Abstract
Studies have found that L1CAM is significantly related to poor survival prognosis of many human cancers, but few studies focused on the prognostic value of L1CAM in lung cancer patients. Therefore, we detected L1CAM protein expression by immunohistochemistry and analyzed associations with survival outcomes of resected non-small cell lung cancer (NSCLC) patients. Overall, a number of 129 patients were enrolled in our study. Totally, 45 (34.9%) out of 129 NSCLC patients had L1CAM-positive expression. L1CAM-positive NSCLC patients had poorer 5-year survival than patients with L1CAM-negative expression by univariate analysis (P=0.05). The same trend manifested among the lung ADC patient set (P<0.05) other than lung SCC (P=0.42). Likewise, univariate analysis showed that L1CAM-positive expression significantly associated with poorer PFS for NSCLC, ADC, and SCC patients (all P<0.05). Multivariate analyses suggested that L1CAM was a predictive factor for patients’ PFS, independent of patients’ clinical features and treatments (P<0.05). However, we failed to observe significant results for NSCLC patients’ OS (P=0.176). Summarized, L1CAM-positive expression associated with poorer survival outcomes, but to elucidate whether L1CAM is an independent predictive factor of poor OS for NSCLC patients, more studies are needed.
Keywords: L1CAM, non-small cell lung cancer, survival, prognosis
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-related death worldwide [1]. Fewer than 20% of lung cancer patients can achieve 5-year survival [2]. About 85% of lung cancer patients have non-small cell lung cancer (NSCLC) stratified by tumor histology [2]. Considering the global disease burden and unfavorable survival of lung cancer, we need to investigate tumor-associated biomarkers, which probably contribute to new targets for cancer treatments.
As a member of the immunoglobulin (Ig) superfamily, L1 cell adhesion molecule (L1CAM) consisting of six Ig-like domains, five fibronectin Type III repeats, a transmembrane region, and a highly conserved cytoplasmic tail [3], has been found to play an important role in the progression and metastasis of human cancers [4], mostly in melanoma [5,6], breast [7,8] and gastrointestinal cancer [9,10]. Relatively, little is known about functions of L1CAM in lung cancers [11,12]. Therefore, we conducted this study to detect the expression of L1CAM protein and find associations between the L1CAM protein expression and survival outcomes of resected NSCLC patients.
Materials and methods
Patients and specimens
NSCLC patients who underwent complete primary tumor resection were consecutively enrolled in our study during the year of 2008 to 2011 from West China Hospital, Sichuan University. All patients received simultaneously standard therapies after surgery according to the non-surgical management for lung cancer of Clinical Oncology Information Network guidelines. Patients with previous malignancies, neo-adjuvant therapy or incomplete clinical data were excluded. Tumor specimens were formalin-fixed and paraffin-embedded immediately after removal from NSCLC patients and then were made into 4 μm-thick sections. We collected patients’ clinical data, including gender, age, smoking status, histological subtype stratified by the WHO classification for NSCLC [13], tumor differentiation, tumor-node-metastasis (TNM) stage on the basis of the TNM staging system of the America Joint Committee on Cancer (AJCC 7th edition) [14].
Follow-up continued until cancer-related death or no less than 5 years after diagnosis. We defined progression-free survival (PFS) as the time interval from the date of operation to the date of first documented disease progression or disease-related death and overall survival (OS) as the time interval between the date of first diagnosis and the date of disease-related death or the end of follow-up. Informed consents were obtained from eligible patients and our study was approved by the Committee on Medical Ethics of West China Hospital, Sichuan University.
Immunohistochemistry assay
We detected L1CAM protein expression in NSCLC tumor sections by using immunohistochemistry as described in our previous study [15]. Briefly, tumor sections first were dewaxed in xylene and dehydrated in the gradient ethanol series, then immersed in sodium citrate buffer (pH 6.0) at 95°C for 16 minutes for antigen retrieval. Subsequently, endogenous peroxidase was blocked with 3% H2O2-methanol solution for 20 minutes. After that sections were incubated with the 1:200 diluted primary antibodies at 4°C overnight. Primary antibodies were recombinant rabbit monoclonal IgG antibodies purchased from HuaAn Biotechnology Corporation (Hangzhou, China, Product ID: JM11-05). Next, secondary antibodies, goat anti-rabbit antibodies purchased from Dako Corporation (Denmark), were incubated for 60 minutes at room temperature. Afterwards, sections were stained with 3,3’-diaminobenzidine and finally counterstained with hematoxylin. Negative controls, primary antibodies were replaced with phosphate-buffered saline (PBS), did not manifest immunoreactivity.
