Abstract
Objective: (1) to investigate the expression of long non-coding RNA (lncRNA) H19 in OVCAR3 and cisplatin-resistant OVCAR3/DDP cells; (2) to explore the effects of lncRNA H19 on cisplatin-resistance in ovarian cancer (OC) cells; (3) to determine the roles of lncRNA H19 on OC cell migration and epithelial to mesenchymal transition (EMT)-related factors. Methods: The human ovarian cancer OVCAR3 cell line was obtained from ATCC; the cisplatin-resistant OVCAR3/DDP cell line was induced from OVCAR3 cells through a progressive cisplatin concentration; OVCAR3 cells that overexpress lncRNA H19 and OVCAR3/DDP cells that silence the lncRNA H19 expression were established by the transfection of a recombinant lentivirus. A cell counting kit-8 (CCK-8) assay was used to determine the cell viability of OVCAR3 and OVCAR3/DDP. A reverse transcription-quantitative polymerase chain reaction (RT-qPCR) demonstrated the expressions of lncRNA H19, E-cadherin, twist, slug, and snail mRNA in OVCAR3 and OVCAR3/DDP cells. A Transwell assay was used to investigate the migration of OVCAR3 and OVCAR3/DDP cells. The expressions of E-cadherin, twist, slug, and snail proteins were determined by Western blot. Results: The cisplatin-resistant OVCAR3/DPP cells were successfully established. The level of lncRNA H19 in the OVCAR3/DDP cells was significantly elevated compared with the OVCAR3 cells (P < 0.05). The overexpression of lncRNA in the OVCAR3 cells improved the cisplatin-resistance, and the inhibition of lncRNA H19 expression in OVCAR3/DDP cells eliminated the cisplatin resistance. Furthermore, the migration ability and the expressions of the EMT positive regulator, twist, slug, snail mRNA, and protein in OVCAR3/DDP were dramatically up-regulated compared with the OVCAR3 group, and the expressions of the EMT negative regulator, E-cadherin mRNA, and protein were decreased compared with the OVCAR3 group, suggesting an increase of migration and EMT ability was observed in the OVCAR3/DDP cells. A gain of lncRNA expression in the OVCAR3 cells promoted migration and EMT-related activity; the loss of lncRNA H19 expression eliminated the enhanced ability of migration and EMT in the OVCAR3/DDP cells. Conclusions: LncRNA H19 is responsible for the cisplatin-resistance, migration, and MET regulation in OVCAR3 cells.
Keywords: Ovarian cancer, lncRNA H19, cisplatin-resistance, migration, EMT
Introduction
Ovarian cancer (OC) is one of the most common causes of morbidity and high mortality among the gynecologic malignancies [1,2]. OC cells can invade or spread to other parts of the body, and there may be no or only vague symptoms in the early stages [3]. This illness is frequently undiagnosed until it is in the advanced stages [4]. Recently, the 5-year survival rate of females with OC has improved, which is only 20% [5]. Cisplatin is used as one of the first-lines of treatment after surgical resection in OC [6]. However, recent studies have shown that OC is increasingly resistant to cisplatin [7]. In order to improve the prognosis of OC patients, it is important to improve the sensitivity of OC cells to cisplatin.
Although the exact mechanisms of OC cisplatin-resistance are poorly understood, accumulating evidence reveals that the epithelial to mesenchymal transition (EMT) plays critical roles in the development of OC’s resistance to cisplatin [8,9]. EMT is a major process for the conversion of early stage OC cells to invasive and metastatic malignancies, due to the loss of epithelial phenotypes and the acquisition of mesenchymal features [10]. Thus, EMT was considered a critical event in cancer progression and metastasis [11]. The induction of EMT is accomplished by several transcription factors including twist, slug, and snail that inhibit the expression of E-cadherin in various human cancers and are responsible for aggressive tumour behaviors and poor prognoses [12-16].
Recent research demonstrates that long non-coding RNA (lncRNA), longer than 200 nucleotides, can regulate gene expression to promote cancer metastasis using several unclear mechanisms [17]. Some studies have shown that the level of lncRNA H19 is abnormally elevated in OC tissues, and its high expression promotes tumor cell migration and invasion [18]. Furthermore, some studies suggest that lncRNA H19 may be involved in the cisplatin-resistance of OC cells [19]. These results indicate that lncRNA H19 is closely related to OC cell migration, invasion and cisplatin-resistance. However, the specific mechanisms and associations of lncRNA H19 with OC cell migration and cisplatin-resistance remains to be investigated. Thus, the aims of this study were: (1) to investigate the expression of lncRNA H19 in OC (OVCAR3) and cisplatin-resistant OC (OVCAR3/DDP) cells; (2) to explore the effects of lncRNA H19 on cisplatin-resistance in OC cells; (3) to determine the roles of lncRNA H19 on OC cell migration and EMT-related factors.
