Abstract
Previous studies showed that the dysregulation of miRNAs was closely associated with cancer progression. The aim of this study was to verify whether miR-134, miR-10a, miR-29c, miR-942, miR-93, and miR-218 could inhibit esophageal squamous cell carcinoma (ESCC) cell invasion and migration. ESCC tissue and normal esophageal tissue adjacent to carcinoma from patients (54 cases) undergoing surgery were collected. RT-PCR was used to test the expression of miR-134, miR-10a, miR-29c, miR-942, miR-93, and miR-218 in these tissues. In addition, western blot was applied to test the expression of MMP-2, MMP-9, COL1A1, COL1A5 and FOXM1. In the vitro experiment, EC9706 cells were transfected with miR-134 mimics, then wound healing was employed to test the migratory ability of EC9706 cells. Transwell chambers was used to test the invasion ability of cells. The expression of MMP-2, MMP-9, COL1A1, COL1A5, and FOXM1 waas detected by western blot. In order to confirm whether FOXM1-3’-UTR was the target gene of miR-134, we performed a luciferase assay. FOXM1 over-expression plasmid was transfected to further confirm miR-134 played its role by targeting FOXM1. Our results showed that the expression of miR-134 was decreased in the ESCC tissue compared with normal esophageal tissue, (P<0.01), but the expression of MMP-2, MMP-9, COL1A1, COL1A5 and FOXM1 were significantly increased (P<0.01). In an in vitro experiment, compared with the mimic control, the expression of MMP-2, MMP-9, COL1A1, COL1A5 and FOXM1 were decreased in the miR-134 mimic-transfected EC9706 cells (P<0.01). The migration and invasion activity of EC9706 cells was also decreased after transfection with miR-134 mimics (P<0.01). The luciferase activity of the FOXM1-3’-UTR plasmid was significantly suppressed by miR-134 (P<0.01). Overexpression of FOXM1 abrogated miR-134-mediated inhibition of EC9706 cell migration and invasion. In conclusion, miR-134 inhibited EC9706 cell migration and invasion by targeting FOXM1. miR-134 may be a novel treatment target for ESCC.
Keywords: Esophageal squamous cell carcinoma, miR-134, forkhead box M1, migration, invasion
Introduction
Esophageal squamous cell carcinoma (ESCC) is aggressive and has poor prognosis. It is the sixth leading cause of cancer death worldwide [1]. As the most prevalent type of esophageal cancer, the incidence of ESCC is increasing in the recent years [2,3]. Thus, it is important to further discover the molecular mechanisms implicated in ESCC development and progression. It is well known that migration and invasion ability are necessary for the tumor cells to depart from the original location and invade other tissues and organs [4]. In this regard, it is urgent to develop a new drug or find a molecular target that participates in ESCC cell migration and invasion in order to improve the prognosis of ESCC.
MicroRNAs are small, non-coding RNAs that post-transcriptionally down-regulate the expression of multiple genes by binding to the 3’-untranslated region (UTR) of target mRNAs [5-8]. MicroRNA expression is related to numerous human diseases including cancer and is associated with altered malignant potential, affecting migration, proliferation, invasion, apoptosis, and survival [9]. In recent years, some studies found that microRNAs were promising biomarkers for ESCC and could play important roles in ESCC development and progression. Xu et al. demonstrated that miR-10b, miR-29c, and miR-205 in the serum had great potential to be noninvasive screening tools for ESCC detection [10]. Wang et al. reported that miR-218 expression was significantly reduced in ESCC tissues and miR-218 played an important role as a tumor-suppressing gene by targeting BMI1 [11]. Furthermore, Geet al. showed that miR-942 promoted cancer stem cell-like traits in ESCC through activation of the Wnt/beta-catenin signaling pathway [12]. O’Brien et al. showed that miR-134 in extracellular vesicles reduced cancer aggression and increased drug sensitivity [13]. However, the expression and function of these miRNAs in human ESCC are uncertain.
Forkheadbox M1 (FOXM1) gene is a member of the FOX family, which has been shown to have important roles in cell fate. Some studies showed that FOXM1 was significantly increased in diverse human cancers such as breast cancer, esophageal cancer, hepatocellular carcinoma, colorectal cancer, and lung cancer [14-17]. Its overexpression was closely correlated with tumor metastasis and progression [16].
In this study, we chose six mircoRNAs which have been reported in other cancers and tested their expression in ESCC and normal esophageal tissue. We found that miR-134 was decreased in the ESCC tissues and did further research to explore the effect of miR-134 in the migration and invasion ability of ESCC cells. We found that over-expression of miR-134 inhibited EC9706 migration and invasion. In addition, we identified FOXM1 as a direct target gene of miR-134 by which miR-134 plays its anti-cancer role in ESCC cells.
