Abstract
Objective: To investigate the expression and clinical significance of Shh, Gli1, FAK, p-FAK and p-AKT in HCC. Methods: Immunohistochemistry was used to measure Shh, Gli1, FAK, p-FAK, and p-AKT expressions in 50 cases of HCC and paracancerous tissues. The Shh, Gli1, and FAK mRNA levels were determined by qRT-PCR in 20 HCCs. The correlations between the expressions of these target genes and the clinicopathological factors were analyzed in HCC. Results: The immunohistochemical results showed that the expressions of Shh, Gli1, FAK, p-FAK, and p-AKT in 50 HCC tissues were significantly higher than those of the paracancerous tissues (P < 0.05). Shh and p-FAK expressions were associated with portal vein invasion, capsular integrity, and distant metastasis (P < 0.05). Gli1, FAK, and p-AKT expressions were closely related to tumor diameter, tumor differentiation, portal vein invasion, capsular integrity, TNM stage and distant metastasis (P < 0.05). Shh was related to Gli1 and p-FAK (r = 0.67, 0.30; P = 0.00, 0.03), Gli1 was positively related to p-FAK and p-AKT (r = 0.52, 0.49; P = 0.00, 0.00), and there was a positive correlation between p-FAK and p-AKT (r = 0.36, P = 0.00). Furthermore, the Shh, Gli1, and FAK mRNA levels in the HCC tissues were significantly higher than those in the paracancerous tissues (P < 0.0001), and the high TNM stages (III and IV) or distant metastasis were significantly higher than those in the low TNM stages (I and II) (P < 0.05) or without distant metastasis (P < 0.05). Conclusion: In HCC, the Hh and PI3K-AKT signaling pathways are both abnormally activated, and Shh, Gli1, FAK, p-FAK and p-AKT can serve as indicators to predict the prognosis of liver cancer.
Keywords: Hepatocellular carcinoma, Shh, Gli1, FAK, p-FAK, p-AKT
Introduction
Primary liver cancer is one of the most common malignant tumors in the world, and it is responsible for the fourth highest death rate among all tumors [1]. Hepatocellular carcinoma (HCC) is the most frequent type of the three histologic types of primary liver cancer, accounting for approximately 90% of primary liver cancer cases [2]. In recent years, with the continuous exploration of new molecular mechanisms in HCC, it was found that the occurrence of HCC is closely related to multiple signaling pathways, including the hedgehog (Hh) and phosphoinositide-3-kinase-protein kinase B (PI3K-AKT) signaling pathways [3,4]. The Hh signaling pathway is a crucial pathway that controls the development and regulation in embryos with a high degree of conservatism. The aberrant activation of the Hh pathway leads to persistent injury, repair and regeneration in hepatocytes, which may eventually lead to the occurrence of liver cancer [5]. The PI3K-AKT signaling pathway is another pivotal pathway in the study of survival signals, and its activation plays a major role in the growth, invasion, and metastasis of various tumor cells, including hepatoma cells [6]. There are several international studies describing how the Hh and PI3K-AKT signaling pathways are activated in liver cancer, but the nature of the crosstalk between them remains elusive.
In the present study, we measured the expressions of the key molecules Shh and Gli1 in the Hh signaling pathway and the key molecule p-AKT and its related molecules FAK, p-FAK and AKT in the PI3K-AKT signaling pathway. We investigated the correlation between the two pathways in the HCC tissues and provide experimental data that may be utilized for the molecular therapy of liver cancer.
Materials and methods
Patients and samples
A total of 50 HCC patients who had undergone hepatectomy without preoperative treatment in the Department of General Surgery of the First Affiliated Hospital of Gannan Medical University (Ganzhou, China) from July 2016 to July 2017 were examined. The age of the patients, including 42 males and 8 females, ranged from 22 to 62 years, with an average age of 51 years. Liver cancer tissues and paired adjacent non-cancerous liver tissues not less than 2 cm away from the HCC were collected. All tumor specimens were pathologically diagnosed as HCC by two pathologists and graded using the WHO grading system. The stages of liver cancer were divided into stages I, II, III and IV in accordance with the American Joint Committee on Cancer (AJCC) standards. According to the results of ultrasound or computed tomography (CT) scanning, intraoperative exploration, the occurrence of vascular invasion and tumor satellite foci, the patients were divided into a distant metastatic HCC group and a non-metastatic HCC group. All 50 tumor specimens were fixed in 10% formalin immediately after being excised and then were paraffin-embedded for immunohistochemical staining. Among these 50 HCC patients, 20 tissue samples from them were rapidly cryopreserved with liquid nitrogen for the extraction of tissue RNA. This study was approved by the Ethics Committee of the First Affiliated Hospital of Gannan Medical University, and all patients agreed to sign an informed consent.