Materials and methods
Lentivirus (LVs) and plasmids preparation
A recombinant lentivirus (LVs) was prepared by GeneChem (Shanghai, China). To overexpress lncRNA H19, lncRNA H19 cDNA was cloned into multiple cloning sites of the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA). An overexpression plasmid and short interference RNA (siRNA) vector-LVs against lncRNA H19 (GenePharma, Shanghai, China) were successfully constructed and were then packaged by 293T cells. The siRNA sequence for lncRNA H19 was: 5’-UAAGUCAUUUGCACUGGUU-3’. Meanwhile, a scrambled locus siRNA or a blank pcDNA3.1 vector was constructed with LVs for the control vector group. The concentrated titer of virus suspension was 2 × 1012 TU/L.
Cell culture and transfection
The human ovarian cancer OVCAR3 cell line was obtained from ATCC (Manassas, USA). Cells were plated on 60 mm dishes (Corning, New York, USA) in RPMI-1640 medium (Gibco, Waltham, USA) and supplemented with 10% fetal bovine serum (FBS, Gibco) and a 1% penicillin-streptomycin solution (Invitrogen, Carlsbad, USA), and cultured at 37°C in a humidified atmosphere containing 5% CO2 until confluence reached ~80%. Transfection was performed by transfecting the lncRNA H19-LVs or the silncRNA H19-LVs following the manufacturer’s protocol.
OVCAR3/DDP cell establishment
The OVCAR3/DDP cell line was induced through a progressive cisplatin concentration. Briefly, OVCAR3 cells in a logarithmic growth phase were first treated with 2.5 μmol/L cisplatin for 48-72 h. The cisplatin was then removed, and the cells were cultured into a normal RPMI-1640 medium without cisplatin until they recovered. Next, the cells were treated with 5 μmol/L and 10 μmol/L, respectively for 48-72 h. Finally, the cisplatin-resistant OVCAR3/DDP cell line was successfully induced when the cells survived in a medium containing 10 μmol/L cisplatin for 2 months with normal activity.
Cell counting kit-8 (CCK-8) assay
OVCAR3 and OVCAR3/DDP cells were seeded into 96-well plates (Corning) at a concentration of 1 × 104 cells/mL. Cell viability was assessed using a cell counting kit-8 (CCK-8; DOJINDO, Tokyo, Japan). Absorbance at 450 nm was measured with a microplate reader (BioTek Synergy 2, Vermont, USA). The means of the optical density (OD) measurements from 6 wells of the indicated groups were used to calculate the percentage of cell viability. Growth inhibition rate (%) = [1 - (OVCAR3/DDP OD - blank OD)/(OVCAR3 OD - blank OD)] × 100%. Resistance index = OVCAR3/DDP IC50/OVCAR3 IC50. IC50: half-maximal inhibitory concentration.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen, Carlsbad, USA) was used to extract the total RNA from OVCAR3 and OVCAR3/DDP cells following the manufacturer’s protocol. The RNA was then subjected to reverse transcription to synthesize cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China). Afterwards, the 20-uL reactions with lncRNA H19, E-cadherin, twist, slug, or snail primers (GENEWIZ, South Plainfield, USA) were measured using a PikoReal 96 Real-Time PCR System (Thermo Fisher Scientific, Waltham, USA) with SYBR Green PCR Master Mix (Applied Biosystems, Waltham, USA). Relative quantitative analysis in mRNA expression was obtained using the ΔΔCT method and normalized to β-actin. The sequences of the primers are as follows: LncRNA H19 forward: 5’-AGGACCGCCTATCCAACA-3’; LncRNA H19 reverse: 5’-TCTCATTGCCGAACACCT-3’; E-cadherin forward: 5’-CGTAGCAGTGACGAATGTGG-3’; E-cadherin reverse: 5’-CTGGGCAGTGTAGGATGTGA-3’; twist forward: 5’-GGACAGTGATTCCCAGACGG-3’; twist reverse: 5’-CCTTTCAGTGGCTGATTGGC-3’; slug forward: 5’-CCTGGTTGCTTCAAGGACAC-3’; slug reverse: 5’-AGCAGCCAGATTCCTCATGT-3’; snail forward: 5’-GTTTACCTTCCAGCAGCCCTAC-3’; snail reverse: 5’-GCCTTTCCCACTGTCCTCATCT-3’; β-actin forward: 5’-TAAAGACCTCTATGCCAACACAGT-3’; β-actin reverse: 5’-CACGATGGAGGGCCGGACTCATC-3’.
Boyden chamber Transwell migration assay
The migration abilities of the OVCAR3 and OVCAR3/DDP cells were determined by a Transwell chamber culture system (8 μm pore; Corning). Briefly, the OVCAR3 and OVCAR3/DDP cells were seeded in a Boyden Transwell chamber without Matrigel-coating at a concentration of 2 × 104 cells/well in a serum-free Opti-MEM medium (Gibco). Then, the complete growth medium supplemented with 10% FBS was added to the lower chamber. After incubation at 37°C for 24 h, the cells attached to the lower insert filter were stained with 0.1% crystal violet (Solarbio, Beijing, China) at room temperature for 10 mins. The migrated cells were measured and counted by Image-Pro Plus 6 software (Media Cybernetics, Rockville, USA).
