Abstract
Objective: Isatin has gained attention in recent years owing to its anticancer properties and is thought to offer medical benefits. Isatin is an endogenous oxidized indole with various behavioral and metabolic properties and is commonly found in mammalian tissues and fluids. It has several plausible applications in biomedical research and has also been investigated as a potential anticancer agent. However, its effects on neuroblastoma (NB) cells are unclear. Here, we evaluate the effects of isatin on neuroblastoma cell metastasis and invasion and reveal the underlying mechanism. Methods: NB cell viability was evaluated with the cell counting kit (CCK)-8 assay. NB cell invasion and migration abilities were tested with transwell and wound healing experiments. The relative mRNA expression of associated molecules was detected with real-time polymerase chain reaction (RT-PCR) and quantitative PCR. The expression level of related proteins was detected with western blotting. Results: Isatin inhibited the proliferation, invasion, and migration of neuroblastoma cells in a dose-dependent manner. Isatin increased the expression level of H3K4m1 and phosphatase and tensin homolog (PTEN) and decreased the phosphorylation level of PTEN downstream proteins phosphoinositide 3-kinase, protein kinase B, mammalian target of rapamycin, focal adhesion kinase, and SHC. Together, these results support the potential anti-metastatic effects of isatin on NB cells.
Keywords: Isatin, neuroblastoma, PTEN, LSD1, invasion
Introduction
Neuroblastoma (NB), one of the most common solid extracranial neoplasms in children, accounts for more than 7% of malignancies in patients younger than 15 years old and is responsible for around 15% of all pediatric cancer-related deaths [1,2]. Although substantial improvement has been observed in the outcomes of a few well-defined subsets of patients during the past few decades, not much improvement has been reported for those with a high-risk clinical phenotype [3,8]. NB is a disease of the sympatico-adrenal lineage of the neural crest, wherein the tumors may develop anywhere in the sympathetic nervous system. About half of all cases are classified under a high risk for disease relapse, and the overall survival rate is less than 40% despite intensive multimodal therapy [4,9]. The leading cause of death in patients with NB is metastasis, which in most cases is observed to inexplicably migrate to the bone marrow. Therefore, it is important to understand the specific mechanism underlying NB invasion and metastasis and [5,10] to discover safer and better compounds to inhibit NB [4,6] invasion and metastasis.
Monoamine oxidase (MAO) is localized in the outer membrane of the mitochondria and catalyzes the oxidative deamination of neuroactive cells. An increase in MAO activity was shown to be accompanied by apoptotic cell death of various neuronal cells following growth factor deprivation [7,11]. Monoamine oxidase A (MAOA) was reported to induce the epithelial to mesenchymal transition (EMT) and consequently mediate the growth, invasiveness, and metastasis of tumor cells.
As a member of the MAO family [8,12], lysine-specific demethylase 1 (LSD1) which specifically removes the dimethyl and monomethyl modifications of H3K4 in the presence of flavine adenine dinucleotide (FAD) [9,13] is strongly associated with the development, invasiveness, and metastasis of tumor cells [10,14]. For instance, LSD1 could be recruited by the SNAG domain of Snail to the E-cadherin promoter for transcription suppression and EMT [11,15]. An epigenetic marker, LSD1 overexpression is one of the characteristics of malignant tumors [12,16] and has been correlated with the malignant progression of multiple cancers, including primary neuroblastic tumors, estrogen receptor-negative breast cancer [13,17], and poorly differentiated NBs [14,18]. LSD1 may also form a co-inhibitory complex with the SNAG domain of Snail and allow the recruitment of LSD1 to the phosphatase and tensin homolog (PTEN) promoter for H3K4 demethylation [15,19] and PTEN transcription repression [16,20].
