Abstract
Introduction: Solitary fibrous tumor (SFT) is a rare mesenchymal tumor. Due to the rarity of malignant solitary fibrous tumor of the liver, information regarding the disease is currently limited. We present herein a case of malignant liver SFT in a 17-year-old female, who was misdiagnosed with hepatoblastoma preoperatively. Case report: A 17-year-old female who was diagnosed with hepatoblastoma preoperatively The patient presented with pain in the upper abdomen and an abdominal mass. Tumor markers were normal and imaging findings were atypical. The tumor was successfully removed by surgery. Postoperative pathological examination and immunohistochemistry confirmed malignant solitary fibrous tumor. The patient recovered uneventfully and is disease-free without recurrence at the time of this report (14 months post-surgery). Conclusion: SFT originates in the liver and is a rare tumor. Differential diagnosis should be considered for liver lesions with atypical imaging findings. More data are needed to understand the disease’s long-term outcome and identify clinical and radiologic features that can be useful for its diagnosis. The best choice for treatment is complete surgical resection, and definitive diagnosis based on histologic and immunohistochemical characteristics. Tumor biology is unclear, and long-term follow-up of SFT patients is critical.
Keywords: Malignant, solitary fibrous tumor, liver, mesenchymal neoplasm, surgical resection
Introduction
Solitary fibrous tumor is a rare mesenchymal tumor. SFTs reported in the literature mostly occurred in the thoracic cavity and pleura, but there were cases of SFTs involving extrthoracic organs [1-3]. More than 80% of SFTs were benign, asymptomatic and slow-growing tumors [4,5]. However, excessive involvement of important structures may lead to accessory tumors and local symptoms [1,2,6,7]. The diagnosis is usually made by histopathologic examination and immunohistochemical examination of the excised sample. Preoperative examination of SFT is difficult because of its nonspecific radiologic characteristics. Biopsy of radiologic liver lesions remains controversial due to the risk of inconclusive results [2,3] or seeding of the biopsy tract [6]. Surgery is still the best option for treatment, and the benefits of adjuvant therapy are poorly understood in rare cases with malignant histologic manifestations [2,8,9]. Herein, we report a very rare case of a malignant SFT of the liver.
Case report
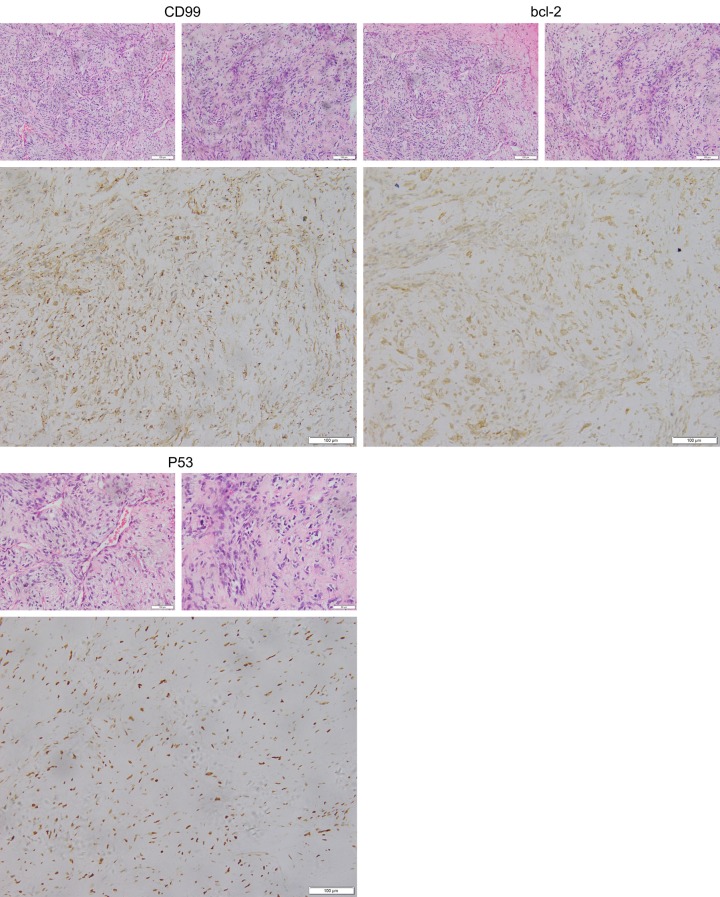
