Abstract
Intrahepatic cholangiocarcinoma (ICC) has had an increasing incidence in recent years. It exhibits high recurrence and metastasis rates, so it is a global health problem. LncRNAs have long been thought to have a role in regulating tumorigenesis and tumor progression and in playing a carcinogenic or tumor suppressor role in malignant cells. Lnc-LFAR1 is enriched in liver tissue, but its role and mechanism in ICC have not been elucidated. ICC cell line QBC939 cells were randomly divided into 3 groups, including a control group, an lnc-LFAR1 overexpression group, and an lnc-LFAR1 siRNA group, the cells of which were transfected with pcDNA3.1-LFAR1 plasmid and lnc-LFAR1 siRNA, respectively. Real-time PCR was used to quantify lnc-LFAR1 expression. A tetrazolium salt (MTT) colorimetric assay was adopted to assess cell viability. A cell scratch assay was selected to assess cell migration. A transwell chamber assay was used to test cell invasion. E-cadherin and vimentin expressions were detected using Western blot. The TGFβ/Smad signaling pathway was measured using real-time PCR. The transfection of pcDNA3.1-LFAR1 significantly increased the expression of lnc-LFAR1, promoted cell proliferation, facilitated cell migration and invasion, reduced E-cadherin levels, enhanced vimentin expression, upregulated TGF-β1 and Smad2 and Smad4 expressions (P < 0.05). Lnc-LFAR1 siRNA transfection clearly downregulated lnc-LFAR1 expression, inhibited cell proliferation, suppressed cell migration and invasion, increased E-cadherin expression, and decreased vimentin, TGF-β1, Smad2, and Smad4 levels (P < 0.05). Targeting lnc-LFAR1 can inhibit cell proliferation, migration, invasion, and EMT by regulating the TGFβ/Smad pathway to affect the occurrence and development of cholangiocarcinoma.
Keywords: Cholangiocarcinoma, lnc-LFAR1, TGFβ/Smad signaling pathway, proliferation, invasion, EMT
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the most common malignant tumor in the intrahepatic biliary epithelium. Its incidence is second only to hepatocellular carcinoma in primary liver malignancy, with an increasing trend [1,2]. The onset of ICC is concealed, with no obvious symptoms or signs in the early stages. Most patients are not found in time, leading to rapid development and a high mortality rate, which seriously threatens global health [3,4]. Adenocarcinoma is the main pathological type of ICC, which can easily invade blood vessels and lymphatic vessels, resulting in high recurrence and metastasis rates [5]. There are many factors involved in the pathogenesis of ICC, including viral infections, genetic factors, and physical and chemical factors [6]. At present, surgical resection or liver transplantation is the main treatment method for ICC, but the prognosis of patients with surgical resections is poor, and the availability of livers for liver transplantation is rare. The long-term survival rate is still low, which causes great pain and economic burden to patients and their families [7,8]. Understanding the mechanism of ICC is a difficult medical challenge. At present, the molecular markers of ICC have not been elucidated, so there is no effective molecular target [9]. The elucidation of the molecular markers and molecular targets of ICC is conducive to its early prevention and treatment.
The protein encoding genes encode fewer than 2% of the human genome. Most transcripts are composed of non-coding RNAs. LncRNAs, transcripts over 200 nt in length, are not involved in encoding proteins that are abundant in the genome [10,11]. Despite their poor conservation, lncRNAs can be regulated by transcription factors with tissue or cell specificity. They are involved in cell differentiation and somatic regulation [12,13]. Recent studies found that lncRNAs regulate gene expression through epigenetic and transcriptional methods, and thus participate in various biological processes such as cell proliferation, survival, and differentiation [14,15]. Lnc-LFAR1 is a newly discovered lncRNA with a 734 nt transcript containing only one exon and is enriched in liver tissue [16]. However, its role and mechanism in ICC has not been elucidated. Therefore, this study aimed to investigate the role and possible mechanism of lnc-LFAR1 in the biological behavior of ICC cell QBC939.