PTEN exerts its role as a tumor suppressor through the downregulation of the phosphoinositide 3-kinase (PI3K)/protein kinase (AKT) signaling pathway, which is highly related to the invasion and metastasis of cancer cells [17,18,21,22]. For instance, PTEN inhibits the migration and invasion of HepG2 cells by decreasing the expression of matrix metalloproteinase (MMP) via the PI3K/AKT pathway [19,23]. PTEN also interacts with focal adhesion kinase (FAK) by reducing its tyrosine phosphorylation and negatively regulates the interaction between the cell and the extracellular matrix [20,24] to inhibit cell migration, cell spreading, and focal adhesion formation [21,25].
Previous studies have shown that isatin is an endogenous indole that inhibits MAOB and may induce SH-SY5Y cell death in a dose- and time-dependent manner [22,26]. Isatin was recently shown to inhibit SH-SY5Y cell migration and invasion through various pathways, especially through the downregulation of MMP-2/MMP-9 expression [23,27]. However, the precise mechanism involved in the anti-metastasis activity of isatin is incompletely understood.
We thought that the inhibition of LSD1 activity may cause H3K4 demethylation and isatin may upregulate the expression of PTEN, which would negatively regulate SH-SY5Y cell invasion and metastasis through the PI3K/AKT and PTEN/p-SHC/p-FAK signaling pathways. To test our assumption, we applied real-time polymerase chain reaction (RT-PCR), western blotting, and transwell assays and determined the molecules involved in this process. The results obtained confirm our hypothesis.
Materials and methods
Cell culture
SH-SY5Y cells (Chinese Academy of Sciences) were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/high glucose (HyClone, USA) supplemented with 10% fetal bovine serum (FBS; BI, California, USA) at 37°C with 5% CO2 and 98% relative humidity in a culture incubator. The cells were incubated with different concentrations (0, 50, 100, and 200 μmol/L) of isatin (99.0%; Sigma, California, USA) for 24, 48, or 72 h after reaching 80% confluency. The cells (about 9 × 105 cells/well) were harvested and used for proliferation, migration, and protein analyses.
Cell viability assay
The viability of the cells was measured with a cell counting kit-8 (CCK-8) assay. The cells were seeded in a 96-well plate in a final volume of 100 μL of complete culture medium containing 1 × 104 cells/well and exposed to different concentrations (0, 25, 50, 100, 200, 300, 400, 500, 600, and 700 μmol/L) of isatin (six wells for each concentration) for 48/72 h at 37°C. After treatment, 10 μL of CCK-8 solution was added to each well and the cells were incubated at 37°C for 3 h. The absorbance of the samples was measured at a wavelength of 450 nm with a microplate reader (Synergy H1; BioTek, Vermont, USA). Each experiment was performed thrice.
Cell migration and invasion assay
SH-SY5Y cells were seeded in a serum-free medium in a six-well plate and exposed to isatin (0, 50, 100, and 200 μmol/L) for 48 h. A micropipette tip was used to scratch and create a wound each well. The cells were monitored during regrowth, and images were captured at different time points (0, 12, 24, and 48 h). The transwell invasion assay was performed in Boyden chambers (Millipore, California, USA). About 2 × 105 cells/well seeded in 200 μL of serum-free medium and pretreated with isatin for 24 h were added to the upper chamber coated with Matrigel, while the medium supplemented with 10% FBS was added to the lower chamber. At the end of the incubation, the cells from the upper surface were completely removed, and the filter was fixed in methanol and stained with crystal violet. Cells invading the Matrigel were counted under an inverted microscope. Data were expressed as the average cell number in five fields and the experiment was repeated thrice.