Materials and methods
Patients and samples
Human ESCC tissues and the corresponding non-tumor tissues were collected during surgical resection from 54 patients with ESCC in the Affiliated Hospital of Qingdao University. The demographic information and clinical features for the included 54 patients are provided in Table 1. Non-tumor tissues were more than 3 cm from the tumor. All specimens were confirmed pathologically. The ESCC and normal esophagus tissues were frozen in liquid nitrogen immediately after surgical resection and stored at -80°C. This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and informed consent was signed by every patient.
Table 1.
Clinicopathologic features and the expression of miR-134 in patients with esophageal squamous cell carcinoma
| Characteristic | Case | miR-134 | P valuea | |
|---|---|---|---|---|
|
| ||||
| Low expression | High expression | |||
| Age (years) | 0.365 | |||
| <60 | 28 | 14 | 14 | |
| ≥60 | 26 | 9 | 17 | |
| Gender | 0.534 | |||
| Male | 33 | 16 | 117 | |
| Female | 21 | 9 | 12 | |
| Pathologic type | 0.298 | |||
| Ulcerative type | 18 | 10 | 8 | |
| Medullary type | 16 | 7 | 9 | |
| Fungating type | 12 | 5 | 7 | |
| Constrictive type | 5 | 0 | 5 | |
| Plaque type | 3 | 1 | 2 | |
| Other | 1 | 1 | 0 | |
| Differentiation | 0.135 | |||
| Well | 7 | 2 | 5 | |
| Moderate | 24 | 12 | 12 | |
| Poor | 23 | 16 | 7 | |
| Tumor size | 0.033 | |||
| ≤4 cm | 26 | 10 | 16 | |
| >4 cm | 28 | 21 | 7 | |
| TNM Classification | 0.009 | |||
| I | 7 | 4 | 3 | |
| II | 13 | 9 | 4 | |
| III | 25 | 16 | 9 | |
| IV | 9 | 7 | 2 | |
| Lymph node status | <0.001 | |||
| Negative | 25 | 13 | 12 | |
| Positive | 29 | 20 | 9 | |
One-way ANOVA and t-test were used to analyze the correlation between the expression of miR-134 and clinicopathologic features of the patients.
Cell culture and transfection
The human ESCC cell line (EC9706) was bought from the Chinese Academy of Sciences (Shanghai, China). The EC9706 cells were kept in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; CA, USA) and penicillin-streptomycin (100 unit/ml-100 μg/ml). The cells were cultured in humidified incubators with 5% CO2 at 37°C. miR-134 mimics and mimic control were chemically synthesized by Ribo Bio Co. Ltd (Guangzhou, China). The sequences were as follows: 5’-UGUGACUGGUUGACCAGAGGGG-3’ (mimics) [18]; 5’-UGAGACUGGAUGACCAGAGUGG-3’ (mimics control). The overexpression oligonucleotides for Human FOXM1 and negative control vector (p-vector) was generated by Ribo Bio and were loaded into lentivirus vectors (Lv-FOXM1 and Lv-NC, respectively). Cells were transfected with DNA plasmids using TransFast transfection reagent (Promega; Madison, WI, USA). For transfection, after seeding the cells into the 6-well plate, and the cells reaching 70%-80% confluence the corresponding vectors were transfected into the EC9706 cells by using Lipofectamine 3000 (Invitrogen, CA, USA) according to the manufacturer’s instructions. The final transfection concentration was 100 nM and the transfection effects were assessed by qRT-PCR after transfection for 24 h. The cells were then subjected to further functional assays.
Quantitative real-time PCR
Total RNA was extracted from tissues or cells by using RNeasy plus mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The 20 μL RT reactions were performed using a PrimeScript® RT reagent kit (Takara, Dalian, China) and incubated for 30 min at 37°C, 5 s at 85°C. For qRT-PCR, 2 μL of diluted RT product was mixed with 23 μL reaction buffer provided by Takara (Takara Inc., Dalian, China) to a final volume of 25. All reactions were carried out using an Eppendorf Mastercycler EP Gradient S (Eppendorf, Germany) under the following conditions: 95°C for 30 s, followed by 45 cycles of 95°C for 5 s and 60°C for 30 s. The expression of miR-134, miR-10a, miR-29c, miR-942, miR-93 and miR-218 was normalized to U6 using the comparative 2-ΔΔCq method [19]. All experiments were done in triplicate. The following primers were used for qRT-PCR:
miR-134: Forward: 5’-ACAGGCCGGGACAAGTGCAATA-3’; Reverse: 5’-GCTGTCA ACGATACGCTACGTA ACG-3’. miR-10a: Forward: 5’-ACGTACCCTGTAGATCCG-3’; Reverse: 5’-GTGCAGGGTCCGAGGT-3’. miR-29c: Forward: 5’-ACACTC-CAG CTG G GTGA-3’; Reverse: 5’-CTCAACTGGTTGA GAGGGATTC-3’. miR-942: Forward: 5’-CCCAAGAAGTCCGAGACACG-3’; Reverse: 5’-TGTTGGCTCGTTCATGGGAT-3’. miR-93: Forward: 5’-CGTTATATCCC AAAGTGCTGTTC-3’; Reverse: 5’-TATGGTTGTTCT CGTCTCCTTCTC-3’. miR-218: Forward: 5’-CGGGCTTGTGCTTGATCTA-3’; Reverse: 5’-GTGCAGGGTCCGAGGT-3’. U6 Forward: 5’-CTCGCTTCGGCAGCACA-3’; Reverse: 5’-AACGCTTCACGAATTTGCGT-3’.