A 17-year-old female complained of recurrent pain and discomfort in the upper abdomen for half a year, and had an abdominal mass for 2 months. Upon admission, physical examination revealed a large, hard mass of about coconut size in the upper abdomen. Laboratory tests showed that tumor-related antigen test, surface markers of hepatitis B and C, blood clotting test, liver function and blood glucose were normal. Contrast-enhanced CT angiography and three-dimensional angiography of the abdomen suggested that there was a large mass shadow in the left lobe of the liver, the size was about 21.0 × 17.3 × 11.8 cm, the edge was clear, and the enhancement was uneven. Multiple enhanced vascular shadows were seen internally, with invasion of the left portal branch. The hepatic, pancreatic and gastric cavities were compressed, and there was no definite abnormality in the pancreatic density (Figures 1, 2). The presence of distant metastasis was excluded by chest CT and whole-body bone scan. Open surgery was performed. During the operation, it was found that the tumor communicated extensively with the left liver and did not penetrate the adjacent organs, suggesting hepatic origin. Intraoperative findings: the tumor originated from the outer lobe of the left liver, with intact capsule, about 21 × 15 × 12 cm, with obvious compression of the gastric cavity and pancreas, adhesion to part of the stomach, smooth surface of the tumor, and visible vascular distention. The tumor was cut open and the cut surface was like fish flesh, with several compartments of different sizes (Figure 3). Postoperative pathologic examination revealed a malignant tumor in the left extrahepatic interlobular tissue, which was adjacent to the liver capsule (without invasion). Immunohistochemical detection: CD99, bcl-2, desmin, and p53 were positive, ki-67 (+, 20%), CK, CD117 and CD34 were negative. The results were more supportive of left extrahepatic malignant solitary fibrous tumor (Figure 4). The patient recovered uneventfully and is disease-free without recurrence at the time of this report (14 months post-surgery).
Figure 1.

A. Non-enhanced CT suggests that there was a huge occupying mass in the abdominal cavity, the boundary between the tumor and the left hepatic lobe was unclear, and the tumor did not break through the capsule. The low density of the cyst was seen in the tumor, which was considered to be accompanied by hemorrhage. B. Enhanced CT indicated mild heterogeneous enhancement of solid components around the tumor in arterial stage, a partial enhanced capsule was observed, and tortuous vascular shadows were found in the tumor. C. Enhanced CT venous phase indicated further expansion of tumor enhancement, with irregular necrotic areas and no contrast agent was found in the left portal vein. D. Enhanced CT suggested that the tumor density was not uniform in the delayed stage, and there was no enhancement in the low-density area, accompanied by a large number of irregular necrotic areas.
Figure 2.
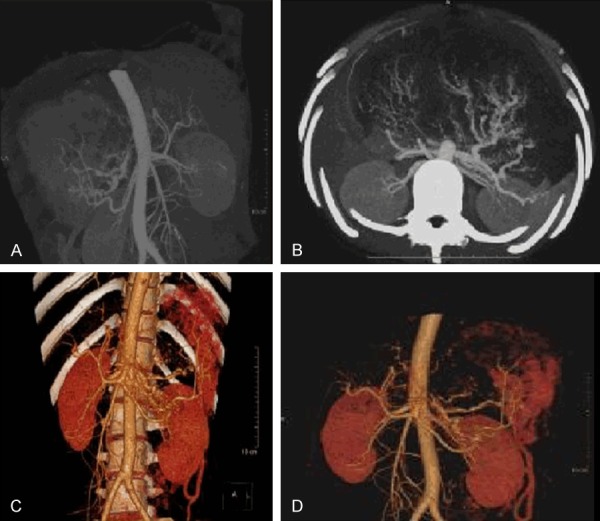
(A, B) Angiography and (C, D) Abdominal angiography and three-dimensional vascular reconstruction suggested mild heterogeneous enhancement of solid components around the tumor, a partial enhanced capsule was observed, multiple enhanced vascular shadows were found in the tumor, and the tumor did not invade other abdominal organs.