Western blot
OVCAR3 and OVCAR3/DDP whole cell proteins were extracted using a Cell Total Protein Extraction Kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions. Protein concentrations were measured using a bicinchoninic acid (BCA) assay. An equivalent amount of proteins was prepared and separated by 8-12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and electro-transferred to nitrocellulose membranes (Millipore, Darmstadt, Germany). Then they were probed with E-cadherin (1:1000), twist (1:50), slug (1:1000), snail (1:500), or β-actin (1:1000) (Abcam, Cambridge, UK) antibodies at 4°C overnight and incubated with a relative secondary antibody at room temperature for 2 h. Finally, the signals were detected using an Odyssey Infrared Imaging System (Li-Cor, Lincoln, USA). The digitized images were analyzed using Image J software (NIH, Bethesda, USA). Protein expression levels were calculated from the ratio of corresponding protein/β-actin × 100%.
Statistical analysis
For all quantitative analyses, data are expressed as the means ± SEM. The statistical analysis was performed using PASW Statistic 21 (SPSS Inc., Chicago, USA). The statistical comparison was carried out with a two-tailed Student’s t test, a one-way analysis of variance, and a chi-square test. The analyses were performed using GraphPad prism 6 (La Jolla, USA). Significance was defined as P < 0.05.
Results
Establishment of the cisplatin-resistant human OVCAR3/DDP ovarian cancer cell line
The cisplatin-resistant OVCAR3/DDP cells were successfully established. The cell viability of OVCAR3/DDP and the parental cells were determined using a CCK-8 assay at different cisplatin concentrations (0-8 μmol/L). As shown in Figure 1A, the cell viability of OVCAR3 gradually decreased with increasing cisplatin concentration, and the viability was almost inhibited when the concentration of cisplatin was 8 μmol/L, but the decline of cell viability in OVCAR3/DDP was significantly reduced compared with OVCAR3 at the same cisplatin concentration (P < 0.01). Furthermore, the growth inhibition rate of these two cells were measured and calculated with 5 different concentrations of cisplatin (0.25, 2.5, 25, 250, and 2,500 μmol/L) (Figure 1B). The inhibition rate of OVCAR3 cells was significantly higher than the rate of the OVCAR3/DDP cells at the same cisplatin concentrations (P < 0.05), and at 2,500 μmol/L, both of the cells were entirely inhibited. The IC50 values of OVCAR3 and OVCAR3/DDP and the RI values of OVCAR3/DDP were calculated as 53.63 ± 3.35, 138.40 ± 3.16, and 2.60, respectively (Figure 1C).
Figure 1.
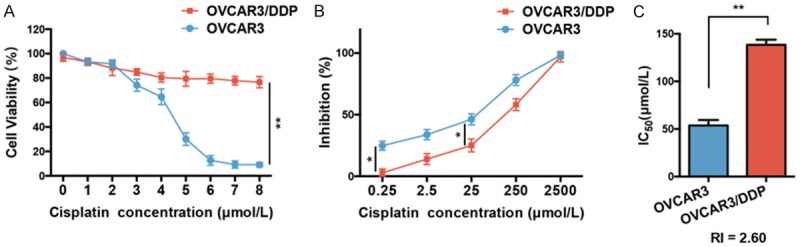
Establishment of the cisplatin-resistant OVCAR3/DDP cell line. A. The cell viability of OVCAR3 and OVCAR3/DDP cells were demonstrated by CCK-8 with different cisplatin concentrations (0-8 μmol/L), n = 5. B. The inhibition rate of cisplatin in OVCAR3 and OVCAR3/DDP groups, n = 5. C. The IC50 in each OVCAR3 and OVCAR3/DDP group, n = 5. The RI was 2.60. *P < 0.05, **P < 0.01 vs. indicated group. IC50: half maximal inhibitory concentration; RI: resistance index. Data were compared using a chi-square test and a one-way analysis of variance.
LncRNA H19 is related to the cisplatin-resistance of OVCAR3 and OVCAR3/DDP
In order to explore whether lncRNA H19 is involved in the cisplatin-resistance effects of OVCAR3 and OCAR3/DDP cells, lncRNA H19-LVs were transfected into OVCAR3 to overexpress lncRNA H19 (OVCAR3 lncRNA H19 group), and silncRNA H19-LVs were transfected into OVCAR3/DDP to silence lncRNA H19 expression (OVCAR3/DDP silncRNA H19 group). As shown in Figure 2A, we examined the expression levels of lncRNA H19 in each group. The level of lncRNA H19 in the OVCAR3/DDP was significantly elevated compared with OVCAR3 (P < 0.05). In addition, lncRNA H19 overexpression in the OVCAR3 lncRNA H19 group sharply increased the lncRNA H19 expression compared with the OVCAR3 group (P < 0.01); and the silencing of lncRNA H19 in the OVCAR3/DDP silncRNA H19 group reduced the expression when compared with the OVCAR3/DDP group (P < 0.05). The cell viability was then examined in these groups with various cisplatin concentrations (0-16 μmol/L). The cell viability of the OVCAR3 lncRNA H19 group was dramatically increased compared with OVCAR3 at the same cisplatin concentrations (P < 0.05 at 3 μmol/L; P < 0.01 at 8 μmol/L) (Figure 2B). Furthermore, the cell viability of the OVCAR3/DDP silncRNA H19 group was sharply decreased when compared with the OVCAR3/DDP group at the same cisplatin concentrations (P < 0.01 at 3 μmol/L) (Figure 2C). These results indicated that the overexpression of lncRNA H19 contributes to cisplatin-resistance in OVCAR3 cells, and the loss of lncRNA H19 expression leads to a failure of resistance to cisplatin in OVCAR3/DDP cells.