Western blotting
Tissues or cells were lysed by RIPA lysis buffer (Beyotime, China) for total protein extraction. The protein concentration was determined using a BCA protein assay kit (Beyotime). Then, the protein lysates were separated by 10% SDS-PAGE and transferred to PVDF membranes, (Millipore, MA, USA). After blocking with bovine serum albumin for 1 h at room temperature, the PVDF membranes were incubated with the primary antibodies (Abcam, Cambridge, UK): COL1A1 (1:1000), COL1A5 (1:1000), MMP-2 (1:1000), MMP-9 (1:2000), FOXM1 (1:1000), β-actin (1:1000) overnight at 4°C. Then the membranes were incubated in HRP-linked secondary antibodies (1:5000, room temperature, Beyotime, China) for 1 h. The bands were detected using the ECL plus Kit (Beyotime, China) and Image J software version 1.4 was used to quantify the bands.
Cell migration assay
Cell migration was evaluated by performing wound healing assay. First, the cells were cultured in serum-free medium containing hydroxyurea (1.8 mM) for 12 h to synchronize cells and suppress cells proliferation. After each treatment, wounds were scratched on the monolayer of cells using 20 μL pipette tips. Plates were washed once with fresh medium to remove non-adherent cells and the cells were continued to be incubated at 37°C for 24 h. Digital camera system (Olympus Corp., Tokyo, Japan) was used to acquire images of the scratches of the cells after incubating for 0 and 24 h.
Cell invasion assay
Transwell chamber was used to test cell invasion capability. Polycarbonate membrane coated trans-well plates with 8 μm pores (Corning, USA) were applied for the experiments. After 24 h of cultivation, cells were cultured in serum-free medium containing hydroxyurea (1.8 mM) for 12 h to synchronize cells and suppress cells proliferation. After each treatment, the cells were trypsinized and reseeded in serum free DMEM medium, then transferred to the upper chamber (5×104 cells/well). DMEM medium supplemented with 20% FBS was then poured into the lower chamber. After 12 h incubation, the migratory cells were fixed by 4% paraformaldehyde and stained blue by DAPI (2 ug/ml). The penetrated cells were counted with an immunofluorescence microscope.
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org/), a computer-aided algorithm, was used to predict the potential binding site of FOXM1 for miR-134. The sequence containing the predicted binding sites was inserted into the pmirGLO plasmid (Promega, USA). The forward primer sequences for the mRNA 3’-UTR of FOXM1 was 5’-ATACGTGGATTGAGGACCCACT-3’ and reverse primer sequences was 5’-TCCAATGTCAAGTAGGGGTTG-3’. FOXM1 mRNA 3’-UTR fragment containing putative binding sites for miR-134 was chemically synthesized by JIKAI Gene Co., Ltd. (Shanghai, China) to obtain the wild type (WT) construct. Mutations in binding sites were introduced by site-directed mutagenesis to obtain a mutant (Mut). All constructs were verified by sequencing. For luciferase reporter assay, EC9706 cells were seeded in 96-well plates, co-transfected with miR-134 mimics (or mimics control) and the WT (or MUT) constructed luciferase reporter by using Lipofectamine 3000. After 48 h transfection, the GloMax-Multi Jr Single Tube Multimode Reader (Promega, Madison, WI, USA) was used to test the Firefly and Renilla luciferase activities.
Statistical analysis
Statistical analysis was conducted by using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.0 (La Jolla, CA). Data are presented as mean ± SD. The comparison between the variances of the two groups was analyzed using 2-tailed Student t-test or one-way ANOVA. Difference between the patients’ number in the two groups were analyzed by Chi-square test. p-value <0.05 was considered significant. All experiments were all repeated at least three times.
Results
The expression of miR-134 was decreased in human ESCC
As miR-134, miR-10a, miR-29c, miR-942, miR-93, and miR-218 were reported to be related with ESCC in the previous studies, we first detected the expression of these miRNAs in the collected ESCC tissues and normal esophageal tissues by RT-qPCR analysis. As shown in Figure 1A, there was no significant difference in miR-10a, miR-29c, miR-942, miR-93 and miR-218 between the two groups. The expression of miR-134 was significantly decreased in the ESCC group, P<0.01. This result prompted us to further focus on miR-134 in the following study.