Materials and methods
Main reagents and instruments
The ICC QBC939 cell line was preserved in the Hunan University laboratory. DMEM medium, fetal bovine serum (FBS), and penicillin-streptomycin were purchased from Hyclone. Dimethyl sulfoxide (DMSO) and MTT powder was purchased from Gibco. Trypsin-EDTA digest was purchased from Sigma. PVDF membrane was purchased from Pall Life Sciences. Western blot related chemical reagent was purchased from Beyotime. ECL reagent was purchased from Amersham Biosciences. Rabbit anti-human E-cadherin monoclonal antibody, rabbit anti-human vimentin monoclonal antibody, and goat anti-rabbit horseradish peroxidase (HRP) labeled IgG secondary antibody were purchased from Cell Signaling. An RNA extraction kit, a reverse transcription kit, and lipo2000 reagent were purchased from Invitrogen. The pcDNA3.1-LFAR1 plasmid and lnc-LFAR1 siRNA were synthesized by Genepharma. Transwell chambers were purchased from Hyclone. The Labsystem Version 1.3.1 microplate reader was purchased from the Bio-rad Corporation. The ABI 7700 Fast Quantitative PCR Reactor was purchased from ABI. Other commonly used reagents were purchased from Sangon.
Methods
QBC939 cell culture and grouping
The QBC939 cells preserved in liquid nitrogen were water bathed at 37°C until the cells were completely thawed. The cells were cultured at 37°C and 5% CO2 for 24-48 h. Next, the QBC939 cells were seeded in a culture dish at a density of 1 × 107 cells/cm2 with complete medium including 10% FBS, 90% high glucose DMEM medium, 100 U/ml penicillin, and 100 μg/ml streptomycin. QBC939 cells were cultured in vitro and randomly divided into 3 groups, including a control group, an lnc-LFAR1 overexpression, and an lnc-LFAR1 siRNA group, the cells of which were transfected with pcDNA3.1-LFAR1 plasmid and lnc-LFAR1 siRNA, respectively.
pcDNA3.1-LFAR1 and lnc-LFAR1 siRNA transfection
The pcDNA3.1-LFAR1 plasmid and lnc-LFAR1 siRNA were transfected into QBC939 cells, respectively. The plasmid sequence of pcDNA3.1-LFAR1 is 5’-TAAACCATAGAGCAATCATA-3’. The lnc-LFAR1 siRNA sequence is 5’-AUCGUGUCAGGUCUGAGA-3’. pcDNA3.1-LFAR1, and the lnc-LFAR1 siRNA liposomes were separately added to 200 μl of serum-free DMEM medium and incubated at room temperature for 15 min. The mixed lipo2000 was separately mixed and incubated at room temperature for 30 min. The mixture was added to the cells in a 6-well plate together with 1.6 ml of serum-free DMEM medium and cultured in a 5% CO2 incubator at 37°C for 6 hours. The medium was replaced and the cells were further cultured for 48 hours.
Real-time PCR
Total RNA was extracted from the cells by Trizol and reverse transcribed to cDNA. The primers were designed by PrimerPremier 6.0 software and synthetized by Invitrogen (Table 1). Real-time PCR was performed at 55°C for 1 min, followed by 35 cycles of 92°C for 30 s, 58°C for 45 s, and 72°C for 35 s. GAPDH was selected as an internal reference. The relative expressions of the mRNA were calculated using the 2-ΔCt method.
Table 1.
Primer sequences
| Gene | Forward 5’-3’ | Reverse 5’-3’ |
|---|---|---|
| GADPH | AGTCTGACCAGTTTGCTGG | TAACCCGTAGTCAGATGTGGT |
| lnc-LFAR1 | TCCTAGACTCCCAGTTTCT | GTGTATAGTAGTGATGGT |
| TGF-β1 | CTAGACTCCTAACCTATAG | ATAGTTGATCTCAGTATA |
| Smad2 | TAGATAATCACCGATCATC | ACAGTAGACCAACAGT |
| Smad4 | CTCAATTCATCCACTCGAAG | GCACAGTGCTCGATGCTT |
MTT assay
The QBC939 cells in the logarithmic phase were seeded in a 96-well plate at 3000 cells/well, and 20 μl MTT was added for 4 h. Next, 150 μl DMSO was added to the plate for 10 min and tested at 570 nm to obtain the absorbance value (A).