Figure 3.

Intact tumor capsule was cut open in the tumor body, with a section in the shape of fish flesh. A large number of vascular epithelial cell tumor-like expanded thin-walled vessels were observed, and several compartments of different sizes were observed in the tumor body, with liquefaction and necrosis in the center.
Figure 4.
The pathologic examination report indicated that “left extrahepatic lobe” was considered as an interlobular malignant tumor, with a size of 21 × 15 × 10 cm and adjacent to the liver capsule (no invasion). The report hints that abundant spindle cells with abundant mitoses were observed under the microscope. CD99, bcl-2 and desmin showed positive expression of p53 and negative expression of CD34 (H&E, × 400).
Discussion
SFT is also known as localized fibrous mesothelioma, single-hair mesothelioma, benign fibrous mesothelioma, localized fibroma, localized fibroma, or pleural fibroma. These classifications are based on histologic characteristics and reflect that the tumor is mainly located in the thoracic or pleural cavity [10,11]. In the liver, the origin of this tumor may be mesenchymal cells. Because of the proliferation and formation of Glisson’s capsule or intrahepatic connective tissue, it can present as a pedicle tumor [12]. Eighty percent of patients are asymptomatic at the time of diagnosis, but in some cases, as described in this article, abdominal fullness and palpable masses are present. When symptoms are present, SFT symptoms are associated with tumor effects, including pain, weight loss, and nausea. Weakness, fever, jaundice, and hypoglycemia are the less common symptoms, and the latter is thought to be a paraneoplastic syndrome of some patients. Laboratory tests are usually nonspecific and do not aid in the diagnosis of SFT. Advanced cases can lead to liver insufficiency or failure [2,8,13-22].
The imaging findings of SFT are non-specific, suggesting that other space-occupying liver lesions such as hepatocellular carcinoma, sarcoma, and inflammatory pseudotumor may have similar imaging characteristics [23]. Abdominal ultrasound imaging may reveal a definite solid heterogeneous mass. In some cases, the tumor is uniformly hyperechoic and may manifest as a cystic area [11]. Abdominal non-enhanced CT scan can reveal a definite low-attenuation and heterogeneous tumor. After contrast medium administration, CT shows hypervascular neoplasms and progressive heterogeneous enhancement. Cystic/necrotic areas within the tumor and external pseudocapsule can be distinguished. Tumors often shift adjacent organs, and compression of adjacent arteries and veins, can cause bile duct obstruction and dilation [2,8,13-20,22]. After contrast media was used, MRI findings were similar to abdominal CT findings with necrotic/cystic areas. SFT has low to medium signal intensity on T1-weighted images, and uneven signal intensity on T2-weighted images. Areas with low T2 signal intensity correspond to fibrotic components [2,8,13-20,22]. Catia Esteves et al. proposed that radiologists should identify isolated lobulated clear masses with fibrous components (low signal intensity and progressive enhancement of T2-weighted images) [24]. On PET-CT, the uptake of glucose by tumors is described as heterogeneous, and the more the uptake, the more likely the tumor is to be malignant [15]. In this case, MRI was not performed after admission, and non-enhanced abdominal CT indicated that there was a huge occupying mass in the abdominal cavity, the boundary between the tumor and the left hepatic lobe was unclear, and the tumor did not break through the capsule. Aow-density area of cyst could be seen in the tumor, which was considered to be accompanied by hemorrhage (Figure 1A). Abdominal contrast-enhanced CT showed uneven enhancement of tumor blood vessels in the arterial phase, and the range of vascular enhancement in the portal vein phase expanded from the periphery of the tumor to the center; however, there was no enhancement in the low-density necrotic area, accompanied by invasion of the left portal vein (Figure 1B-D). Abdominal angiography and three-dimensional vascular reconstruction suggested mild heterogeneous enhancement of solid components around the tumor, the partial enhanced capsule was observed, multiple enhanced vascular shadows were found in the tumor, and the tumor did not invade other abdominal organs (Figure 2). In order to confirm the preoperative diagnosis and evaluation, some scholars suggest radiotherapy-guided percutaneous liver biopsy. However, since this invasive examination is performed only on tumor biopsies or partial marginal specimens, it may be missed or misdiagnosed if the tumor is proliferating. In addition, because of its unclear nature, biopsy can lead to tumor spread along the biopsy tract [2,7,17,25]. Therefore, percutaneous liver biopsy is not recommended for the diagnosis and evaluation of SFT.
