Abstract
Objective: To construct a recombinant adenovirus with artificial microRNA targeting the epidermal growth factor receptor (EGFR) to inhibit the proliferation and induce the apoptosis of tumor cells. Methods: An artificial microRNA (amiR) targeting EGFR was designed and cloned into an adenovirus shuttle plasmid to obtain pDC312-SLPI (secretory leukoprotease inhibitor)-EGFR amiR-pA. Then it was co-transfected into 293 cells with the adenovirus backbone plasmid pBHGloxΔE1, 3Cre to pack the recombinant adenovirus Ad-SLPI-EGFR amiR. After the recombinant adenovirus infected Hep-2 cells by Ad-SLPI-EGFR amiR, a morphological change was observed, and an MTT assay was performed to assess the proliferation of the tumor cells. The expression of EGFR was determined by western blotting analyses to assess the effect of Ad-SLPI-EGFR amiR. Results: The sequence of artificial microRNA targeting EGFR was confirmed by DNA sequencing. Ad-SLPI-EGFR amiR significantly downregulated EGFR expression at the protein level and exerted an inhibitory effect on proliferation in Hep-2 cells. The transfected cells became round, shrunken, partly grape-like and floating under a lighted microscope. Conclusion: The recombinant adenovirus of Ad-SLPI-EGFR amiR was successfully constructed, and it was able to sufficiently decrease the expression of EGFR and inhibit the proliferation of Hep-2 cells.
Keywords: EGFR, artificial microRNA
Introduction
In the last two decades, the epidemiologic evidence suggests that EGFR and aberrant signaling pathways have an essential role in many human epithelial malignancies, such as skin squamous-cell carcinoma, head and neck squamous-cell carcinoma (HNSCC) and breast cancer [1]. EGFR increases the proliferation rate and reduces cell apoptosis in tumor cells. The EGFR-targeting tumor therapy strategy is a promising approach, the most representative of which are the monoclonal antibodies and the small molecule tyrosine kinase (TK) inhibitors of EGFR [2-5]. However, small molecule inhibitors including gefitinib have no clear effect on head and neck cancer. The monoclonal antibodies, including cetuximab, cannot be widely used due to their limited response rate, drug resistance, and side effects, including skin toxicity and gastrointestinal symptoms, although they have an adjuvant role in traditional radiotherapy and chemotherapy.
With the progress of molecular cloning technology, the virus-mediated gene therapy that downregulates the expression of EGFR by RNA interference (RNAi) is a promising approach for the treatment of tumors. The second-generation shRNA, amiR, containing a hairpin structure and the natural framework of microRNA, can be regulated by the mammalian gene promoter and show a high efficacy compared with siRNA and the first-generation shRNA [6]. The amiR makes it possible to use tumor tissue-specific promoters to regulate the expression of EGFR.
In this study, we constructed a recombinant adenovirus with amiR targeting EGFR and explored the effect of gene therapy on inhibiting tumor proliferation using a recombinant adenovirus.
Materials and methods
Cells, cell culture, and reagents
Human embryonic kidney cells (293 cells), human umbilical vein endothelial cells (HuVEC), and the human laryngeal squamous cell carcinoma cell line Hep-2 were provided by the Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Hep-2 cells and HuVEC were seeded into 6-well plates at a density of 5 × 105 cells/well and maintained in RPMI1640 containing 10% fetal bovine serum for 24 h. Hep-2 cells and HuVEC were transduced with Ad-SLPI-EGFRamiR or Ad-SLPI-GFP at a multiplicity of infection (MOI) of 50 plaque forming units (pfu)/cell. After 48 h or 72 h of transduction, the cell morphology was observed under a lighted microscope. The pDC312 vector, pBGHloxΔE1, 3Cre (Microbix Biosystems, Ontario, Canada), pGEM-T Easy vector (Promega, Beijing, China), adenovirus expression vector pDC312-SLPI-pA vector with SLPI promoter, and the BGH poly A sequence were constructed and saved by our lab.