Transwell assay
The Transwell chamber was coated with 50 mg/L Matrigel diluted at 1:5 and air-dried at 4°C. 100 μl of tumor cell suspension prepared by serum-free medium and 10% FBS DMEM medium were added to the inner and outer chambers with 3 replicate wells in each group. Then the chamber was placed in a 24-well plate. After 48 hours of culture, the Transwell chamber was washed with PBS, and the cells above the membrane were removed. Next, the membrane was fixed in ice ethanol. After staining with crystal violet, the cells in the lower layer of the microporous membrane were counted. The experiment was repeated three times.
Cell scratch assay
5 × 105 cells were passaged and inoculated into a 6-well plate. A marker pen was placed behind the 6-well plate to evenly draw a horizontal line, which was passed through the hole every 0.5 to 1 cm. At least 5 lines were drawn through each hole. After 24 hours, the tip was used to draw a line as far as possible. The cells were washed 3 times with PBS and cultured in serum-free DMEM medium at 37°C and 5% CO2. The results were observed after 48 hours.
Western blot
The cells were lysed on ice and quantified using the BCA method. The isolated proteins were electrophoresed using 10% SDS-PAGE. The gel was transferred to PVDF membrane using the semi-dry transfer method at 100 mA for 2 h. After being blocked by 5% skim milk for 2 h, the membrane was incubated in E-cadherin and vimentin primary antibodies at 4°C overnight. After being incubated in a secondary antibody in the dark for 30 min, the membrane was imaged using a chemiluminescence reagent for 1 min and analyzed by image processing system software and Quantity One software. The experiment was repeated four times (n = 4).
Statistical analysis
All data analyses were performed using SPSS 16.0 software. The measurement data were presented as the mean ± standard deviation and compared using one-way ANOVA. P < 0.05 was considered statistically significant.
Results
Lnc-LFAR1 expression in QBC939 cells
Real-time PCR was used to test lnc-LFAR1 expression in ICC QBC939 cells. pcDNA3.1-LFAR1 plasmid transfection significantly promoted the expression of lnc-LFAR1 compared with the control group (P < 0.05). Lnc-LFAR1 siRNA transfection obviously downregulated the expression of lnc-LFAR1 compared with the control group (P < 0.05) (Figure 1).
Figure 1.
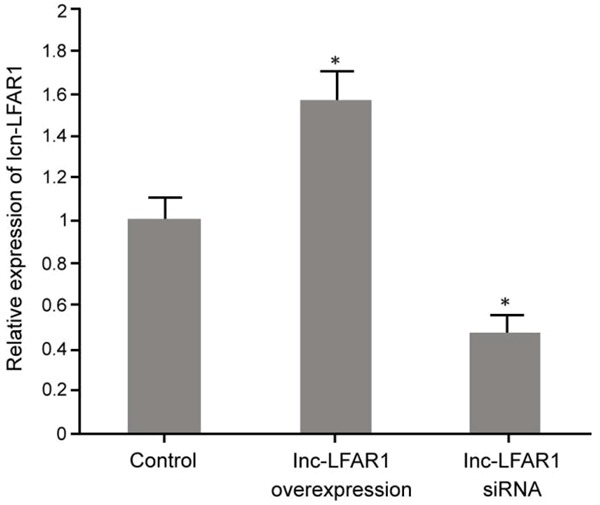
Lnc-LFAR1 expression in ICC QBC939 cells. *P < 0.05, compared with the control.
The impact of lnc-LFAR1 on QBC939 cell proliferation
The effect of pcDNA3.1-LFAR1 plasmid and lnc-LFAR1 siRNA transfection on QBC939 cell proliferation was determined using an MTT assay. It showed that the upregulation of lnc-LFAR1 expression by the pcDNA3.1-LFAR1 plasmid markedly promoted the proliferation of KB cells compared with the control group (P < 0.05). A decreased expression of lnc-LFAR1 markedly inhibited QBC939 cell proliferation compared with the control group (P < 0.05) (Figure 2).
Figure 2.