Figure 1.
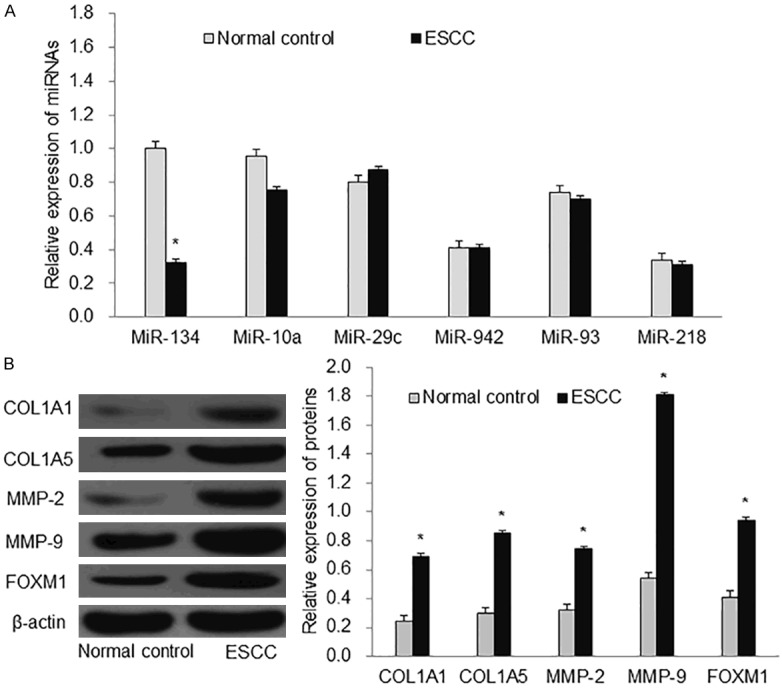
Expression of ESCC-related miRNAs and proteins. A: The expressions of six miRNAs (miR-134, miR-10a, miR-29c, miR-942, miR-93 and miR-218) in normal esophageal tissue and ESCC tissues. B: The protein expression of MMP-2, MMP-9, COL1A1, COL1A5 and FOXM1. ESCC: esophageal squamous cell carcinoma. *P<0.01 compared with normal control.
miR-134 suppressed EC9706 cell migration and invasion
In order to explore how miR-134 played its role in ESCC progression. We first tested the tumor cell migration related proteins (COL1A1, COL5A1, MMP-2, MMP-9) in the ESCC tissues and normal esophageal tissues by western blot. We found that the expression of COL1A1, COL5A1, MMP-2 and MMP-9 was increased in the ESCC compared with the normal control (Figure 1B, P<0.01). This data suggested that miR-134 could inhibit the migration and invasion of ESCC cells. After miR-134 was successfully transfected into the EC9706 cells (Figure 2A), we further conducted wound healing assay and transwell assay to test it. In the wound healing assay, we found that miR-134 significantly decreased the cell migration area after 24 h compared with the control group (Figure 2B, P<0.01). Transwell assay demonstrated that miR-134 inhibited cell invasion ability (Figure 2C). In addition, we also detected the expression of COL1A1, COL5A1, MMP-2, and MMP-9 in the cell experiment and found that miR-134 inhibited their expression in EC9706 cells (Figure 2D). Taken together, these results showed that miR-134 suppressed EC9706 cell migration and invasion.
Figure 2.
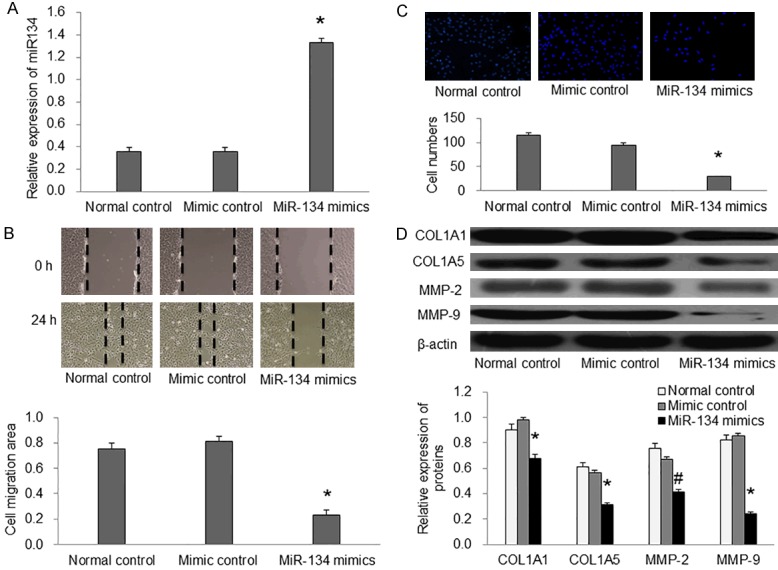
miR-134 suppressed EC9706 cell migration and invasion in vitro. A: Relative expression of miR-134 in each group. B: Wound-healing assay showed that miR-134 inhibited EC9706 cell migration. C: Transwell chamber assay showed that miR-134 inhibited EC9706 cell invasion. D: The expression of COL1A1, COL5A1, MMP-2 and MMP-9 were measured by western blotting in EC9706 cells. *P<0.01 compared with mimic control.
