Abstract
We determined the effects of the BCL-2, C-MYC, and BCL-6 gene aberrations and their protein expressions on the prognosis of primary central nervous system diffuse B-cell lymphoma (PCNS-DLBCL) patients. The pathological and clinical information of 47 immunocompetent patients was reviewed, and the immunohistochemical markers for BCL2, CD10, BCL6, MUM1, and MYC were reevaluated. Genetic abnormalities included increased copy number, translocation, gene amplification, and double aberration and were detected by fluorescence in situ hybridization (FISH). A survival analysis showed that elevated protein levels in the cerebrospinal fluid (CSF), increased the IPI score, and EBV infection adversely affected survival. However, high BCL2 (≥70%) and positive MYC expressions (≥40%) showed no significant influence on survival or BCL-2 gene abnormality, and BCL2/MYC double expression and BCL-2/C-MYC double aberrations were associated with adverse outcomes for PCNS-DLBCL patients.
Keywords: Primary central nervous system diffuse B-cell lymphoma, BCL2, MYC, BCL6, prognosis, gene aberration
Introduction
Primary central nervous system diffuse large B-cell lymphoma (PCNS-DLBCL) represents a rare subgroup of diffuse large B-cell lymphomas occurring in the brain, eyes, meninges, or spinal cord without simultaneous systemic involvement [1]. The lymphomas account for 2-3% of all primary malignant brain tumors and approximately 1% of non-Hodgkin’s lymphomas in adults [1]. PCNS-DLBCL is usually highly aggressive and predominantly occurs in immunodeficient patients, including those with acquired immunodeficiency syndrome and organ transplant recipients.
In recent years, the adverse effects of C-MYC/BCL-2 double aberration (double-hit, DH) and MYC/BCL2 double-expression (DE) have been well-documented in systemic DLBCL [2-7]; however, their influence on PCNS-DLBCL is poorly understood. Hence, to evaluate the effects of BCL-2 and C-MYC aberrations and BCL2 and MYC expression in PCNS-DLBCL cases, we reviewed the clinical and pathological data of 47 well-documented cases of PCNS-DLBCL in immunocompetent patients.
Materials and methods
Patients
Electronic archival files of 66 patients diagnosed with PCNS-DLBCL and treated at the Zhejiang Cancer Hospital from 01/01/2008 to 06/01/2018 were analyzed. Data were retrieved for 47 cases, for which the pathological material was sufficient for the performance of in situ hybridization (ISH) and fluorescence in situ hybridization (FISH) examinations. All pathological samples were reviewed by two experienced pathologists. None of the patients had a history of primary or secondary immunodeficiency disease. Informed consent was obtained from all the patients, and the study was approved by the Medical Ethics Committee of Zhejiang Cancer Hospital (ethical approval document: IRB-2018-98).
IHC
Immunohistochemistry (IHC) for BCL2, CD10, BCL6, MUM1, and MYC was performed. Formalin-fixed paraffin-embedded (FFPE) tissue blocks were cut into 3-5-μm-thick sections, deparaffinized, rehydrated, blocked with 3% hydrogen peroxide, and retrieved in a water bath at 95°C and pH 9.0 for 20 min. Antibodies against BCL2, CD10, BCL6, MUM1 (DAKO, Denmark) and MYC (Maxim Biotec Ltd, Fuzhou, China) were then used in the EnVision Two-Step method. Tonsillitis tissue was used as the control. Expression was considered positive as per prior studies [8,9].
ISH
ISH testing for Epstein-Barr virus (EBV)-encoded small RNA (1/2 EBER) was performed on 3~5-μm-thick sections from FFPE tissue blocks. The sections were deparaffinized, hydrated, and dried at 37°C. A fluorescein isothiocyanate (FITC)-labeled oligonucleotide probe (Y5200; Dako) was hybridized on the sections in the thermostat at 55°C for 3 h after a 10-min digestion; the sections were then incubated with an anti-FITC antibody (Y5201, Dako) for 30 min. The staining was visualized with the ISH iView system by using alkaline phosphatase and the nitrate tetrazole blue/5-Bromo-4-Chloro-3-Indolyl Phosphate (NBT/BCIP) substrate, with neutral red for contrast. Nasopharyngeal carcinoma tissue sections were used as the positive control and buffer fluid as the blank control. For each case, the number of positive cells in three representative microscopic fields was counted with a 20× objective lens, and the average number was calculated. The presence of a median of ≥25 positive cells per media power field was defined as ‘positive’ [10].