Immunoreactive evaluation
Immunoreactive evaluation was independently finalized by two pathologists blind to patients’ clinical data. Three fields in tumor sections were randomly chosen under light microscope to evaluate areas of brown-stained tumor cells. Averagely, more than 1% brown-stained cells on sections were defined as positive expression. Divergences about immunoreactive evaluation were resolved through discussion between pathologists.
Statistical analysis
Statistical analysis were conducted using the software SPSS version 19.0 (Chicago, USA) and graphs were drawn using Graphpad prism version 6 (La Jolla, USA). Chi-square (χ2) tests were adopted to analyze correlations among patients’ clinical data and L1CAM expression. Kaplan-Meier curves and log-rank tests were used to evaluate correlations among patients’ survival and L1CAM expression. Multivariate Cox regression models were built to evaluate independent prognostic factors. P values ≤0.05 were considered statistically significant.
Results
Clinical features of enrolled patients in our study
In total, NSCLC tumor specimens of 129 cases were enrolled for immumohistochemistry. Clinical features of the 129 patients were demonstrated in Table 1 in detail. Altogether, 90 cases (69.8%) were male and the mean age of all was 56.09 years old. Of the patients, 82 cases (63.6%) were ever or current smokers. Our study included 72 cases (55.8%) of adenocarcinoma (ADC), 50 cases (38.8%) of squamous cell carcinoma and 7 cases (5.4%) of other histologic subtypes, consisting of 3 cases of adenosquamous carcinoma, 1 case of adenoid cystic carcinoma, 1 case of lymphoepithelioma-like carcinoma, 1 case of large cell carcinoma, and 1 case of mucoepidermoid carcinoma, data not shown in Table 1.
Table 1.
Baseline data of eligible patients
| Variables | Data (n=129) |
|---|---|
| Age (mean ± SD, range, year) | 56.09±10.08 |
| Gender | |
| Male | 90 (69.8%) |
| Female | 39 (30.2%) |
| Smoking status | |
| Yes | 82 (63.6%) |
| No | 47 (36.4%) |
| Histologic subtype | |
| ADC | 72 (55.8%) |
| SCC | 50 (38.8%) |
| Others | 7 (5.4%) |
| Differentiation | |
| Poor | 68 (52.7%) |
| Moderate-well | 61 (47.3%) |
| T stage | |
| T1-2 | 88 (68.2%) |
| T3-4 | 41 (31.8%) |
| N stage | |
| N0 | 69 (53.5%) |
| N1-3 | 60 (46.5%) |
| M stage | |
| M0 | 122 (94.6%) |
| M1 | 7 (5.4%) |
| Radiotherapy | |
| Yes | 35 (27.1%) |
| No | 94 (72.9%) |
| Chemotherapy | |
| Yes | 96 (74.4%) |
| No | 33 (25.6%) |
ADC, Adenocarcinoma. SCC, Squamous cell carcinoma.
Correlations among clinical features and L1CAM expression
L1CAM was positively stained in cytoplasm and cytomembrane in tumor cells (brown-stained, Figure 1). Of the patients, 45 (34.9%) were positive expression. Correlations among L1CAM expression and clinical features were demonstrated in Table 2. L1CAM was significantly correlated with patients’ age (P=0.006), whereas no statistical significance was observed among L1CAM expression and other clinical variables (all P>0.05).
Figure 1.
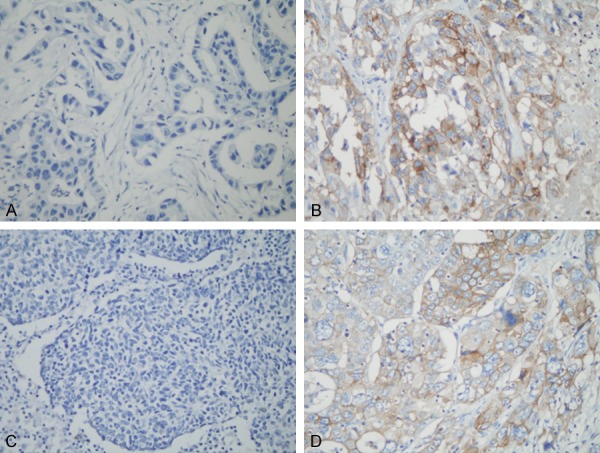
Immunohistochemistry staining showed the L1CAM expression in lung adenocarcinoma (A, negative; B, positive) and lung squamous cell carcinoma (C, negative; D, positive). Original magnification: ×400.