Western blot analysis
SH-SY5Y cells were lysed in a radioimmunoprecipitation assay (RIPA) buffer (Solarbio, Beijing, China) supplemented with a protease inhibitor cocktail (Sigma, Germany) on ice for 1 h and centrifuged for 20 min at 4000 × g. The supernatant was collected and the protein concentration was measured with bicinchoninic acid (BCA) assay (Beyotime, Jiangsu, China). DTT from the kit was mixed with 40 μL of deionized water to obtain a 400 mM solution. The fluorescent 5 × master mix was mixed with 20 μL of 10 × sample buffer and 20 μL of the prepared DTT solution. The biotinylated ladder provided with the kit was mixed with 16 μL of deionized water, 2 μL of 10 × sample buffer, and 2 μL of the prepared DTT solution and gently mixed. The entire volume of the ladder was transferred to a 0.6 mL tube (blue), and the 10 × sample buffer was diluted 1:100 with water to obtain 0.1 × sample buffer. The protein lysate was diluted with 0.1 × sample buffer and the final concentration of the protein sample was adjusted to 0.2 mg/mL. The samples and biotinylated ladder were denatured at 95°C for 5 min and stored on ice. The primary antibody was diluted with antibody diluent 2 (1:50). The secondary horseradish peroxidase (HRP) conjugate was provided in the detection module and was ready to use. About 200 μL Lumino-S and 200 μL peroxide supplied in the detection module were mixed in a microcentrifuge tube and gently pipetted and stored on ice. All the reagents were added to the wells of the plate (Figure 1A lower panel) for the immunoassay. The assay was loaded in Compass software and a capillary cartridge was inserted into the cartridge holder. After the change in the interior light from orange to blue, the assay plate lid was removed, and the plate was held firmly on bench and its evaporation seal was carefully peeled-off. Any bubbles observed in the separation matrix wells were removed with a pipette tip. The assay plate was placed on the plate holder, the door was closed, and the run was started. After run completion, the plate and cartridge were discarded. Data were collected and analyzed by Compass software.
Figure 1.
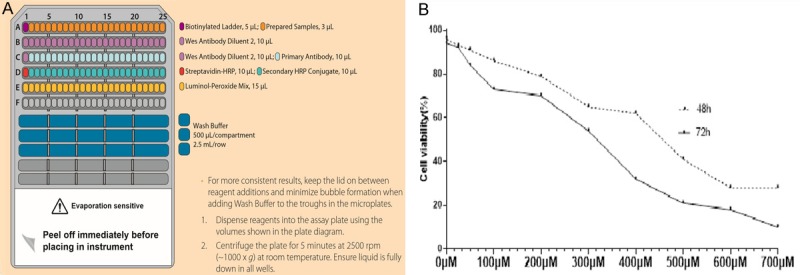
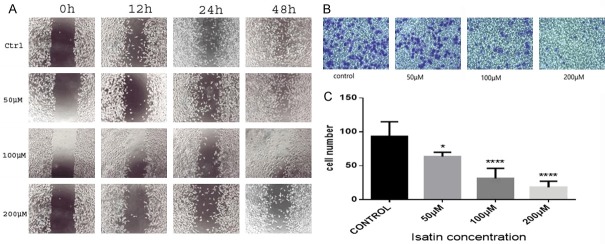
Isatin inhibits SH-SY5Y cell growth. All western reagents were added to the wells of the plate, as shown in (A). The effect of isatin on the proliferation of neuroblastoma cells. The cells were incubated with 0, 25, 50, 100, 200, 300, 400, 500, 600, and 700 μmol/L of isatin for 48 or 72 h in a CCK-8 assay (B). *P < 0.05.
Quantitative RT-PCR assay
Total RNA was extracted from the cultured SH-SY5Y cells with Trizol reagent (Solarbio, Beijing, China). A reverse transcription kit (Transgene, Beijing, China) was used to construct the template cDNA for real-time PCR with Trans Start Probe RT-PCR Super Mix (Transgene, Beijing, China). The data were obtained on Bio-Rad (California, USA) One-Step Plus system. The primer sequences used are shown in Table 1.
Table 1.