Tissue microarray construction
Representative tumor regions and their matched adjacent non-cancerous liver tissues were carefully selected using a microscope, and then the columnar microtissue specimens (0.8 × 0.8 × 4.0 mm) were obtained using a special sampler. Microtissue specimens were encapsulated with a semi-solid support and then fixed in a suitable mold at a certain distance (1 mm). Subsequently, they were embedded in paraffin and serially sectioned at 5 μm thickness and finally attached to a glass slide to obtain a tissue microarray block. Each sample was arrayed in duplicate.
Immunohistochemical staining and scoring
The tissue microarray blocks were incubated at 60°C for 1-2 h before they were deparaffinized and rehydrated. Antigen retrieval was performed in a citrate buffer (pH 6.0) in a pressure cooker. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide (H2O2) for 15 min at room temperature. The slides were then incubated with primary antibodies overnight at 4°C as follows: anti-Shh (Solarbio, Beijing, 1:150 dilution), anti-Gli1 (Solarbio, Beijing, 1:150 dilution), anti-FAK (Abcam, USA, 1:200 dilution), anti-p-FAK (Abcam, USA, 1:25 dilution) and anti-p-AKT (Abcam, USA, 1:150 dilution) antibodies. The negative control group was treated with phosphate-buffered saline (PBS) instead of a primary antibody. After incubation at 37°C for 30 min with secondary biotinylated rabbit antibody horseradish peroxidase (HRP, Sigma, USA, 1:1000 dilution), the slides were developed with the 3,3-diaminobenzidine solution and then counterstained with hematoxylin.
The immunohistochemical staining intensities of the Shh, Gli1, FAK, p-FAK, and p-AKT expressions were categorized as negative (-) or positive (+), which were determined by two independent observers without any knowledge of the patients’ clinical characteristics.
Quantitative Real-Time PCR (qRT-PCR)
The mRNA expression levels in HCC tissues and paired adjacent normal liver tissues were determined by qRT-PCR. According to the manufacturer’s instructions, total RNA was extracted with TRIzol Reagent (Invitrogen, USA) and then cDNA was synthesized using the FastQuant RT Kit (with gDNase; TIANGEN, Beijing). qRT-PCR was performed on an ABI 7500 Real-time PCR system (Thermo, USA) using SuperReal Premix Plus (with SYBR Green; TIANGEN, Beijing). The optimal amplification conditions were as follows: 95°C denaturation for 15 min, and then 40 cycles of 95°C for 10 s and 60°C for 30 s. The expressions of the target genes were normalized to β-actin expression levels in each sample. The following primers used in the study were present: Shh: forward 5’-CGGGCAGATAGGAAGGTGAT-3’, Shh: reverse 5’-GGGCCATAATGAACCACGTC-3’, Gli1: forward 5’-CCTGGATCGGATAGGTGGTC-3’, Gli1: reverse 5’-GGCCCTTGAACCTCTGGACT-3’, FAK: forward 5’-CTACAGCCTTATGACGAAATGC-3’, FAK: reverse 5’-CTTCTCTTCCTCCAGGATTGTG-3’, β-actin: forward 5’-GTGGACATCCGCAAAGAC-3’, β-actin: reverse 5’-GAAAGGGTGTAACGCAACT-3’.
Statistical analysis
SPSS v.18.0 software was used for the statistical analysis. The measurement data were expressed as the mean ± standard deviation (x ± s). A comparison between the data was performed using a chi-square (χ2) test. The correlation between each protein was obtained using the Spearman correlation analysis. P < 0.05 was considered to indicate a significant difference between values.
