Abstract
Objective: Carbonic anhydrase XII (CA XII) is a key mediator of several signaling pathways that are involved in cancer development. This study was designed to investigate the clinical significance and prognostic value of postoperative CA XII expression in patients with hepatocellular carcinoma (HCC). Methods: Immunohistochemistry (IHC) was performed on HCC tissue (n = 90), and the relationships between CA XII expression in the HCC tissue, the clinicopathologic features, and survival were further evaluated. The mRNA expression of CA XII and clinicopathologic characteristics of patients with hepatocellular carcinoma were extracted from The Cancer Genome Atlas (TCGA) database. Results: CA XII was overexpressed in hepatocellular carcinoma tissues compared to normal liver tissues from the TCGA database. Moreover, CA XII mRNA expression was significantly associated with several clinicopathologic factors of hepatocellular carcinoma including sex (P = 0.011) and pathologic grade (P = 0.012). For HCC tissue samples in a tissue microarray (TMA), high CA XII protein expression was closely related to age (P = 0.013), tumor size (P = 0.014), and pathological grade (P = 0.015). A Kaplan-Meier analysis showed that elevated CA XII expression was associated with short disease-free survival (DFS) (P = 0.002) and overall survival (OS) (P = 0.006). In addition, a multivariate analysis showed that high CA XII expression was an independent prognostic indicator for disease-free survival (P = 0.018), but not overall survival, in HCC patients. Conclusion: This study showed that high CA XII expression was associated with poor prognosis in HCC patients.
Keywords: CA XII, HCC, TCGA, prognosis, survival
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world and accounts for 5.6% of all cancer [1]. The survival rate of HCC patients has improved in the past 30 years, but the 5-year survival rate remains low at ~15% in the US [2] and ~5% in underdeveloped countries [3]. The GLOBOCAN estimates showed that in 2012, over 700,000 patients died from HCC worldwide, making HCC the second deadliest cancer [3]. Common treatments for HCC, such as chemotherapy, radiotherapy, and surgery, have led to significant progress; however, most HCC patients are already at an advanced stage of disease at the time of diagnosis due to the lack of apparent symptoms in the early stage. Moreover, HCC tends to progress rapidly with early infiltration, which results in a poor prognosis [4-8]. Therefore, prognostic evaluation plays a key role in detecting the natural progression of these tumors [9]. To date, few studies have investigated prognostic biomarkers of HCC [10-12]. Thus, more reliable diagnostic and prognostic biomarkers of HCC must be investigated to provide better treatment options for patients with HCC.
Carbonic anhydrase (CA) XII is a transmembrane zinc metalloenzyme that promotes the reversible hydration of carbon dioxide to form bicarbonates and is thus involved in acidification of the microenvironment. CA XII has been shown to contribute to cancer cell survival, promote cancer cell invasion and migration, and maintain cancer cell pluripotency [13-15]. Anomalous high CA XII expression has been observed in a variety of cancers, such as non-small cell lung cancer [16], estrogen-dependent breast cancer [17], brain tumors [18], and hepatic fibrolamellar carcinoma (FLC) [19]. Studies have shown that CA XII upregulation is associated with an adverse prognosis in patients with colorectal cancer, oral squamous cell carcinoma, renal cancer, and brain cancer [13,18,20-22]. Recent studies have shown that the expression of CA XII is also one of the most important independent postoperative prognostic factors after radical esophagectomy in patients with advanced esophageal squamous cell carcinoma (ESCC) [23]. Studies have shown that CA XII expression may be a potential prognostic factor, but no consensus has been reached about its prognostic value in cancers; for certain types of cancers, such as lung cancer, cervical cancer, and breast cancer, CA XII is associated with a favorable prognosis [16,24-26]. Recent studies have shown that CA XII expression is high in normal endometrium and low in endometrial adenocarcinoma [27]. Moreover, CA XII expression has no prognostic value in other cancers such as gastric cancer [28]. Therefore, more research is needed to investigate the prognostic value of CA XII in human cancers, including HCC.
No studies have investigated the associated clinicopathologic characteristics and prognostic value of CA XII expression in HCC. We hypothesized that CA XII may be a prognostic factor in HCC. This study was designed to explore the relationship between CA XII expression and poor prognosis in HCC patients. In this study, we examined the relationship between CA XII expression and clinicopathologic features using the The Cancer Genome Atlas (TCGA) database and tissue microarrays (TMAs) and evaluated the prognostic value of CA XII by Kaplan-Meier and Cox regression analyses. For the first time, CA XII was shown to be an independent prognostic factor for disease-free survival (DFS) in HCC, which indicates that CA XII may be a new potential marker related to HCC progression and warrants further study.