Primer sequences for LSD1, PTEN, and GAPDH
| Gene | Forward | Reverse |
|---|---|---|
| LSD1 | 5’-TGGTGGTAACAGGTCTTGGAGG-3’ | 5’-GGCTTCATAAAGTGGGCATTTTTG-3’ |
| PTEN | 5’-AGTTCCCTCAGCCGTTACCT-3’ | 5’-ATTTGACGGCTCCTCAACTG-3’ |
| GAPDH | 5’-GGAGCCAAAAGGGTCATCATCT-3’ | 5’-AGGGGCCATCCACAGTCTTCT-3’ |
Statistical analysis
The data were obtained from three independent experiments and represented as the mean ± standard deviation (SD). The corresponding data were compared with a one-way analysis of variance (ANOVA) using GraphPad Prism 6 statistical software (GraphPad, La Jolla, CA, USA), and significance was set at P < 0.05.
Results
Detection of cell viability with the CCK-8 assay
Isatin inhibited the invasion and metastasis of NB cells in vitro. The growth inhibitory effects of isatin on SH-SY5Y cells were evaluated with a CCK-8 assay. As shown in Figure 1B, isatin at various concentrations influenced the survival rate of SH-SY5Y cells after treatment for 48/72 h. Isatin was nontoxic at concentrations below 200 μmol/L, as evident from the survival of 80% of the cells. So the concentrations of 0 μmol/L, 50 μmol/L, 100 μmol/L, 200 μmol/L were used in subsequent experiments.
Decrease in the invasion and migration ability of isatin-treated cells as compared with control cells
The wound healing rate was significantly lower in isatin-treated SH-SY5Y cells than in the untreated cells. At 48 h, the wound closure was almost complete in the control cells, but the isatin-treated cells showed a noticeable wound gap (Figure 2A). In addition, isatin at 200 μmol/L concentration decreased the invasion of cells to 20% as compared with the control cells (Figure 2B and 2C). Isatin also exerted an anti-proliferation effect on the SH-SY5Y cells (Figure 1B). All these results suggest that isatin exhibits a potentially inhibitory effect on NB cell metastasis.
Figure 2.
Isatin inhibits SH-SY5Y cell invasion and migration. Isatin inhibits SH-SY5Y cell migration and invasion (A). The effect of isatin on neuroblastoma cell migration at × 100 magnification under an inverted microscope (B). Statistical analysis of SH-SY5Y cell Matrigel invasion counted in five random fields (C). *P < 0.05, **P < 0.01 as compared with the control.
Increase in the expression of H3K4m1 and PTEN
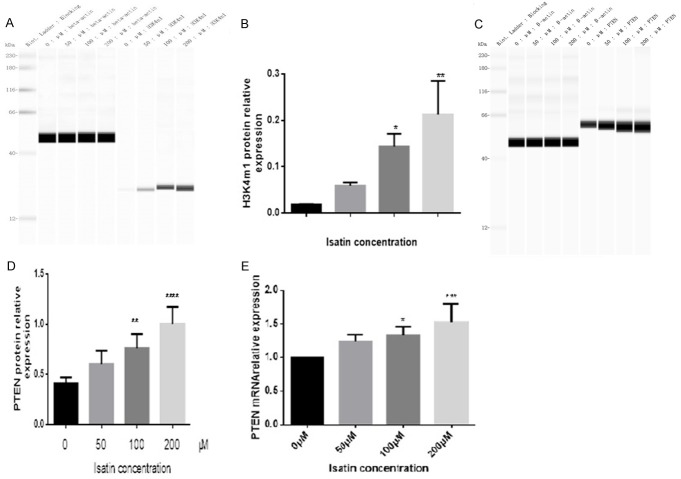
As reported in previous studies, isatin inhibits MAOA activity in SH-SY5Y cells [24,28]. Here, we investigated whether isatin modulates LSD1 activity by evaluating the expression of its target protein H3K4m1 [9,13]. The expression of the H3K4m1 protein significantly increased in the cells incubated with 200 μmol/L of isatin as compared with the control cells (Figure 3A and 3B), indicating that isatin may influence SH-SY5Y cell invasion and metastasis through the inhibition of LSD1 activity. LSD1 is known to maintain the proliferation of carcinoma cells through the PI3K/AKT pathway [25,29]. Whether LSD1 decreases the expression of PTEN, a typical inhibitor of PI3K/AKT pathway [26,27,30,31], remains unclear in NB cells. In this direction, we tested the expression of related molecules through western blot analysis and RT-PCR. As a result, we found that the protein (Figure 3C and 3D) and mRNA (Figure 3E) expression levels of PTEN in SH-SY5Y cells exposed to isatin increased in a dose-dependent manner (Figure 3E).