Table 2.
The correlations among L1CAM expression and clinical features of patients
| Variables | L1CAM (n=129) | P value | |
|---|---|---|---|
|
| |||
| Positive (n=45) | Negative (n=84) | ||
| Age (years) | 0.006* | ||
| ≤65 | 30 (66.7%) | 73 (86.9%) | |
| >65 | 15 (33.3%) | 11 (13.1%) | |
| Gender | 0.519 | ||
| Male | 33 (73.3%) | 57 (67.9%) | |
| Female | 12 (26.7%) | 27 (32.1%) | |
| Smoking history | 0.592 | ||
| Yes | 30 (66.7%) | 52 (61.9%) | |
| No | 15 (33.3%) | 32 (38.1%) | |
| Histologic subtype | 0.615 | ||
| ADC | 23 (51.1%) | 49 (58.3%) | |
| SCC | 20 (44.4%) | 30 (35.7%) | |
| Others | 2 (4.4%) | 5 (6.0%) | |
| Differentiation | 0.524 | ||
| Poor | 22 (48.9%) | 46 (54.8%) | |
| Moderate-well | 23 (51.1%) | 38 (45.2%) | |
| T stage | 0.284 | ||
| T1-2 | 28 (62.2%) | 60 (71.4%) | |
| T3-4 | 17 (37.8%) | 24 (28.6%) | |
| N stage | 0.256 | ||
| N0 | 21 (46.7%) | 48 (57.1%) | |
| N1-3 | 24 (53.3%) | 36 (42.9%) | |
| M stage | 0.204 | ||
| M0 | 41 (91.1%) | 81 (96.4%) | |
| M1 | 4 (8.9%) | 3 (3.6) | |
| Radiotherapy | 0.359 | ||
| Yes | 10 (22.2) | 25 (29.8) | |
| No | 35 (77.8) | 59 (70.2) | |
| Chemotherapy | 0.522 | ||
| Yes | 35 (77.8%) | 61 (72.6%) | |
| No | 10 (22.2%) | 23 (27.4%) | |
Statistically significant.
NSCLC, non-small cell lung cancer. ADC, Adenocarcinoma. SCC, Squamous cell carcinoma.
Correlations between L1CAM expression and patients’ survival
About 53.3% of NSCLC patients with L1CAM-positive expression achieved five-year survival, significantly lower than 70.1% for L1CAM-negative patients (P=0.05, Figure 2A). No significant difference was observed among lung squamous cell carcinoma (SCC) patients (P=0.42, Figure 2B), whereas we observed L1CAM-positive expression was significantly related to poorer OS for the lung adenocarcinoma (ADC) patients set, the same trend as that of the NSCLC patients set (P<0.05, Figure 2C). More detailed data are demonstrated in Table 3.
Figure 2.
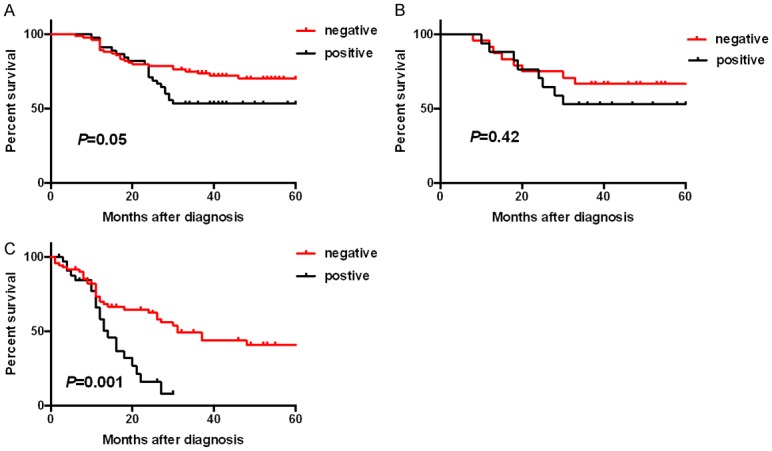
Kaplan-Meier survival curves showed the correlations between L1CAM expression and overall survival in non-small cell lung cancers (NSCLC) (A), lung squamous-cell carcinomas (SCC) (B), and lung adenocarcinomas (ADC) (C).