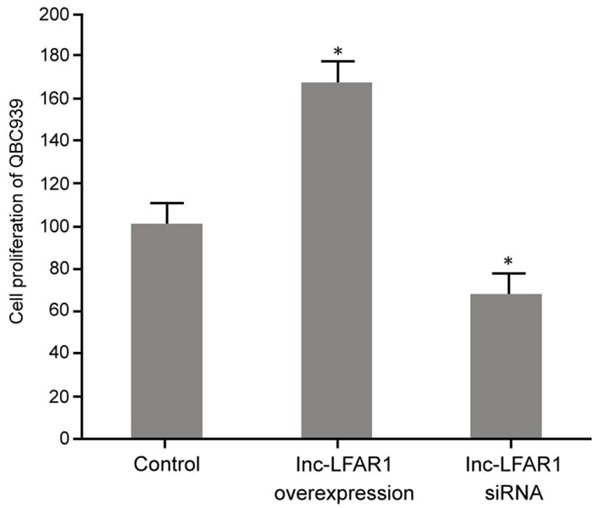
The influence of regulating lnc-LFAR1 on QBC939 proliferation. *P < 0.05, compared with control.
The influence of lnc-LFAR1 on QBC939 cell migration
A cell scratch assay was used to determine the effect of the pcDNA3.1-LFAR1 plasmid and lnc-LFAR1 siRNA transfection on the migration ability of QBC939 cells. It was demonstrated that the upregulation of lnc-LFAR1 expression markedly promoted the migration of QBC939 cells compared with the control group (P < 0.05). The transfection of lnc-LFAR1 siRNA apparently decreased the lnc-LFAR1 expression and inhibited QBC939 cell migration compared with the control group (P < 0.05) (Figure 3).
Figure 3.
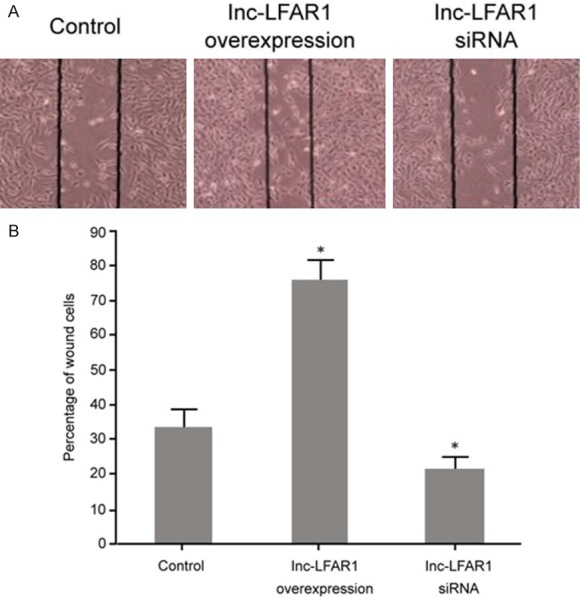
The influcence of lnc-LFAR1 on QBC939 cell migration. A. Cell scratch assay detection of the impact of regulating lnc-LFAR1 on QBC939 cell migration. B. Migration ability analysis. *P < 0.05, compared with control.
The effect of lnc-LFAR1 on QBC939 cell invasion
The Transwell assay was selected to determine the effect of the pcDNA3.1-LFAR1 plasmid and lnc-LFAR1 siRNA on the invasion of QBC939 cells. It was revealed that the upregulation of the lnc-LFAR1 expression after pcDNA3.1-LFAR1 plasmid transfection significantly enhanced KB cell invasion compared with the control group (P < 0.05). Transfection of lnc-LFAR1 siRNA clearly decreased the expression of lnc-LFAR1 and restrained QBC939 cell invasion compared with the control group (P < 0.05) (Figure 4).
Figure 4.
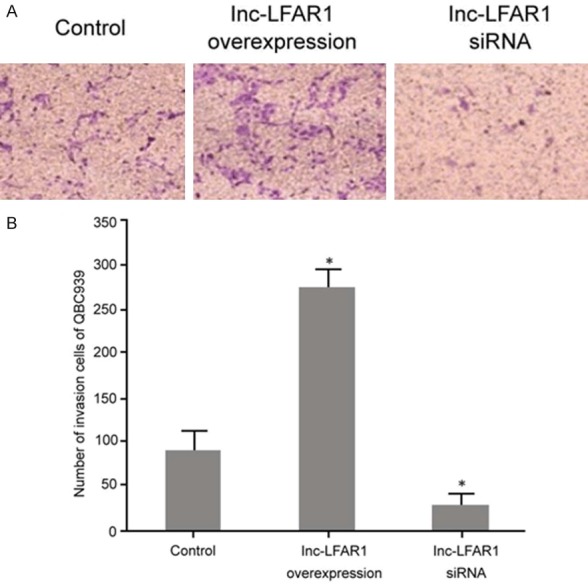
The effect of lnc-LFAR1 on QBC939 cell invasion. A. Transwell assay detection of the impact of regulating lnc-LFAR1 on QBC939 cell invasion. B. Migration ability analysis. *P < 0.05, compared with control.