Construction of amiR targeting EGFR
Using miR-30 as the basic framework, EGFR miRNA was designed according to the EGFR gene sequence (http://codex.cshl.edu/scripts/newmain.pl). The microRNA-binding sequence 5’-AGA TCT GAT CCA AGA AGG TAT ATT GCT GTT GAC AGT GAG CGA CCT CCA GAG GAT GTT CAA TAA TAG TGA AGC CAC AGA TGT ATT ATT GAA CAT CCT CTG GAG GCT GCC TAC TGC CTC GGA CTT CAA GGG CTA CGA TGG ATC C-3’ contains the EGFR-target sequence, BglII and BamHI restriction sites for cloning, and flanking sequence of miR-30. The EGFR miRNA was synthesized according to the following steps: 1, the forward primer 5’-TGCTGTTGACAGTGAGCGACCTCCAGAGGATGTTCAATAATAGT GAAGCCACAGATGTA-3’, and the reverse primer 5’-TCCGZAGGCAGTAGGCA GCCTCCAGAGGATGTTCAATAATACATCTGTGGCTTCACTATT-3’, cycle conditions were as follows: 95°C for 5 min, followed by 25 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s, then an extension at 72°C for 7 min; 2, the above polymerase chain reaction (PCR) product as a template was added BglII restriction site at the forward primer 5’end and BamHI restriction enzyme sites at the reverse primer 5’end for sequence recognition and cloning, the forward primer PS: 5’-AGATCTGATCCAAGAAGGTATATTGCTGTTGACAGTGAGCG-3’, and the reverse primer PA: 5’-GGATCCATCGTAGCCCTTGAAGTCCGAGGCAGTAG GCA-3’, cycle conditions were as follows: 95°C for 5 min, followed by 25 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s, then an extension at 72°C for 7 min. The EGFR amiR was cloned into a pGEM-T Easy vector for sequencing. After identification of the correct pGEM-T Easy-EGFR miRNA vector, the artificial EGFR miRNA with multiple repeat EGFR miRNA sequences was obtained by using BglII and BamHI restriction sites for the enhancement of the inhibitory effect of the target sequence.
Generation of Ad-SLPI EGFR amiR
The EGFR amiR was cloned into pDC312-SLPI-pA to obtain the adenovirus shuttle plasmid pDC312-SLPI-EGFR amiR-pA [7]. Then it was co-transfected into 293 cells with the adenovirus backbone plasmid pBHGloxΔE1, 3Cre to pack the recombinant adenovirus Ad-SLPI-EGFR amiR. After identification, the 293 cells were repeat transfected with a recombinant adenovirus for amplification of the virus. Then the adenovirus was purified by CsCl density gradient centrifugation and determined by TCID50 for viral titer, then used in subsequent experiments.
Western blot assay
After virus transduction, Hep2 cells were collected from 6-well plates, and were treated with Ad-SLPI-EGFRamiR or Ad-SLPI-GFP at different MOI for 72 h. An equal amount of protein cell lysate (40 μg) was used for the analysis. Anti-EGFR (1:10,000, Cell Signaling Technology, MA, USA) and anti-GAPDH (1:5000, Santa Cruz, CA, USA) antibodies were used for protein detection.
MTT assay
Recombinant adenovirus Ad-SLPI-EGFR amiR and control adenovirus Ad-SLPI-GFP were independently diluted to MOIs of 200, 100, 50, and 0 pfu/cell. Then, both viruses were independently used to transduce Hep-2 cells (2 × 103/100 μL) for 72 h. The MTT assay was performed to detect cell proliferation by measuring the optical density (OD) based on the manufacturer’s instructions, and the cell proliferation inhibition rate was calculated.
Results
Construction and identification of pGEM-T Easy-EGFR amiRNA
The EGFR miRNA sequencing: AGA TCT GAT CCA AGA AGG TAT ATT GCT GTT GAC AGT GAG CGA CCT CCA GAG GAT GTT CAA TAA TAG TGA AGC CAC AGA TGT ATT ATT GAA CAT CCT CTG GAG GCT GCC TAC TGC CTC GGA CTT CAA GGG CTA CGA TGG ATC C, exactly matches the design. The EGFR amiR was cloned into the pGEM-T Easy vector. After identification of the correct pGEM-T Easy-EGFR miRNA vector, the artificial EGFR miRNA with multiple repeat EGFR miRNA sequences was obtained using BglII and BamHI restriction sites. After digestion with BamHI and BglII, the macrorestriction map showed an 852 bp band (Figure 1), which confirmed that the EGFR amiR we constructed contained 6 repeats of a single EGFR amiR (142 bp).