FISH
FISH testing for c-myc, bcl-2, and bcl-6 was performed in all cases on formalin-fixed, paraffin-embedded tissue sections according to procedures previously described [11]. Dual color break-apart probes for c-myc, bcl-6, and dual color fusion probes for bcl-2/IGH were obtained from Abbott Molecular/Vysis (Des Plaines, IL). The scoring criteria included analysis of only single nuclei with distinct nuclear borders and the avoidance of overlapping cells. The presence of at least one green and one red signal/cell was required for the analysis. For the break-apart probe, two yellow signals were found in normal cells, and one yellow signal and a pair of separated red and green signals indicated gene break-apart, the presence of 3-5 overlapping yellow signals indicated an increased copy number (ICN), and the presence of ≥6 yellow signals indicated gene amplification. For the fusion probe, the normal cells harbored two pairs of separated red and green signals, cells with bcl-2/IGH gene translocation showed two yellow fusion signals and a pair of red and green signals, and cells with ICN of the bcl-2 gene displayed ≥3 red signals and two green signals. c-myc gene abnormality along with BCL-2 or BCL-6 gene aberration (including translocation, ICN and amplification) was defined as a double hit (DH) [4,5].
Statistical methods
An association analysis was performed for all of the 47 cases, and a survival analysis was done for the 33 cases who received HD-MTX-based chemotherapy using SAS 9.4 Software.
Results
Clinical and treatment-related data
Forty-seven immunocompetent patients were included in the study. Their mean age was 58.98±2.23 years (37-84 years), and the male to female ratio was 27:20. Twenty-three patients had multiple lesions and deep brain areas were affected in 24. The most common presentations were headache (80.85%), dizziness (63.83%), dyskinesia (44.68%) and ataxia (27.65%); other symptoms, including vomiting (14.89%), cognition dysfunction (8.51%), aphasia (6.38%) and paralysis (6.38%) were less frequently observed. The protein concentration in the cerebrospinal fluid (CSF) and the lactate dehydrogenase (LDH) levels in the serum were elevated in 23 and 15 cases, respectively. Nineteen patients had high Eastern Cooperative Oncology Group (ECOG) scores (>1).
Forty-one patients underwent total or partial tumor resection, and 6 consented to undergo stereotactic biopsies. Thirty-three patients received high-dose methotrexate (HD-MTX)-based chemotherapy in which 13 cases subsequently received whole brain radiotherapy (WBRT) as a supplementary treatment. Overall, 18 cases received rituximab in addition to chemotherapy. Six patients only underwent WBRT after their surgeries, and 8 patients did not receive any form of therapy after surgery for personal reasons or due to their weak physical conditions. The median follow-up interval was 10 months (range: 1-100 months). The 3-year overall survival (OS) rate was 33.47%, and the 2-year survival rate was 43.03%. The 3-year and 2-year OS for the 33 patients with HD-MTX-based chemotherapy was 49.57% and 63.74% respectively. The clinical and treatment-related data are provided in Table 1.
Table 1.
Patients’ clinical and treatment information
| General information (47 cases) | Survival analysis (33 cases with HD-MTX)* | |||
|---|---|---|---|---|
|
|
|
|||
| Variables | Cases (%) | Case (%) | 3-year OS | p value |
| Sex | 0.4944 | |||
| Female | 20 (42.55%) | 13 (39.39%) | 51.95% | |
| Male | 27 (57.45%) | 20 (40.61%) | 52.84% | |
| Age (years) | 0.6283 | |||
| >60 | 24 (51.06%) | 18 (54.55%) | 39.84% | |
| ≤60 | 23 (48.94%) | 15 (45.45%) | 60.76% | |
| Lesion site | 0.0932 | |||
| Superficial | 24 (51.06%) | 15 (45.45%) | 57.44% | |
| Deep region | 23 (48.94%) | 18 (54.55%) | 24.40% | |
| Lesion Num. | 0.5728 | |||
| Multiple | 23 (48.94%) | 18 (54.55%) | 36.29% | |
| Single | 24 (51.06%) | 15 (45.45%) | 44.46% | |
| CSF Protein | 0.0173 | |||
| Normal | 24 (57.50%) | 17 (51.52%) | 68.36% | |
| Elevated | 23 (42.50%) | 16 (48.48%) | 0.00% | |
| LDH | 0.763 | |||
| Normal | 32 (68.09%) | 21 (63.63%) | 45.45% | |
| Elevated | 15 (31.91%) | 12 (36.37%) | 38.79% | |
| ECOG score | 0.3933 | |||
| ≤1 | 40 (85.11%) | 30 (90.90%) | 52.20% | |
| >1 | 7 (14.89%) | 3 (9.10%) | Not gain | |
| IPI (scores) | 0.0042 | |||
| Low risk (0-1) | 36 (76.60%) | 26 (78.79%) | 54.26% | |
| Medium (2-3) | 11 (23.40%) | 7 (21.21%) | 0.00% | |
| Rituximab | 0.2832 | |||
| Yes | 18 (38.30%) | 18 (54.55%) | 51.24% | |
| No | 29 (41.70%) | 15 (45.44%) | 47.28% | |
| WBRT | 0.5297 | |||
| Yes | 19 (40.43%) | 13 (39.39%) | 40.00% | |
| No | 28 (59.57%) | 20 (60.61%) | 65.68% | |
| HD-MTX | ||||
| Yes | 33 (70.21%) | |||
| No | 14 (29.18%) | |||
The survival analysis only included patients with high dose MTX-based chemotherapy, which is now a standard treatment scheme for PCNS-DLBCL.