Materials and methods
Data source and pretreatment
The hepatocellular carcinoma mRNA expression data were acquired from the TCGA (https://portal.gdc.cancer.gov/) database, from which, 374 hepatocellular carcinoma samples and 50 normal liver samples were obtained. After excluding cases with inadequate clinical information, the data from 335 patients with hepatocellular carcinoma was combined with their complete detailed clinical information. The R language package edgeR (http://bioconductor.org/biocLite.R,biocLite (“edgeR”)) was used to calculate the differentially expressed genes (DEGs) using the settings fold-change > 1 and P < 0.05 as the cutoff lines. The TCGA original HTSeq-Count data were processed using the trimmed mean of M-values (TMM) method for homogenization with edgeR. We used the log2 transformation to convert expression levels of CA XII for further analysis. The gene microarray data (GSE39791, GSE63898, GSE14323, GSE89377, GSE54236) were downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/).
HCC tissue microarray construction
Ninety patients with HCC were enrolled to construct TMAs, provided by Shanghai Outdo Biotech Co., Ltd. (Shanghai, China), to perform IHC analysis. Notable clinical data were provided such as sex, age, tumor size, tumor encapsulation, number of tumors, pathologic grade, hepatitis B virus infection, liver cirrhosis and tumor node metastasis (TNM) stage from the TMA data provided by the Shanghai Outdo Biotech Co., Ltd. No patients received any form of treatment (radiation therapy, chemotherapy, or immunotherapy) before surgery. We obtained informed consent from each patient, and ethical approval to conduct the research was provided by the ethical research committee of each local hospital.
IHC analysis of the HCC TMAs
Immunohistochemical detection was performed as described in a previous study [29,30]. Rabbit polyclonal anti-CA XII antibody (1:200, ab230515, Abcam, Cambridge, MA) was first added to the plates and incubated with the TMA. After washing with phosphate-buffered saline (PBS), the slides were incubated with anti-rabbit secondary IgG antibody. PBS was used instead of the primary anti-CA XII antibody as a negative control. The IHC score ranged from 0-12, which was generated by the number of stained cells multiplied by the cell staining intensity. The density of CA XII in the stained cells was graded as follows: 0 (negative staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The CA XII staining percentage was categorized as follows: 0 (0%), 1 (1%-10%), 2 (11%-50%), 3 (51%-75%), and 4 (76%-100%). Finally, the median value of the IHC score (4.5) was chosen as the cutoff value to define the high and low expression groups. A score of 0-4 points was considered low CA XII expression, while a score > 4 points was considered high CA XII expression.
Statistical analysis
The SPSS software package, version 18 (SPSS Inc., Chicago, IL, USA) and STATA 14.0 (Stata Corporation, College Station, TX, USA) were used for statistical analyses. Data are presented as the mean ± standard deviation. Statistical analysis was performed on the differences between the two groups using a paired or unpaired t-test, and the non-normally distributed data were analyzed using the Mann-Whitney U test. The Kruskal-Wallis test, followed by the Mann-Whitney U test, was performed to compare multiple groups. The Kaplan-Meier method was used to generate survival curves, and the differences were analyzed using the log-rank test. A Cox regression model was established for the multivariate survival analysis to determine the prognostic factors that were significant in the univariate analysis for either DFS or overall survival (OS). Significance was considered P < 0.05.
Results
Elevated CA XII mRNA expression in HCC
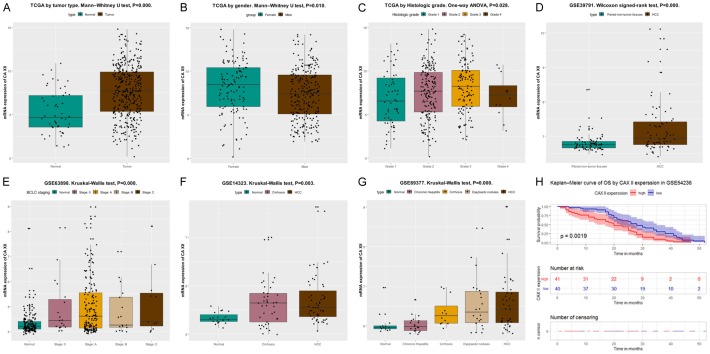
To investigate the role of CA XII in HCC, we evaluated CA XII mRNA expression in HCC based on the TCGA database. RNA-Seq data showed that CA XII expression was significantly elevated in HCC tissues compared to normal liver tissues (P = 0.000) (Figure 1A). There were also differences in CA XII expression between groups stratified by sex (Figure 1B, P = 0.010) and tumor pathologic grade (Figure 1C, P = 0.028). CA XII mRNA expression differed for different types of liver tissues in the GSE39791 (Figure 1D, P = 0.000), GSE63898 (Figure 1E, P = 0.000), GSE14323 (Figure 1F, P = 0.003), and GSE89377 (Figure 1G, P = 0.000) datasets.
Figure 1.