The impact of lnc-LFAR1 on EMT in QBC939 cells
The effects of transfection of the pcDNA3.1-LFAR1 plasmid and the lnc-LFAR1 siRNA on the EMT of QBC939 cells were detected by Western blot. It was shown that the upregulation of lnc-LFAR1 expression after transfection of pcDNA3.1-LFAR1 plasmid significantly decreased E-cadherin and vimentin expressions compared with the control group (P < 0.05). The decreased expression of lnc-LFAR1 clearly enhanced the E-cadherin and vimentin levels compared with the control (P < 0.05) (Figure 5).
Figure 5.
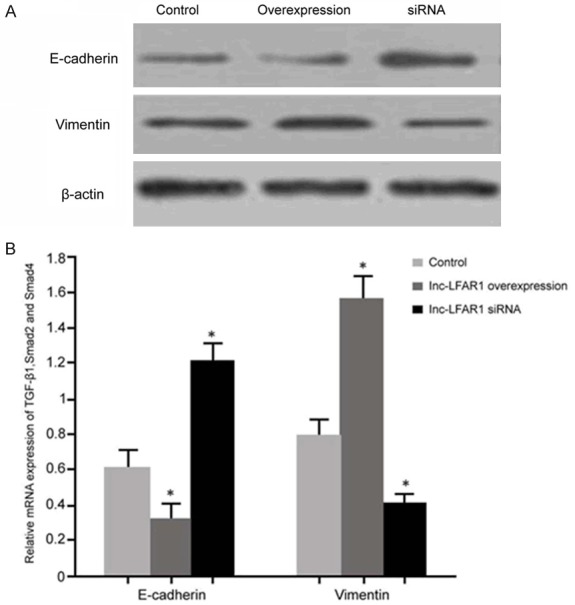
The impact of lnc-LFAR1 on EMT in QBC939 cells. A. Western blot detection of EMT protein expression; B. E-cadherin and vimentin protein expression analyses. *P < 0.05, compared with the control.
The influence of lnc-LFAR1 on the TGFβ/Smad signaling pathway in QBC939 cells
The effects of the pcDNA3.1-LFAR1 plasmid and of lnc-LFAR1 siRNA transfection on the TGFβ/Smad signaling pathway in the QBC939 cells was analyzed by real-time PCR. It was revealed that the upregulation of lnc-LFAR1 expression after the transfection of pcDNA3.1-LFAR1 markedly upregulated TGF-β1, Smad2, and Smad4 levels compared with the control group (P < 0.05). A decreased expression of lnc-LFAR1 apparently inhibited TGF-β1, Smad2, and Smad4 expressions compared with the control (P < 0.05) (Figure 6).
Figure 6.
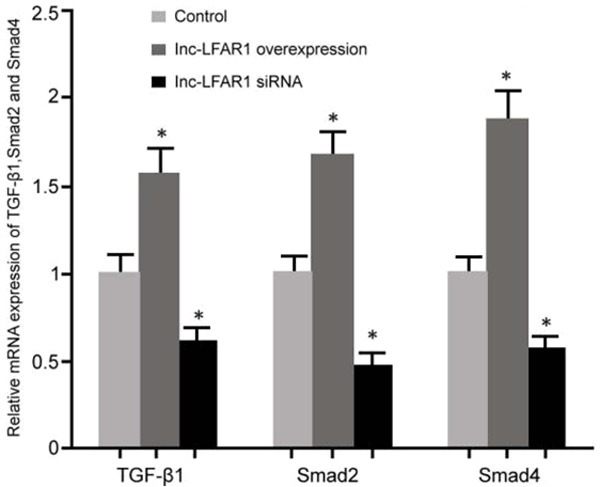
The influence of lnc-LFAR1 on the TGFβ/Smad signaling pathway in the QBC939 cells. *P < 0.05, compared with the control.