Results
Immunohistochemical analysis of Shh, Gli1, FAK, p-FAK and p-AKT protein expression in HCC and nonmalignant tissues and correlations between different proteins
Immunohistochemical staining showed that Shh was mainly localized in the cytoplasms and/or plasma membranes of hepatoma cells (Figure 1A, 1B). Positive staining of Shh was detected in 31 cases (31/50) of HCC tissues, and Shh was observed in 17 cases (17/50) of adjacent normal tissues. Gli1 was mainly located in the cytoplasms and/or nuclei of the liver cancer cells (Figure 1C, 1D), and Gli1 positive expression was found in 29 cases (29/50) of liver cancer tissues, but 18 cases (18/50) were in the adjacent normal tissues. The positive expression of FAK was primarily localized in the cytoplasms (Figure 1E, 1F), with 26 cases (26/50) and 15 cases (15/50) in which the FAK were positively stained in both the HCC and paired adjacent normal tissues.
Figure 1.
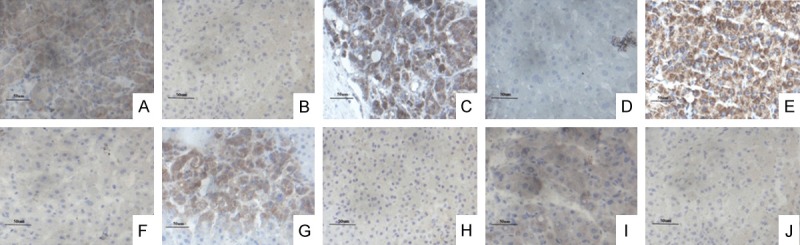
The distribution of Shh, Gli1, FAK, p-FAK, and p-AKT by immunohistochemistry (IHC) (400 ×). A, C, E, G and I represent the distribution of Shh, Gli1, FAK, p-FAK, and p-AKT in the HCC tissues, respectively; B, D, F, H and J represent the distribution of Shh, Gli1, FAK, p-FAK, and p-AKT in the paracancerous tissues, respectively.
The results of the immunohistochemistry also showed that p-FAK was prevailingly localized in the cytoplasms and cell membranes (Figure 1G, 1H), and positive staining of p-FAK in the HCC and paraneoplastic tissues was found in 20 cases (20/50) and 7 cases (7/50) respectively. A positive expression of p-AKT was detected in the cytoplasms and plasma membranes (Figure 1I, 1J), and there were 24 (24/50) and 2 (2/50) cases in the HCC and adjacent normal tissues respectively. Taken together, the statistical analysis revealed that positive expressions of Shh, Gli1, FAK, p-FAK, and p-AKT in the HCC tissues were significantly higher than they were in the paracancerous tissues (P < 0.05, Table 1).
Table 1.
Positive protein expressions of Shh, Gli1, FAK, p-FAK, and p-AKT in HCC and adjacent normal tissues
| Group | Case | Shh* | Gli1* | FAK* | p-FAK* | p-AKT* |
|---|---|---|---|---|---|---|
| HCC | 50 | 31 (31/50) | 29 (29/50) | 26 (26/50) | 20 (20/50) | 24 (24/50) |
| Paracancer | 50 | 17 (17/50) | 18 (18/50) | 15 (15/50) | 7 (7/50) | 2 (2/50) |
Note: The HCC group is compared with the paired adjacent normal group;
P < 0.05.
Shh, sonic hedgehog; Gli1, hedgehog transcription factor glioma-associated oncogene 1; FAK, focal adhesion kinase; p-FAK, phosphorylated focal adhesion kinase; p-AKT, phosphorylated protein kinase B; HCC, hepatocellular carcinoma.
A Spearman correlation analysis was performed on the protein expression of Shh, Gli1, FAK, p-FAK, and p-AKT in the 50 cases of HCC tissues. The results indicated that Shh protein expression was positively correlated with Gli1 (r = 0.67, P = 0.00,) and p-FAK (r = 0.30, P = 0.03) protein expression, but there was no marked correlation between FAK (r = -0.09, P = 0.52) and p-AKT (r = -0.07, P = 0.61) protein expression. The Gli1 expression was positively correlated with p-FAK (r = 0.52, P = 0.00) and p-AKT (r = 0.49, P = 0.00) protein expression, but it was not related to FAK (r = 0.08, P = 0.61) protein expression. Additionally, there was a significant correlation between the p-FAK and p-AKT (r = 0.36, P = 0.00) protein expression.