CA XII mRNA is overexpressed in the HCC cohort from the TCGA and GEO databases. The mRNA levels of CA XII in HCC samples and normal counterpart tissues were compared in a cohort of patients from the TCGA (A, P = 0.000). CA XII expression in the groups stratified by sex (B, P = 0.010) and tumor pathologic grade (C, P = 0.028). CA XII mRNA expression in HCC is significantly elevated compared with that in normal tissues, as observed in the microarrays GSE39791 (D, P = 0.000), GSE63898 (E, P = 0.000), GSE14323 (F, P = 0.003), and GSE89377 (G, P = 0.000). The original data were plotted using the statistical software R (version 3.5.2). The impact of CA XII expression on OS in HCC patients in the GSE54236 dataset (H, P = 0.002, log-rank test). HCC, hepatocellular carcinoma; GEO: Gene Expression Omnibus; TCGA: The Cancer Genome Atlas; OS, overall survival.
Correlation between CA XII and the clinicopathological features of HCC
We investigated the relationship between CA XII expression in HCC samples and the widely recognized clinicopathologic features to explore the role of CA XII in HCC. We collected data from 335 patients with hepatocellular carcinoma, after excluding cases with inadequate clinical information, from the TCGA database. The results from the TCGA cohort indicated that CA XII expression was associated with sex (P = 0.011) and pathologic grade (P = 0.012) (Table 1). Similar results were found in the TMAs. High CA XII expression in HCC was related to age (P = 0.013), tumor size (P = 0.014), and pathologic grade (P = 0.015) (Table 1). In contrast, high CA XII expression was unrelated to tumor encapsulation, liver cirrhosis, HBsAg level, number of tumors, and TNM stage (all P > 0.05). IHC showed that positive staining for CA XII was primarily localized to the cytoplasm and cell membrane (Figure 2).
Table 1.
Relationship between CA XII overexpression and clinicopathologic characteristics of HCC patients in TMA specimens and TCGA data
| Clinical feature | TMA | TCGA | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Case | High, n (%) | χ2 | P-value | Case | Mean ± SD | P-value | |
| 90 | 37 (41.1) | 335 | |||||
| Gender | |||||||
| Male | 74 | 32 (43.2) | 0.782 | 0.377a | 227 | 1053.97±2689.60 | 0.011*,b |
| Female | 16 | 5 (31.3) | 108 | 1786.45±4145.06 | |||
| Age, years | |||||||
| < 60 | 68 | 23 (33.8) | 6.102 | 0.013*,a | 159 | 1609.59±4148.86 | 0.483b |
| ≥ 60 | 22 | 14 (63.6) | 176 | 1001.49±2086.70 | |||
| Race category | |||||||
| White | na | na | na | na | 164 | 1796.99±4097.35 | 0.297c |
| Black or African American | na | na | na | 14 | 492.52±700.84 | ||
| Asian | na | na | na | 155 | 770.56±1937.35 | ||
| American Indian or Alaska native | na | na | na | 2 | 5574.21±7879.17 | ||
| Tumor size (cm) | |||||||
| > 5 | 42 | 23 (54.8) | 6.061 | 0.014*,a | na | na | na |
| ≤ 5 | 48 | 14 (29.2) | na | na | |||
| Tumor encapsulation | |||||||
| None | 43 | 17 (39.5) | 0.085 | 0.771a | na | na | na |
| Complete | 47 | 20 (42.6) | na | na | |||
| Tumor number | |||||||
| Multiple | 16 | 10 (62.5) | 3.677 | 0.055a | na | na | na |
| Solitary | 74 | 27 (36.5) | na | na | |||
| Hepatitis B virus infection | |||||||
| Yes | 77 | 34 (44.2) | 2.041 | 0.153a | na | na | na |
| No | 13 | 3 (23.1) | na | na | |||
| Liver cirrhosis | |||||||
| Yes | 78 | 33 (42.3) | 0.346 | 0.556a | na | na | na |
| No | 12 | 4 (33.3) | na | na | |||
| Pathologic grade | |||||||
| Grade 1 | 2 | 0 (0.00) | 8.380 | 0.015*,a | 45 | 854.22±1785.68 | 0.012*,c |
| Grade 2 | 63 | 21 (33.3) | 161 | 1232.96±3525.96 | |||
| Grade 3 | 25 | 16 (64.0) | 117 | 1627.08±3408.58 | |||
| Grade 4 | 0 | 12 | 406.06±572.01 | ||||
| TNM stage | |||||||
| Stage I | 61 | 22 (36.1) | 1.991 | 0.158a | 164 | 1058.96±2840.99 | 0.130c |
| Stage II | 29 | 15 (51.7) | 83 | 1125.12±2447.09 | |||
| Stage III | 0 | 83 | 1488.47±3216.30 | ||||
| Stage IV | 0 | 5 | 8318.16±12210.66 | ||||
P < 0.05;
χ2;
Mann-Whitney U test;
Kruskal-Wallis test;
Adequate amounts of clinical data for 39 HCC patients from the TCGA were unavailable. CA XII, carbonic anhydrase XII; HCC, hepatocellular carcinoma; TMA, tissue microarray; TCGA, The Cancer Genome Atlas; SD, standard deviation; TNM, Tumor Node Metastasis.