Discussion
As a tumor with a high incidence in hepatobiliary surgery, ICC is highly difficult to diagnose and treat. At present, no molecular markers or treatments have been found. Surgery is the only treatment method, and chemotherapy is not effective in ICC patients in the advanced stages [17]. As a transcriptional and post-transcriptional regulator, lncRNA has potential as a therapeutic target and can be used as an indicator of tumor prognosis [18,19]. It was found that lnc-LFAR1 has a short open reading frame (ORF) that plays an important role in translation initiation [20]. In liver fibrosis, lnc-LFAR1 is involved in disease progression by regulating the TGFβ/Smad signaling pathway [16]. However, the role and mechanism of lnc-LFAR1 in ICC has not been elucidated. Our results confirmed that the transfection of the pcDNA3.1-LFAR1 plasmid overexpressed the lnc-LFAR1 level, promoted cell proliferation, and enhanced the cell migration and invasion abilities in ICC cells. Conversely, the transfection of lnc-LFAR1 siRNA significantly reduced lnc-LFAR1 expression, restrained cell proliferation, and attenuated cell migration and invasion. This suggests that the up-regulation of lnc-LFAR1 can promote the development and progression of intrahepatic cholangiocarcinoma, and the down-regulation of lnc-LFAR1 can inhibit its proliferation, invasion, and migration.
The epithelial-mesenchymal transition (EMT) refers to epithelial cells transforming into mesenchymal cells during tumor invasion and metastasis [21]. EMT is accompanied by decreased E-cadherin expression and upregulated vimentin levels [22,23]. This study further aimed to analyze the regulation of lnc-LFAR1 on EMT in intrahepatic cholangiocarcinoma. The up-regulation of lnc-LFAR1 inhibited E-cadherin expression and facilitated vimentin levels in intrahepatic cholangiocarcinoma. However, the down-regulation of lnc-LFAR1 suppressed the expression of vimentin and enhanced the expression of E-cadherin in intrahepatic cholangiocarcinoma. TGF-β1, an important biological factor, can bind to its receptor to regulate the expression of downstream signaling factors and activate intracellular transcription factors. The TGF-β1 receptor is Smad, which can be associated with Smad2 and Smad4 to form a heteromeric complex. The activated Smad factor enters the nucleus and regulates the transcription of target genes to play an important role in cell biological behavior [24,25]. This study confirmed that the overexpression of lnc-LFAR1 promotes the expression of TGF-β1, Smad2, and Smad4 in ICC cells, thereby upregulating the TGFβ/Smad signaling pathway. Inhibiting the expression of lnc-LFAR1 suppresses the expression of TGF-β1, Smad2 and Smad4 and further down-regulates the TGFβ/Smad signaling pathway, suggesting that it can regulate ICC proliferation, invasion, and EMT. Further research, should deeply analyze the expression of lnc-LFAR1 in the tumor tissues of ICC patients and its correlation with their clinicopathological features, thus providing a reference for a clinical therapeutic target.
Conclusion
Targeting lnc-LFAR1 can inhibit cell proliferation, migration, invasion, and EMT by regulating the TGFβ/Smad pathway to affect the occurrence and development of cholangiocarcinoma.
Acknowledgements
This work was supported by the research fund of the Hunan Provincial Health and Family Planning Commission (grant no. C2016005), the scientific research fund of the Changsha Municipal Science and Technology Bureau (grant no. k15zd006-34), and the Hunan Natural Science Foundation (grant no. 2017JJ2152).
Disclosure of conflict of interest
None.