miR-134 directly targeted FOXM1 in EC9706 cells
In order to find the potential target gene of miR-134 in ESCC, we used a computer-aided algorithm, TargetScan (http://www.targetscan.org/), to predict it. As shown in (Figure 3A), we found that there was a potential seed sequence of miR-134 in the 3’UTR of FOXM1. Moreover, miR-134 mimic decreased the expression of FOXM1 in the EC9706 cells (Figure 3C).
Figure 3.
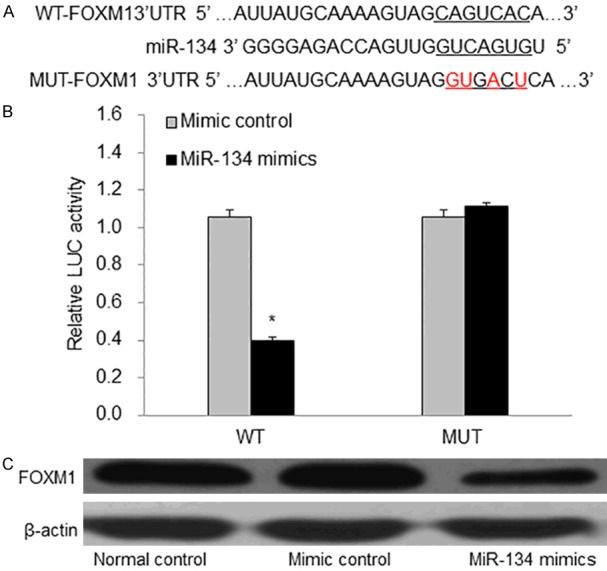
FOXM1 is a direct target gene of miR-134 in EC9706 cells. A: The binding site between miR-134 and FOXM1. B: miR-134 decreased the luciferase activity of the FOXM1. C: The expression of FOXM1 in each group. WT: wild type; MUT: mutant. LUC: luciferase. *P<0.01 vs mimic control.
In addition, the result of Dual-luciferase reporter assay showed that miR-134 mimics inhibited luciferase activity in the WT vector (P<0.01), and this effect of miR-134 mimics disappeared in the MUT vector (Figure 3B). Therefore, these data suggested that FOXM1 was the direct target of miR-134 in EC9706 cells. At last, we conducted the FOXM1 over-expression study to test the effect of FOXM1 in miR-134’s inhibition of EC9706 cell migration. pcDAN3.1+HA-FOXM1 was successfully transfected and expressed in EC9706 cells (Figure 4A). Over-expression of FOXM1 partly reversed the inhibitory effect of miR-134 on EC9706 cell migration and invasion (Figure 4B-D). These results showed that miR-134inhibited EC9706 cell migration and invasion by targeting FOXM1 in EC9706 cells.
Figure 4.
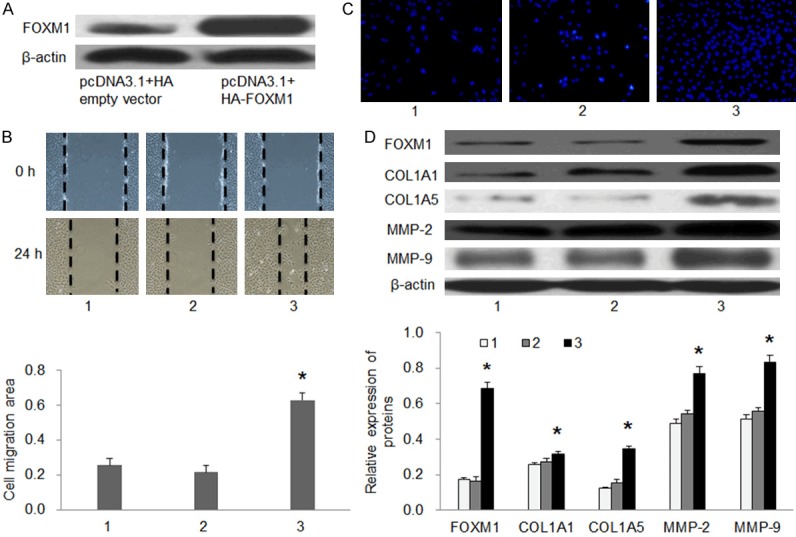
Overexpression of FOXM1 partly abrogated miR-134 induced inhibitory effects on EC9706 cells. A: The expression of FOXM1 in each group. B: Cell migration ability was detected by wound-healing assay in EC9706 cells. C: Cell invasion ability was detected by Transwell chamber assay in EC9706 cells. D: The expression of FOXM1, COL1A1, COL5A1, MMP-2, and MMP-9 in each group. 1: miR-134 mimics; 2: miR-134 mimics+pcDNA3.1+HA empty vector; 3: miR-134 mimics+pcDNA3.1+HA-FOXM1. #P<0.05 and *P<0.01 compared with miR-134 mimics+pcDNA3.1+HA empty vector.