HD-MTX: High dose MTX-based chemotherapy. WBRT: whole brain radiotherapy.
IHC, ISH, and FISH results
Morphologically, all the tumors showed diffuse infiltration and a replacement of the brain parenchyma by large lymphoid cells, often in a sheet-like pattern. The morphological characteristics included a diffuse and sheet-like growth of big tumor cells with large pleomorphic nuclei and prominent nucleoli (Figure 1A); perivascular cuffing of the tumor cells and areas of geographic necrosis were also noted (Figure 1B). As per available patient records, tumors were positive for CD20 but negative for both CD3 and CD5. Thirty-six cases (76.60%) were positive for BCL2, in which 23 (42.50%) showed high expressions (≥70%, Figure 1C). CD10 was positive in 11 cases (23.40%). Most of the samples were positive for BCL6 (34/47, 72.34%) and MUM1 (44/47, 93.61%). All cases expressed MYC protein, with expressions ranging from 1-60% of the tumor cells, but only 18 cases were defined as positive (≥.0%, Figure 1D). Eleven (23.40%) cases were defined as presenting with BCL2/MYC DE. Four tumor samples (8.5%) showed positive signals for EBER. In accordance with the Hans model [8], 11 cases (23.40%) were classified as the germinal center B cell (GCB) subtype, and the remaining 36 cases as the non-GCB subtype.
Figure 1.
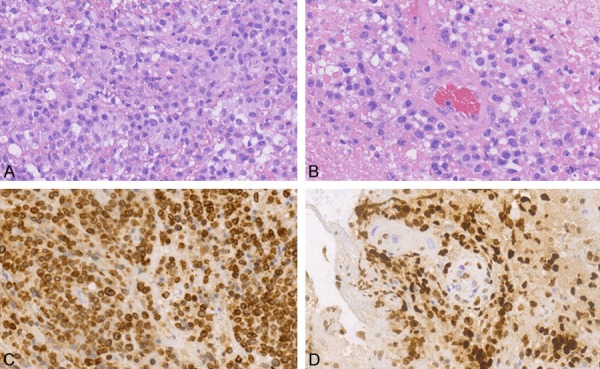
Histopathology and immunohistochemical analysis in a representative PCNS-DLBCL tissue. A. Large tumor cells with a diffuse growth pattern and neutrophil cell invasion. B. Cuffing structure as tumor cells invade the perivascular space on a background of geographic necrosis. C. Diffuse expression of BCL2 in the nuclear membrane of tumor cells. D. MYC expression in the nuclei of tumor cells invading the perivascular space. PCNS-DLBCL, primary central nervous system diffuse B-cell lymphoma.
On FISH, 43 cases showed satisfactory signals for BCL-2/IGH and C-MYC and 42 for BCL-6. Figure 2A and 2B are representative of negative signals for all 3 genes. Overall, 13 patients showed BCL-2 gene abnormality, including 10 with ICN (Figure 2C) and 3 with translocations (Figure 2D). BCL-6 gene alterations were observed in 8 cases, with 2 cases of translocation (Figure 2E), 4 with ICN (Figure 2F) and 2 with amplifications (Figure 2G). C-MYC ICN was observed in 9 cases (Figure 2H), and 5 cases harbored bcl-2/c-myc DH. Detailed data on the IHC, ISH, and those on the FISH results are provided in Table 2.
Figure 2.
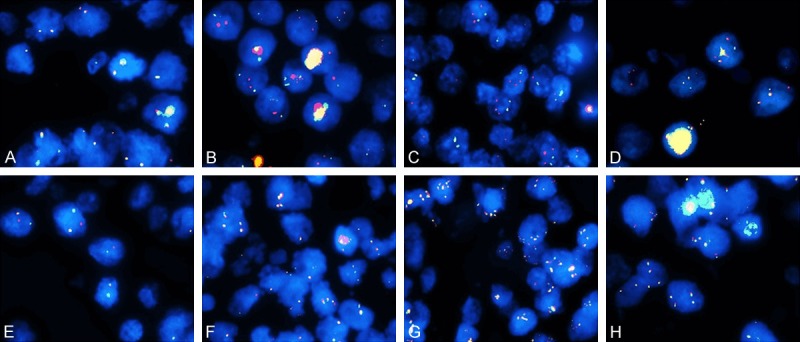
FISH analysis of the representative samples (all the samples were obtained at ×1000 magnification). A. Tumor cells with normal C-MYC as shown by the two yellow fusion signals in one nucleus. B. Tumor with normal BCL-2/IGH harboring two pairs of red and green signals in one nucleus. C. Tumor with BCL-2 ICN showing ≥3 red signals and two green signals in one nucleus. D. Tumor with BCL-2/IGH translocation with two yellow signals and a pair of red and green signals in one nucleus. E. Tumor with break-apart BCL-6 has one yellow signal and a pair of separated red and green signals in one nucleus. F. 3~5 yellow signals in one nucleus in a tumor with BCL-6 ICN. G. Tumor with BCL-6 gene amplification shows ≥6 yellow signals in one nucleus. H. 3~5 yellow signals in one nucleus of a tumor with C-MYC ICN. FISH, fluorescence in situ hybridization; ICN, increased copy number.