Figure 1.
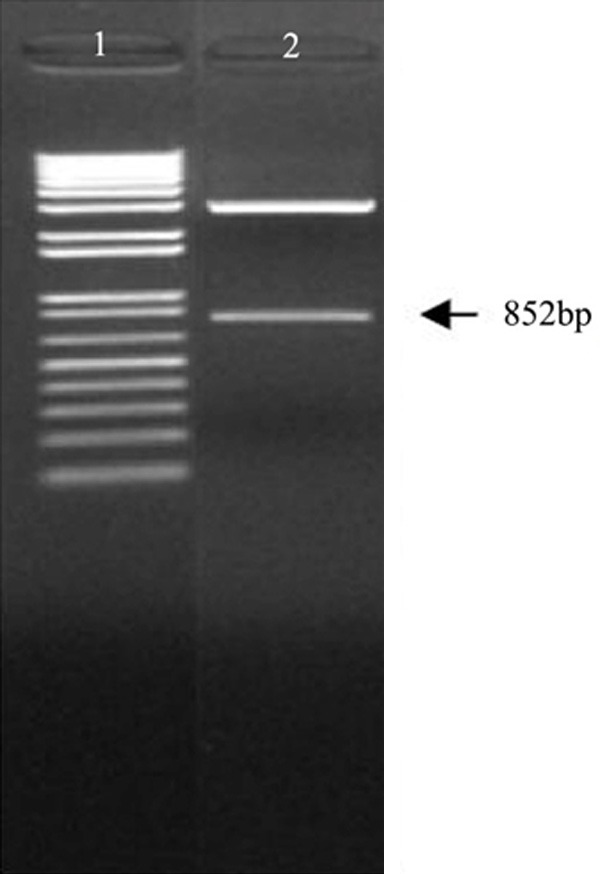
Macrorestriction map of pGEM-T Easy-EGFRamiR. 1.1 kb Plus DNA Marker; 2. Restriction map of pGEM-T Easy-EGFRamiR by BamH I/BglII.
Construction of adenovirus shuttle plasmid pDC312-SLPI-EGFR amiR-Pa
After digestion with EcoR I and Sac I/BamH I, the macrorestriction map of pDC312-SLPI-EGFR amiR-pA showed an 852 bp and a 1492 bp band (Figure 2). This confirmed a single forward insertion of the EGFR amiR gene into the pDC312-SLPI-pA vector, which confirmed the successful construction of the adenovirus shuttle plasmid pDC312-SLPI-EGFR amiR-pA.
Figure 2.
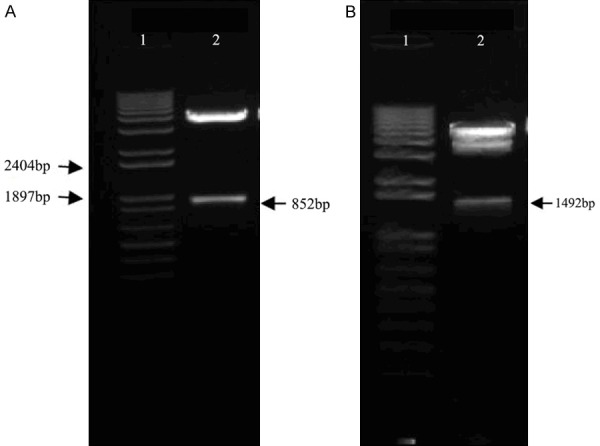
Macrorestriction map of the adenovirus shuttle plasmid vector. A. Macrorestriction map of pDC312-SLPI-EGFR amiR-pA.1. 1 Kb Plus DNA Marker; 2. Restriction map of pDC312-SLPI-EGFRamiR-pA by EcoR I. B. Macrorestriction map of pDC312-SLPI-EGFRamiR-pA.1. 1Kb Plus DNA Marker; 2. Restriction map of pDC312-SLPI-EGFRamiR-pA by Sac I/BamH I.