Correlations of Shh, Gli1, FAK, p-FAK and p-AKT expression with the clinicopathological characteristics of HCC
As shown in Table 2, of the 50 HCC patients, the expressions of Shh, Gli1, FAK, p-FAK, and p-AKT had no significant correlation with gender, age, HBV infection, or AFP. The expressions of Shh and p-FAK were not associated with tumor diameter or tumor differentiation (P > 0.05) but had a positive association with the invasion of the portal vein, cancer capsular integrity, and distant metastasis (P < 0.05). In addition, the Gli1, FAK, and p-AKT overexpressions correlated significantly with tumor diameter, degree of tumor differentiation, invasion of the portal vein, cancer capsular integrity, TNM stage, and distant metastasis (P < 0.05). In the HCC tissues, with an increase in tumor diameter, the degree of tumor differentiation was worse; and with the invasion of the portal vein, invasion of the cancer capsule, increase of the TNM stage (III, IV) and distant tumor metastasis, the positive rate of each protein showed an elevated trend (P < 0.05).
Table 2.
Correlations between the protein expression of Shh, Gli1, FAK, p-FAK, and p-AKT with the clinicopathological parameters of hepatocellular carcinoma
| Group | Case | Shh | Gli1 | FAK | p-FAK | p-AKT | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| + | P | + | P | + | P | + | P | + | P | ||
| Gender | |||||||||||
| Male | 43 | 25 | 0.23 | 26 | 0.64 | 25 | 0.08 | 16 | 0.56 | 22 | 0.48 |
| Female | 7 | 6 | 3 | 1 | 4 | 2 | |||||
| Age | |||||||||||
| ≤ 50 | 15 | 11 | 0.35 | 11 | 0.15 | 10 | 0.17 | 5 | 0.53 | 8 | 0.62 |
| > 50 | 35 | 20 | 18 | 16 | 15 | 16 | |||||
| Tumor diameter (cm) | |||||||||||
| ≤ 3 | 13 | 6 | 0.20 | 2 | 0.00 | 3 | 0.15 | 4 | 0.43 | 2 | 0.01 |
| > 3 | 37 | 25 | 27 | 23 | 16 | 22 | |||||
| HBV infection | |||||||||||
| - | 6 | 2 | 0.27 | 2 | 0.39 | 3 | 0.92 | 5 | 0.06 | 4 | 0.59 |
| + | 44 | 29 | 27 | 23 | 15 | 20 | |||||
| AFP (μg/L) | |||||||||||
| ≤ 400 | 15 | 12 | 0.12 | 6 | 0.09 | 7 | 0.62 | 8 | 0.21 | 10 | 0.08 |
| > 400 | 35 | 19 | 23 | 19 | 12 | 14 | |||||
| Differentiation | |||||||||||
| High | 31 | 21 | 0.29 | 14 | 0.02 | 8 | 0.00 | 11 | 0.41 | 7 | 0.00 |
| Low | 19 | 10 | 15 | 18 | 9 | 13 | |||||
| Cancer capsular integrity | |||||||||||
| Intact | 26 | 12 | 0.02 | 7 | 0.00 | 9 | 0.01 | 5 | 5 | 0.00 | |
| Violated | 24 | 19 | 22 | 17 | 15 | 0.00 | 19 | ||||
| Portal vein invasion | |||||||||||
| - | 30 | 12 | 0.00 | 14 | 0.04 | 10 | 0.00 | 8 | 0.02 | 6 | 0.00 |
| + | 20 | 19 | 15 | 16 | 12 | 18 | |||||
| TNM stage | |||||||||||
| I, II | 33 | 17 | 0.03 | 15 | 0.01 | 13 | 0.01 | 22 | 0.18 | 10 | 0.00 |
| III, IV | 17 | 14 | 14 | 13 | 8 | 14 | |||||
| Metastasis | |||||||||||
| Absence | 22 | 9 | 0.00 | 4 | 0.00 | 7 | 0.01 | 18 | 0.01 | 6 | 0.00 |
| Presence | 28 | 22 | 25 | 19 | 12 | 18 | |||||
Note: P < 0.05 was considered to indicate a significant difference between values. Shh, sonic hedgehog; Gli1, hedgehog transcription factor glioma-associated oncogene 1; FAK, focal adhesion kinase; p-FAK, phosphorylated focal adhesion kinase; p-AKT, phosphorylated protein kinase B; HBV, Hepatitis B virus; AFP, a-fetoprotein.