Discussion
Advances in diagnostic techniques and therapeutic means have improved the early detection and reduced the mortality rate of ESCC [10]. However, despite the progress in clinical treatment, the overall 5-year survival rate of ESCC patient still remains low (around 10%) due to delayed diagnosis and high recurrence rate. The major reason for its mortality and relapse is that ESCC cells have a strong ability to metastasize. In recent years, progress was made in understanding of tumor mechanisms, but tumor metastasis is a complex process, and molecular mechanisms that regulate metastasis in ESCC cells are still poorly understood [20].
First discovered in 1993, miRNAs have been found to be involved in various processes of many human diseases by regulating cell migration, invasion, proliferation, apoptosis, and epithelial to mesenchymal transition (EMT) [21,22]. Recently, it has been estimated that miRNAs could regulate at least 30% of all gene expression in humans [23]. Shuang et al. found that the expression of miR-134 was decreased in ovarian cancer [24]. Bao et al. revealed a tumor-suppressive role of miR-134 which was decreased in human osteosarcoma [25]. In this study, we found that miR-134 expression was down-regulated in ESCC tissues compared with normal controls. COL1A1, COL5A1, MMP-2 and MMP-9 which had been reported to be involved in cell migration and invasion, were also increased in the ESCC tissue [26-28]. We further investigated the roles of miR-134 on ESCC cell migration and invasion in vitro and found that overexpression of miR-134 significantly inhibited the migration and invasion ability of ESCC cells. These results suggested that miR-134 may be a tumor suppressor in the development and progression of ESCC.
At the molecular level, O’Brien et al. reported that miR-134 in extracellular vesicles reduced triple-negative breast cancer aggressiveness and increased drug sensitivity by down-regulation of HSP90 [13]. Additionally, Liu et al. showed that in renal cell carcinoma cells, miR-134 functioned as a tumor suppressor in cell proliferation and EMT by targeting KRAS. In the field of lung cancer, Li et al. found that miR-134 inhibited non-small cell lung cancer cell EMT by targeting FOXM1 [29]. Recently, Wu et al. demonstrated that miR-134 modulated the proliferation of human cardiomyocyte progenitor cells by down-regulation of Meis2. Similar to the above research results, in this study, we found that over-expression of miR-134 significantly decreased the expression of FOXM1 in EC9706 cells and we further identified that FOXM1 was a direct target of miR-134 in EC9706 cells by using bioinformatics and luciferase reporter gene assays.
FOXM1 has been shown to regulate multiple intracellular signaling cascades [30]. FOXM1 binds to sequence specific motifs on DNA (C/TAAACA) through its DNA-binding domain (DBD) and activates proliferation, migration, and EMT associated genes. Aberrant overexpression of FOXM1 has been shown to be involved in cancer progression in a variety of tumor types including ESCC [31]. Research shows that down-regulation of FOXM1 could suppresses PLK1-regulated cell cycle progression in renal cancer cells [32]. In addition, microRNA-24-1 through FOXM1 inhibited bladder cancer cell proliferation [5]. Consistent with the results of these studies, we found that over-expression of FOXM1 could reverse miR-134 induced-migration and invasion of EC9706 cells. Therefore, we thought that FOXM1 may be involved in miR-134 mediated ESCC metastasis.
In view of the above findings, we found that miR-134 was down-regulated, while MMP-2, MMP-9, COL1A1, COL1A5, and FOXM1 were up-regulated in ESCC tissue. We further investigated the role of miR-134 on EC9706 cells and found that miR-134 inhibited EC9706 cell migration and invasion by targeting FOXM1. Our result provides new information about the mechanism responsible for the development of ESCC, which may also benefit the development of miRNA-directed diagnosis and therapy against ESCC.
Acknowledgements
This study was supported by Youth Science Foundation of The Affiliated Hospital of Qingdao University.
Disclosure of conflict of interest
None.
