Abstract
Aims: The following study examines the FXR and HRG expression in benign and malignant lesions of the pancreas and evaluates the association between FXR and HRG expression with clinicopathological features and prognosis of pancreatic cancer. Materials and methods: Immunohistochemistry of FXR and HRG was performed with EnVision™ in 106 pancreatic ductal adenocarcinoma (PDAC) specimens, 35 paracancer samples (2 cm away from the tumor, when possible or available), 55 benign lesions and 13 normal tissue samples. Results: The percentage of cases with positive FXR and negative HRG expression was significantly higher in PDAC compared to pericancerous tissues, benign lesions and normal tissues (P<0.05 or P<0.01). In pancreatic tissues with benign lesions, tissues with positive FXR and/or negative HRG protein expression exhibited dysplasia or intraepithelial neoplasia. The percentage of cases with positive FXR and negative HRG expressions was significantly higher in PDAC with lymph node metastasis, invasion, and TNM stage III+IV disease (P<0.05 or P<0.01). The expression of FXR was negatively correlated with HRG (P<0.05). In addition, the univariate Kaplan-Meier analysis showed that positive FXR and negative HRG expression, poor differentiation, large tumor size, high TNM stage, lymph node metastasis, and invasion were closely associated with decreased overall survival in PDAC patients (P<0.05 or P<0.01). Moreover, multivariate Cox regression analysis identified that positive FXR and negative HRG expression were independent factors for poor prognosis in PDAC. The AUC for FXR was (AUC=0.709, 95% CI: 0.632-0.787), and for HRG was (AUC=0.719, 95% CI: 0.643-0.796) in PDAC compared to benign lesions. Conclusions: Positive FXR and negative HRG expression are closely associated with the carcinogenesis, clinical, pathological and biological behaviors, and poor prognosis in PDAC.
Keywords: Pancreatic ductal adenocarcinoma, dysplasia, pancreatic intraepithelial neoplasia, FXR, HRG, immunohistochemistry
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly malignant tumor of the pancreas and the fourth-leading cause of cancer-related deaths in the USA with an estimated 39,590 pancreatic cancer deaths in 2014 [1]. The 5-year survival rate of PDAC is less than 5% [2], and annual deaths from PDAC continue to rise [1]. This poor prognosis results from late diagnosis due to the lack of specific signs and biomarkers for early diagnosis, the metastatic potential of PDAC, and resistance to chemotherapy. Despite recent advancements in diagnostic technology, over 85-90% of patients present with advanced and metastatic disease, thus losing the option of surgical resection [3]. Unfortunately, most surgical patients still relapse despite adjuvant systemic therapies [4]. Metastasis is a natural feature of pancreatic cancer, responsible for over 90% of patients’ deaths [5]. The search for diagnostic and targeting therapy biomarkers continues, since few of them have reached clinical application.
The Farnesoid X-receptor (FXR; NR1H4) is an adopted nuclear receptor with oxysterols, such as primary and secondary bile acids, as its endogenous ligands [6,7]. Bile acids are the metabolic products of cholesterol that can be toxic to the body at high concentrations. FXR prevents the accumulation of bile acids by inducing expression of a second nuclear receptor small heterodimer partner (SHP, NR0B2), which in turn inhibits expression of CYP7A1, the rate-limiting enzyme in bile acid synthesis [8]. The main sites of bile acid production and secretion are the liver and intestine, respectively, which is mirrored by high levels of FXR expression in these organs [9]. Yet, as approximately 70% of bile acids are re-absorbed by the body, they can reach micromolar concentrations in the plasma, which may underlie the expression of FXR in a number of other tissues [10,11]. FXR is highly expressed in the entero-hepatic system where it transcriptionally regulates bile acid and lipid metabolism [12]. Recently, some studies have demonstrated that FXR is overexpressed in multiple human cancers, including breast cancer [13], lung cancer [14], gastric cancer [15,16], colorectal cancer [17,18], pancreatic cancer [19] and hepatocellular carcinoma [20-22]. Nonetheless, some authors have found that overexpression of FXR could inhibit the carcinogenesis and progression of prostate cancer [23,24].
Histidine-rich glycoprotein (HRG) is a heparin-binding plasma protein that modulates immune, hemostatic, and vascular functions and the activity of degradative enzymes such as the plasminogen/plasmin to regulate angiogenesis [25]. HRG is a 75 kDa single chain heparin-binding plasma protein produced by the liver and is present at high levels in plasma (100-200 lg/ml) [26]. HRG, which is a host-produced antiangiogenic and immunomodulatory factor, regulates tumor vessel abnormalization and inflammation for various reasons. First, HRG is a multidomain protein that binds thrombospondins (TSPs), heparin, FcgR receptors and other molecules implicated in tumorigenesis [27]. Second, HRG is deposited in the tumor stroma from plasma or platelets; nonetheless, the tumor HRG levels have been analyzed in only a few human cancers [28]. Third, binding of HRG to its ligands is facilitated by Zn2+ and low pH, conditions found in the tumor milieu [29]. Fourth, HRG stimulates phagocytosis of dying cells [30], but it is unknown if it regulates TAM polarization. Fifth, HRG inhibits tumor growth [31], while its precise underlying mechanisms remain incompletely understood. Moreover, a role for HRG in metastasis has not yet been documented.