Figure 2.
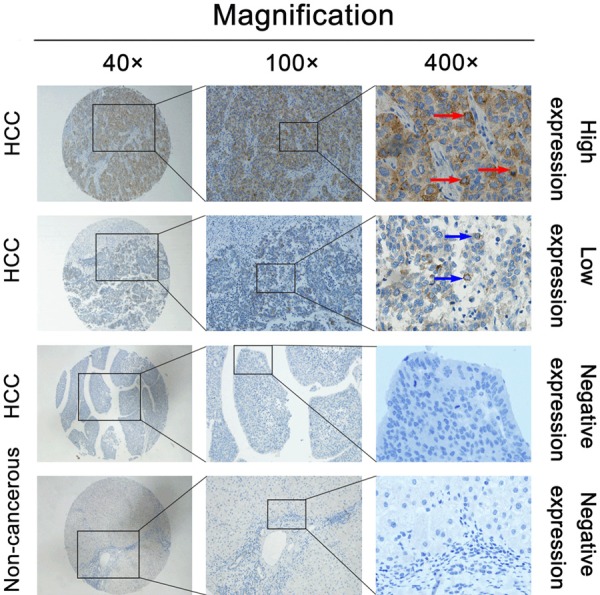
IHC detection of CA XII expression in paired HCC tissues and adjacent nontumor tissues. CA XII expression was significantly upregulated in HCC tissues. The first and second line in the figure show the high and low expression levels of CA XII in HCC tissues, respectively. The third line in the figure indicates the negative expression of CA XII protein in HCC tissues. The last line in the figure shows the negative expression of the CA XII protein in nontumor tissues adjacent to the tumor tissues. The middle and right panels contain high-magnification images of the corresponding boxed areas in the left panels. The red and blue arrows show positive staining in the cytoplasm and cell membrane of the cancer cells. IHC, immunohistochemistry.
Impact of CA XII proteins on the survival of HCC patients
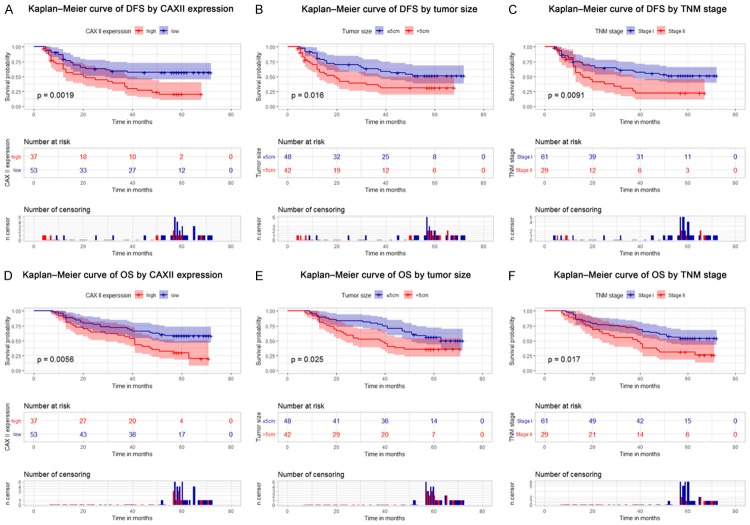
We further analyzed the relationship between CA XII protein expression and the prognosis of HCC patients. The results showed that HCC patients with high CA XII expression presented a significantly unfavorable DFS time (P = 0.002) and OS time (P = 0.006) (Figure 3A and 3D). HCC patients with tumors > 5 cm or TNM stage II had low DFS rates (P = 0.016 and P = 0.009) (Figure 3B and 3C) and OS rates (P = 0.025 and P = 0.017) (Figure 3E, 3F). A Cox regression univariate analysis demonstrated that among the clinicopathologic features, CA XII expression, tumor size, and TNM stage were related to DFS and OS (Tables 2 and 3). A multivariate analysis revealed that CA XII (P = 0.018) indicated poor DFS (Table 2), but CA XII expression (P = 0.060) was not an independent risk factor for OS in HCC patients (Table 3). Validation of the survival analysis with the GSE54236 dataset is shown in Figure 1H.
Figure 3.
High CA XII expression is associated with poor outcomes for patients with HCC. The Kaplan-Meier analysis revealed significant differences in DFS (A, P = 0.002) and OS (D, P = 0.006) between postoperative patients with high and low CA XII expression in the HCC cohort. The survival analysis of HCC patients was performed by the Kaplan-Meier method. Patients with tumors > 5 cm or TNM stage II experienced poor DFS (B, P = 0.016 and C, P = 0.009) and OS rates (E, P = 0.025 and F, P = 0.017). The P-values were determined using the log-rank test. DFS, disease-free survival.
Table 2.