Figure 3.
Isatin increases the protein expression of H3K4m1 (A and B) and PTEN (C and D) in SH-SY5Y cells after 48 h of treatment, as detected with a western blot analysis. Statistical analysis of RT-PCR data for the expression of PTEN mRNA (E). Values are expressed as mean ± SD. *P < 0.05, **P < 0.01 as compared with control.
Decrease in the phosphorylation of PI3K, AKT, mTOR, FAK, and SHC
We observed a decrease in the phosphorylation levels of PTEN downstream molecules, such as PI3K (Figure 4A and 4C), AKT (Figures 4B and 5D), mTOR (Figure 4E and 4F), FAK (Figure 5A and 5C), and SHC (Figure 5B and 5D), as confirmed with western blotting.
Figure 4.
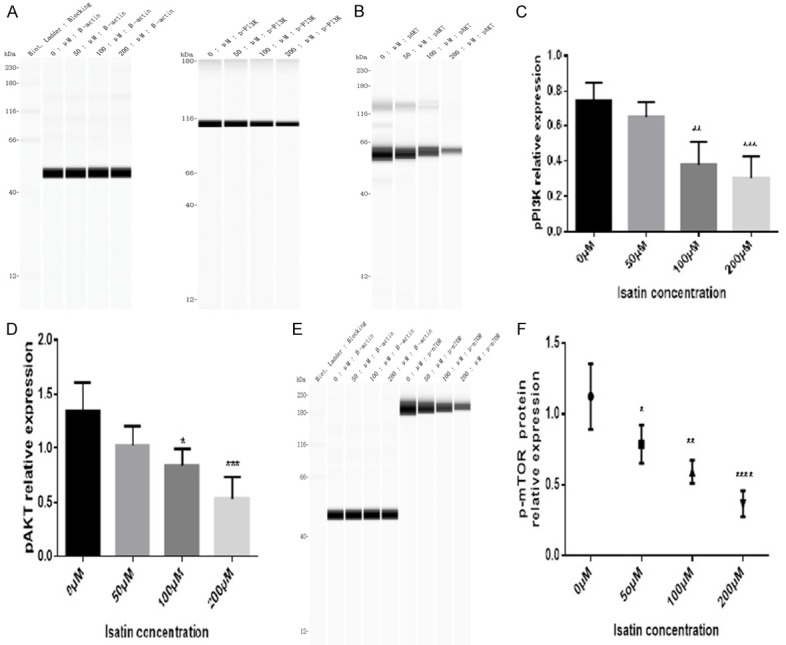
The phosphorylation level of PI3K (A and C), AKT (B and D), and mTOR (E and F) in SH-SY5Y cells decreased after 48 h of treatment with isatin, as detected with western blot analysis. Values are expressed as mean ± SD. *P < 0.05, **P < 0.01 as compared with control.
Figure 5.