Table 2.
Patients’ IHC, ISH, FISH results
| General information (47 cases) | Survival analysis (33 cases with HD-MTX) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Variables | Cases (%) | Cases (%) | 3-year OS | P value | |
| BCL2 | 0.0507 | ||||
| 0~69% | 24 (57.50%) | 23 (69.70%) | 62.93% | ||
| ≥70% | 23 (42.50%) | 10 (30.30%) | 0.00% | ||
| BCL6 | 0.9367 | ||||
| Neg. | 13 (27.50%) | 9 (27.27%) | 47.71% | ||
| Pos. | 34 (72.34%) | 24 (72.73%) | 56.56% | ||
| IHC | MUM1 | 0.3053 | |||
| Neg. | 3 (6.39%) | 2 (6.06%) | Not gain | ||
| Pos. | 44 (93.61%) | 31 (93.94%) | 52.33% | ||
| CD10 | 0.7164 | ||||
| Neg. | 36 (76.60%) | 24 (72.73%) | 46.75% | ||
| Pos. | 11 (23.40%) | 9 (27.27%) | 71.43% | ||
| MYC | 0.7133 | ||||
| <40% | 29 (61.70%) | 22 (66.67%) | 53.57% | ||
| ≥40% | 18 (38.30%) | 11 (33.33%) | 35.35% | ||
| ISH | EBER | 0.0020 | |||
| Neg. | 43 (91.49%) | 30 (90.90%) | 52.56% | ||
| Pos. | 4 (8.51%) | 3 (9.10%) | Not gain | ||
| BCL-2 | 0.0064 | ||||
| Nor. | 30 (69.77%) | 24 (77.42%) | 60.22% | ||
| Abnor*. | 13 (30.23 %) | 7 (22.48%) | 0.00% | ||
| FISH | BCL-6 | 0.7015 | |||
| Nor. | 34 (80.95 %) | 25 (80%) | 50.65% | ||
| Abnor†. | 8 (19.05%) | 5 (20%) | Not gain | ||
| C-MYC | 0.1291 | ||||
| Nor. | 34 (79.07%) | 24 (77.42%) | 60.75% | ||
| ICN | 9 (21.93%) | 7 (22.48%) | 26.76% | ||
Including break-apart and ICN.
Including gene break-apart and ICN and amplification.
EBER, Epstein-Barr virus-encoded small RNA; OS, overall survival; ISH, in situ hybridization; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; ICN, increased copy number; OS, overall survival.
Statistical analysis
The statistical analysis was performed using the SAS 9.4 software. A univariate survival analysis (Kaplan-Meier curves, log-rank test) indicated that elevated protein levels in the CSF (P=0.0173, Figure 3A) increased the IPI core (P=0.042, Figure 3B) and the EBV infection (P=0.0020, Figure 3C) and were associated with poor survival. Furthermore, we found that the bcl-2 gene abnormality (P=0.0064; Figure 3D) BCL2/MYC DE (P=0.0476, Figure 3E) and the BCL-2/c-myc DH (P=0.0019, Figure 3F) were clearly associated with adverse outcomes (Detailed information about DE and DH is listed in Table 3). Other clinical and pathological factors, including sex, age, number of lesions, deep brain region involvement, LDH, ECOG score, WBRT, Rituximab plus to chemotherapy, CD10, BCL6 and MUM1 expression levels, different immunophenotypes according to the Hans model, ICN of the c-myc gene, and the bcl-6 abnormality did not have a significant influence on overall survival (OS). A high expression of BCL2 was associated with bcl-2 gene abnormality (Fisher’s exact test, P=0.0041), but there was no relation between MYC overexpression and C-MYC ICN (Fisher’s exact test, P=0.0571).
Figure 3.