Univariate and multivariate analyses of the predictors for disease-free survival in HCC patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | P > |z| | 95% CI | HR | P > |z| | 95% CI | |
| CA XII expression | 2.35 | |||||
| High versus Low | 0.003* | 1.337-4.140 | 2.01 | 0.018* | 1.126-3.584 | |
| Gender | ||||||
| Male versus Female | 1.75 | 0.172 | 0.784-3.896 | |||
| Age (years) | 1.04 | |||||
| ≥ 60 versus < 60 | 0.894 | 0.561-1.940 | ||||
| Tumor size (cm) | ||||||
| > 5 versus ≤ 5 | 1.96 | 0.020* | 1.114-3.445 | 1.55 | 0.139 | 0.868-2.767 |
| Tumor encapsulation | ||||||
| None versus Complete | 1.11 | 0.718 | 0.632-1.944 | |||
| Tumor number | ||||||
| Multiple versus Solitary | 1.70 | 0.124 | 0.866-3.329 | |||
| Hepatitis B virus infection | ||||||
| Yes versus No | 1.24 | 0.578 | 0.581-2.649 | |||
| Liver cirrhosis | ||||||
| Yes versus No | 1.07 | 0.868 | 0.480-2.383 | |||
| Pathologic grade | ||||||
| Grade versus and 2 versus Grade 3 | 0.83 | 0.521 | 0.475-1.459 | |||
| TNM stage | ||||||
| Stage I versus Stage II | 0.48 | 0.012* | 0.272-0.852 | 0.54 | 0.039* | 0.306-0.971 |
P < 0.05.
HR, hazard ratio; 95% CI, 95% confidence interval.
Table 3.
Univariate and multivariate analyses of the predictors of overall survival in HCC patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | P > |z| | 95% CI | HR | P > |z| | 95% CI | |
| CA XII expression | ||||||
| High versus Low | 2.17 | 0.007* | 1.232-3.818 | 1.77 | 0.060 | 0.976-3.213 |
| Gender | ||||||
| Male versus Female | 1.65 | 0.221 | 0.740-3.672 | |||
| Age (years) | ||||||
| ≥ 60 versus < 60 | 1.27 | 0.445 | 0.684-2.372 | |||
| Tumor size (cm) | ||||||
| > 5 versus ≤ 5 | 1.88 | 0.029* | 1.068-3.315 | 1.50 | 0.183 | 0.826-2.713 |
| Tumor encapsulation | ||||||
| None versus Complete | 1.01 | 0.967 | 0.577-1.774 | |||
| Tumor number | ||||||
| Multiple versus Solitary | 1.56 | 0.197 | 0.795-3.053 | |||
| Hepatitis B virus infection | ||||||
| Yes versus No | 1.36 | 0.429 | 0.636-2.899 | |||
| Liver cirrhosis | ||||||
| Yes versus No | 1.26 | 0.576 | 0.563-2.805 | |||
| Pathologic grade | ||||||
| Grade 2 versus Grade 3 | 0.90 | 0.713 | 0.513-1.578 | |||
| TNM stage | ||||||
| Stage I versus Stage II | 0.51 | 0.020* | 0.287-0.898 | 0.57 | 0.053 | 0.319-1.007 |
P < 0.05.
Discussion
Human carbonic anhydrases (hCAs, EC 4.2.1.1) are metalloenzymes that regulate certain physiologic functions, such as electrolyte secretion, pH, biosynthesis, and carbon dioxide/bicarbonate transport, and therefore affect many metabolic processes [31,32]. hCAs are classified into at least 15 subtypes based on their tissue specificity, subcellular localization, and molecular characteristics. CA XII is a hypoxia-induced membrane-bound enzyme that is highly expressed in various types of solid tumors such as colon cancer, breast cancer, and glioma [33]. Hypoxia shifts metabolism to glycolysis and anaerobic fermentation and increases the level of lactic acid [33]. CA XII promotes tumor progression by maintaining an acidic extracellular pH and an alkaline intracellular pH in quiescent cells, which upregulates the expression of proteases, angiogenic factors, and cell growth factors and compromises immune function [34-38]. Liu et al. [39] showed that CA XII was one of the key factors involved in the development of laryngeal cancer by different pathways, such as glycolysis/gluconeogenesis and nitrogen metabolism, which suggests that CA XII plays a key role in laryngeal cancer. Tafreshi et al. [34] performed RT-PCR and immunocytochemistry (ICC) to determine CA XII expression levels in human breast cancer cell lines (MCF7, MDA-mb231, ZR-75.1, MCF10A, and DCIS) under normoxic and hypoxic conditions. The results showed that CA XII expression on the cell surface of tumor cells was related to tumor hypoxia. Gondi et al. [40] used a monoclonal antibody (6A10) that binds CA XII to show that this antibody inhibited CA surface activity in CA XII-expressing cancer cells. In vitro studies also demonstrated that the antibody exerted a growth inhibitory effect by acting on the catalytic process rather than by acting on antigen binding. Moreover, 6A10 significantly delayed tumor growth in a mouse xenograft model of human tumors. These results demonstrate that CA XII plays a key role in the physiology of cancer cells [40,41]. Recent studies have shown that CA XII expression was increased on the surface of chemotherapy-resistant cells, which indicates that CA XII may be a potential therapeutic target that may be used to overcome chemotherapy resistance in tumor cells [42]. Recently, CA XII has been considered a prognostic marker or a potential drug target in tumors due to its confirmed role in tumor progression, metastasis, acidification, and tumor cell signaling during tumorigenesis [43,44]. Many preclinical studies have been conducted to investigate CA XII-targeted inhibitors and biologics, and many promising anticancer agents have been developed to inhibit CA activity [45,46]. Nevertheless, detailed information about the role of CA XII in HCC, especially its prognostic value, is still limited.