References
- 1.Faouder JL, Gigante E, Léger T, Albuquerque M, Beaufrère A, Soubrane O, Dokmak S, Camadro JM, Cros J, Paradis V. Proteomic landscape of cholangiocarcinomas reveals 3 different subgroups according to their localization and the aspect of non-tumor liver. Proteomics Clin Appl. 2018;13:e1800128. doi: 10.1002/prca.201800128. [DOI] [PubMed] [Google Scholar]
- 2.Meadows V, Francis H. MicroRNAs and cholangiocarcinoma: elucidating the effects of tiny giants. AME Med J. 2018:3. doi: 10.21037/amj.2018.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mammola CL, Vetuschi A, Pannarale L, Sferra R, Mancinelli R. Epidermal growth factor-like domain multiple 7 (EGFL7): expression and possible effect on biliary epithelium growth in cholangiocarcinoma. Eur J Histochem. 2018;62 doi: 10.4081/ejh.2018.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair A, Ingram N, Verghese ET, Wijetunga I, Markham AF, Wyatt J, Prasad KR, Coletta PL. Neutrophil gelatinase-associated lipocalin as a theragnostic marker in perihilar cholangiocarcinoma. Anticancer Res. 2018;38:6737–6744. doi: 10.21873/anticanres.13043. [DOI] [PubMed] [Google Scholar]
- 5.Chang AI, Kim YK, Min JH, Lee J, Kim H, Lee SJ. Differentiation between inflammatory myofibroblastic tumor and cholangiocarcinoma manifesting as target appearance on gadoxetic acid-enhanced MRI. Abdom Radiol (NY) 2019;44:1395–1406. doi: 10.1007/s00261-018-1847-y. [DOI] [PubMed] [Google Scholar]
- 6.Altman AM, Kizy S, Marmor S, Huang JL, Denbo JW, Jensen EH. Current survival and treatment trends for surgically resected intrahepatic cholangiocarcinoma in the United States. J Gastrointest Oncol. 2018;9:942–952. doi: 10.21037/jgo.2017.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Liang Y, Yang G, Lan Y, Han J, Wang J, Yin D, Song R, Zheng T, Zhang S, Pan S, Liu X, Zhu M, Liu Y, Cui Y, Meng F, Zhang B, Liang S, Guo H, Liu Y, Hassan MK, Liu L. Tetraspanin 1 promotes epithelial-to-mesenchymal transition and metastasis of cholangiocarcinoma via PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37:300. doi: 10.1186/s13046-018-0969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan KM, Tsai CY, Yeh CN, Yeh TS, Lee WC, Jan YY, Chen MF. Characterization of intrahepatic cholangiocarcinoma after curative resection: outcome, prognostic factor, and recurrence. BMC Gastroenterol. 2018;18:180. doi: 10.1186/s12876-018-0912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martviset P, Chaijaroenkul W, Muhamad P, Na-Bangchang K. Bioactive constituents isolated from atractylodes lancea (Thunb.) DC. rhizome exhibit synergistic effect against cholangiocarcinoma cell. J Exp Pharmacol. 2018;10:59–64. doi: 10.2147/JEP.S177032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu H, Chen J, Song Y, Shao H. Gastric adenocarcinoma predictive long intergenic non-coding RNA promotes tumor occurrence and progression in non-small cell lung cancer via regulation of the miR-661/eEF2K signaling pathway. Cell Physiol Biochem. 2018;51:2136–2147. doi: 10.1159/000495831. [DOI] [PubMed] [Google Scholar]
- 11.Lv P, Qiu X, Gu Y, Yang X, Xu X, Yang Y. Long non-coding RNA SNHG6 enhances cell proliferation, migration and invasion by regulating miR-26a-5p/MAPK6 in breast cancer. Biomed Pharmacother. 2018;110:294–301. doi: 10.1016/j.biopha.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Jiao Q, Yin RH, Zhao SJ, Wang ZY, Zhu YB, Wang W, Zheng YY, Yin XB, Guo D, Wang SQ, Zhu YX, Bai WL. Identification and molecular analysis of a lncRNA-HOTAIR transcript from secondary hair follicle of cashmere goat reveal integrated regulatory network with the expression regulated potentially by its promoter methylation. Gene. 2018;688:182–192. doi: 10.1016/j.gene.2018.11.084. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y, Li S, Wang S, Rubegni P, Tognetti L, Zhang J, Yan L. Long noncoding RNA HAND2-AS1 inhibits cancer cell proliferation, migration, and invasion in esophagus squamous cell carcinoma by regulating microRNA-21. J Cell Biochem. 2019;120:9564–9571. doi: 10.1002/jcb.28233. [DOI] [PubMed] [Google Scholar]