Packaging, identification, and virus titer determination of recombinant adenovirus Ad-SLPI-EGFR amiR
The 293 cells were transfected with the shuttle plasmid pDC312-SLPI-EGFR amiR-pA and the backbone plasmid pBHGloxΔE1, 3Cre. After the cells transfected for 10 days, we found that 5% of cells seemed to be swollen, round, and floating with the complete shape under a lighted microscope (Figure 3A). The cytopathic effect (CPE) was observed with more than 80% of the cells, that is, grape-like and floating (Figure 3B). The cells were collected, frozen, and thawed repeatedly, and the virus titer was determined after amplification and purification. The number of CPE holes that appeared at each gradient under the microscope were calculated, and the results were as follows:
Figure 3.
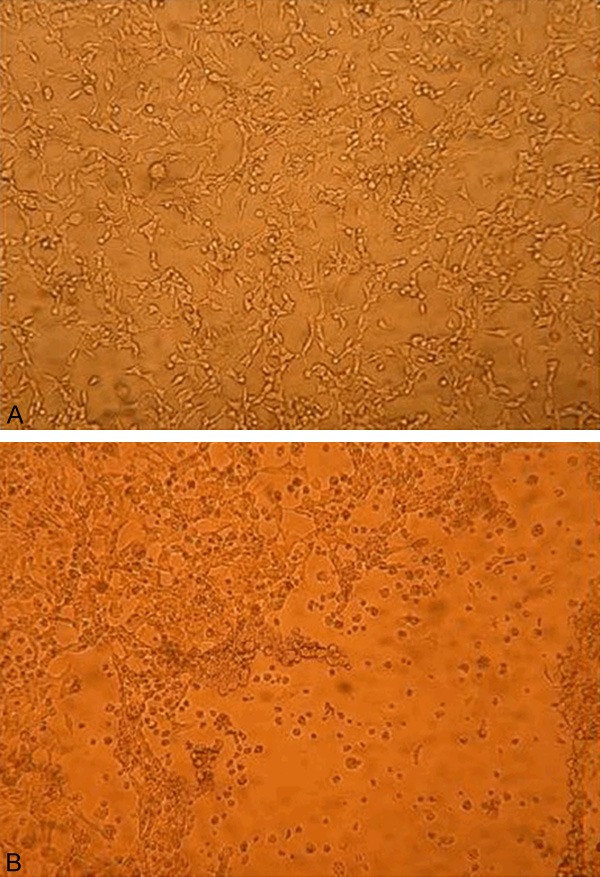
Cytopathic effect of 293 cells after being co-transfected with plasmids. A. Plasmids co transfected with 293 cells for 10 days; B. Plasmids co transfected with 293 cells for 13 days.
S(Ad-SLPI-EGFRamiR) = 10/10+10/10+10/10+10/10+10/10+10/10+10/10+10/10+5/10 = 8.5, T(Ad-SLPI-EGFRamiR) = 101+(s-0.5)/0.1 pfu/ml = 109/0.1 pfu/ml = 1 × 1010 pfu/ml.
The adenovirus supernatant was saved and digested by proteinase K, and 2 μL was taken as a template. PCR was performed using PS and PA of EGFR amiR as primers. We found 142 bp, 284 bp and 426 bp fragments of Ad-SLPI-EGFRamiR were amplified by electrophoresis on agarose gel after real-time quantification (Figure 4).
Figure 4.