In this study, we first downloaded the hepatocellular carcinoma RNA-Seq data from the TCGA database. Then, microarray data were downloaded from the GEO database. The results showed that the HCC specimens had higher mRNA levels of CA XII than normal nontumor tissues. Next, we constructed an HCC TMA, and IHC showed that CA XII expression was drastically higher in HCC cells than in noncancerous cells. Dinh et al. [19] performed western blotting and IHC and showed that CA XII was specifically overexpressed in fibrolamellar carcinoma (FLC). These results are consistent with and support the findings of this study. In addition, we employed TMAs to further investigate the relationship between CA XII expression and the HCC clinicopathologic features. At the mRNA level, CA XII expression was significantly correlated with sex and pathological grade. At the protein level, CA XII expression was significantly associated with age, tumor size, and pathologic grade. Ochi et al. [23] conducted a study with 70 patients with primary ESCC and demonstrated that CA XII was related to the PT category and venous infiltration.
The Kaplan-Meier analysis revealed that high CA XII expression was associated with short DFS and OS in HCC patients, which suggests that CA XII may be a promising prognostic biomarker for predicting DFS and OS. With the survival analysis, this study showed that CA XII expression was a risk factor for a poor prognosis in terms of DFS for HCC patients. Viikilä et al. [20] showed that CA XII expression was related to the survival rate of patients with colorectal cancer and that high CA XII expression was associated with a poor prognosis, consistent with the findings of this study.
This study has some limitations. For example, we did not collect clinical data on the history of alcohol consumption or HCV infections for the HCC patients, and these factors are considered important in the etiology of HCC. In addition, this study was based on TMA specimens, which were limited and lacked information on the TNM stage III and IV specimens; we will improve the clinical data collection methods in future studies. More research is needed to determine the molecular mechanisms of CA XII in the development of HCC.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Malek NP, Schmidt S, Huber P, Manns MP, Greten TF. The diagnosis and treatment of hepatocellular carcinoma. Dtsch Arztebl Int. 2014;111:101–106. doi: 10.3238/arztebl.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, Mazzaferro V, Wiest R, Reig M, Wagner A, Bolondi L. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412–3419. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 7.Daher S, Massarwa M, Benson AA, Khoury T. Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J Clin Transl Hepatol. 2018;6:69–78. doi: 10.14218/JCTH.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gramont A, Watson S, Ellis LM, Rodon J, Tabernero J, de Gramont A, Hamilton SR. Pragmatic issues in biomarker evaluation for targeted therapies in cancer. Nat Rev Clin Oncol. 2015;12:197–212. doi: 10.1038/nrclinonc.2014.202. [DOI] [PubMed] [Google Scholar]
- 10.Giannini EG, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M, Benvegnu L, Caturelli E, Zoli M, Borzio F, Chiaramonte M, Trevisani F. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015;61:184–190. doi: 10.1002/hep.27443. [DOI] [PubMed] [Google Scholar]
- 11.Li KW, Li X, Wen TF, Lu WS. The effect of postoperative TACE on prognosis of HCC: an update. Hepatogastroenterology. 2013;60:248–251. doi: 10.5754/hge12665. [DOI] [PubMed] [Google Scholar]
- 12.Petrizzo A, Mauriello A, Tornesello ML, Buonaguro FM, Tagliamonte M, Buonaguro L. Cellular prognostic markers in hepatitis-related hepatocellular carcinoma. Infect Agent Cancer. 2018;13:10. doi: 10.1186/s13027-018-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lounnas N, Rosilio C, Nebout M, Mary D, Griessinger E, Neffati Z, Chiche J, Spits H, Hagenbeek TJ, Asnafi V, Poulsen SA, Supuran CT, Peyron JF, Imbert V. Pharmacological inhibition of carbonic anhydrase XII interferes with cell proliferation and induces cell apoptosis in T-cell lymphomas. Cancer Lett. 2013;333:76–88. doi: 10.1016/j.canlet.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh MJ, Chen KS, Chiou HL, Hsieh YS. Carbonic anhydrase XII promotes invasion and migration ability of MDA-MB-231 breast cancer cells through the p38 MAPK signaling pathway. Eur J Cell Biol. 2010;89:598–606. doi: 10.1016/j.ejcb.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Parkkila S, Parkkila AK, Saarnio J, Kivela J, Karttunen TJ, Kaunisto K, Waheed A, Sly WS, Tureci O, Virtanen I, Rajaniemi H. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem. 2000;48:1601–1608. doi: 10.1177/002215540004801203. [DOI] [PubMed] [Google Scholar]