Some authors have found that HRG expression is significantly decreased in breast cancer [32], lung cancer [33], gastric cancer [34], colorectal cancer [35], hepatocellular cancer [36], pancreatic ductal adenocarcinoma [37], ovarian cancer [38] and prostate cancer [39]. HRG might be an independent diagnostic indicator and improved prognostic indicator.
The role of FXR and HRG expression in PDAC remain to be clarified. In this study, FXR and HRG expressions in benign and malignant pancreatic lesions were measured by immunohistochemistry. The clinicopathologic significance of FXR and HRG expressions and their associations with the prognosis of PDAC were analyzed.
Material and methods
Case selection
One hundred and six PDACs, thirty-five peritumoral tissues, fifty-five precursor pancreatic tissues, and thirteen normal pancreatic tissues were obtained at the Second and third Xiangya Hospitals, Central South University. This study was pre-approved by the Ethics Committee for Human Study of Central South University. Among the one hundred and six PDACs, sixty-one came from male patients (57.5%) and forty-five came from female patients (42.5%) with an average age of 54.50±11.53 years. Histopathologic subtypes of PDAC include: thirty-eight well-differentiated adenocarcinomas (35.8%), thirty-five moderately-differentiated adenocarcinomas (33%), and thirty-three poorly-differentiated adenocarcinomas (31.1%). Invasion and lymph node metastases were evaluated according to standard criteria. Among the one hundred and six PDAC, eleven cases (10.4%) were TNM stage I, forty-one cases (39.6%) were TNM stage II, thirty-seven cases (34.9%) were TNM stage III, and sixteen cases (15.1%) were TNM stage IV tumors. Among the one hundred and six PDACs, twenty-nine cases (27.5%) had regional lymph node metastasis, and sixty-four cases (60.4%) had invasion to surrounding organs and tissues. Survival information was obtained through letters and phone calls from all patients with PDAC.
Thirty-five peritumoral tissues were collected ≥2 cm from the tumors of above mentioned PDAC patients. Twelve of the 35 peritumoral tissues were normal, ten were PanINs (pancreatic intraepithelial neoplasms) grade I, eight were PanINs grade II, and five were PanINs grade III.
Fifty-five precursor pancreatic tissues were collected from twenty-nine (52.7%) males and twenty-six (47.3%) females. Among the fifty-five patients who supplied the precursor specimens, thirteen (23.6%) were ≤45 years old and forty-two (76.4%) were >45 years old. The fifty-five precursor tissues included twenty chronic pancreatitis tissues (36.4%), twenty adenomas (36.4%), and fifteen intraepithelial neoplasias (27.3%). Ten, six, and four of the twenty chronic pancreatitis tissues were mild, moderate, and severe pancreatitis, respectively. The twenty adenomas included five mucinous adenomas and fifteen serous adenomas. Four, three, and two of the twenty adenomas had mild, moderate, and severe dysplasia, respectively. Among the fifteen intraepithelial neoplasias, six had grade I, five had grade II, and four had grade III intraepithelial neoplasia. Thirteen normal pancreatic tissues were collected during surgery of the twenty pancreatic adenomas.
All tissues were treated with 4% formaldehyde for 24 to 48 hours, with 10% formalin solution, and were then embedded in paraffin.
Immunohistochemistry
Rabbit anti-human FXR and HRG polyclonal antibody were purchased from Dako Corporation (Carpentaria, CA, USA). EnVisionTM Detection Kit was purchased from Dako Laboratories (CA, USA). Positive controls were provided with the EnVisionTM Detection Kit. EnVision immunohistochemistry of FXR and HRG was performed following the user manual. Briefly, 4 μM-thick sections were cut from paraffin-embedded tissues. The sections were deparaffinized and then incubated with 3% H2O2 in the dark for 15 min. The heat-induced epitope retrieval was conducted with sodium citrate buffer (10 mM Sodium citrate, 0.05% Tween 20, pH 6.0) at 96°C for 30 min. The sections were incubated with rabbit anti-human FXR and HRG primary antibody (1:100 dilution) for 1 hr after they were soaked in PBS for 3×5 min. The sections were incubated with several drops of Solution A (ChemMateTMEnVison+/HRP) for 30 min followed by DAB staining and hematoxylin counter-staining. The sections were dehydrated, soaked in xylene, and mounted with neutral balsam. Five hundred cells from ten random fields were examined per section by 2 observers independently. An average of the percentages from these two observers was used for final evaluation. Cases with positive cells ≥25% were considered positive whereas other cases were considered negative.