Figure 2.
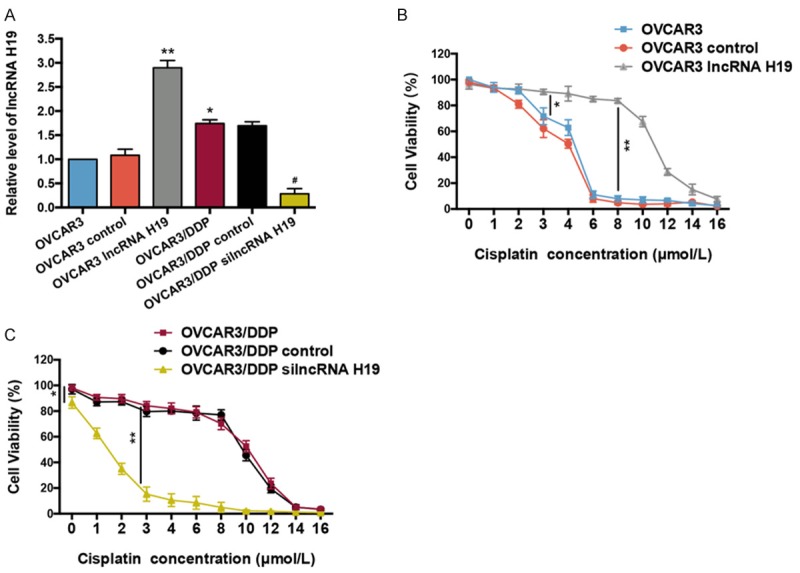
LncRNA H19 contributes to cisplatin-resistance in OVCAR3 and OVCAR3/DDP cells. A. LncRNA H19 expression levels in OVCAR3 and OVCAR3/DDP cells were analyzed by RT-qPCR, n = 6. B. Cell viability of OVCAR3, and OVCAR3 transfected with lncRNA H19-LVs (lncRNA H19 overexpression) and blank vector (control) with various concentrations of cisplatin (0-16 μmol/L), n = 6. C. Cell viability of OVCAR3/DDP, and OVCAR3/DDP transfected with silncRNA H19-LVs (silence of lncRNA H19 expression) and blank vector (control) with various concentrations of cisplatin, n = 6. *P < 0.05, **P < 0.01 vs. OVCAR3 or indicated group, #P < 0.05 vs. OVCAR3/DDP group. LVs: recombinant lentivirus. Data were compared using a one-way analysis of variance and a chi-square test.
Expression of lncRNA H19 modulates the migration of OVCAR3 and OVCAR3/DDP cells
The migration ability of OVCAR3/DDP cells was significantly increased compared to the OVCAR3 cells (P < 0.01) (Figure 3A and 3B). The overexpression of lncRNA H19 dramatically increased the migration ability of the OVCAR3 cells (P < 0.01), even more than the migration ability of the OVCAR3/DPP cells (P < 0.05) (Figure 3C and 3D). The silencing of lncRNA H19 expression significantly decreases the migration ability of OVCAR3/DDP cells (P < 0.01), even less than the migration ability of OVCAR3 cells (P < 0.05).
Figure 3.