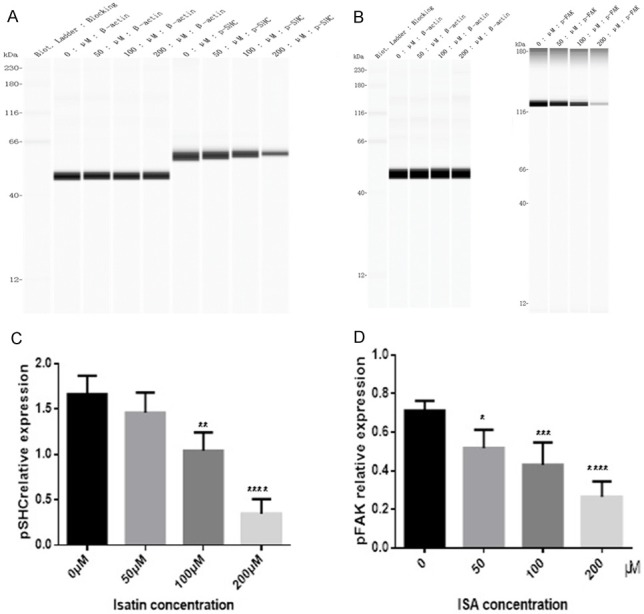
The phosphorylation level of p-SHC (A) and p-FAK (B) in SH-SY5Y cells decreased after 48 h of treatment with isatin, as detected with a western blot analysis. Statistical analysis of the expression of p-SHC (C) and p-FAK protein (D). Values are expressed as the mean ± SD. *P < 0.05, **P < 0.01 as compared with control.
No change in the expression of LSD1
We failed to observe any change in the expression of LSD1 at the protein (Figure 6A and 6B) and mRNA (Figure 6C) levels, suggesting that isatin may inhibit cell invasion not by decreasing the expression of LSD1 but through the inhibition of LSD1 activity.
Figure 6.
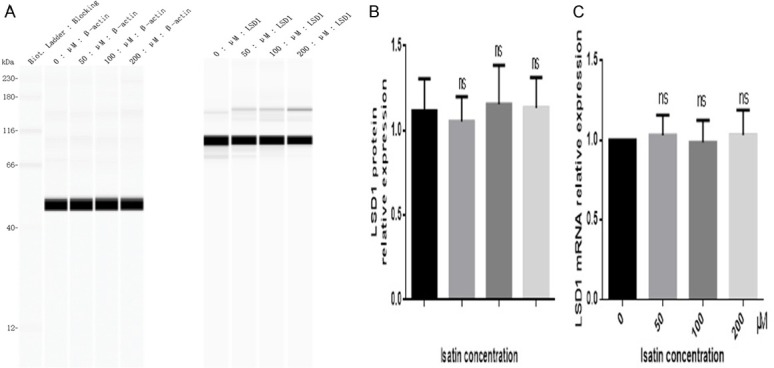
The protein (A and B) and mRNA (C) expressions levels of LSD1 in SH-SY5Y cells showed no change after 48 h of treatment with isatin, as detected with a western blot analysis. Values are expressed as the mean ± SD. *P < 0.05, **P < 0.01 as compared with control.
All these results suggest that isatin may inhibit LSD1 activity and increase PTEN expression, leading to the inhibition of SH-SY5Y cell invasion and metastasis through the PI3K/AKT and PTEN/p-SHC/p-FAK signaling pathways.