Shh, Gli1, and FAK mRNA expressions in HCC and adjacent normal liver tissues
To further verify whether the Shh, Gli1 and FAK expressions in the HCC tissues were higher than the expressions in the paracancerous tissues and to investigate whether the mRNA expression was correlated with the clinicopathological parameters, we used qRT-PCR to detect their mRNA levels in 20 cases of HCC and paired adjacent non-tumor tissues, and the results showed that the mRNA expression levels of Shh, Gli1, and FAK in the HCC tissues were significantly higher than those in the adjacent normal tissues (P < 0.0001, Figure 2A, 2D, 2G). Furthermore, their mRNA expressions in the HCC tissues with high TNM stages (III, IV) were significantly higher than the expressions with low TNM stages (I, II) (P < 0.05, Figure 2B, 2E, 2H), and HCC patients with distant tumor metastases had higher mRNA expression levels than those without distant metastasis (P < 0.05, Figure 2C, 2F, 2I).
Figure 2.
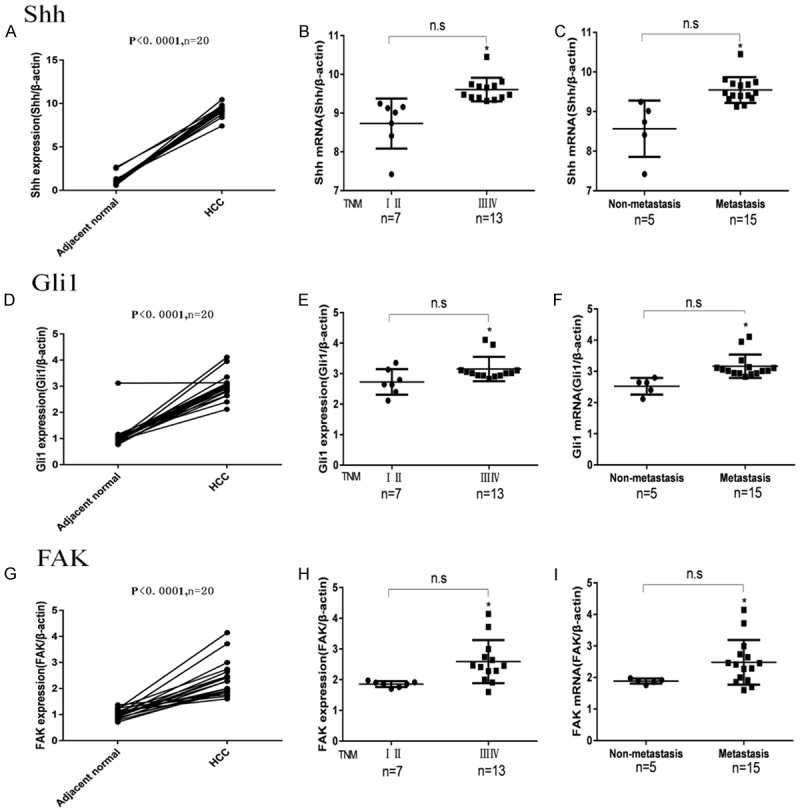
The mRNA expression levels of Shh, Gli1 and FAK in 20 HCC patients were determined by qRT-PCR. A, D and G represent the mRNA expression levels of Shh, Gli1, and FAK in HCC or in the adjacent normal liver tissues, respectively; B, E and H represent the mRNA expression levels of Shh, Gli1 and FAK in the HCC tissues with high (III, IV) or low (I, II) TNM stages, respectively; C, F and I represent the mRNA expression levels of Shh, Gli1, and FAK in HCC tissues with or without distant metastasis, respectively. The mRNA expression levels were normalized against β-actin.