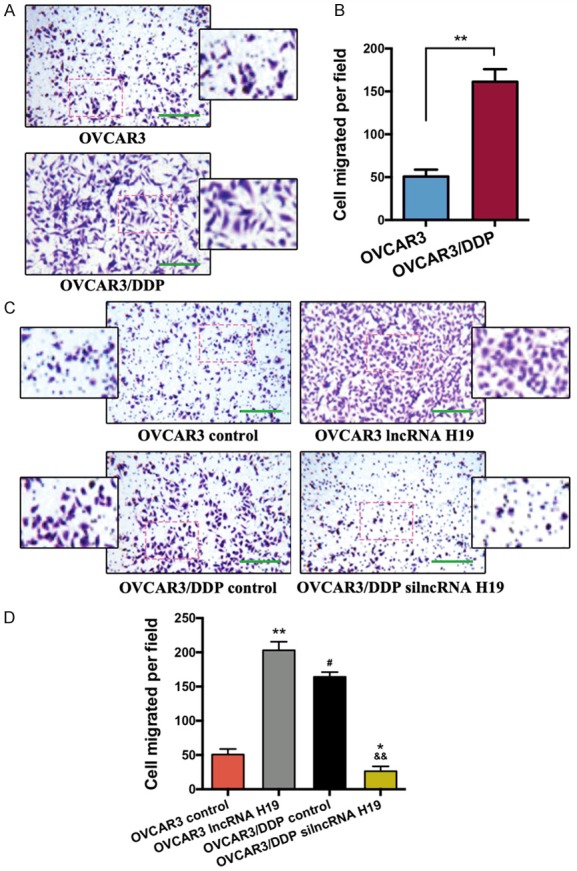
LncRNA H19 regulates OVCAR3 and OVCAR3/DDP cell migration. A. The migration activities of the OVCAR3 and OVCAR3/DDP cells were determined by using a Transwell assay, bar = 500 μm. B. Bar graph of the Transwell results; the migrated cells were measured by Image-Pro Plus 6.0, n = 5. C. Representative images of the migration activity in each group, bar = 500 μm. D. Bar graph of the Transwell results, n = 5. *P < 0.05, **P < 0.01 vs. indicated or the OVCAR3 control group, #P < 0.05 vs. OVCAR3 lncRNA H19 group, &&P < 0.01 vs. OVCAR3/DDP control group. Data were compared using a two-tailed Student’s t test.
EMT-related E-cadherin, twist, slug, and snail mRNA expression in OVCAR3 and OVCAR3/DDP cells
Finally, we examined the EMT-related regulators, E-cadherin, twist, slug, and snail mRNA and protein expression in OVCAR3 and OVCAR3/DDP cells. The E-cadherin mRNA level was decreased both in the OVCAR3 lncRNA H19 and the OVCAR3/DDP groups compared with the OVCAR3 group (P < 0.01 and P < 0.05, respectively), whereas the mRNA level of E-cadherin in the OVCAR3/DDP silncRNA H19 group was significantly elevated compared with the OVCAR3/DDP group (P < 0.01) (Figure 4A). As shown in Figure 4B-D, the mRNA expression of twist, slug, and snail in OVCAR3 lncRNA H19 and OVCAR3/DDP (except snail) groups were all increased compared with the OVCAR3 group (P < 0.01 and P < 0.05, respectively). Furthermore, the twist, slug, and snail mRNA expressions in the OVCAR3/DDP silncRNA H19 group were dramatically decreased when compared with the OVCAR3/DDP group (P < 0.01; snail in OVCAR3/DDP silncRNA H19 vs. OVCAR3/DDP: P < 0.05).
Figure 4.
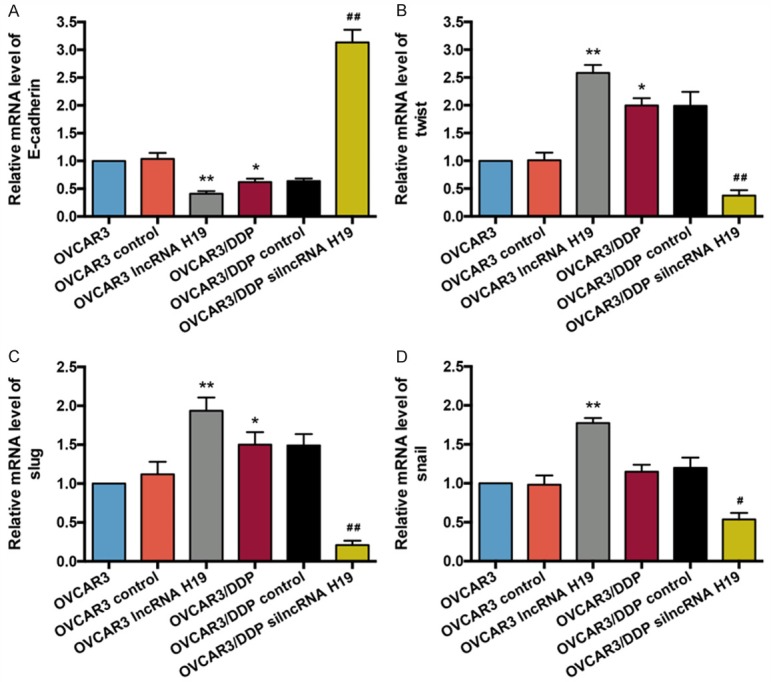
E-cadherin, twist, slug, and snail mRNA levels in the OVCAR3 and OVCAR3/DDP cells. A. The E-cadherin mRNA expression in the OVCAR3 cells of each group was analyzed by RT-qPCR, n = 5. B. RT-qPCR was used to detect twist mRNA levels in each group, n = 5. C. The slug mRNA expression in the OVCAR3 cells of each group was analyzed by RT-qPCR, n = 5. D. The snail mRNA expression in the OVCAR3 cells of each group, n = 5. *P < 0.05, **P < 0.01 vs. OVCAR3 group, #P < 0.05, ##P < 0.01 vs. OVCAR3/DDP group. Data were compared using a one-way analysis of variance.