Statistical analysis
Data were analyzed using the SPSS 17.0 (statistical package for the Social Sciences, Version 17.0). The inter-relationship of FXR and HRG with histological or clinical factors was analyzed using χ2 test or Fisher’s exact test. The overall survival of patients with PDAC was analyzed using Kaplan-Meier univariate survival analysis and log-rank tests. Multivariate analysis was performed with Cox proportional hazards model and the 95% confidence interval was calculated. A P<0.05 was considered significant.
Results
FXR and HRG protein expression in adenocarcinoma, peritumoral tissues, precursor, and normal pancreatic tissues
Immunohistochemical staining showed that positive FXR and HRG expression were located in the cytoplasm (Figures 1 and 2). In one hundred and six PDACs, sixty and forty-seven were FXR (56.6%) and HRG (44.3%) positive, respectively. In the thirty-five peritumoral tissues, ten and twenty-eight were FXR (28.6%) and HRG (80.0%) positive, respectively. In fifty-five precursor pancreatic lesions, ten and forty-seven were FXR (18.2%) and HRG (85.5%) positive, respectively. In all thirteen normal tissues, FXR was negative and HRG was positive. The positive rates of FXR were significantly higher in PDAC than those in peritumoral, precursor, and normal pancreatic tissues (P<0.05 or P<0.01; Table 1). The positive rate of HRG was significantly lower in PDAC than in peritumoral, precursor, and normal pancreatic tissues (P<0.05 or P<0.01; Table 1). Peritumoral tissues and precursor pancreatic lesions with positive FXR and/or negative HRG expression exhibited moderate to severe dysplasia and grade II or III intraepithelial neoplasia. Among the fifty-five precursor lesions, the positive rate of FXR in chronic pancreatitis, adenomas, and intraepithelial neoplasia were 10.0% (2/20) and 20.0% (4/20), 26.7% (4/15); the positive rate of HRG in chronic pancreatitis, adenomas, and intraepithelial neoplasia were 90.0% (18/20) and 85.0% (17/20), 80.0% (12/15), respectively. No significant differences in the positive rate of FXR and HRG were observed between three types of precursor lesions (P>0.05).
Figure 1.
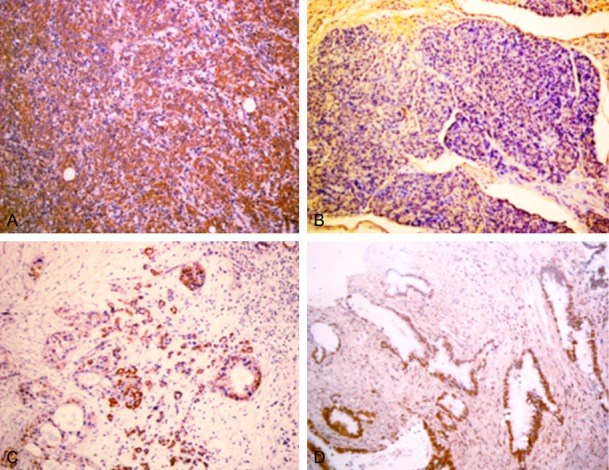
A. Positive expression of FXR, poorly differentiated PDAC, ×200. B. Negative expression of FXR, moderately differentiated PDAC, ×200. C. Positive expression of FXR, chronic pancreatitis, ×200. D. Positive expression of FXR, adenoma, ×200.
Figure 2.
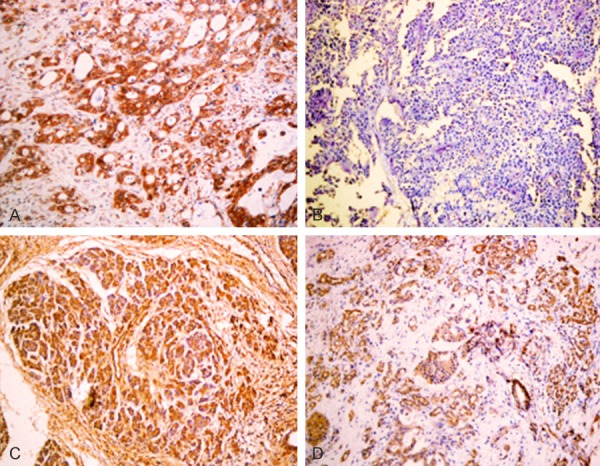
A. Positive expression of HRG, well differentiated PDAC, ×200. B. Negative expression of HRG, poorly differentiated PDAC, ×200. C. Positive expression of HRG, intraepithelial neoplasia II, ×200. D. Positive expression of HRG, pericancerous tissue, ×200.
Table 1.
Comparison of FXR and HRG expression in normal, benign, and malignant pancreatic tissues
| Tissue type | Case No. | FXR positive (%) | HRG positive (%) |
|---|---|---|---|
| PDAC | 106 | 60 (56.6) | 47 (44.3) |
| Peritumoral tissues | 35 | 10 (28.6)* | 28 (80.0)* |
| Benign tissues | 55 | 10 (18.2)** | 47 (85.5)** |
| Normal pancreatic tissues | 13 | 0 (0.0)** | 13 (100.0)** |
Compared to PDAC:
P<0.05;
P<0.01.