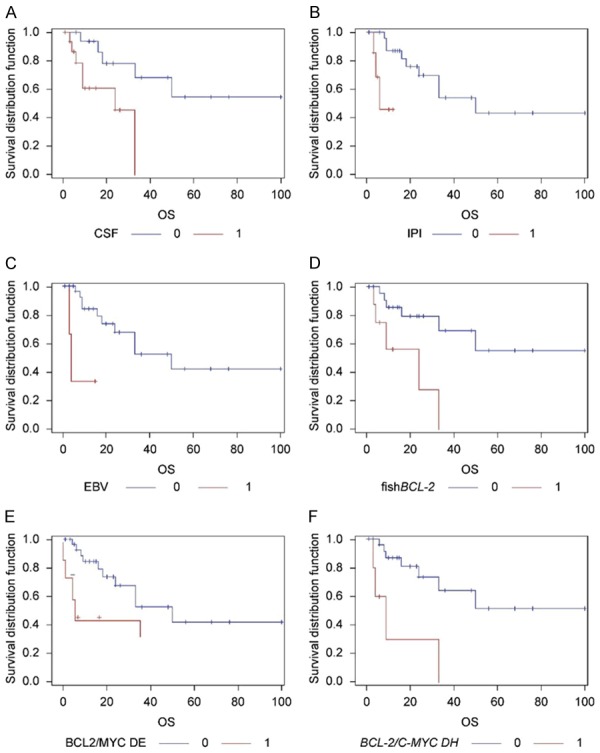
Kaplan Meier graphs for overall survival. Elevated protein levels in the CSF (A), high expressions of IPI (B) and EBV infection (C) had significant negative effects on overall survival, The BCL-2 aberrations (D) were associated with worse prognoses in PCNS-DLBCL patients. Both BCL2/MYC DE (E) and BCL-2/C-MYC DH (F) led to the shortest survival period, on comparing patients without these two proteins DE and without two genes DH. PCNS-DLBCL, primary central nervous system diffuse B-cell lymphoma; HD-MTX, high-dose methotrexate.
Table 3.
BCL-2, C-MYC BCL-6 gene and protein information and survival analysis
| Gene and protein information | |||||||||||||||||
|
| |||||||||||||||||
| Protein | BCL2 | BCL6 | Gene | BCL-2 | BCL-6 | ||||||||||||
|
|
|
|
|
||||||||||||||
| <70% | ≥70% | Neg. | Pos. | Nor. | Abnor. | Nor. | Abnor. | ||||||||||
|
| |||||||||||||||||
| CMYC | Neg. | 17 | 12 | 9 | 20 | C-MYC | Nor. | 26 | 8 | 27 | 6 | ||||||
| Pos. | 7 | 11 | 4 | 14 | Abnor. | 4 | 5 | 7 | 2 | ||||||||
|
| |||||||||||||||||
| Survival analysis (33 cases with HD-MTX) | |||||||||||||||||
|
| |||||||||||||||||
| Cases (%) | 3-year OS | P value | Case (%) | 3-year OS | P value | ||||||||||||
|
| |||||||||||||||||
| DE | Yes | 4 (12.12%) | Not gain | 0.0476 | DH | Yes | 5 (16.13%) | 0.00% | 0.0019 | ||||||||
| No | 29 (87.88%) | 52.66% | No | 26 (83.87%) | 64.35% | ||||||||||||
DE, BCL2/MYC Double expression; DH, BCL-2/c-myc gene Double-hit.
Discussion
PCNS-DLBCL, a rare subgroup of DLBCL, accounts for more than 95% of primary central nervous lymphomas (PCNSLs) and appears to have an aggressive clinical course. The molecular mechanisms of PCNS-DLBCL are still unclear. We designed this study to evaluate the role of the bcl-2 and c-myc aberrations and BCL2 and MYC expressions in PCNS-DLBCL. A cohort of 47 patients with no history of primary or secondary immunodeficiency disease was included in this study. The overall prognosis was poor, and the 2-year and 3-year OS were 43.07% and 33.47%, which is a little lower than what is reported in studies, and the 2-year and 3-year OS for patients with HD-MTX-based chemotherapy was 63.74% and 49.54% respectively, which is similar to the OS reported in previous studies [12,13].
The International Extranodal Lymphoma Study Group identified five clinical variables that are correlated with prognoses in PCNSL: elevated LDH level, age >60 years, ECOG performance status >1, high CSF protein concentration, and location of the tumor in deep brain regions [12]. In the current study, high CSF protein levels were associated with poor outcomes and a lower rate of survival. High serum LDH levels, age >60 years, deep brain region involvement, and ECOG score >1 had no significant prognostic effect.
The influence of BCL6-a zinc-finger transcriptional repressor-on PCNS-DLBCL is not clear. Earlier studies have reported that the expression of BCL6 is favorable to survival in patients with PCNS-DLBCL [14,15]; however, two recent reports showed that high BCL6 expression was correlated with decreased survival [13,16]. In the current study, patients with BCL6 expression had a better 5-year OS and mean survival than BCL6-negative patients, but the difference was not significant. Most patients were classified into the non-GCB group, according to the Hans algorithm, although no difference in survival between the GCB and non-GCB groups was found, which is consistent with previous reports [17-20].