Immunohistochemistry remains the most important tool for the final diagnosis of SFT. The expression of CD34, CD99 and Bcl-2 was consistent with SFT. Immunohistochemistry was of great help in discrimination of SFT from other diseases, such as leiomyoma (SMA positive, CD34 negative), inflammatory pseudotumor (vimentin positive, CD34 negative), fibrosarcoma (CD34 negative) and gastrointestinal stromal tumor (CD117 positive, CD34 positive) [26]. The SFT of final diagnosis depends on histology and immunohistochemistry. Histologically, the tumor capsule of the patient in this case was intact, with a section in the shape of fish flesh. A large number of vascular epithelial cell tumor-like expanded thin-walled vessels were observed, and several compartments of different sizes were observed in the tumor, with liquefactive necrosis in the center (Figure 3). Immunohistochemistry showed spindle cell proliferation with abundant cells, and 5 mitoses figures per 10 high porwer field could be seen. CD99, Bcl-2, Desmin, and p53 were positively expressed, while CD34 was negative (Figure 4). SFT was diagnosed in this case according to the immunohistochemical and histologic features of these features. Although most liver SFT are benign, they sometimes exhibit malignant behavior; the current World Health Organization (WHO) classification criteria of soft tissue tumors is used to identify malignant SFT. These criteria include hypercellularity, cytologic atypia, tumor necrosis, high mitotic rate (four or more mitotic figures per 10 high-power fields), and/or infiltrative margins. The resected tumor specimen from our case showed features of high cellularity with nuclear crowding, moderate to marked cellular atypia, up to five mitotic figures per 10 high-power fields, and tumor necrosis, fulfilling the WHO criteria for malignant SFT.
The treatment option for hepatic SFT is complete surgical resection. If the marginal negative excision is successful, no further treatment is required. For inoperable or incompletely resectable tumors, hepatic targeted therapy can be achieved by transcatheter arterial chemoembolization, but there is no strong evidence to support this approach [27]. Beyer et al. described a patient with SFT, initially thought to be a desmoid fibroma, who received hormone replacement therapy before the imatinib trial, but did not respond. The patient was eventually surgically resected with no apparent malignancy [28]. Maccio et al. reported a case in which two liver SFT patients received chemotherapy, but metastatic spread to the lungs was not successful, and both patients died within 5 months [22]. Chen et al. presented a case of malignant SFTL in which local extensive recurrence and metastasis occurred 6 years after radical hepatectomy [9]. Due to lack of experience and understanding of the biologic nature of the disease, the prognosis of SFT is not clear and difficult to measure, so follow-up is recommended. Surgical removal of the free edge is still the standard treatment for SFT [2,8,13-20,22]. Radiotherapy, chemotherapy, and transcatheter arterial chemoembolization have been proposed in cases where the tumor has not been complete or is unresectable [13,20,22]. Currently, there is no large series of non-surgical treatment, namely the efficacy verification of malignant SFT [2,8,13-22]. The response rates of radiotherapy and systemic chemotherapy were different [16,19,20,22]. The sensitivity of SFT to conventional chemotherapy was low [13,20,22]. Some trials using anti-angiogenic tyrosine kinase inhibitors (such as sunitinib and pazopanil) to treat SFT in different sites have achieved promising results [13,29]. The prognosis of malignant SFT is poor, and postoperative follow-up was strongly recommended [2,8,13-22].
SFT originating in the liver is a rare tumor. Differential diagnosis should be considered for liver lesions with atypical imaging findings. More data are needed to understand the disease’s long-term outcome and identify clinical and radiological features that can be useful for its diagnosis. The best choice for treatment is complete surgical resection, and definitive diagnosis based on histological and immunohistochemical characteristics. Tumor biology is unclear, and long-term follow-up of SFT patients is critical.