FXR and HRG protein expression was associated with clinicopathological characteristics of PDAC
As shown in Table 2, the positive rates of FXR and negative rates of HRG expression were significantly lower in cases with no metastasis in lymph node, no invasion to surrounding tissues and organs, and TNM I+II stage disease compared to cases with lymph node metastasis, invasion, and TNM III or IV stage disease (P<0.05 or P<0.01). The expressions of FXR and HRG exhibited no significant association with sex, age, differentiation degree,or tumor diameter (P>0.05). Among the sixty cases with positive FXR expression, twenty-one cases had positive HRG expression. Among the forty-six cases with negative FXR expression, nineteen cases had negative HRG expression. The expression of FXR was negatively correlated with HRG (χ2=4.887, P<0.05).
Table 2.
Correlations of FXR and HRG protein expression with the clinicopathologic characteristics of pancreatic ductal adenocarcinoma
| CPC | Case No. | FXR | HRG | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Pos No. (%) | P value | Pos No. (%) | P value | ||||
| Age (year) | |||||||
| ≤45 years | 22 | 16 (72.7) | 2.938 | 0.087 | 8 (36.4) | 0.716 | 0.398 |
| >45 years | 84 | 44 (52.4) | 39 (46.4) | ||||
| Sex | |||||||
| Male | 61 | 35 (54.7) | 0.035 | 0.852 | 31 (50.8) | 2.445 | 0.118 |
| Female | 45 | 25 (55.6) | 16 (35.6) | ||||
| Differentiation | |||||||
| Well | 38 | 17 (44.7) | 4.486 | 0.106 | 18 (47.4) | 0.221 | 0.895 |
| Moderately | 35 | 20 (57.1) | 15 (42.9) | ||||
| Poorly | 33 | 23 (69.7) | 14 (42.4) | ||||
| Tumor size | |||||||
| ≤3 cm | 13 | 6 (46.2) | 5.136 | 0.077 | 9 (69.2) | 4.807 | 0.090 |
| 3-5 cm | 68 | 35 (51.5) | 30 (44.1) | ||||
| >5 cm | 25 | 19 (76.0) | 8 (32.0) | ||||
| Lymphnode metastasis | |||||||
| No | 77 | 33 (42.9) | 21.652 | 0.000 | 41 (53.2) | 9.048 | 0.003 |
| Yes | 29 | 27 (93.1) | 6 (20.7) | ||||
| Invasion | |||||||
| No | 42 | 12 (28.6) | 22.253 | 0.000 | 28 (66.7) | 14.051 | 0.000 |
| Yes | 64 | 48 (75.0) | 19 (29.7) | ||||
| TNM stage | |||||||
| I+II | 53 | 20 (27.3) | 15.362 | 0.000 | 33 (81.8) | 13.799 | 0.001 |
| III+IV | 53 | 40 (40.5) | 14 (57.1) | ||||
FXR and HRG protein expressions correlated with overall survival in patients with PDAC
Survival information of all patients was collected. Twenty-nine patients survived over one year, but seventy-seven patients died within one year with a mean overall survival time of 9.44±0.69 months. Kaplan-Meier survival analysis revealed that the differentiation, tumor size, lymph node metastasis, invasion, and TNM stage were significantly associated with the average overall survival time of patients with PDAC (P<0.05 or P<0.01) (Table 3). Average overall survival time for FXR positive patients or HRG negative ones was significantly lower than in those with negative FXR or positive HRG expression (P=0.000) (Figure 3). Cox multivariate analysis showed that tumor mass >5 cm, poor differentiation, lymph node metastasis, invasion, and high TNM stage (III or IV) were negatively correlated with overall survival and positively correlated with mortality. Positive FXR and negative HRG expression negatively correlated with overall survival and positively correlated with mortality. Both the positive FXR and negative HRG expressions were independent prognostic factors (Table 4). Finally, we calculated the AUC for FXR (AUC=0.709, 95% CI: 0.632-0.787), or HRG (AUC=0.719, 95% CI: 0.643-0.796) in PDAC compared to benign lesions, respectively (Figure 4).
Table 3.