The expressions of E-cadherin, twist, slug, and snail proteins in OVCAR3 and OVCAR3/DDP cells
Similar to the results of RT-qPCR, the western blot results indicated that the protein expression of E-cadherin was reduced both in the OVCAR3 lncRNA H19 and the OVCAR3/DDP groups compared with the OVCAR3 group (P < 0.01 and P < 0.05, respectively), and there was a sharp increase of E-cadherin expression in the OVCAR3/DDP silncRNA H19 group compared with the OVCAR3/DDP group (P < 0.01) (Figure 5A and 5B). In addition, the expressions of twist, slug, snail in the OVCAR3 lncRNA H19 and OVCAR3/DDP groups were dramatically elevated compared with the OVCAR3 group (P < 0.01; twist in OVCAR3/DDP vs. OVCAR3: P < 0.05) (Figure 5A, 5C-E). A decrease in the twist, slug, and snail mRNA expressions was observed in the OVCAR3/DDP silncRNA H19 group when compared with the OVCAR3/DDP group (P < 0.01).
Figure 5.
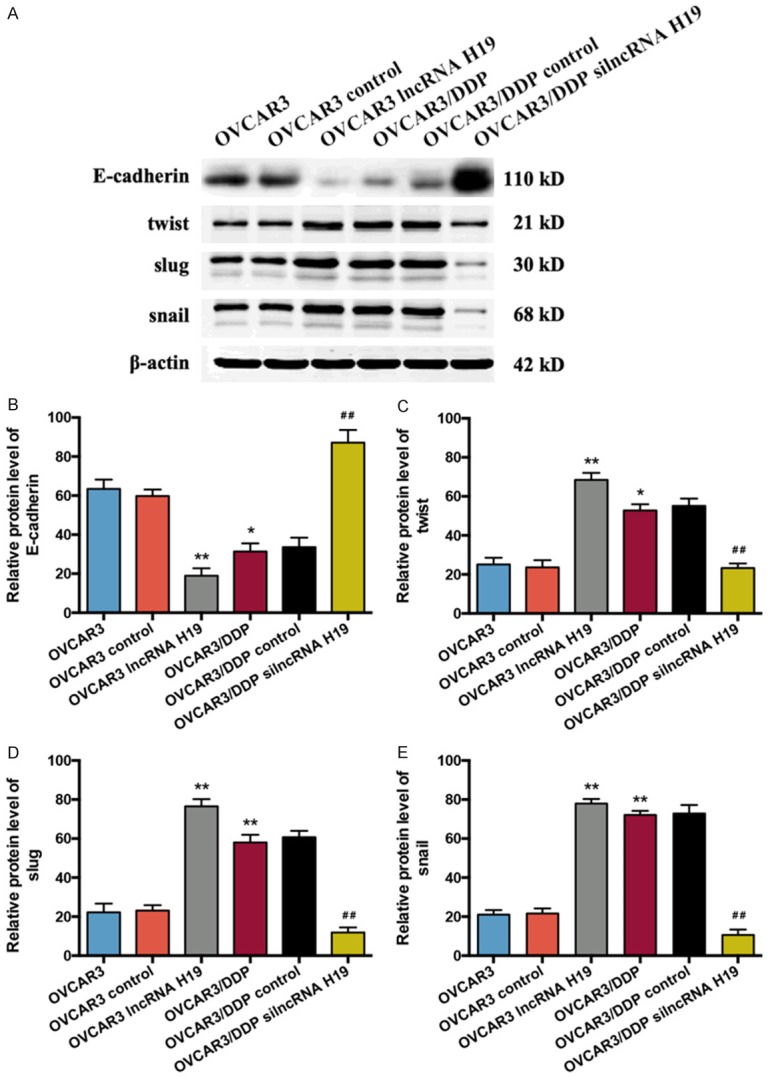
Expression of E-cadherin, twist, slug, and snail proteins in OVCAR3 and OVCAR3/DDP cells. A. The protein expressions of E-cadherin, twist, slug, and snail in the OVCAR3 and OVCAR3/DDP cells were determined by western blot. B. Bar graph of western blot results of the E-cadherin expression in each group, n = 5. C. Bar graph of the western blot results of the twist expression in each group, n = 5. D. Bar graph of the western blot results of the slug expression in each group, n = 5. E. Bar graph of the western blot results of the snail expression in each group, n = 5. *P < 0.05, **P < 0.01 vs. OVCAR3 group, #P < 0.05, ##P < 0.01 vs. OVCAR3/DDP group. Data were compared using a one-way analysis of variance.
