetic study in young rats. Genes Nutr. 2017;12:10. doi: 10.1186/s12263-017-0558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung SH, Lee HC, Hwang HJ, Park HA, Moon YA, Kim BC, Lee HM, Kim KP, Kim YN, Lee BL, Lee JC, Ko YG, Park HJ, Lee JS. Acyl-CoA thioesterase 7 is involved in cell cycle progression via regulation of PKCζ-p53-p21 signaling pathway. Cell Death Dis. 2017;8:e2793. doi: 10.1038/cddis.2017.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung JY, Chiang SR, Liu KT, Tsai MJ, Huang MS, Shieh JM, Yen MC, Hsu YL. Overexpression and proliferation dependence of acyl-CoA thioesterase 11 and 13 in lung adenocarcinoma. Oncol Lett. 2017;14:3647. doi: 10.3892/ol.2017.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Shibata K, Koya Y, Kajiyama H, Senga T, Yamashita M, Kikkawa F. B7-H4 overexpression correlates with a poor prognosis for cervical cancer patients. Mol Clin Oncol. 2014;2:219–225. doi: 10.3892/mco.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsiaousidou A, Tsaroucha AK, Lambropoulou M, Pitiakoudis M, Polychronidis A, Chatzitheoklitos E, Romanidis K, Simopoulos C. Increased B7H4 tissue expression correlates with high CA19.9 serum levels and a worse prognosis of pancreatic adenocarcinoma. Clin Exp Med. 2016;16:351–6. doi: 10.1007/s10238-015-0352-7. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Xu C, Wang Y, Yu L, Zhang X. Prognostic values of B7-H4 in non-small cell lung cancer. Biomarkers. 2016:1–16. doi: 10.1080/1354750X.2016.1203997. [DOI] [PubMed] [Google Scholar]
- 33.Podojil JR, Liu LN, Marshall SA, Chiang MY, Goings GE, Chen L, Langermann S, Miller SD. B7-H4Ig inhibits mouse and human T-cell function and treats EAE via IL-10/Treg-dependent mechanisms. J Autoimmun. 2013;44:71–81. doi: 10.1016/j.jaut.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Lee Y, Li Y, Jiang Y, Lu H, Zang W, Zhao X, Liu L, Chen Y, Tan H, Yang Z, Zhang MQ, Mak TW, Ni L, Dong C. Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8+ T cells. Immunity. 2018;48:773–786. e5. doi: 10.1016/j.immuni.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Lee M, Yoon JH. Metabolic interplay between glycolysis and mitochondrial oxidation: the reverse Warburg effect and its therapeutic implication. World J Biol Chem. 2015;6:148–61. doi: 10.4331/wjbc.v6.i3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warmoes M, Jaspers JE, Xu G, Sampadi BK, Pham TV, Knol JC, Piersma SR, Boven E, Jonkers J, Rottenberg S, Jimenez CR. Proteomics of genetically engineered mouse mammary tumors identifies fatty acid metabolism members as potential predictive markers for cisplatin resistance. Mol Cell Proteomics. 2013;12:1319–34. doi: 10.1074/mcp.M112.024182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JH, Vithayathil S, Kumar S, Sung PL, Dobrolecki LE, Putluri V, Bhat VB, Bhowmik SK, Gupta V, Arora K, Wu D, Tsouko E, Zhang Y, Maity S, Donti TR, Graham BH, Frigo DE, Coarfa C, Yotnda P, Putluri N, Sreekumar A, Lewis MT, Creighton CJ, Wong LC, Kaipparettu BA. Fatty acid oxidation-driven src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep. 2016;14:2154–2165. doi: 10.1016/j.celrep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, Eyob H, Kajimura S, Tward A, Krings G, Nomura DK, Goga A. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22:427–32. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastò A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, Rasola A, Frasson C, Nardo G, Zulato E, Nicoletto MO, Manicone M, Indraccolo S, Amadori A. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5:4305–19. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khosravi-Maharlooei M, Pakyari M, Jalili RB, Salimi-Elizei S, Lai JC, Poormasjedi-Meibod M, Kilani RT, Dutz J, Ghahary A. Tolerogenic effect of mouse fibroblasts on dendritic cells. Immunology. 2016;148:22–33. doi: 10.1111/imm.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iizuka A, Kondou R, Nonomura C, Ashizawa T, Ohshima K, Kusuhara M, Isaka M, Ohde Y, Yamaguchi K, Akiyama Y. Unstable B7-H4 cell surface expression and T-cell redirection as a means of cancer therapy. Oncol Rep. 2016;36:2625–2632. doi: 10.3892/or.2016.5084. [DOI] [PubMed] [Google Scholar]
- 42.Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 43.Ramapriyan R, Caetano MS, Barsoumian HB, Mafra ACP, Zambalde EP, Menon H, Tsouko E, Welsh JW, Cortez MA. Altered cancer metabolism in mechanisms of immunotherapy resistance. Pharmacol Ther. 2019;195:162–171. doi: 10.1016/j.pharmthera.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Pons-Tostivint E, Thibault B, Guillermet-Guibert J. Targeting PI3K signaling in combination cancer therapy. Trends Cancer. 2017;3:454–469. doi: 10.1016/j.trecan.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]