Disclosure of conflict of interest
None.
References
- 1.Neeff H, Obermaier R, Technau-Ihling K, Werner M, Kurtz C, Imdahl A, Hopt UT. Solitary fibrous tumour of the liver: case report and review of the literature. Langenbecks Arch Surg. 2004;389:293–8. doi: 10.1007/s00423-004-0488-5. [DOI] [PubMed] [Google Scholar]
- 2.Peng L, Liu Y, Ai Y, Liu Z, He Y, Liu Q. Skull base metastases from a malignant solitary fibrous tumor of the liver. Diagn Pathol. 2011;6:127. doi: 10.1186/1746-1596-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Zaheer A, Sandrasegaran K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: cross-sectional imaging spectrum. Radiographics. 2011;31:393–408. doi: 10.1148/rg.312105080. [DOI] [PubMed] [Google Scholar]
- 4.Filosso PL, Asioli S, Ruffini E, Rovea P, Macri’ L, Sapino A, Bretti S, Lyberis P, Oliaro A. Radical resection of a giant, invasive and symptomatic malignant solitary fibrous tumour (SFT) of the pleura. Lung Cancer. 2009;64:117–20. doi: 10.1016/j.lungcan.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JA, Rekhtman N, Chun J, Wakely PE Jr, Ali SZ. Malignant solitary fibrous tumor: cytopathologic findings and differential diagnosis. Cancer Cytopathol. 2010;118:83–9. doi: 10.1002/cncy.20069. [DOI] [PubMed] [Google Scholar]
- 6.Moran CA, Ishak KG, Goodman ZD. Solitary fibrous tumor of the liver: a clinicopathologic and immunohistochemical study of nine cases. Ann Diagn Pathol. 1998;2:19–24. doi: 10.1016/s1092-9134(98)80031-2. [DOI] [PubMed] [Google Scholar]
- 7.Sun K, Lu JJ, Teng XD, Ying LX, Wei JF. Solitary fibrous tumor of the liver: a case report. World J Surg Oncol. 2011;9:37. doi: 10.1186/1477-7819-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terkivatan T, Kliffen M, de Wilt JH, van Geel AN, Eggermont AM, Verhoef C. Giant solitary fibrous tumour of the liver. World J Surg Oncol. 2006;4:81. doi: 10.1186/1477-7819-4-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Slater K. Solitary fibrous tumour of the liver-report on metastasis and local recurrence of a malignant case and review of literature. World J Surg Oncol. 2017;15:27. doi: 10.1186/s12957-017-1102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perini MV, Herman P, D’Albuquerque LA, Saad WA. Solitary fibrous tumor of the liver: report of a rare case and review of the literature. Int J Surg. 2008;6:96–9. doi: 10.1016/j.ijsu.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.El-Khouli RH, Geschwind JF, Bluemke DA, Kamel IR. Solitary fibrous tumor of the liver: magnetic resonance imaging evaluation and treatment with transarterial chemoembolization. J Comput Assist Tomogr. 2008;32:769–71. doi: 10.1097/RCT.0b013e3181557453. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann C, Mourra N, Tubiana JM, Arrivé L. Tumeur fibreuse solitaire du foie. J Radiol. 2006;87:139–42. doi: 10.1016/s0221-0363(06)73986-5. [DOI] [PubMed] [Google Scholar]
- 13.Beltrán MA. Solitary fibrous tumor of the liver: a review of the current knowledge and report of a new case. J Gastrointest Cancer. 2015;46:333–42. doi: 10.1007/s12029-015-9769-1. [DOI] [PubMed] [Google Scholar]
- 14.Debs T, Kassir R, Amor IB, Martini F, Iannelli A, Gugenheim J. Solitary fibrous tumor of the liver: report of two cases and review of the literature. Int J Surg. 2014;12:1291–4. doi: 10.1016/j.ijsu.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Soussan M, Felden A, Cyrta J, Morère JF, Douard R, Wind P. Case 198: solitary fibrous tumor of the liver. Radiology. 2013;269:304–8. doi: 10.1148/radiol.13121315. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz S, Kirimlioglu V, Ertas E, Hilmioglu F, Yildirim B, Katz D, Mizrak B. Giant solitary fibrous tumor of the liver with metastasis to the skeletal system successfully treated with trisegmentectomy. Dig Dis Sci. 2000;45:168–174. doi: 10.1023/a:1005438116772. [DOI] [PubMed] [Google Scholar]