Discussion
Prior work has revealed the effectiveness of isatin in the prevention of cancer cell proliferation and progression. A study by Havrylyuk [28,32] showed that isatin exhibits a remarkable antiproliferative effect on cancer. However, these studies have either focused only on the anti-proliferation property of isatin or have not clarified the anti-invasive mechanism isatin’s underlying effects in NB cells. In the present study, we investigated the anti-invasive effects of isatin on NB cells and revealed the underlying mechanism using western blotting and RT-PCR. As a result, we found that isatin treatment increased the expression of H3K4m1 in SH-SY5Y cells, wherein H3K4m1 acts as a substrate of LSD1. The expression of LSD1, however, showed no change, indicating that isatin is likely to downregulate H3K4m1 expression by inhibiting the activity of LSD1 and not by decreasing LSD1 expression. The upregulation in H3K4m1 expression resulted in an increase in the level of PTEN. We also observed a decrease in the expression of the downstream molecules involved in PTEN signaling, including p-SHC, p-FAK, p-PI3K, p-AKT, and p-mTOR. These findings indicate that isatin exerts its anti-invasion and anti-metastasis effects on SH-SY5Y cells by increasing the expression level of H3K4m1, which then activates PTEN signaling through the inhibition of LSD1 activity. To our knowledge, this is the first study to systematically investigate the influence of isatin on PTEN signaling-related molecules in NB cells. However, our study has a few limitations. Although our hypotheses were statistically supported by the results of biochemical experiments in vitro, whether the mechanism is reproducible in animals or humans is questionable. Further studies are warranted to evaluate the effects of isatin on tumors in animal models.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81472542, 201501-201812), the Qingdao Startup and Innovation Leader Talent Plan (13-CX-3, 201409-201709), and the “Clinical Medicine+X” Plan, Qingdao University (201707201906).
Disclosure of conflict of interest
None.
References
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamo A, Sese B, Boue S, Castano J, Paramonov I, Barrero MJ, Belmonte JC. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol. 2011;13:652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993;11:1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 4.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 5.Song J, Hou L, Ju C, Zhang J, Ge Y, Yue W. Isatin inhibits proliferation and induces apoptosis of SH-SY5Y neuroblastoma cells in vitro and in vivo. Eur J Pharmacol. 2013;702:235–241. doi: 10.1016/j.ejphar.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 6.da Silva JFM, Garden SJ, Pinto AC. The chemistry of isatins: a review from 1975 to 1999. J Braz Chem Soc. 2001;12:273–324. [Google Scholar]
- 7.Fitzgerald JC, Ufer C, Billett EE. A link between monoamine oxidase-A and apoptosis in serum deprived human SH-SY5Y neuroblastoma cells. J Neural Transm (Vienna) 2007;114:807–810. doi: 10.1007/s00702-007-0692-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Yang YT, Wang F, Wan K, Yarnane K, Zhang Y, Lei M. Crystal structure of human histone lysine-specific demethylase 1 (LSD1) Proc Natl Acad Sci U S A. 2006;103:13956–13961. doi: 10.1073/pnas.0606381103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Escriva M, Peiro S, Herranz N, Villagrasa P, Dave N, Montserrat-Sentis B, Murray SA, Franci C, Gridley T, Virtanen I, Garcia de Herreros A. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol. 2008;28:1528–1540. doi: 10.1128/MCB.02061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y, Wu Y, Li J, Dong C, Ye X, Chi YI, Evers BM, Zhou BP. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010;29:1803–1816. doi: 10.1038/emboj.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao GB, Huang XJ, Gong AH, Zhang ZJ, Lu RZ, Sang JR. [Histone demethylase LSD1 and its biological functions] . Yi Chuan. 2010;32:331–338. doi: 10.3724/sp.j.1005.2010.00331. [DOI] [PubMed] [Google Scholar]