The negative influence of c-myc and bcl-2 gene aberrations and the overexpression of MYC and BCL2 in systemic highly aggressive B cell lymphoma has been widely reported [2,4-7,21], but their effect in PCNS-DLBCL is controversial. Tapia et al. discovered that MYC expression is associated with poor prognoses in PCNS-DLBCL cases; however, in another study, there was no association between MYC expression and prognosis [22]. Recently, Kim et al. reported that MYC and BCL2 overexpression is associated with a higher class in the Memorial Sloan-Kettering Cancer Center prognostic model and poor clinical outcomes in primary diffuse large B-cell lymphoma of the central nervous system. MYC/BCL2 DE was reported to be a strong prognostic factor in PCNS-DLBCL in some studies [23], but not in others [16,24]. In this study, neither the overexpression of BCL2 nor MYC affected survival significantly, though BCL2 overexpression showed a hint of poor survival (P=0.0507). However, it was clear that BCL2/MYC DE adversely impacted prognoses (P=0.0476).
Cady et al. found that the prevalence rates of translocations in bcl-6 and c-myc were 17% and 3% in PCNS-DLBCL cases, respectively, and that bcl-6 translocation was associated with decreased OS [25]. Close to 40% of the cases in our study showed Bcl-2 aberrations, and our analysis revealed that such aberrations were associated with poor outcomes in PCNS-DLBCL cases, like in the case of systemic DLBCL [5,26]. In systemic DLBCL, c-myc ICN is associated with poor outcomes [5], but its relationship with prognoses in PCNS-DLBCL is not well-known. We did not observe any relationship between C-myc ICN and/or BCL-6 gene aberration and prognoses. BCL-2/c-myc DH was also a negative factor for OS. To our knowledge, our research is the first to report the effect of C-MYC, BCL-2 and BCL-6 abnormalities on the prognosis of PCNS-DLBCL; our findings may serve as a reference guide for other scientists in this field.
EBV infection is closely associated with PCNS-DLBCL in patients with human immunodeficiency virus infection [27]. The EBV-positivity rate is around 6% in immunocompetent patients with PCNS-DLBCL [28]. Studies have shown that the incidence of EBV infection in the Japanese population is a little higher than the incidence in Western populations [10,28-30]. In the current study, the positivity rate (8.51%) for EBV infection was similar to that reported in the Japanese population and a little higher than that in the Western population. Our study confirmed the finding that EBV infection suggests adverse clinical outcomes in PCNS-DLBCL patients, as reported previously [28].
WBRT has been criticized for a long time for its inadequate efficacy and severe neurotoxicity [31]. HD-MTX-based chemotherapy has been suggested as a first-line treatment option for PCNS-DLBCL due to its effectiveness in prolonging survival and improving the quality of life [13,32,33]. Our results further proved that HD-MTX-based chemotherapy, with or without WBRT, is an effective treatment option in such patients following surgery.
Rituximab, a targeted drug for B cell lymphoma, has been widely used in the treatment of systemic highly aggressive B cell lymphoma, but its usage in PCNS-DLBCL is controversial, due its inability to access the CNS because of the blood-brain barrier (BBB) [34]. However, several recent clinical studies have provided evidence stating that rituximab can promote complete remission and prolong survival [13,31,32,35,36]. Eighteen cases in this cohort received rituximab along with HD-MTX-based chemotherapy, but we observed little difference in prognosis between the groups with and without rituximab. Therefore, it stands to reason that the therapeutic effect of rituximab on PCNS-DLBCL patients needs to be further studied with larger cohorts.
In conclusion, we found that bcl-2 gene aberrations, BCL2/MYC DE, and bcl-2/c-myc gene DH were associated with adverse outcomes in PCNS-DLBCL cases, providing new insights into the prognostic value of these factors in PCNS-DLBCL.
Acknowledgements
We would like to thank Editage [www.editage.cn] for the English language editing. This study was supported in part by the Zhejiang Medical Science and Technology Project, General Program under grant nos. 2018245519, 2018283987, and 2017207606.
Disclosure of conflict of interest
None.