- 17.Fuksbrumer MS, Klimstra D, Panicek DM. Solitary fibrous tumor of the liver: imaging findings. AJR Am J Roentgenol. 2000;175:1683–1687. doi: 10.2214/ajr.175.6.1751683. [DOI] [PubMed] [Google Scholar]
- 18.Chan G, Horton PJ, Thyssen S, Lamarche M, Nahal A, Hill DJ, Marliss EB, Metrakos P. Malignant transformation of a solitary fibrous tumor of the liver and intractable hypoglycemia. J Hepatobiliary Pancreat Surg. 2007;14:595–9. doi: 10.1007/s00534-007-1210-0. [DOI] [PubMed] [Google Scholar]
- 19.Brochard C, Michalak S, Aubé C, Singeorzan C, Fournier HD, Laccourreye L, Calès P, Boursier J. A not so solitary fibrous tumor of the liver. Gastroenterol Clin Biol. 2010;34:716–720. doi: 10.1016/j.gcb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Jakob M, Schneider M, Hoeller I, Laffer U, Kaderli R. Malignant solitary fibrous tumor involving the liver. World J Gastroenterol. 2013;19:3354–3357. doi: 10.3748/wjg.v19.i21.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song L, Zhang W, Zhang Y. (18)F-FDG PET/CT imaging of malignant hepatic solitary fibrous tumor. Clin Nucl Med. 2014;39:662–4. doi: 10.1097/RLU.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 22.Maccio L, Bonetti LR, Siopis E, Palmiere C. Malignant metastasizing solitary fibrous tumors of the liver: a report of three cases. Pol J Pathol. 2015;66:72–6. doi: 10.5114/pjp.2015.51156. [DOI] [PubMed] [Google Scholar]
- 23.Ali SZ, Hoon V, Hoda S, Heelan R, Zakowski MF. Solitary fibrous tumor. A cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Cancer. 1997;81:116–21. doi: 10.1002/(sici)1097-0142(19970425)81:2<116::aid-cncr5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Esteves C, Maia T, Lopes JM, Pimenta M. Malignant solitary fibrous tumor of the liver: airp best cases in radiologic-pathologic correlation. Radiographics. 2017;37:2018–2025. doi: 10.1148/rg.2017160200. [DOI] [PubMed] [Google Scholar]
- 25.Chen JJ, Ong SL, Richards C, Garcea G, Pollard C, Berry D, Dennison A. Inaccuracy of fine-needle biopsy in the diagnosis of solitary fibrous tumour of the liver. Asian J Surg. 2008;31:195–8. doi: 10.1016/S1015-9584(08)60085-8. [DOI] [PubMed] [Google Scholar]
- 26.Changku J, Shaohua S, Zhicheng Z, Shusen Z. Solitary fibrous tumor of the liver: retrospective study of reported cases. Cancer Invest. 2006;24:132–5. doi: 10.1080/07357900500524348. [DOI] [PubMed] [Google Scholar]
- 27.Shinde RS, Gupta A, Goel M, Patkar S. Solitary fibrous tumor of the liver-an unusual entity: a case report and review of literature. Ann Hepatobiliary Pancreat Surg. 2018;22:156–158. doi: 10.14701/ahbps.2018.22.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyer L, Delpero JR, Chetaille B, Sarran A, Perrot D, Moureau-Zabotto L, Guiramand J, Bertucci F. Solitary fibrous tumor in the round ligament of the liver: a fortunate intraoperative discovery. Case Rep Oncol. 2012;5:187–94. doi: 10.1159/000338616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruzzo M, Martin-Liberal J, Messiou C, Miah A, Thway K, Alvarado R, Judson I, Benson C. Pazopanib as first line treatment for solitary fibrous tumours: the royal marsden hospital experience. Clin Sarcoma Res. 2015;5:5. doi: 10.1186/s13569-015-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]