- 13.Lim S, Janzer A, Becker A, Zimmer A, Schule R, Buettner R, Kirfel J. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31:512–520. doi: 10.1093/carcin/bgp324. [DOI] [PubMed] [Google Scholar]
- 14.Schulte JH, Lim S, Schramm A, Friedrichs N, Koster J, Versteeg R, Ora I, Pajtler K, Klein-Hitpass L, Kuhfittig-Kulle S, Metzger E, Schule R, Eggert A, Buettner R, Kirfel J. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy. Cancer Res. 2009;69:2065–2071. doi: 10.1158/0008-5472.CAN-08-1735. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Kang T, Zhou BP. Doxorubicin enhances Snail/LSD1-mediated PTEN suppression in a PARP1-dependent manner. Cell Cycle. 2014;13:1708–1716. doi: 10.4161/cc.28619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun G, Alzayady K, Stewart R, Ye P, Yang S, Li W, Shi Y. Histone demethylase LSD1 regulates neural stem cell proliferation. Mol Cell Biol. 2010;30:1997–2005. doi: 10.1128/MCB.01116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama A, Igarashi K, Sato T, Takagi K, Otsuka IM, Shishido Y, Baba T, Ito R, Kanno J, Ohkawa Y, Morohashi K, Sugawara A. Identification of myelin transcription factor 1 (MyT1) as a subunit of the neural cell type-specific lysine-specific demethylase 1 (LSD1) complex. J Biol Chem. 2014;289:18152–18162. doi: 10.1074/jbc.M114.566448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 19.Tian T, Nan KJ, Guo H, Wang WJ, Ruan ZP, Wang SH, Liang X, Lu CX. PTEN inhibits the migration and invasion of HepG2 cells by coordinately decreasing MMP expression via the PI3K/Akt pathway. Oncol Rep. 2010;23:1593–1600. doi: 10.3892/or_00000800. [DOI] [PubMed] [Google Scholar]
- 20.Tamura M, Gu JG, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 21.Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci. 2001;114:2375–2382. doi: 10.1242/jcs.114.13.2375. [DOI] [PubMed] [Google Scholar]
- 22.Igosheva N, Lorz C, O’Conner E, Glover V, Mehmet H. Isatin, an endogenous monoamine oxidase inhibitor, triggers a dose- and time-dependent switch from apoptosis to necrosis in human neuroblastoma cells. Neurochem Int. 2005;47:216–224. doi: 10.1016/j.neuint.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Xu PP, Hou L, Ju CX, Zhang Z, Sun WY, Zhang L, Song JL, Lv YQ, Liu L, Chen ZX, Wang YH. Isatin inhibits the proliferation and invasion of SH-SY5Y neuroblastoma cells. Mol Med Rep. 2016;13:2757–2762. doi: 10.3892/mmr.2016.4850. [DOI] [PubMed] [Google Scholar]
- 24.Sun WY, Zhang L, Hou L, Ju CX, Zhao SM, Wei YY. Isatin inhibits SH-SY5Y neuroblastoma cell invasion and metastasis through MAO/HIF-1a/CXCR4 signaling. Anticancer Drugs. 2017;28:645–653. doi: 10.1097/CAD.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Wang Y, Wang S, Liu Y, Zhang J, Xu Y, Zhang Z, Bao W, Wu S. LSD1 sustains estrogen-driven endometrial carcinoma cell proliferation through the PI3K/AKT pathway via di-demethylating H3K9 of cyclin D1. Int J Oncol. 2017;50:942–952. doi: 10.3892/ijo.2017.3849. [DOI] [PubMed] [Google Scholar]
- 26.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1 alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 27.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Havrylyuk D, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R. Synthesis of new 4-thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activity. J Med Chem. 2012;55:8630–8641. doi: 10.1021/jm300789g. [DOI] [PubMed] [Google Scholar]
- 29.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Xia F, Zhang Y, Xie L, Jiang H, Zeng H, Chen C, Liu L, He X, Hao X, Fang X, Liu X, Zhang F, Gu H, Wan J, Cheng Y, Zhang CC, Chen GQ, Lu Y, Yu Z, Zheng J. B7-H4 enhances the differentiation of murine leukemia-initiating cells via the PTEN/AKT/RCOR2/RUNX1 pathways. Leukemia. 2017;31:2260–2264. doi: 10.1038/leu.2017.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Xie J, Fei Y, Gao P, Xie Q, Gao W, Xu Z. GDNF family receptor alpha 2 promotes neuroblastoma cell proliferation by interacting with PTEN. Biochem Biophys Res Commun. 2019;510:339–344. doi: 10.1016/j.bbrc.2018.12.169. [DOI] [PubMed] [Google Scholar]
- 32.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]