Correlations of clinicopathologic characteristics, FXR, and HRG expression with the mean survival in patients with PDAC
| Group | Case No. (n) | Mean survival (month) | Chi-square | P value |
|---|---|---|---|---|
| Sex | ||||
| Male | 61 | 9.98 (2-24) | 1.656 | 0.198 |
| Female | 45 | 8.61 (2-21) | ||
| Age (year) | ||||
| ≤45 | 22 | 8.18 (3-19) | 2.144 | 0.143 |
| >45 | 84 | 9.73 (2-24) | ||
| Differentiation | ||||
| Well | 38 | 11.27 (3-24) | ||
| Moderately | 35 | 9.74 (3-21) | 17.786 | 0.000 |
| Poorly | 33 | 6.86 (2-14) | ||
| Tumor size | ||||
| <3 cm | 13 | 13.46 (5-21) | 7.504 | 0.023 |
| 3~5 cm | 68 | 9.34 (2-22) | ||
| >5 cm | 25 | 7.40 (3-24) | ||
| TNM stage | ||||
| I | 11 | 16.46 (11-24) | ||
| II | 42 | 11.37 (3-22) | 80.807 | 0.000 |
| III | 37 | 7.14 (2-17) | ||
| IV | 16 | 4.56 (2-8) | ||
| Lymph node metastasis | ||||
| No | 77 | 10.64 (2-24) | 27.120 | 0.000 |
| Yes | 29 | 6.35 (2-12) | ||
| Invasion | ||||
| No | 42 | 13.33 (5-24) | 46.949 | 0.000 |
| Yes | 54 | 6.83 (2-17) | ||
| FXR | ||||
| - | 46 | 12.74 (6-24) | 38.310 | 0.000 |
| + | 60 | 6.83 (2-18) | ||
| HRG | ||||
| - | 59 | 7.23 (2-19) | 24.956 | 0.000 |
| + | 47 | 12.07 (3-24) |
Figure 3.
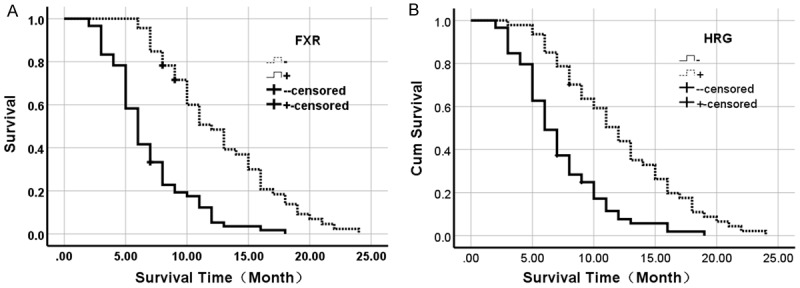
Kaplan-Meier plots of overall survival in patients with PDAC and with positive and negative FXR and HRG expression. A. Kaplan-Meier plots of overall survival in patients with PDAC and with positive and negative FXR expression. B. Kaplan-Meier plots of overall survival in patients with PDAC and with positive and negative HRG expression.
Table 4.
Multivariate Cox regression analysis of survival rate in patients with pancreatic ductal adenocarcinoma and FXR and HRG expression
| Groups | Factors | B | SE | wald | P | RR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower | Upper | |||||||
| Differentiated degree | Well/moderately/poorly | .456 | .153 | 8.883 | .003 | 1.578 | 1.169 | 2.129 |
| Tumor size | <3 cm/3-5 cm/>5 cm | .240 | .205 | 1.371 | .242 | 1.271 | .851 | 1.900 |
| Lymph node metastasis | No/yes | .818 | .301 | 7.385 | .007 | 2.266 | 1.256 | 4.088 |
| Invasion | No/yes | .929 | .348 | 7.126 | .008 | 2.532 | 1.280 | 5.008 |
| TNM stage | I/II/III/IV | .728 | .243 | 8.975 | .003 | 2.071 | 1.286 | 3.334 |
| FXR | -/+ | .840 | .258 | 10.600 | .001 | 2.316 | 1.397 | 3.841 |
| HRG | -/+ | -.749 | .242 | 9.579 | .002 | .473 | .294 | .760 |
Figure 4.
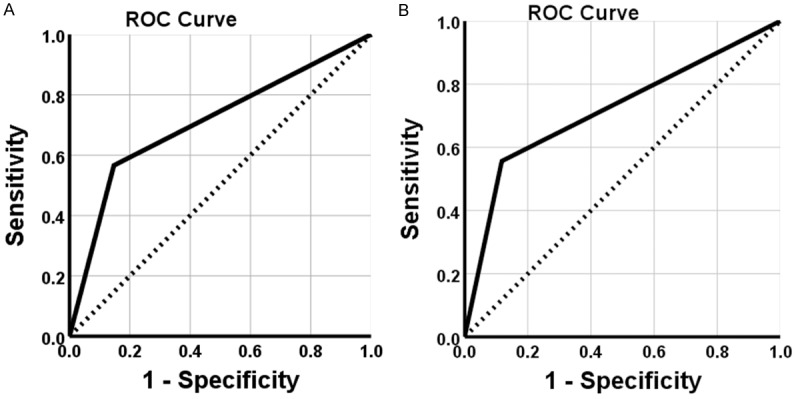
Receiver operating characteristic curve analysis. A. ROC shows the ability of FXR in diagnosis of PDAC. B. ROC shows the ability of HRG in PDAC diagnosis.
Discussion
The expression of FXR and HRG in PDAC has not been previously reported, although their expression has been associated with the progression and prognosis of a variety of tumors. This study investigated FXR and HRG protein expression in PDAC tumors, peritumoral tissues, benign pancreatic lesions, and normal pancreatic tissues using immunohistochemistry. A significant increase in FXR and decrease in HRG expression in PDAC tumors were observed. The positive FXR and negative HRG expressions were associated with TNM stage, invasion, metastasis, and poor prognosis of PDAC.