References
- 1.Ansari MH, Irani S, Edalat H, Amin R, Mohammadi Roushandeh A. Deregulation of miR-93 and miR-143 in human esophageal cancer. Tumour Biol. 2016;37:3097–103. doi: 10.1007/s13277-015-3987-9. [DOI] [PubMed] [Google Scholar]
- 2.Hu HB, Jie HY, Zheng XX. Three circulating LncRNA predict early progress of esophageal squamous cell carcinoma. Cell Physiol Biochem. 2016;40:117–125. doi: 10.1159/000452529. [DOI] [PubMed] [Google Scholar]
- 3.Zhou CL, Li JJ, Ji P. Propofol suppresses esophageal squamous cell carcinoma cell migration and invasion by down-regulation of sex-determining region Y-box 4 (SOX4) Med Sci Monit. 2017;23:419–427. doi: 10.12659/MSM.899732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kogo R, How C, Chaudary N, Bruce J, Shi W, Hill RP, Zahedi P, Yip KW, Liu FF. The microRNA-218~Survivin axis regulates migration, invasion, and lymph node metastasis in cervical cancer. Oncotarget. 2015;6:1090–1100. doi: 10.18632/oncotarget.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoguchi S, Seki N, Chiyomaru T, Ishihara T, Matsushita R, Mataki H, Itesako T, Tatarano S, Yoshino H, Goto Y, Nishikawa R, Nakagawa M, Enokida H. Tumour-suppressive microRNA-24-1 inhibits cancer cell proliferation through targeting FOXM1 in bladder cancer. FEBS Lett. 2014;588:3170–3179. doi: 10.1016/j.febslet.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 6.Mirzadeh Azad F, Naeli P, Malakootian M, Baradaran A, Tavallaei M, Ghanei M, Mowla SJ. Two lung development-related microRNAs, miR-134 and miR-187, are differentially expressed in lung tumors. Gene. 2016;577:221–226. doi: 10.1016/j.gene.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 7.Mima K, Nishihara R, Yang J, Dou R, Masugi Y, Shi Y, da Silva A, Cao Y, Song M, Nowak J, Gu M, Li W, Morikawa T, Zhang X, Wu K, Baba H, Giovannucci EL, Meyerhardt JA, Chan AT, Fuchs CS, Qian ZR, Ogino S. MicroRNA MIR21 (miR-21) and PTGS2 expression in colorectal cancer and patient survival. Clin Cancer Res Clin Cancer Res. 2016;22:3841–8. doi: 10.1158/1078-0432.CCR-15-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupaimoole R, Ivan C, Yang D, Gharpure KM, Wu SY, Pecot CV, Previs RA, Nagaraja AS, Armaiz-Pena GN, McGuire M, Pradeep S, Mangala LS, Rodriguez-Aguayo C, Huang L, Bar-Eli M, Zhang W, Lopez-Berestein G, Calin GA, Sood AK. Hypoxia-upregulated microRNA-630 targets Dicer, leading to increased tumor progression. Oncogene. 2016;35:4312–20. doi: 10.1038/onc.2015.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu N, Jiang F, He TL, Zhang JK, Zhao J, Wang C, Jiang GX, Cao LP, Kang PC, Zhong XY, Lin TY, Cui YF. The roles of microRNA-122 overexpression in inhibiting proliferation and invasion and stimulating apoptosis of human cholangiocarcinoma cells. Sci Rep. 2015;5:16566. doi: 10.1038/srep16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H, Yao Y, Meng F, Qian X, Jiang X, Li X, Gao Z, Gao L. Predictive value of serum miR-10b, miR-29c, and miR-205 as promising biomarkers in esophageal squamous cell carcinoma screening. Medicine (Baltimore) 2015;94:e1558. doi: 10.1097/MD.0000000000001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu K, Li C, Zheng X, Yang W, Yao Y, Liu Q. Prognostic significance of miR-218 in human hepatocellular carcinoma and its role in cell growth. Oncol Rep. 2014;32:1571–1577. doi: 10.3892/or.2014.3386. [DOI] [PubMed] [Google Scholar]
- 12.Ge C, Wu S, Wang W, Liu Z, Zhang J, Wang Z, Li R, Zhang Z, Li Z, Dong S, Wang Y, Xue Y, Yang J, Tan Q, Wang Z, Song X. miR-942 promotes cancer stem cell-like traits in esophageal squamous cell carcinoma through activation of Wnt/beta-catenin signalling pathway. Oncotarget. 2015;6:10964–10977. doi: 10.18632/oncotarget.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien K, Lowry MC, Corcoran C, Martinez VG, Daly M, Rani S, Gallagher WM, Radomski MW, MacLeod RA, O’Driscoll L. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget. 2015;6:32774–32789. doi: 10.18632/oncotarget.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders DA, Ross-Innes CS, Beraldi D, Carroll JS, Balasubramanian S. Genome-wide mapping of FOXM1 binding reveals co-binding with estrogen receptor alpha in breast cancer cells. Genome Biol. 2013;14:R6. doi: 10.1186/gb-2013-14-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed M, Hussain AR, Siraj AK, Uddin S, Al-Sanea N, Al-Dayel F, Al-Assiri M, Beg S, Al-Kuraya KS. Co-targeting of Cyclooxygenase-2 and FoxM1 is a viable strategy in inducing anticancer effects in colorectal cancer cells. Mol Cancer. 2015;14:131. doi: 10.1186/s12943-015-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N, Xie Y, Li B, Ning Z, Wang A, Cui X. FoxM1 influences mouse hepatocellular carcinoma metastasis in vitro. Int J Clin Exp Pathol. 2015;8:2771–2778. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Zhang J, Cui X, Yang Y, Li M, Qu J, Li J, Wang J. FoxM1: a novel tumor biomarker of lung cancer. Int J Clin Exp Med. 2015;8:3136–3140. [PMC free article] [PubMed] [Google Scholar]