Discussion
Gli1, as one of the three nuclear transcription factors, has the most significant effect on the downstream target genes of the Hh signaling pathway [7,8]. After blocking the entry of Gli1 into the nucleus, the transcription of the target genes is inhibited [9]. Previous studies have shown that the expression of Gli1 in the nucleus plays a central role in the Hh signaling pathway, so Gli1 is widely considered to be an indicator of its activation [10]. Therefore, an abnormal expression of Gli1 may reflect the activity of the whole pathway to a certain extent, which is closely related to the occurrence, invasion, and metastasis of tumors [11]. The expressions of the Hh signaling pathway’s key components in HCC have been previously reported [12-15], but their correlations with the clinicopathological factors, especially the malignant biological behavior factors, such as invasion and metastasis, remain controversial.
In the present study, the protein expression of Shh in HCC tissues was significantly higher than it was in the paracancerous tissues. Shh was associated with the invasion of the portal vein, the integrity of the tumor capsule, TNM grade and distant metastasis, but it was not related to the patients’ gender, ages, AFP values, or HBV infections. These results are similar to those of Lin Song’s et al. [16-18]. In addition, our results revealed that the protein expression of Gli1 in the HCC tissues was significantly higher than it was in the adjacent normal liver tissues. In the HCC group, the expression of Gli1 was related to tumor diameter, differentiation, portal invasion, capsular integrity, TNM stage, and distant metastasis. Similarly, there are some researchers who have demonstrated that the protein expression of Gli1 was closely related to tumor invasion, TNM stage, and distant metastasis, and the invasiveness was enhanced because of the overexpression of Gli1 [19,20]. Furthermore, we detected the mRNA levels of Shh and Gli1 by qRT-PCR, and the results showed that the mRNA expression levels of Shh and Gli1 in the HCC tissues were significantly higher than those of the adjacent normal tissues, and they were also significantly higher in TNM stages III and IV as compared to the tissues with low TNM stages (I, II). According to Qiuran Xu’s et al. [21] study, the aberrant expression of Gli1 was due to the effect by Shh, thus activating the Hh signaling pathway, which indicated that there may be some correlations between them. Therefore, we used statistical methods to analyze the association between Shh and Gli1 and found that the positive protein expression of Gli1 was positively correlated with the protein expression of Shh.
The PI3K-AKT pathway is an important signaling pathway in cells. It is abnormally expressed in a variety of human malignant tumors [22-24]. Phosphorylated AKT (p-AKT) is closely related to cell movement, proliferation and apoptosis, so it is widely regarded as the core component in the PI3K-AKT signaling pathway and the most important downstream protein of PI3K. After inhibition of the PI3K-AKT pathway in a rat model, it was found that the expression level of p-AKT was down-regulated, and the invasiveness of the tumor cells was also weakened [25]. What’s more, some researchers have found that the expression level of p-AKT is significantly related to the degree of colorectal cancer differentiation and TNM stage [26-28]. Similar to their results, our findings also implied that the expression of p-AKT in HCC was significantly higher than it was in the paracancerous tissues. The expression of p-AKT in HCC is related to tumor diameter, tumor differentiation, invasion of the portal vein, tumor capsular integrity and distant metastasis.
FAK is an upstream molecule in the PI3K-AKT pathway, which could greatly promote tumor migration and invasion after its phosphorylation [29]. We conducted an additional study on FAK and p-FAK, and the results revealed that the expression levels of FAK and p-FAK in HCC tissues were higher than those of the adjacent normal tissues and also indicated that the expressions of FAK and p-FAK were significantly correlated with TNM stage, portal vein invasion, tumor capsular integrity, and distant metastasis, which had been further verified by qRT-PCR. At present, it has been found that the abnormal expression of p-FAK amplified the extracellular survival signals and promoted tumor proliferation, migration, invasion, and metastasis [30-32]. Interestingly, our Spearman correlation analysis showed that there were positive correlations between FAK, p-FAK, and p-AKT. Therefore, we have sufficient evidence to speculate that FAK, p-FAK, and p-AKT may act as key molecules in the PI3K-AKT pathway to jointly regulate the occurrence, development, invasion, and metastasis of HCC.
The Hh signaling pathway component of Gli1 could activate the PI3K-AKT pathway [25]. In our study, the expression of Shh, Gli1, FAK, p-FAK, and p-AKT were associated with portal vein invasion, tumor capsular integrity, and distant metastasis. These indicated that the Hh and PI3K-AKT pathways are both involved in malignant biological processes such as the invasion and metastasis of HCC. In addition, Lingyun Wei’s et al. [33] study revealed that the Hh and PI3K-AKT pathways are simultaneously activated in esophageal cancer which is similar to our findings.