Most of the studies identified bile acids as risk factors in human cancers, especially for the gastrointestinal tract cancers [40], despite some of the existing data arguing that bile acids have anti-tumoral factors [41]. FXR is a nuclear receptor responsible for bile acid homeostasis by regulating the expression of genes involved in the process [8-11]. However, various reports have shown that FXR is elevated in multiple human cancers responsible for their initiation and progression [13-22]. For instance, it has been proven that FXR can suppress the proliferation of liver cancer by inhibiting mTOR/S6K signaling [42]. On the other hand, some of the reports have proposed the opposite; i.e. they have shown that FXR promotes the carcinogenesis and progression of prostate cancer [23,24] and esophageal cancer growth [43]. The role of FXR in gallbladder cancer remains largely unknown, which is why the identification of its functional significance may shed new light into its pathogenic mechanism.
HRG was initially identified from proteomic studies on identification of breast cancer biomarkers [44]. HRG, which is a 2-glycoprotein synthesized by liver and present in plasma and platelets, is potentially involved in numerous biologic processes. It can inhibit rosette formation and interact with heparin, thrombospondin and plasminogen. There have been conflicting reports on both enhancing and inhibitory effects of HRG in angiogenesis [45].
Proteomics approaches and other experimental methods have been employed to determine that HRG could be useful as a prognostic tool in some types of cancers [32-39]. Previous studies have confirmed the importance of HRG in hemostasis, angiogenesis, and immunity, which in turn can greatly affect tumor control and metastasis. In vivo, HRG can significantly affect tumor growth and metastasis through modulation of TAMs towards an M1 phenotype, activation of platelets, and normalization of angiogenesis. Some studies have tested whether HRG might be used as an independent diagnostic and improved prognostic indicator in patients [32-39].
In the present study, the percentage of cases with positive FXR and negative HRG expression was significantly higher in PDAC patients than that in pericancerous tissues, benign lesions and normal tissues. In pancreatic tissues with benign lesions, tissues with positive FXR and/or negative HRG protein expression exhibited dysplasia or intraepithelial neoplasia. The percentage of cases with positive FXR and negative HRG expression was significantly higher in PDAC patients with poor-differentiation, lymph node metastasis, invasion, and TNM stage III+IV disease than in patients with well-differentiation, no lymph node metastasis and invasion, and TNM stage I+II disease. The expression of FXR was negatively correlated with HRG in PDAC. The univariate Kaplan-Meier analysis showed that positive FXR and negative HRG, poor-differentiation, large tumor size, high TNM stage, lymph node metastasis, invasion and surgical curability, were closely associated with a decreased overall survival in PDAC patients. The multivariate Cox regression analysis identified that positive FXR and negative HRG expression were independent factors for poor-prognosis with PDAC. Yet, the AUC for FXR was (AUC=0.709, 95% CI: 0.632-0.787), and for HRG was (AUC=0.719, 95% CI: 0.643-0.796) in PDAC compared to benign lesions, respectively. These findings strongly suggested that FXR and HRG might have important roles in the carcinogenesis, progression, biological behaviors and prognosis of PDAC.
In conclusion, the elevated expression of FXR and lowered expression of HRG in PDAC samples indicated that they were significant markers for progression, clinical biologic behavior, and prognosis. The involvement of FXR and HRG in chemoresistance indicated that these two markers have a strong potential to be developed as targets for gene therapy, which may sensitize chemotherapy and radiotherapy. In addition, patients with positive FXR and negative HRG expression in their tumors were more likely to suffer from invasion and metastatic recurrence. These patients may require close monitoring for clinical signs of relapse, so that therapeutic inventions can be applied early enough for optimal outcomes.
Disclosure of conflict of interest
None.