Discussion
OC is the most lethal gynecologic malignancy in females, and in the United States in 2017 there were an estimated 22,500 new cases and ~14,100 mortalities due to OC [20]. The causes of the high mortality of OC are primarily related to its diagnosis after it has reached an advanced stage and the high rate of therapy resistance [3,21]. The chemoresistance of platinum-based combinations in OC is one of the obstacles limiting cancer drug treatments and weakening the effectiveness of chemotherapy in a large number of patients [6]. In addition, evidence reveals that the ability of migration and invasion in OC cells is elevated through the loss of epithelial characteristics and the gain of a mesenchymal characteristics known as EMT [22]. Tumor cells become invasive, enter into the circulatory system, and move from the systemic circulation to new host tissue. The cell adhesion molecule E-cadherin is required for stable adherence formation and the maintenance of epithelial characteristics. Loss of E-cadherin expression is one of the indicators of EMT in tumour cells [12]. Studies have reported that E-cadherin expression is decreased in various cancers and is associated with tumour progression and metastasis [23]. The transcriptional factors snail and slug have been considered as direct suppressors of E-cadherin [24]. Another trigger EMT is twist [25], and the overexpress of twist results in an enhanced level of N-cadherin, leading to a further decline of E-cadherin expression [26].
Non-coding RNA (ncRNA) is an RNA molecule that does not encode protein expression. Recently, some studies demonstrated that ncRNA plays important roles in cancer development and progression [27]. LncRNA are ncRNA longer than 200 nucleotides, and lncRNA functions in a broad range of cell activities such as proliferation, apoptosis, and migration [28]. An elevated expression of 15 kinds of lncRNA was demonstrated in OC tissues, but their functions were not determined [29]. Moreover, an increase of one lncRNA expression, ANRIL, leads to the up-regulation of proliferation, migration, and invasion in OC cells [30]. Recently, 11 lncRNAs were shown to modulate the migration, EMT, and metastasis in many types of cancer [31]. LncRNA H19 is encoded by the H19 gene, and several studies have revealed that its function may be related to the migration and chemoresistance of OC cells [18,19]. However, the roles and mechanisms of lncRNA H19 in OC are still unknown. Therefore, much about lncRNA H19 and OC needs to be investigated.
In the present study, we first established a stable cisplatin-resistant OVCAR3/DDP cell line. The cell viability of OVCAR3/DDP cells is significantly increased compared with normal OVCAR3 cells under the same concentration of cisplatin treatment (P < 0.01). The growth inhibition rate in OVCAR3/DDP is lower (P < 0.05) and the IC50 is higher (P < 0.01) than in the OVCAR3 group. And the RI of OVCAR/DDP is 2.60. These results suggest that a cisplatin-resistant OVCAR3/DDP was successfully established. Next, we measured the level of lncRNA H19 in OVCAR3 and OVCAR3/DDP cells. The expression of lncRNA H19 was elevated in the OVCAR3/DDP cells compared with the OVCAR3 cells (P < 0.05). The overexpression of lncRNA H19 in the OVCAR3 group enhanced the cisplatin-resistance of the OVCAR3 cells; the upregulation of lncRNA H19 levels in the OVCAR3/DDP group eliminated the cisplatin-resistance ability of the OVCAR3/DDP cells. These results demonstrated that lncRNA H19 is responsible for the cisplatin-resistance of OVCAR3 and OVCAR3/DDP cells. Furthermore, the migration of OC cells was determined. The migration ability of OVCAR3/DDP is stronger than the migration ability of OVCAR3 (P < 0.01). An increase of lncRNA H19 expression in the OVCAR3 group enhanced cell migration and the loss of lncRNA HH19 levels in the OVCAR3/DDP group lowered the migration, indicating that lncRNA H19 is related to cell migration in OVCAR3 and OVCAR3/DDP cells. Finally, we determined the mRNA and protein expressions of the EMT-related regulators, E-cadherin, twist, slug, and snail. Both E-cadherin mRNA and protein levels are decreased in OVCAR3/DDP compared with OVCAR3, and twist, slug, and snail mRNA and protein expression are significantly promoted in OVCAR3/DDP cells compared with OVCAR3 cells. In addition, the overexpression of lncRNA H19 in the OVCAR3 group sharply lowered the E-cadherin mRNA and protein expressions and increased the twist, slug, and snail mRNA and protein levels. The silencing of lncRNA H19 in the OVCAR3/DDP group dramatically elevated the mRNA and protein expressions of E-cadherin and reduced the mRNA and protein levels of twist, slug, and snail. These results revealed that lncRNA H19 is associated with the EMT process in OVCAR3 and OVCAR3/DDP cells. Limitations of our study include the restriction of investigating the effects of lncRNA H19 in OC cells rather than exploring its downstream signaling pathways. And in addition to the OVCAR3 cell line, it is unclear whether lncRNA H19 has a similar role on other OC cell lines.
In summary, this study provided a novel insight into understanding the mechanism underlying the chemoresistance and migration of OC cells during EMT. Furthermore, our study reveals for the first time that lncRNA H19 is indeed involved in the cisplatin-resistance, migration, and EMT of OC cells. It was also shown that lncRNA H19 may be promising as a novel therapeutic target for overcoming OC drug resistance and metastasis.
Acknowledgements
This work was supported by the project of Shanghai Committee of Science and Technology (15DZ1941001).