References
- 1.Kluin PM, Deckert M, Ferry JA. Primary diffuse large B-cell lymphoma of the CNS. In: Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumors haematopoietic and lymphoid Tissues. 4th edition. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2008. pp. 240–241. [Google Scholar]
- 2.Kawamoto K, Miyoshi H, Yoshida N, Nakamura N, Ohshima K, Sone H, Takizawa J. MYC translocation and/or BCL 2 protein expression are associated with poor prognosis in diffuse large B-cell lymphoma. Cancer Sci. 2016;107:853–861. doi: 10.1111/cas.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, Liu WM, Visco C, Li Y, Miranda RN, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A, Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WW, Zhao X, van Krieken JH, Huang Q, Huh J, Ai W, Ponzoni M, Ferreri AJ, Zhou F, Slack GW, Gascoyne RD, Tu M, Variakojis D, Chen W, Go RS, Piris MA, Møller MB, Medeiros LJ, Young KH. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from the international DLBCL rituximab-CHOP consortium program. Blood. 2013;121:4021–4031. doi: 10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Lin P, Young KH, Kanagal-Shamanna R, Yin CC, Medeiros LJ. MYC/BCL2 double-hit high-grade B-cell lymphoma. Adv Anat Pathol. 2013;20:315–326. doi: 10.1097/PAP.0b013e3182a289f2. [DOI] [PubMed] [Google Scholar]
- 5.Lu TX, Fan L, Wang L, Miao KR, Liang JH, Gong QX, Wang Z, Young KH, Xu W, Zhang ZH, Li JY. MYC or BCL2 copy number aberration is a strong predictor of outcome in patients with diffuse large B-cell lymphoma. Oncotarget. 2015;6:18374–18388. doi: 10.18632/oncotarget.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry AM, Alvarado-Bernal Y, Laurini JA, Smith LM, Slack GW, Tan KL, Sehn LH, Fu K, Aoun P, Greiner TC, Chan WC, Bierman PJ, Bociek RG, Armitage JO, Vose JM, Gascoyne RD, Weisenburger DD. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol. 2014;165:382–391. doi: 10.1111/bjh.12763. [DOI] [PubMed] [Google Scholar]
- 7.Yan LX, Liu YH, Luo DL, Zhang F, Cheng Y, Luo XL, Xu J, Cheng J, Zhuang HG. MYC expression in concert with BCL2 and BCL6 expression predicts outcome in Chinese patients with diffuse large B-cell lymphoma, not otherwise specified. PLoS One. 2014;9:e104068. doi: 10.1371/journal.pone.0104068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakhleh RE, Manivel JC, Copenhaver CM, Sung JH, Strickler JG. In situ hybridization for the detection of epstein-barr virus in central nervous system lymphomas. Cancer. 1991;67:444–448. doi: 10.1002/1097-0142(19910115)67:2<444::aid-cncr2820670221>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Foot NJ, Dunn RG, Geoghegan H, Wilkins BS, Neat MJ. Fluorescence in situ hybridisation analysis of formalin-fixed paraffin-embedded tissue sections in the diagnostic work-up of non-burkitt high grade B-cell non-hodgkin’s lymphoma: a single centre’s experience. J Clin Pathol. 2011;64:802–808. doi: 10.1136/jclinpath-2011-200015. [DOI] [PubMed] [Google Scholar]
- 12.Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A, Devizzi L, Berger F, Ponzoni M, Borisch B, Tinguely M, Cerati M, Milani M, Orvieto E, Sanchez J, Chevreau C, Dell’Oro S, Zucca E, Cavalli F. Prognostic scoring system for primary CNS lymphomas: the international extranodal lymphoma study group experience. J. Clin. Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, Cheson BD, Kaplan LD. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (alliance 50202) J. Clin. Oncol. 2013;31:3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN, Harris NL, Batchelor TT. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9:1063–1069. [PubMed] [Google Scholar]
- 15.Song MK, Chung JS, Joo YD, Lee SM, Oh SY, Shin DH, Yun EY, Kim SG, Seol YM, Shin HJ, Choi YJ, Cho GJ. Clinical importance of Bcl-6-positive non-deep-site involvement in non-HIV-related primary central nervous system diffuse large B-cell lymphoma. J Neurooncol. 2011;104:825–831. doi: 10.1007/s11060-011-0555-z. [DOI] [PubMed] [Google Scholar]
- 16.Kreher S, Johrens K, Strehlow F, Martus P, Borowiec K, Radke J, Heppner F, Roth P, Thiel E, Pietsch T, Weller M, Korfel A. Prognostic impact of B-cell lymphoma 6 in primary CNS lymphoma. Neuro Oncol. 2015;17:1016–1021. doi: 10.1093/neuonc/nov046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhagavathi S, Sharathkumar A, Hunter S, Sung L, Kanhere R, Venturina MD, Wilson JD. Activated B-cell immunophenotype might be associated with poor prognosis of primary central nervous system lymphomas. Clin Neuropathol. 2008;27:13–20. doi: 10.5414/npp27013. [DOI] [PubMed] [Google Scholar]