In conclusion, the Hh and PI3K-AKT signaling pathways are generally activated in HCC; in particular, the positive correlation between Gli1 and p-AKT indicates that there may be some connections between the two pathways, which are closely related to malignant biological behaviors such as the invasion and metastasis of liver cancer. This may be a potential molecular mechanism for the initiation and development of HCC. An investigation of the expression of the two signaling pathway components in HCC tissues and the relationship between them was also conducted. It may provide important evidence for exploring the choice among treatment strategies in HCC, and for targeting the development of new drugs that could jointly inhibit the Hh and PI3K-AKT pathways.
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangxi Province (20151BAB205044) and the Science and Technology Research Project of Jiangxi Provincial Education Department (GJJ13687).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Wang L, Geng Z, Wan Y, Meng F, Meng X. Expression of PI3K-Akt-PAK1 pathway in the process of metastasis of hepatocellular carcinoma. J Prac Hepatol. 2017;20:307–311. [Google Scholar]
- 4.Della Corte CM, Viscardi G, Papaccio F, Esposito G, Martini G, Ciardiello D, Martinelli E, Ciardiello F, Morgillo F. Implication of the hedgehog pathway in hepatocellular carcinoma. World J Gastroenterol. 2017;23:4330–4340. doi: 10.3748/wjg.v23.i24.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 6.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–96. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 7.Sabol M, Trnski D, Musani V, Ozretic P, Levanat S. Role of GLI transcription factors in pathogenesis and their potential as new therapeutic targets. Int J Mol Sci. 2018;19:58–64. doi: 10.3390/ijms19092562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabol M, Car D, Musani V, Ozretic P, Oreskovic S, Weber I, Levanat S. The Hedgehog signaling pathway in ovarian teratoma is stimulated by sonic hedgehog which induces internalization of Patched. Int J Oncol. 2012;41:1411–1418. doi: 10.3892/ijo.2012.1554. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz i Altaba A. Catching a Gli-mpse of hedgehog. Cell. 1997;90:193–196. doi: 10.1016/s0092-8674(00)80325-6. [DOI] [PubMed] [Google Scholar]
- 10.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 11.Guo L, Tang R, Zhong C, Zhang Y, Lin M, Yu H, Gong E. Expression of Hedgehog pathway in hepatocellular carcinoma cell lines and in liver cancerous tissues. J Clin Hepatol. 2009;12:5–7. [Google Scholar]
- 12.Cheng W, Hu Z, Tian D. Expression of hedgehog signaling pathway in hepatoma cell lines and its relation with snail. Acta Med Univ Sci Technol Huazhong. 2010;39:212–215. [Google Scholar]
- 13.Tsai CL, Hsu FM, Tzen KY, Liu WL, Cheng AL, Cheng JC. Sonic Hedgehog inhibition as a strategy to augment radiosensitivity of hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:1317–1324. doi: 10.1111/jgh.12931. [DOI] [PubMed] [Google Scholar]
- 14.Al-Bahrani R, Nagamori S, Leng R, Petryk A, Sergi C. Differential expression of sonic hedgehog protein in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Pathol Oncol Res. 2015;21:901–908. doi: 10.1007/s12253-015-9918-7. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q, Liu X, Zheng X, Yao Y, Wang M, Liu Q. The transcriptional activity of Gli1 is negatively regulated by AMPK through hedgehog partial agonism in hepatocellular carcinoma. Int J Mol Med. 2014;34:733–741. doi: 10.3892/ijmm.2014.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Chen Z, Zhong W. SHH protein expression in pancreatic cancer tissue and its clinical significance. Chinese Journal of General Surgery. 2016;25:1266–1270. [Google Scholar]