- 16.Ilie MI, Hofman V, Ortholan C, Ammadi RE, Bonnetaud C, Havet K, Venissac N, Mouroux J, Mazure NM, Pouyssegur J, Hofman P. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int J Cancer. 2011;128:1614–1623. doi: 10.1002/ijc.25491. [DOI] [PubMed] [Google Scholar]
- 17.Tozlu S, Girault I, Vacher S, Vendrell J, Andrieu C, Spyratos F, Cohen P, Lidereau R, Bieche I. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr Relat Cancer. 2006;13:1109–1120. doi: 10.1677/erc.1.01120. [DOI] [PubMed] [Google Scholar]
- 18.Proescholdt MA, Mayer C, Kubitza M, Schubert T, Liao SY, Stanbridge EJ, Ivanov S, Oldfield EH, Brawanski A, Merrill MJ. Expression of hypoxia-inducible carbonic anhydrases in brain tumors. Neuro Oncol. 2005;7:465–475. doi: 10.1215/S1152851705000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinh TA, Vitucci EC, Wauthier E, Graham RP, Pitman WA, Oikawa T, Chen M, Silva GO, Greene KG, Torbenson MS, Reid LM, Sethupathy P. Comprehensive analysis of the cancer genome atlas reveals a unique gene and non-coding RNA signature of fibrolamellar carcinoma. Sci Rep. 2017;7:44653. doi: 10.1038/srep44653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viikila P, Kivela AJ, Mustonen H, Koskensalo S, Waheed A, Sly WS, Pastorek J, Pastorekova S, Parkkila S, Haglund C. Carbonic anhydrase enzymes II, VII, IX and XII in colorectal carcinomas. World J Gastroenterol. 2016;22:8168–8177. doi: 10.3748/wjg.v22.i36.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien MH, Ying TH, Hsieh YH, Lin CH, Shih CH, Wei LH, Yang SF. Tumor-associated carbonic anhydrase XII is linked to the growth of primary oral squamous cell carcinoma and its poor prognosis. Oral Oncol. 2012;48:417–423. doi: 10.1016/j.oraloncology.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Haapasalo J, Hilvo M, Nordfors K, Haapasalo H, Parkkila S, Hyrskyluoto A, Rantala I, Waheed A, Sly WS, Pastorekova S, Pastorek J, Parkkila AK. Identification of an alternatively spliced isoform of carbonic anhydrase XII in diffusely infiltrating astrocytic gliomas. Neuro Oncol. 2008;10:131–138. doi: 10.1215/15228517-2007-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochi F, Shiozaki A, Ichikawa D, Fujiwara H, Nakashima S, Takemoto K, Kosuga T, Konishi H, Komatsu S, Okamoto K, Kishimoto M, Marunaka Y, Otsuji E. Carbonic anhydrase XII as an independent prognostic factor in advanced esophageal squamous cell carcinoma. J Cancer. 2015;6:922–929. doi: 10.7150/jca.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi M, Matsumoto T, Ryuge S, Yanagita K, Nagashio R, Kawakami Y, Goshima N, Jiang SX, Saegusa M, Iyoda A, Satoh Y, Masuda N, Sato Y. CAXII is a sero-diagnostic marker for lung cancer. PLoS One. 2012;7:e33952. doi: 10.1371/journal.pone.0033952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo CW, Nam BH, Kim JY, Shin HJ, Lim H, Lee S, Lee SK, Lim MC, Song YJ. Carbonic anhydrase XII expression is associated with histologic grade of cervical cancer and superior radiotherapy outcome. Radiat Oncol. 2010;5:101. doi: 10.1186/1748-717X-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnett DH, Sheng S, Charn TH, Waheed A, Sly WS, Lin CY, Liu ET, Katzenellenbogen BS. Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res. 2008;68:3505–3515. doi: 10.1158/0008-5472.CAN-07-6151. [DOI] [PubMed] [Google Scholar]
- 27.Hynninen P, Parkkila S, Huhtala H, Pastorekova S, Pastorek J, Waheed A, Sly WS, Tomas E. Carbonic anhydrase isozymes II, IX, and XII in uterine tumors. APMIS. 2012;120:117–129. doi: 10.1111/j.1600-0463.2011.02820.x. [DOI] [PubMed] [Google Scholar]
- 28.Leppilampi M, Saarnio J, Karttunen TJ, Kivela J, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S. Carbonic anhydrase isozymes IX and XII in gastric tumors. World J Gastroenterol. 2003;9:1398–1403. doi: 10.3748/wjg.v9.i7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu M, Fan W, Pu X, Ni H, Zhang W, Chang F, Gong L, Xiong L, Wang J, Gu X. Elevated expression of SHIP2 correlates with poor prognosis in non-small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2185–2191. [PMC free article] [PubMed] [Google Scholar]