Table 3.
The correlations among L1CAM expression and patients’ survival by univariate analysis
| Variables | OS | PFS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| L1CAM in NSCLC (P/N) | 1.75 | 1.00-3.47 | 0.05* | 2.26 | 1.58-5.58 | <0.05* |
| L1CAM in SCC (P/N) | 1.49 | 0.56-4.11 | 0.42 | 2.56 | 1.22-9.52 | 0.03* |
| L1CAM in ADC (P/N) | 3.40 | 2.06-12.90 | <0.05* | 2.40 | 1.44-7.57 | <0.05* |
Statistically significant.
OS, overall survival. PFS, progression-free survival. P, positive. N, negative.
Further analyses showed that 16.0% of L1CAM-positive NSCLC patients achieved 2-year progression-free survival (PFS), significantly lower than 62.4% for the L1CAM-negative set (P<0.05, Figure 3A). Subgroup analyses showed around 23.2% of L1CAM-positive lung SCC patients achieved 2-year PFS, significantly lower than 73.0% for LCAM1-negative set (P=0.028, Figure 3B). Likewise, the trend was observed among the lung ADC patients set (P=0.007, Figure 3C).
Figure 3.
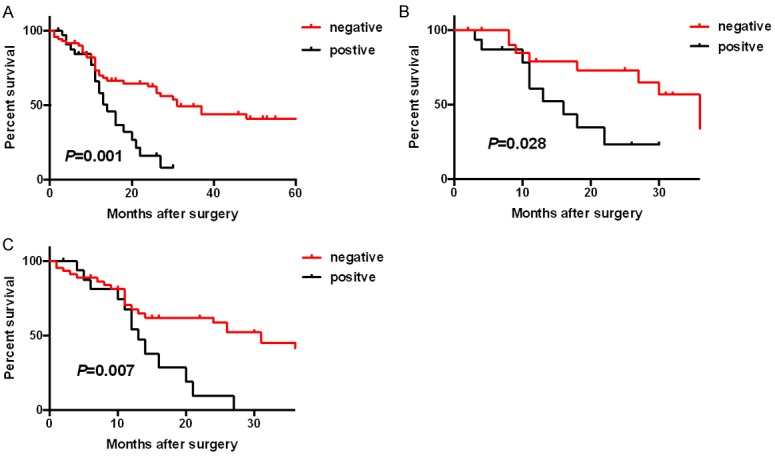
Kaplan-Meier survival curves showed the correlations between L1CAM expression and progression-free survival in non-small cell lung cancers (NSCLC) (A), lung squamous-cell carcinomas (SCC) (B), and lung adenocarcinomas (ADC) (C).
More interestingly, multivariate Cox regression models including L1CAM expression, clinical variables and treatments, including age, gender, smoking status, T stage, N stage, M stage, tumor differentiation, radiotherapy, and chemotherapy manifested that L1CAM-positive NSCLC patients had poorer PFS (P=0.003). Unfortunately, our study did not suggest L1CAM was an independent prognostic factor for OS among NSCLC patients (P=0.176), detailed data shown in Table 4.
Table 4.
The correlations among L1CAM expression and NSCLC patients’ survival by multivariate analysis
| Variables | OS | PFS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| L1CAM in NSCLC (P/N) | 1.553 | 0.820-2.939 | 0.176 | 2.709 | 1.399-5.245 | 0.003* |
Statistically significant.
Discussion
Our study found L1CAM was positive expression mostly in the membrane and cytoplasm of NSCLC tumor cells and about 45 (34.9%) out of 129 NSCLC patients had L1CAM-positive expression. L1CAM-positive expression was significantly associated with age in resected NSCLC patients (P<0.05). L1CAM-positive NSCLC patients had poorer 5-year survival than patients with L1CAM-negative expression by univariate analysis (P=0.05). The trend was strongest among the lung ADC patients group (P<0.05) other than lung SCC (P=0.42). Likewise, univariate analysis showed that L1CAM-positive expression significantly associated with poorer PFS for NSCLC, ADC, and SCC patients (all P<0.05). Multivariate analyses suggested that L1CAM was a predictive factor for patients’ PFS, independent of patients’ clinical features and treatments (P<0.05). However, we failed to observe significant results for NSCLC patients’ OS (P=0.176).