- 18.Min M, Lin L, Bi CF, Wang XQ, Luo TY, Zhao S, Zhang WY, Liu WP. Analysis of the immunohistochemical subtypes and prognosis of primary diffuse large B cell lymphoma of the central nervous system. Zhonghua Zhong Liu Za Zhi. 2012;34:110–116. doi: 10.3760/cma.j.issn.0253-3766.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Hattab EM, Martin SE, Al-Khatib SM, Kupsky WJ, Vance GH, Stohler RA, Czader M, Al-Abbadi MA. Most primary central nervous system diffuse large B-cell lymphomas occurring in immunocompetent individuals belong to the nongerminal center subtype: a retrospective analysis of 31 cases. Mod Pathol. 2010;23:235–243. doi: 10.1038/modpathol.2009.164. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri-Broët S, Crinière E, Broët P, Delwail V, Mokhtari K, Moreau A, Kujas M, Raphaël M, Iraqi W, Sautès-Fridman C, Colombat P, Hoang-Xuan K, Martin A. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107:190–196. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 21.Valera A, López-Guillermo A, Cardesa-Salzmann T, Climent F, González-Barca E, Mercadal S, Espinosa I, Novelli S, Briones J, Mate JL, Salamero O, Sancho JM, Arenillas L, Serrano S, Erill N, Martínez D, Castillo P, Rovira J, Martínez A, Campo E, Colomo L Grup per l’Estudi dels Limfomes de Catalunya i Balears (GELCAB) MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554–1562. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill KZ, Iwamoto F, Allen A, Hoehn D, Murty VV, Alobeid B, Bhagat G. MYC protein expression in primary diffuse large B-cell lymphoma of the central nervous system. PLoS One. 2014;9:e114398. doi: 10.1371/journal.pone.0114398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi QY, Feng X, Bao W, Ma J, Lv JH, Wang X, Rao Q, Shi QL. MYC/BCL2 co-expression is a stronger prognostic factor compared with the cell-of-origin classification in primary CNS DLBCL. J Neuropathol Exp Neurol. 2017;76:942–948. doi: 10.1093/jnen/nlx083. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Wang Y, Liu Y, Liu Z, Cui Q, Ji N, Sun S, Wang B, Wang Y, Sun X, Liu Y. Immunohistochemical profile and prognostic significance in primary central nervous system lymphoma: analysis of 89 cases. Oncol Lett. 2017;14:5505–5512. doi: 10.3892/ol.2017.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cady FM, O’Neill BP, Law ME, Decker PA, Kurtz DM, Giannini C, Porter AB, Kurtin PJ, Johnston PB, Dogan A, Remstein ED. Del (6) (q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. J. Clin. Oncol. 2008;26:4814–4819. doi: 10.1200/JCO.2008.16.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obermann EC, Csato M, Dirnhofer S, Tzankov A. BCL2 gene aberration as an IPI-independent marker for poor outcome in non-germinal-centre diffuse large B cell lymphoma. J Clin Pathol. 2009;62:903–907. doi: 10.1136/jcp.2009.066597. [DOI] [PubMed] [Google Scholar]
- 27.Raphael L, Said J, Borisch B, et al. Lymphomas associated with HIV infection. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO classification of tumors haematopoietic and lymphoid tissues. 4th edition. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2008. pp. 340–342. [Google Scholar]
- 28.Utsuki S, Oka H, Miyajima Y, Kijima C, Yasui Y, Fujii K. Epstein-Barr virus (EBV)-associated primary central nervous system lymphoma: is incidence of EBV expression associated with median survival time? Brain Tumor Pathol. 2011;28:145–149. doi: 10.1007/s10014-011-0020-x. [DOI] [PubMed] [Google Scholar]
- 29.Kitai R, Matsuda K, Adachi E, Saito Y, Nakajima T, Takeuchi H, Sato K, Imamura Y, Kubota T. Epstein-barr virus-associated primary central nervous system lymphoma in the Japanese population. Neurol Med Chir (Tokyo) 2010;50:114–118. doi: 10.2176/nmc.50.114. [DOI] [PubMed] [Google Scholar]
- 30.Jamal SE, Li S, Bajaj R, Wang Z, Kenyon L, Glass J, Pang CS, Bhagavathi S, Peiper SC, Gong JZ. Primary central nervous system epstein-barr virus-positive diffuse large B-cell lymphoma of the elderly: a clinicopathologic study of five cases. Brain Tumor Pathol. 2014;31:265–273. doi: 10.1007/s10014-013-0173-x. [DOI] [PubMed] [Google Scholar]
- 31.Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, Röth A, Hertenstein B, von Toll T, Hundsberger T, Mergenthaler HG, Leithäuser M, Birnbaum T, Fischer L, Jahnke K, Herrlinger U, Plasswilm L, Nägele T, Pietsch T, Bamberg M, Weller M. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 32.Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B, Grimm S, Lai RK, Reiner AS, Panageas K, Karimi S, Curry R, Shah G, Abrey LE, DeAngelis LM, Omuro A. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J. Clin. Oncol. 2013;31:3971–3979. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, Gavrilovic IT, Nolan C, Pentsova E, Grommes CC, Panageas KS, Baser RE, Faivre G, Abrey L, Sauter CS. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125:1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, Ignoffo R, Aldape K, Shen A, Lee D, Grillo-Lopez A, Shuman MA. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- 35.Holdhoff M, Ambady P, Abdelaziz A, Sarai G, Bonekamp D, Blakeley J, Grossman SA, Ye X. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology. 2014;83:235–239. doi: 10.1212/WNL.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyakita Y, Ohno M, Takahashi M, Muragaki Y, Katai H, Narita Y. Immunochemotherapy using rituximab (RTX) and high-dose methotrexate (HD-MTX): an evaluation of the addition of RTX to HD-MTX in recurrent primary central nervous system lymphoma (PCNSL) Jpn J Clin Oncol. 2017;47:919–924. doi: 10.1093/jjco/hyx095. [DOI] [PubMed] [Google Scholar]