Disclosure of conflict of interest
None.
References
- 1.Kujawa KA, Lisowska KM. [Ovarian cancer--from biology to clinic] . Postepy Hig Med Dosw (Online) 2015;69:1275–1290. doi: 10.5604/17322693.1184451. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez L, Kim MK, Lyle LT, Bunch KP, House CD, Ning F, Noonan AM, Annunziata CM. Characterization of ovarian cancer cell lines as in vivo models for preclinical studies. Gynecol Oncol. 2016;142:332–340. doi: 10.1016/j.ygyno.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, Sessa C, Castiglione M ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v23–30. doi: 10.1093/annonc/mdq244. [DOI] [PubMed] [Google Scholar]
- 6.Foster T, Brown TM, Chang J, Menssen HD, Blieden MB, Herzog TJ. A review of the current evidence for maintenance therapy in ovarian cancer. Gynecol Oncol. 2009;115:290–301. doi: 10.1016/j.ygyno.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Morgan RJ Jr, Alvarez RD, Armstrong DK, Burger RA, Chen LM, Copeland L, Crispens MA, Gershenson DM, Gray HJ, Hakam A, Havrilesky LJ, Johnston C, Lele S, Martin L, Matulonis UA, O’Malley DM, Penson RT, Powell MA, Remmenga SW, Sabbatini P, Santoso JT, Schink JC, Teng N, Werner TL, Dwyer MA, Hughes M National comprehensive cancer networks. Ovarian cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11:1199–1209. doi: 10.6004/jnccn.2013.0142. [DOI] [PubMed] [Google Scholar]
- 8.Davidson B, Trope CG, Reich R. Epithelial-mesenchymal transition in ovarian carcinoma. Front Oncol. 2012;2:33. doi: 10.3389/fonc.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchini S, Fruscio R, Clivio L, Beltrame L, Porcu L, Fuso Nerini I, Cavalieri D, Chiorino G, Cattoretti G, Mangioni C, Milani R, Torri V, Romualdi C, Zambelli A, Romano M, Signorelli M, di Giandomenico S, D’Incalci M. Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Eur J Cancer. 2013;49:520–530. doi: 10.1016/j.ejca.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 14.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 15.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 17.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoshani O, Massalha H, Shani N, Kagan S, Ravid O, Madar S, Trakhtenbrot L, Leshkowitz D, Rechavi G, Zipori D. Polyploidization of murine mesenchymal cells is associated with suppression of the long noncoding RNA H19 and reduced tumorigenicity. Cancer Res. 2012;72:6403–6413. doi: 10.1158/0008-5472.CAN-12-1155. [DOI] [PubMed] [Google Scholar]
- 19.Tanos V, Prus D, Ayesh S, Weinstein D, Tykocinski ML, De-Groot N, Hochberg A, Ariel I. Expression of the imprinted H19 oncofetal RNA in epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 1999;85:7–11. doi: 10.1016/s0301-2115(98)00275-9. [DOI] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 21.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 22.Cao L, Shao M, Schilder J, Guise T, Mohammad KS, Matei D. Tissue transglutaminase links TGF-beta, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012;31:2521–2534. doi: 10.1038/onc.2011.429. [DOI] [PubMed] [Google Scholar]
- 23.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Kurrey NK, K A, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155–165. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Karreth F, Tuveson DA. Twist induces an epithelial-mesenchymal transition to facilitate tumor metastasis. Cancer Biol Ther. 2004;3:1058–1059. doi: 10.4161/cbt.3.11.1302. [DOI] [PubMed] [Google Scholar]
- 26.Alexander NR, Tran NL, Rekapally H, Summers CE, Glackin C, Heimark RL. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res. 2006;66:3365–3369. doi: 10.1158/0008-5472.CAN-05-3401. [DOI] [PubMed] [Google Scholar]
- 27.Marshall L, White RJ. Non-coding RNA production by RNA polymerase III is implicated in cancer. Nat Rev Cancer. 2008;8:911–914. doi: 10.1038/nrc2539. [DOI] [PubMed] [Google Scholar]
- 28.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Fu Z, Dai C, Cao J, Liu X, Xu J, Lv M, Gu Y, Zhang J, Hua X, Jia G, Xu S, Jia X, Xu P. LncRNAs expression profiling in normal ovary, benign ovarian cyst and malignant epithelial ovarian cancer. Sci Rep. 2016;6:38983. doi: 10.1038/srep38983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu JJ, Wang Y, Liu YL, Zhang Y, Ding JX, Hua KQ. The long non-coding RNA ANRIL promotes proliferation and cell cycle progression and inhibits apoptosis and senescence in epithelial ovarian cancer. Oncotarget. 2016;7:32478–32492. doi: 10.18632/oncotarget.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhamija S, Diederichs S. From junk to master regulators of invasion: lncRNA functions in migration, EMT and metastasis. Int J Cancer. 2016;139:269–280. doi: 10.1002/ijc.30039. [DOI] [PubMed] [Google Scholar]