L1CAM is a 200-220 kDa transmembrane glycoprotein composed of six Ig-like domains, five fibronectin Type III repeats, a transmembrane region and a highly conserved cytoplasmic tail [3]. A previous study showed that L1CAM expression was positively related to epithelial-mesenchymal transition (EMT) markers such as slug, vimentin, and beta-catenin, but inversely with E-cadherin through double IHC and IF. TGF-beta1 induced increased L1CAM expression in A549 and H1395 cell lines, and L1CAM knockdown led to significantly reduced Matrigel invasion [12]. Another study also found that L1CAM knockdown led to reduced migration and invasion in three NSCLC cell lines, whereas overexpression of L1CAM enhances invasion in noninvasive cells. L1CAM induced sustained Erk activation in vitro and in vivo experiments. Downregulation of L1CAM significantly retarded tumor growth in SCID mice and reduced metastasis in nude rats [11]. The findings above indicate L1CAM is involved in lung cancer progression, which may explain our results that L1CAM is significantly related to poorer survival in resected NSCLC patients. Tischler et al found that L1CAM expression was associated with unfavorable OS and PFS of NSCLC patients in univariate analysis and L1CAM was an independent predictor of survival in a multivariate analysis including pT, pN, and pM category, and tumor differentiation grade for PFS [12], which is consistent with results in our study. However, they also showed L1CAM was an independent predictor of OS in a multivariate analysis including pT, pN, and pM category, and tumor differentiation grade [12]. Our study did not show L1CAM was an independent predictive factor for OS. Our multivariate analysis models includes age, gender, smoking status, T stage, N stage, M stage, tumor differentiation, radiotherapy and chemotherapy, which may contributes to the different results. In addition, Hai et al analyzed 4 published microarray studies among NSCLC patients and revealed that L1CAM was significantly associated with poorer PFS or OS in 3 study cohorts, however they did not observed the same results for PFS in 1 study cohorts. Furthermore, multivariate analyses indicated L1CAM was an independent predictive factor for PFS or OS in 2 study cohorts, but in another 2 cohorts they did not observed the same results [11]. All these divided results indicate that more prospective studies are needed to confirm the prognostic significances of L1CAM among NSCLC patients.
Actually, though L1CAM has not been widely reported in lung cancer, overwhelming evidence has shown that L1CAM expression was associated with poor prognosis, tumor progression and metastasis to lymph nodes in nearly all cancers and Altevogt et al have reviewed these studies [4]. Based on studies on functions of L1CAM in human cancers, further animal studies have shown that L1CAM monoclonal antibodies led to a significant tumor reduction in ovarian carcinoma, cholangiocarcinoma, and pancreatic cancer [16-18]. A recent study also found that L1CAM knockdown led to a reduced expression of EMT-related genes and increased p53/p21 and P38 activity, resulting in reduced lung metastasis in a melanoma xenograft model [5]. These results suggested that L1CAM might be a promising new target for antibody-based therapy of human advanced malignancies. More interestingly, another study found that injection of a mouse specific L1CAM-mAbs multiple times into normal mice did not generate behavioral changes or other detectable side effects, which further enables L1CAM to be a putative anti-cancer target [19].
Summarized, L1CAM-positive expression associated with poorer survival outcomes, but to elucidate whether L1CAM is an independent predictive factor of poor OS for NSCLC patients, more studies are needed.