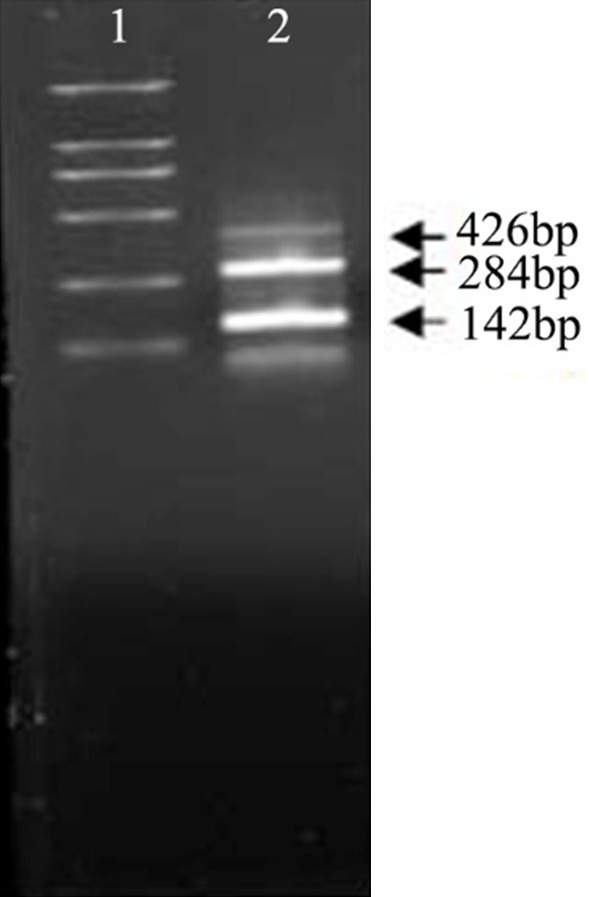
Characterization of EGFR amiR by PCR. 1. DL2000 DNA Ladder 2. Adenovirus supernatant of Ad-SLPI-EGFRamiR as template.
The recombinant adenovirus Ad-SLPI-EGFR amiR decreases the expression of EGFR
The transfected Hep-2 cells were used to analyze the expression of EGFR. Compared with the lenti-control (Ad-SLPI-GFP) and mock-treated groups (without the virus), the expression of EGFR was significantly decreased in the Hep-2 cells infected with the Ad-SLPI-EGFR amiR (Figure 5).
Figure 5.
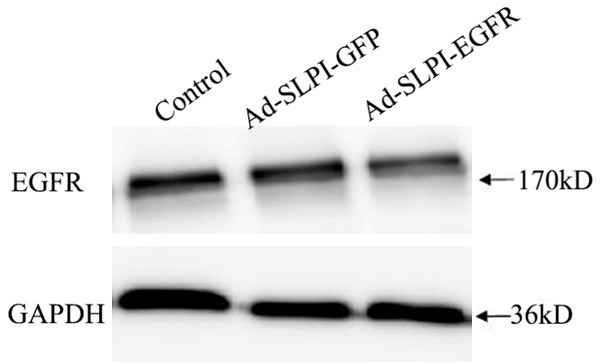
The expression of EGFR in Hep-2 infected by Ad-SLPI-EGFRamiR and Ad-SLPI-GFP. 1. Control (Hep-2 cultured in RPMI1640 for 72 h). 2. Hep-2 infected by Ad-SLPI-GFP virus (MOI = 100) for 72 hours. 3. Hep-2 infected by Ad-SLPI-EGFRamiR virus (MOI = 100) for 72 hours.
The morphological change of Ad-SLPI-EGFR amiR on Hep-2 cells and HuVEC
The Hep-2 cells and HuVEC were transduced with Ad-SLPI-EGFR amiR at a MOI of 50 pfu/cell for 72 hours. The hep-2 cells became round, shrunken, partly grape-like, and floating, but no significant morphological change in HuVEC was observed (Figure 6).
Figure 6.