- 17.Ma XL, Sun HJ, Wang YS, Huang SH, Xie JW, Zhang HW. Study of Sonic hedgehog signaling pathway related molecules in gastric carcinoma. World J Gastroenterol. 2006;12:3965–3969. doi: 10.3748/wjg.v12.i25.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai H, Li H, Li J, Li X, Li Y, Shi Y, Wang D. Sonic hedgehog signaling pathway mediates development of hepatocellular carcinoma. Tumour Biol. 2016;16:5463–5466. doi: 10.1007/s13277-016-5463-6. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 20.Yanai K, Nagai S, Wada J, Yamanaka N, Nakamura M, Torata N, Noshiro H, Tsuneyoshi M, Tanaka M, Katano M. Hedgehog signaling pathway is a possible therapeutic target for gastric cancer. J Surg Oncol. 2007;95:55–62. doi: 10.1002/jso.20606. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Zheng X, Zan X, Yao Y, Yang W, Liu Q. Gli1 expression and its relationship with the expression of Shh, vimentin and ecadherin in human hepatocellular carcinoma. Chin J Cell Mol Immuno. 2012;28:536–539. [PubMed] [Google Scholar]
- 22.Chiu WC, Lee YC, Su YH, Wang YY, Tsai CH, Hou YA, Wang CH, Huang YF, Huang CJ, Chou SH, Hsieh PW, Yuan SF. The synthetic beta-nitrostyrene derivative CYT-Rx20 inhibits esophageal tumor growth and metastasis via PI3K/AKT and STAT3 pathways. PLoS One. 2016;11:1–19. doi: 10.1371/journal.pone.0166453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Fu H, Luo Y, Chen L, Cheng R, Zhang N, Guo H. cPLA2alpha mediates TGF-beta-induced epithelial-mesenchymal transition in breast cancer through PI3k/Akt signaling. Cell Death Dis. 2017;8:1–13. doi: 10.1038/cddis.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ke Z, Caiping S, Qing Z, Xiaojing W. Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med Oncol. 2015;32:368. doi: 10.1007/s12032-014-0368-y. [DOI] [PubMed] [Google Scholar]
- 25.Kim CS, Vasko VV, Kato Y, Kruhlak M, Saji M, Cheng SY, Ringel MD. AKT activation promotes metastasis in a mouse model of follicular thyroid carcinoma. Endocrinology. 2005;146:4456–4463. doi: 10.1210/en.2005-0172. [DOI] [PubMed] [Google Scholar]
- 26.Colakoglu T, Yildirim S, Kayaselcuk F, Nursal TZ, Ezer A, Noyan T, Karakayali H, Haberal M. Clinicopathological significance of PTEN loss and the phosphoinositide 3-kinase/Akt pathway in sporadic colorectal neoplasms: is PTEN loss predictor of local recurrence? Am J Surg. 2008;195:719–725. doi: 10.1016/j.amjsurg.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 27.Xiong B, Cheng Y, Ma L, Zhang C. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int J Oncol. 2013;42:219–228. doi: 10.3892/ijo.2012.1707. [DOI] [PubMed] [Google Scholar]
- 28.Chen JS, Huang XH, Wang Q, Huang JQ, Zhang LJ, Chen XL, Lei J, Cheng ZX. Sonic hedgehog signaling pathway induces cell migration and invasion through focal adhesion kinase/AKT signaling-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9 in liver cancer. Carcinogenesis. 2013;34:10–19. doi: 10.1093/carcin/bgs274. [DOI] [PubMed] [Google Scholar]
- 29.Al-Zahrani KN, Baron KD, Sabourin LA. Ste20-like kinase SLK, at the crossroads. Cell Adh Migr. 2014;7:1–10. doi: 10.4161/cam.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theocharis S, Kotta-Loizou I, Giaginis C, Alexandrou P, Danas E, Tsourouflis G, Tsoukalas N, Coutts RH, Tasoulas J, Klijanienko J. Expression and clinical significance of concomitant FAK/SRC and p-paxillin in mobile tongue squamous cell carcinoma. Anticancer Res. 2017;37:1313–1319. doi: 10.21873/anticanres.11449. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Wang D, Liu Y. SASH1 inhibits cervical cancer cell proliferation and invasion by suppressing the FAK pathway. Mol Med Rep. 2016;13:3613–3618. doi: 10.3892/mmr.2016.4946. [DOI] [PubMed] [Google Scholar]
- 32.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery. 2004;135:555–562. doi: 10.1016/j.surg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Wei L, Xu Z. Cross-signaling among phosphinositide-3 kinase, mitogen-activated protein kinase and sonic hedgehog pathways exists in esophageal cancer. Int J Cancer. 2011;129:275–284. doi: 10.1002/ijc.25673. [DOI] [PubMed] [Google Scholar]