- 18.Wu YH, Zhao H, Zhou LP, Zhao CX, Wu YF, Zhen LX, Li J, Ge DX, Xu L, Lin L, Liu Y, Liang DD, Chen YH. miR-134 modulates the proliferation of human cardiomyocyte progenitor cells by targeting meis2. Int J Mol Sci. 2015;16:25199–25213. doi: 10.3390/ijms161025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuomi JM, Voorbraak F, Jones DL, Ruijter JM. Bias in the Cq value observed with hydrolysis probe based quantitative PCR can be corrected with the estimated PCR efficiency value. Methods. 2010;50:313–322. doi: 10.1016/j.ymeth.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Lin DC, Hao JJ, Nagata Y, Xu L, Shang L, Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, Garg M, Liu LZ, Yang H, Yin D, Shi ZZ, Jiang YY, Gu WY, Gong T, Zhang Y, Xu X, Kalid O, Shacham S, Ogawa S, Wang MR, Koeffler HP. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Liu D, Liang H, Xue L, Su C, Liu M. MiR-1228 promotes breast cancer cell growth and metastasis through targeting SCAI protein. Int J Clin Exp Pathol. 2015;8:6646–6655. [PMC free article] [PubMed] [Google Scholar]
- 22.Hua K, Yang W, Song H, Song J, Wei C, Li D, Fang L. Up-regulation of miR-506 inhibits cell growth and disrupt the cell cycle by targeting YAP in breast cancer cells. Int J Clin Exp Med. 2015;8:12018–12027. [PMC free article] [PubMed] [Google Scholar]
- 23.Iwakawa HO, Tomari Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015;25:651–665. doi: 10.1016/j.tcb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Shuang T, Wang M, Shi C, Zhou Y, Wang D. Down-regulated expression of miR-134 contributes to paclitaxel resistance in human ovarian cancer cells. FEBS Lett. 2015;589:3154–3164. doi: 10.1016/j.febslet.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 25.Bao Y, Peng L, Ma J, Liu K, Li W. Decreased miR-134 expression and its tumor-suppressive function in human osteosarcoma. Genet Mol Res. 2015;14:16771–16781. doi: 10.4238/2015.December.14.4. [DOI] [PubMed] [Google Scholar]
- 26.Onoda T, Ono T, Dhar DK, Yamanoi A, Nagasue N. Tetracycline analogues (doxycycline and COL-3) induce caspase-dependent and -independent apoptosis in human colon cancer cells. Int J Cancer. 2006;118:1309–1315. doi: 10.1002/ijc.21447. [DOI] [PubMed] [Google Scholar]
- 27.Fan HX, Li HX, Chen D, Gao ZX, Zheng JH. Changes in the expression of MMP2, MMP9, and ColIV in stromal cells in oral squamous tongue cell carcinoma: relationships and prognostic implications. J Exp Clin Cancer Res. 2012;31:90. doi: 10.1186/1756-9966-31-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes-Reynosa P, Robledo T, Macias-Silva M, Wu SV, Salazar EP. Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008;27:220–231. doi: 10.1016/j.matbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Li Z, Kong X, Xie D, Jia Z, Jiang W, Cui J, Du Y, Wei D, Huang S, Xie K. Down-regulation of microRNA-494 via loss of SMAD4 increases FOXM1 and beta-catenin signaling in pancreatic ductal adenocarcinoma cells. Gastroenterology. 2014;147:485–497. e418. doi: 10.1053/j.gastro.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 30.Wang CY, Hua L, Sun J, Yao KH, Chen JT, Zhang JJ, Hu JH. MiR-211 inhibits cell proliferation and invasion of gastric cancer by down-regulating SOX4. Int J Clin Exp Pathol. 2015;8:14013–14020. [PMC free article] [PubMed] [Google Scholar]
- 31.Gormally MV, Dexheimer TS, Marsico G, Sanders DA, Lowe C, Matak-Vinkovic D, Michael S, Jadhav A, Rai G, Maloney DJ, Simeonov A, Balasubramanian S. Suppression of the FOXM1 transcriptional programme via novel small molecule inhibition. Nat Commun. 2014;5:5165. doi: 10.1038/ncomms6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Zhang G, Kong C. FOXM1 participates in PLK1-regulated cell cycle progression in renal cell cancer cells. Oncol Lett. 2016;11:2685–2691. doi: 10.3892/ol.2016.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]