Abbreviations
- PDAC
Pancreatic ductal adenocarcinoma
- FXR
Farnesoid X-receptor
- HRG
Histidine-rich glycoprotein
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neoptolemos JP. Adjuvant treatment of pancreatic cancer. Eur J Cancer. 2011;47:S378–380. doi: 10.1016/S0959-8049(11)70210-6. [DOI] [PubMed] [Google Scholar]
- 5.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 6.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 7.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo G, Renga B, Mencarelli A, Pellicciari R, Fiorucci S. Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:289–303. doi: 10.2174/1568008054863781. [DOI] [PubMed] [Google Scholar]
- 9.Mishra GR, Suresh M, Kumaran K, Kannabiran N, Suresh S, Bala P, Shivakumar K, Anuradha N, Reddy R, Raghavan TM, Menon S, Hanumanthu G, Gupta M, Upendran S, Gupta S, Mahesh M, Jacob B, Mathew P, Chatterjee P, Arun KS, Sharma S, Chandrika KN, Deshpande N, Palvankar K, Raghavnath R, Krishnakanth R, Karathia H, Rekha B, Nayak R, Vishnupriya G, Kumar HG, Nagini M, Kumar GS, Jose R, Deepthi P, Mohan SS, Gandhi TK, Harsha HC, Deshpande KS, Sarker M, Prasad TS, Pandey A. Human protein reference database--2006 update. Nucleic Acids Res. 2006;34:D411–D414. doi: 10.1093/nar/gkj141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0--the human metabolome database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alasmael N, Mohan R, Meira LB, Swales KE, Plant NJ. Activation of the Farnesoid X-receptor in breast cancer cell lines results in cytotoxicity but not increased migration potential. Cancer Lett. 2016;370:250–259. doi: 10.1016/j.canlet.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor fxr protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 13.Giaginis C, Karandrea D, Alexandrou P, Giannopoulou I, Tsourouflis G, Troungos C, Danas E, Keramopoulos A, Patsouris E, Nakopoulou L, Theocharis S. High farnesoid X receptor (FXR) expression is a strong and independent prognosticator in invasivebreast carcinoma. Neoplasma. 2017;64:633–639. doi: 10.4149/neo_2017_420. [DOI] [PubMed] [Google Scholar]
- 14.You W, Chen B, Liu X, Xue S, Qin H, Jiang H. Farnesoid X receptor, a novel proto-oncogene in non-small cell lung cancer, promotes tumor growth via directly transactivating CCND1. Sci Rep. 2017;7:591. doi: 10.1038/s41598-017-00698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan JH, Fang L. MicroRNA-92 promotes gastric cancer cell proliferation and invasion through targeting FXR. Tumour Biol. 2014;35:11013–11019. doi: 10.1007/s13277-014-2342-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Ni Z, Li T, Su L, Zhang L, Liu N, Shi Y. Activation of FXR promotes intestinal metaplasia of gastric cells via SHP-dependent upregulation of the expression of CDX2. Oncol Lett. 2018;15:7617–7624. doi: 10.3892/ol.2018.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5:135–44. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Wu LL, Han T, Zhuo M, Lei W, Cui JJ, Jiao F, Wang LW. Correlated high expression of FXR and Sp1 in cancer cells confers a poor prognosis for pancreatic cancer: a study based on TCGA and tissue microarray. Oncotarget. 2017;8:33265–33275. doi: 10.18632/oncotarget.16633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J, Zhao K, Zheng L, Xu Z, Gong W, Chen S, Shen X, Huang G, Gao M, Zeng Y, Zhang Y, He F. Upregulation of microRNA-122 by farnesoid X receptor suppresses the growth of hepatocellular carcinoma cells. Mol Cancer. 2015;14:163. doi: 10.1186/s12943-015-0427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo F, Xu Z, Zhang Y, Jiang P, Huang G, Chen S, Lyu X, Zheng P, Zhao X, Zeng Y, Wang S, He F. FXR induces SOCS3 and suppresses hepatocellular carcinoma. Oncotarget. 2015;6:34606–34616. doi: 10.18632/oncotarget.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Zhang X, Ji L, Gu J, Zhou M, Chen S. Farnesoid X receptor associates with β-catenin and inhibits its activity in hepatocellular carcinoma. Oncotarget. 2015;6:4226–4238. doi: 10.18632/oncotarget.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Tong SJ, Wang X, Qu LX. Farnesoid X receptor inhibits LNcaP cell proliferation via the upregulation of PTEN. Exp Ther Med. 2014;8:1209–1212. doi: 10.3892/etm.2014.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu N, Zhao J, Wang J, Teng H, Fu Y, Yuan H. Farnesoid X receptor ligand CDCA suppresses human prostate cancer cells growth by inhibiting lipid metabolism via targeting sterol response element binding protein 1. Am J Transl Res. 2016;8:5118–5124. [PMC free article] [PubMed] [Google Scholar]
- 25.Blank M, Shoenfeld Y. Histidine-rich glycoprotein modulation of immune/autoimmune, vascular, and coagulation systems. Clin Rev Allergy Immunol. 2008;34:307–312. doi: 10.1007/s12016-007-8058-6. [DOI] [PubMed] [Google Scholar]
- 26.Poon IK, Patel KK, Davis DS, Parish CR, Hulett MD. Histidine-rich glycoprotein: the Swiss army knife of mammalian plasma. Blood. 2011;117:2093–2101. doi: 10.1182/blood-2010-09-303842. [DOI] [PubMed] [Google Scholar]
- 27.Blank M, Shoenfeld Y. Histidine-rich glycoprotein modulation of immune/autoimmune, vascular, and coagulation systems. Clin Rev Allergy Immunol. 2008;34:307–312. doi: 10.1007/s12016-007-8058-6. [DOI] [PubMed] [Google Scholar]