- 30.Fu M, Gu X, Ni H, Zhang W, Chang F, Gong L, Chen X, Li J, Qiu L, Shi C. High expression of inositol polyphosphate phosphatase-like 1 associates with unfavorable survival in hepatocellular carcinoma. Int J Clin Exp Pathol. 2013;6:2515–2522. [PMC free article] [PubMed] [Google Scholar]
- 31.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 32.Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem. 2016;31:345–360. doi: 10.3109/14756366.2015.1122001. [DOI] [PubMed] [Google Scholar]
- 33.Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites. 2017;7 doi: 10.3390/metabo7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tafreshi NK, Lloyd MC, Proemsey JB, Bui MM, Kim J, Gillies RJ, Morse DL. Evaluation of CAIX and CAXII expression in breast cancer at varied O2 levels: CAIX is the superior surrogate imaging biomarker of tumor hypoxia. Mol Imaging Biol. 2016;18:219–231. doi: 10.1007/s11307-015-0885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz L, Seyfried T, Alfarouk KO, Da Veiga Moreira J, Fais S. Out of warburg effect: an effective cancer treatment targeting the tumor specific metabolism and dysregulated pH. Semin Cancer Biol. 2017;43:134–138. doi: 10.1016/j.semcancer.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Spugnini EP, Sonveaux P, Stock C, Perez-Sayans M, De Milito A, Avnet S, Garcia AG, Harguindey S, Fais S. Proton channels and exchangers in cancer. Biochim Biophys Acta. 2015;1848:2715–2726. doi: 10.1016/j.bbamem.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008-2013) Expert Opin Ther Pat. 2013;23:737–749. doi: 10.1517/13543776.2013.798648. [DOI] [PubMed] [Google Scholar]
- 38.Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev. 2014;33:1095–1108. doi: 10.1007/s10555-014-9531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F, Du J, Liu J, Wen B. Identification of key target genes and pathways in laryngeal carcinoma. Oncol Lett. 2016;12:1279–1286. doi: 10.3892/ol.2016.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gondi G, Mysliwietz J, Hulikova A, Jen JP, Swietach P, Kremmer E, Zeidler R. Antitumor efficacy of a monoclonal antibody that inhibits the activity of cancer-associated carbonic anhydrase XII. Cancer Res. 2013;73:6494–6503. doi: 10.1158/0008-5472.CAN-13-1110. [DOI] [PubMed] [Google Scholar]
- 41.Battke C, Kremmer E, Mysliwietz J, Gondi G, Dumitru C, Brandau S, Lang S, Vullo D, Supuran C, Zeidler R. Generation and characterization of the first inhibitory antibody targeting tumour-associated carbonic anhydrase XII. Cancer Immunol Immunother. 2011;60:649–658. doi: 10.1007/s00262-011-0980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopecka J, Campia I, Jacobs A, Frei AP, Ghigo D, Wollscheid B, Riganti C. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget. 2015;6:6776–6793. doi: 10.18632/oncotarget.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cadoni R, Pala N, Lomelino C, Mahon BP, McKenna R, Dallocchio R, Dessi A, Carcelli M, Rogolino D, Sanna V, Rassu M, Iaccarino C, Vullo D, Supuran CT, Sechi M. Exploring heteroaryl-pyrazole carboxylic acids as human carbonic anhydrase xii Inhibitors. ACS Med Chem Lett. 2017;8:941–946. doi: 10.1021/acsmedchemlett.7b00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baig MH, Adil M, Khan R, Dhadi S, Ahmad K, Rabbani G, Bashir T, Imran MA, Husain FM, Lee EJ, Kamal MA, Choi I. Enzyme targeting strategies for prevention and treatment of cancer: implications for cancer therapy. Semin Cancer Biol. 2019;56:1–11. doi: 10.1016/j.semcancer.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 45.De Luca L, Mancuso F, Ferro S, Buemi MR, Angeli A, Del Prete S, Capasso C, Supuran CT, Gitto R. Inhibitory effects and structural insights for a novel series of coumarin-based compounds that selectively target human CA IX and CA XII carbonic anhydrases. Eur J Med Chem. 2018;143:276–282. doi: 10.1016/j.ejmech.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 46.Ivanova J, Carta F, Vullo D, Leitans J, Kazaks A, Tars K, Zalubovskis R, Supuran CT. N-Substituted and ring opened saccharin derivatives selectively inhibit transmembrane, tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem. 2017;25:3583–3589. doi: 10.1016/j.bmc.2017.04.007. [DOI] [PubMed] [Google Scholar]