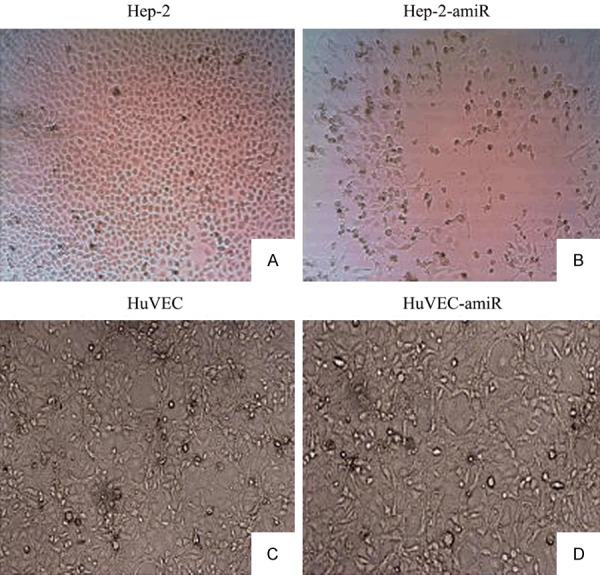
Morphologic changes after the infection with the recombinant adenovirus of Ad-SLPI-EGFRamiR. A. Control (Hep-2 cultured in RPMI1640 for 72 h). B. Hep-2 infected by Ad-SLPI-EGFRamiR (MOI = 50) for 72 hours. C. Control (HuVEC cultured in RPMI1640 for 72 h). D. HuVEC infected by Ad-SLPI-EGFRamiR (MOI = 50) for 72 hours.
Cytotoxicity of Ad-SLPI-EGFR amiR on Hep-2 cells and HuVEC by MTT Assay
Hep-2 cells and HuVEC were transduced with Ad-SLPI-EGFR amiR or Ad-SLPI-GFP at a different MOI for 72 hours. As shown in Figure 7, the inhibition rate of the Hep-2 cells infected with Ad-SLPI-EGFR amiR was significantly increased as the virus titer was increased (MOI 0, 50, 100, 200), but the rates of HuVEC infected with Ad-SLPI-EGFR amiR or Hep-2 and HuVEC infected with Ad-SLPI-GFP were not significant increased. Moreover, Ad-SLPI-EGFR amiR and Ad-SLPI-GFP inhibited the proliferation of Hep-2 cells and HuVEC at a MOI of 400, which may contribute to the toxicity of the virus itself.
Figure 7.
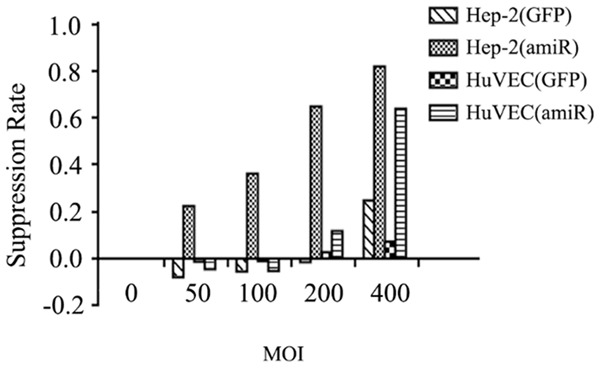
Inhibition of cell growth after infection with Ad-SLPI-EGFRamiR. Hep-2 cells and HuVEC were transduced with Ad-SLPI-EGFR amiR or Ad-SLPI-GFP at a different MOI for 72 hours.
Discussion
In this study, we chose the classic tumor-associated molecule EGFR as a target. The reasons we chose EGFR are: 1, many studies have found that EGFR is overexpressed in multiple epithelial malignancies and has a close relationship with tumor growth, metastasis, and prognosis [1]; 2, the structure, ligands, signal transduction pathways, and mechanisms of EGFR have been well studied [8]; 3, the agents targeting EGFR, including monoclonal antibodies and small molecule TK inhibitors, have been very successful in clinical applications. Cetuximab was approved by the Food and Drug Administration of the United States for HNSCC in 2006 [2]. With the progress of current clinical trials of two popular EGFR-targeted agents in HNSCC, researchers found that small molecule inhibitors have no clear effect on HNSCC [3]. Although the monoclonal antibodies have an adjuvant role in traditional radiotherapy and chemotherapy and are obviously better than the small molecule inhibitors [4,5], they still have a low response rate, high drug resistance, and side effects including skin toxicity and gastrointestinal mucosa inflammation that limit their further use, especially the higher resistance rate [9-11]. Both the small molecule inhibitors and the monoclonal antibodies function at the protein level, so the quaternary structure of protein and frequent interactions of proteins may result in the ineffectiveness of these agents. Moreover, the mutations of EGFR are one of the important causes of resistance.