- 28.Klenotic PA, Huang P, Palomo J, Kaur B, Van Meir EG, Vogelbaum MA, Febbraio M, Gladson CL, Silverstein RL. Histidine-rich glycoprotein modulates the anti-angiogenic effects of vasculostatin. Am J Pathol. 2010;176:2039–2050. doi: 10.2353/ajpath.2010.090782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolny C, Mazzone M, Tugues S, Laoui D, ohansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Åkerud P, De Mol M, Salomäki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Poon IK, Hulett MD, Parish CR. Histidine-rich glycoprotein is a novel plasma pattern recognition molecule that recruits IgG to facilitate necrotic cell clearance via FcgammaRI on phagocytes. Blood. 2010;115:2473–2482. doi: 10.1182/blood-2009-07-234013. [DOI] [PubMed] [Google Scholar]
- 31.Karrlander M, Lindberg N, Olofsson T, Kastemar M, Olsson AK, Uhrbom L. Histidine-rich glycoprotein can prevent development of mouse experimental glioblastoma. PLoS One. 2009;4:e8536. doi: 10.1371/journal.pone.0008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Autenshlyus AI, Brusentsov II, Marinkin IO, Smirnova SA, Rukavishnikov MY, Lyakhovich VV. Messenger RNA of the histidine-rich glycoprotein in breast tumors. Dokl Biochem Biophys. 2018;478:37–40. doi: 10.1134/S1607672918010106. [DOI] [PubMed] [Google Scholar]
- 33.Kristof J, Sakrison K, Jin X, Nakamaru K, Schneider M, Beckman RA, Freeman D, Spittle C, Feng W. Real-time reverse-transcription quantitative polymerase chain reaction assay is a feasible method for the relative quantification of heregulin expression in non-small cell lung cancertissue. Biomark Insights. 2017;12:1177271917699850. doi: 10.1177/1177271917699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguchi H, Sakamoto C, Wada K, Akamatsu T, Uchida T, Tatsuguchi A, Matsui H, Fukui H, Fujimori T, Kasuga M. Expression of heregulin alpha, erbB2, and erbB3 and their influences on proliferation of gastricepithelial cells. Gastroenterology. 1999;117:1119–1127. doi: 10.1016/s0016-5085(99)70397-5. [DOI] [PubMed] [Google Scholar]
- 35.Bogoevska V, Wolters-Eisfeld G, Hofmann BT, El Gammal AT, Mercanoglu B, Gebauer F, Vashist YK, Bogoevski D, Perez D, Gagliani N, Izbicki JR, Bockhorn M, Güngör C. HRG/HER2/HER3 signaling promotes AhR-mediated Memo-1 expression and migration in colorectal cancer. Oncogene. 2017;36:2394–2404. doi: 10.1038/onc.2016.390. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Jiang K, Li Y, Gao D, Sun L, Zhang S, Liu T, Guo K, Liu Y. Histidine-rich glycoprotein function in hepatocellular carcinoma depends on its N-glycosylation status, and it regulates cell proliferation by inhibiting Erk1/2 phosphorylation. Oncotarget. 2015;6:30222–30231. doi: 10.18632/oncotarget.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolb A, Kleeff J, Arnold N, Giese NA, Giese T, Korc M, Friess H. Expression and differential signaling of heregulins in pancreatic cancer cells. Int J Cancer. 2007;120:514–523. doi: 10.1002/ijc.22360. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama H, Soeda S, Watanabe T, Fujimori K. Association between growth factor heregulin1α and receptors in growth of ovarian cancer cell line with high potentiality of peritoneal dissemination. Fukushima J Med Sci. 2012;58:22–32. [PubMed] [Google Scholar]
- 39.Grimsley SJ, Shini S, Underwood MA, Edwards J. Heregulin expression and prognosis in prostate adenocarcinoma. Urol Int. 2011;87:363–368. doi: 10.1159/000328627. [DOI] [PubMed] [Google Scholar]
- 40.Tsuei J, Chau T, Mills D, Wan YJ. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med. 2014;239:1489–1504. doi: 10.1177/1535370214538743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo C, Su J, Li Z, Xiao R, Wen J, Li Y, Zhang M, Zhang X, Yu D, Huang W, Chen WD, Wang YD. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) suppresses gastric cancer cell proliferation and migration through antagonizing STAT3 signaling pathway. Oncotarget. 2015;6:34402–34413. doi: 10.18632/oncotarget.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X, Zeng Y, Wang X, Ma X, Li Q, Li N, Su H, Huang W. FXR blocks the growth of liver cancer cells through inhibiting mTOR-s6K pathway. Biochem Biophys Res Commun. 2016;474:351–356. doi: 10.1016/j.bbrc.2016.04.106. [DOI] [PubMed] [Google Scholar]
- 43.Guan B, Li H, Yang Z, Hoque A, Xu X. Inhibition of farnesoid X receptor controls esophageal cancer cell growth in vitro and in nude mouse xenografts. Cancer. 2013;119:1321–1329. doi: 10.1002/cncr.27910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaub NP, Jones KJ, Nyalwidhe JO, Cazares LH, Karbassi ID, Semmes OJ. Serum proteomic biomarker discovery reflective of stage and obesity in breast cancer patients. J Am Coll Surg. 2009;208:970–978. doi: 10.1016/j.jamcollsurg.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Jain S, Zuka M, Liu J, Russell S, Dent J, Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann B, Ware J. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A. 2010;104:9024–9028. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]