Acknowledgements
We appreciate Li Li and Fei Chen in the Key Laboratory of Transplant Engineering and Immunology, Ministry of Health, West China Hospital, Sichuan University for their aids in the laboratory work.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Noone AM HN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2015. Bethesda, MD: National Cancer Institute; https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 3.Moos M, Tacke R, Scherer H, Teplow D, Fruh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- 4.Altevogt P, Doberstein K, Fogel M. L1CAM in human cancer. Int J Cancer. 2016;138:1565–1576. doi: 10.1002/ijc.29658. [DOI] [PubMed] [Google Scholar]
- 5.Ernst AK, Putscher A, Samatov TR, Suling A, Galatenko VV, Shkurnikov MY, Knyazev EN, Tonevitsky AG, Haalck T, Lange T, Maar H, Schroder-Schwarz J, Riecken K, Schumacher U, Wicklein D. Knockdown of L1CAM significantly reduces metastasis in a xenograft model of human melanoma: L1CAM is a potential target for anti-melanoma therapy. PLoS One. 2018;13:e0192525. doi: 10.1371/journal.pone.0192525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kilsdonk JW, Wilting RH, Bergers M, van Muijen GN, Schalkwijk J, van Kempen LC, Swart GW. Attenuation of melanoma invasion by a secreted variant of activated leukocyte cell adhesion molecule. Cancer Res. 2008;68:3671–3679. doi: 10.1158/0008-5472.CAN-07-5767. [DOI] [PubMed] [Google Scholar]
- 7.Doberstein K, Milde-Langosch K, Bretz NP, Schirmer U, Harari A, Witzel I, Ben-Arie A, Hubalek M, Muller-Holzner E, Reinold S, Zeimet AG, Altevogt P, Fogel M. L1CAM is expressed in triple-negative breast cancers and is inversely correlated with androgen receptor. BMC Cancer. 2014;14:958. doi: 10.1186/1471-2407-14-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Yang F, Ding Y, Zhen L, Han X, Jiao F, Tang J. Overexpression of L1 cell adhesion molecule correlates with aggressive tumor progression of patients with breast cancer and promotes motility of breast cancer cells. Int J Clin Exp Pathol. 2015;8:9240–9247. [PMC free article] [PubMed] [Google Scholar]
- 9.Kato K, Maesawa C, Itabashi T, Fujisawa K, Otsuka K, Kanno S, Tada H, Tatemichi Y, Kotani K, Oikawa H, Sugai T, Wakabayashi G, Masuda T. DNA hypomethylation at the CpG island is involved in aberrant expression of the L1 cell adhesion molecule gene in colorectal cancer. Int J Oncol. 2009;35:467–476. doi: 10.3892/ijo_00000358. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Yamada S, Tanaka C, Ito S, Murai T, Kobayashi D, Fujii T, Nakayama G, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Kodera Y. Overexpression of L1CAM is associated with tumor progression and prognosis via ERK signaling in gastric cancer. Ann Surg Oncol. 2014;21:560–568. doi: 10.1245/s10434-013-3246-5. [DOI] [PubMed] [Google Scholar]
- 11.Hai J, Zhu CQ, Bandarchi B, Wang YH, Navab R, Shepherd FA, Jurisica I, Tsao MS. L1 cell adhesion molecule promotes tumorigenicity and metastatic potential in non-small cell lung cancer. Clin Cancer Res. 2012;18:1914–1924. doi: 10.1158/1078-0432.CCR-11-2893. [DOI] [PubMed] [Google Scholar]
- 12.Tischler V, Pfeifer M, Hausladen S, Schirmer U, Bonde AK, Kristiansen G, Sos ML, Weder W, Moch H, Altevogt P, Soltermann A. L1CAM protein expression is associated with poor prognosis in non-small cell lung cancer. Mol Cancer. 2011;10:127. doi: 10.1186/1476-4598-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Li D, Zhou P, Li WM. Smurf1-positive expression indicates favorable survival for resected non-small cell lung cancer patients. Int J Clin Exp Pathol. 2018;11:399–405. [PMC free article] [PubMed] [Google Scholar]
- 16.Arlt MJ, Novak-Hofer I, Gast D, Gschwend V, Moldenhauer G, Grunberg J, Honer M, Schubiger PA, Altevogt P, Kruger A. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006;66:936–943. doi: 10.1158/0008-5472.CAN-05-1818. [DOI] [PubMed] [Google Scholar]
- 17.Min JK, Kim JM, Li S, Lee JW, Yoon H, Ryu CJ, Jeon SH, Lee JH, Kim JY, Yoon HK, Lee YK, Kim BH, Son YS, Choi HS, Lim NK, Kim DG, Hong HJ. L1 cell adhesion molecule is a novel therapeutic target in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2010;16:3571–3580. doi: 10.1158/1078-0432.CCR-09-3075. [DOI] [PubMed] [Google Scholar]
- 18.Schafer H, Dieckmann C, Korniienko O, Moldenhauer G, Kiefel H, Salnikov A, Kruger A, Altevogt P, Sebens S. Combined treatment of L1CAM antibodies and cytostatic drugs improve the therapeutic response of pancreatic and ovarian carcinoma. Cancer Lett. 2012;319:66–82. doi: 10.1016/j.canlet.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Doberstein K, Harter PN, Haberkorn U, Bretz NP, Arnold B, Carretero R, Moldenhauer G, Mittelbronn M, Altevogt P. Antibody therapy to human L1CAM in a transgenic mouse model blocks local tumor growth but induces EMT. Int J Cancer. 2015;136:E326–339. doi: 10.1002/ijc.29222. [DOI] [PubMed] [Google Scholar]