- 14.Cui L, Dong Y, Wang X, Zhao X, Kong C, Liu Y, Jiang X, Zhang X. Downregulation of long noncoding RNA SNHG1 inhibits cell proliferation, metastasis, and invasion by suppressing the Notch-1 signaling pathway in pancreatic cancer. J Cell Biochem. 2019;120:6106–6112. doi: 10.1002/jcb.27897. [DOI] [PubMed] [Google Scholar]
- 15.Shen C, Yan T, Wang Z, Su HC, Zhu X, Tian X, Fang JY, Chen H, Hong J. Variant of SNP rs1317082 at CCSlnc362 (RP11-362K14.5) creates a binding site for miR-4658 and diminishes the susceptibility to CRC. Cell Death Dis. 2018;9:1177. doi: 10.1038/s41419-018-1222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K, Han X, Zhang Z, Zheng L, Hu Z, Yao Q, Cui H, Shu G, Si M, Li C, Shi Z, Chen T, Han Y, Chang Y, Yao Z, Han T, Hong W. The liver-enriched lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch pathways. Nat Commun. 2017;8:144. doi: 10.1038/s41467-017-00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaeteewoottacharn K, Kariya R, Pothipan P, Fujikawa S, Pairojkul C, Waraasawapati S, Kuwahara K, Wongkham C, Wongkham S, Okada S. Attenuation of CD47-SIRPα signal in cholangiocarcinoma potentiates tumor-associated macrophage-mediated phagocytosis and suppresses intrahepatic metastasis. Transl Oncol. 2018;12:217–225. doi: 10.1016/j.tranon.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su K, Zhao Q, Bian A, Wang C, Cai Y, Zhang Y. A novel positive feedback regulation between long noncoding RNA UICC and IL-6/STAT3 signaling promotes cervical cancer progression. Am J Cancer Res. 2018;8:1176–1189. [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst EH, Nielsen J, Ipsen MB, Villesen P, Lykke-Hartmann K. Transcriptome analysis of long non-coding RNAs and genes encoding paraspeckle proteins during human ovarian follicle development. Front Cell Dev Biol. 2018;6:78. doi: 10.3389/fcell.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao XY, Xiong X, Liu T, Mi L, Peng X, Rui C, Guo L, Li S, Li X, Lin JD. Long noncoding RNA licensing of obesity-linked hepatic lipogenesis and NAFLD pathogenesis. Nat Commun. 2018;9:2986. doi: 10.1038/s41467-018-05383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao H, Hao G, Sun Y, Li L, Wang Y. Long noncoding RNA H19 mediated the chemosensitivity of breast cancer cells via Wnt pathway and EMT process. Onco Targets Ther. 2018;11:8001–8012. doi: 10.2147/OTT.S172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CC, Cheng YC, Chang CY, Lin CM, Chang JY. Alpha-tubulin acetyltransferase/MEC-17 regulates cancer cell migration and invasion through epithelial-mesenchymal transition suppression and cell polarity disruption. Sci Rep. 2018;8:17477. doi: 10.1038/s41598-018-35392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu T, Chen JM, Xiao TG, Shu XB, Xu HC, Yang LL, Xing LJ, Zheng PY, Ji G. Qinggan Huoxue Recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-β1/Smad signaling pathway. World J Gastroenterol. 2016;22:4695–4706. doi: 10.3748/wjg.v22.i19.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XY, Ban GF, Al-Shameri B, He X, Liang DZ, Chen WX. High-temperature requirement protein A1 regulates odontoblastic differentiation of dental pulp cells via the transforming growth factor Beta 1/smad signaling pathway. J Endod. 2018;44:765–772. doi: 10.1016/j.joen.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Qian J, Li X, Chen W, Xu A, Zhao K, Hua Y, Huang Z, Zhang J, Liang C, Su S, Li P, Shao P, Li J, Qin C, Wang Z. Long noncoding RNA BX357664 regulates cell proliferation and epithelial-to-mesenchymal transition via inhibition of TGF-β1/p38/HSP27 signaling in renal cell carcinoma. Oncotarget. 2016;7:81410–81422. doi: 10.18632/oncotarget.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]