In this study, we used RNA interference to inhibit the synthesis of EGFR at the level of message RNA. We used the second-generation shRNA amiR to regulate the inhibitory effect using the laryngeal cancer-specific promoter SLPI, which can be regulated by the second type of promoter rather than by the U6 promoter [6]. We designed the precursor microRNA and processed the final form of single-stranded short-acting RNA, which fully matched the EGFR mRNA untranslated region and the first coding junction region. No mutations have been reported in this region so far, so it can effectively bind and degrade EGFR mRNA regardless of the EGFR mutation and can reduce the synthesis of the EGFR protein. The down-regulating effect was confirmed by western blot analysis and the proliferation rate of Hep-2 cells was significantly inhibited after the EGFR down-regulation.
The AdMax™ System of the Microbix Biosystems Company was used in the recombinant adenovirus packaging of this experiment. The adenovirus shuttle plasmid vector and the backbone plasmid vector were co-transfected into 293 cells to produce the virus. Compared with the traditional method, the recombinase system (Cre-loxp) makes it an easy operation, improves the recombination efficiency, and increases the virus yield.
In summary, we successfully constructed a tissue-specific promoter SLPI regulatory EGFR targeting adenovirus (Ad-SLPI-EGFR amiR), which can significantly decrease the expression of EGFR in Hep-2 cells and inhibit the proliferation of Hep-2 cells. This lays the groundwork for similar future studies.
Acknowledgements
We thank Prof. Jiang Cao for his technical assistance with this work. The work was supported by the funds from Medical Science and Technology Project of Zhejiang Province (no. 2012KYB101) and the National Natural Science Foundation of China (no. 81172561).
Disclosure of conflict of interest
None.
References
- 1.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefèbvre JL. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–50. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 3.Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J. Clin. Oncol. 1988;6:1653–64. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 4.Johnston PG, Geoffrey F, Drake J, Voeller D, Grem JL, Allegra CJ. The cellular interaction of 5-fluorouracil and cisplatin in a human colon carcinoma cell line. Eur J Cancer. 1996;32a:2148–54. doi: 10.1016/s0959-8049(96)00266-3. [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama M, Yamamoto W, Park JS, Okamoto R, Hanaoka H, Takano H, Saito N, Matsukawa M, Shirasaka T, Kurihara M. Low-dose cisplatin and 5-fluorouracil in combination can repress increased gene expression of cellular resistance determinants to themselves. Clin Cancer Res. 1999;5:2620–8. [PubMed] [Google Scholar]
- 6.Zhang J, Lee J, Urba S, Foster J, Worden F. A phase II trial evaluating weekly docetaxel and capecitabine in patients with metastatic or advanced, locally recurrent head and neck cancers. Cancer Invest. 2010;28:910–6. doi: 10.3109/07357907.2010.483502. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Yang B, Zhang S, Ling Y, Ye J, Jia Z, Cao J. Antitumor potential of SLPI promoter controlled recombinant caspase-3 expression in laryngeal carcinoma. Cancer Gene Therapy. 2012;19:328–35. doi: 10.1038/cgt.2012.5. [DOI] [PubMed] [Google Scholar]
- 8.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, van Glabbeke M European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 9.Kish JA, Weaver A, Jacobs J, Cummings G, Al-Sarraf M. Cisplatin and 5-fluorouracil infusion in patients with recurrent and disseminated epidermoid cancer of the head and neck. Cancer. 1984;53:1819–24. doi: 10.1002/1097-0142(19840501)53:9<1819::aid-cncr2820530903>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Amrein PC, Weitzman SA. Treatment of squamous-cell carcinoma of the head and neck with cisplatin and 5-fluorouracil. J. Clin. Oncol. 1985;3:1632–9. doi: 10.1200/JCO.1985.3.12.1632. [DOI] [PubMed] [Google Scholar]
- 11.Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, Ensley J, Forastiere AA Eastern Cooperative Oncology Group. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the eastern cooperative oncology group. J. Clin. Oncol. 2005;23:3562–7. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]


