Abstract
To investigate the association between plasma omentin-1 levels and the risk of colorectal cancer (CRC) and the pathological changes of CRC, a total of 358 colorectal adenocarcinoma patients were included in the experience group, and 286 people were included in the control group. Their levels of omentin-1, adiponectin, visfatin, leptin, and their anthropometric and metabolic parameters were determined, and we analyzed the tertile distributions in the control group, according to the different levels: low, medium, and high. The results showed that the omentin-1 levels in patients with CRC were higher than the levels in the controls [(67.28 ± 32.25) vs (33.16 ± 19.93) ng/mL, P = 0.005]. The patients with the highest concentration of omentin-1 presented significantly higher odds for CRC, adjusted for potential confounding factors for CRC (odds ratio: 5.76; 95% CI 1.81-8.95; P = 0.001). The plasma omentin-1 level in CRC yielded a receiver operating characteristic curve area of 88.4%. The optimal sensitivity and specificity were 81.2% and 69.8% in discriminating CRC from the normal control. A high omentin-1 level was significantly associated the increasing stage of colorectal adenocarcinomas and the depth of invasion (P = 0.005, 0.026, respectively). The present study suggests an increased level of omentin-1 not only was a strong risk factor for CRC but could also represent a potential biomarker for CRC stage progression and CRC diagnosis in Chinese patients.
Keywords: Omentin-1, colorectal cancer, body mass index, biomarker, colonoscopy, Chinese
Introduction
Many epidemiologic studies have shown that obesity is defined as an abnormal increase of adipose tissue, including an increase in the size and number of adipocytes, and is associated with an increased risk of many malignancies, including colorectal cancer (CRC) [1,2]. However, the potential mechanisms and relationship to carcinogenesis have not been fully elucidated.
Adipose tissue is considered an active endocrine organ that secretes active adipocytokines, participates in the regulation of physiological and pathological processes, including appetite, insulin sensitivity and resistance, inflammation, immunity, hematopoiesis, and angiogenesis. Due to the significance of the changes in cellular metabolic status in cancer and stimulation from exogenous factors on cancer cell growth, the potential influence of adipocytokines on carcinogenesis cells has become a research hotspot [3]. A multitude of in vitro tests and epidemiological studies have been carried out on adipocytokines, in order to clarify the association between obesity and CRC, and hormones such as visfatin, adiponectin, leptin, and resistin have been under investigation [4-6].
As a new member of the adipocytokines, omentin-1, also called intelectin-1, is a 34 kda rich-adipocytokine which is selectively expressed in omental adipose tissue and abundantly in plasma. There are two highly homologous isoforms of omentin, omentin-1 and omentin-2. However, omentin-1 is the major circulating form in human plasma. Decreased omentin levels are linked to increasing obesity and insulin resistance [7]. Therefore, omentin levels may be predictive of the metabolic consequence, or co-morbidities associated with obesity [8].
Recently, a series of studies indicated that omentin-1 might participate in gastrointestinal carcinogenesis [9-12]. So far, few epidemiologic studies have examined the association of the circulating levels of omentin-1 with CRC or the interaction effect of several adipocytokines on the development of CRC [13,14]. A recent study showed that higher pre-diagnostic omentin concentrations have an independent association with higher colorectal cancer risk over a mean follow-up time of 10.4 years and suggested that circulating levels of omentin-1 have a close relationship with CRC progression [14]. However, there is no available data to demonstrate the possible associations between omentin-1 and the clinicopathological variables of CRC patients.
Here, we measured the concentration of omentin-1 in plasma in middle-aged and elderly Chinese patients and attempted to investigate the association between omentin-1 levels and the risk of CRC (the known risk factors for CRC were taken into account, including plasma visfatin, adiponectin, leptin, resistin, and the anthropometric and metabolic parameters) and various clinicopathological parameters (including the site, the size, the histological grade and the stage of the tumors).
Materials and methods
Study subjects
A total of 358 Chinese patients of the Han ethnicity, age ≥ 35 years and with pathologically diagnosed colorectal adenocarcinoma confirmed by hospital pathologists, were enrolled in this study. We enrolled patients who successfully completed a total colonoscopy examination by experienced gastrointestinal physicians using video-endoscopy, between September 2007 and September 2011 at the first affiliated Hospital of Anhui Medical University. Patients with familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, previous gastrointestinal tract surgery, inflammatory bowel disease, serious liver and renal dysfunction, acute and chronic infectious disease, and those who were under dietary or drug treatment for diabetes mellitus were excluded from this study. 271 of the 358 patients were diagnosed with colon cancer, and the others were diagnosed with rectal cancer. The controls were 286 Chinese patients of the Han ethnicity, age ≥ 35 years, and without colorectal polyps or inflammatory bowel disease, who accepted a total colonoscopy because of a voluntary health check-up or occult fecal blood loss during the same period and at the same hospital, with the same exclusion criteria. For every eligible case, an attempt was made to randomly identify a control as closely as possible in time to the admission of the corresponding case (± 1 mo) and matched to the case by age (± 5 y).
This study was approved by the Ethics Committee of the first affiliated Hospital of Anhui Medical University. All patients signed an informed written consent in advance. The cancer lesions were treated appropriately by open surgical colectomy. For each case, the localization and size of the tumor, the histological grade, and the clinical and pathological stages were recorded. Histological typing, grading and tumor staging were based on the criteria of the World Health Organization (WHO) classification and the TNM system. According to the tumor localization, the samples were classified as “right-sided” (localized in the caecum or in the ascending or transverse colon) or “left-sided” (set in the descendant or sigmoid colon or in the rectum). According to tumor size, two groups were identified: the first comprised tumors ≤ 4 cm in size, and the second consisted of tumors > 4 cm in size. Local invasion was also classified into two groups, pT1-T2, and pT3-T4, respectively. Moreover, the cases were subdivided into two groups based on their histological grades, the first group comprising grade 1 and grade 2 cases, and the second group consisting of grade 3 adenocarcinomas.
All enrollees in the study were encouraged to complete a questionnaire concerning their lifestyle and personal and family medical history as described in a previous study [15]. In brief, the questionnaire inquired about their smoking habits, alcohol intake, physical activity, vegetable intake, medications (such as antihypertension drug use, aspirin, etc.), and family history of CRC and diabetes. Body weight, height, waist measurement, hip measurement, and blood pressure were recorded, and the body mass index (BMI), as well as the waist/hip ratio (WHR) were calculated.
Laboratory procedures
The blood samples were collected after overnight fasting when the endoscopic check was done, and the blood samples were preserved at -80°C until analysis. The glucose, insulin, omentin-1, visfatin, adiponectin, leptin, and resistin levels were measured in the plasma samples, and total cholesterol (TCH) and triglyceride (TG) were measured in the serum samples. Insulin resistance was calculated by the homeostatic model assessment of insulin resistance (HOMA-IR) method as follows: HOMA-IR = fasting glucose × fasting insulin/22.5, and the result is expressed in microunits/L and glucose in mmol/L.
The plasma/serum level of each factor was as follows: plasma concentrations of omentin-1, adiponectin, leptin, and visfatin were analyzed in one run using a Human ELISA Kit (enzyme-linked immunosorbent assay) (Biovision Inc., USA), respectively. The kit manufacturer has reported that intra-assay and inter-assay coefficients of variation for omentin-1 are 5.1-7.6%, 5.6-7.1%, for visfatin 4.8-8.9%, 4.6-9.3%, for adiponectin 4.9-6.8%, 5.4-7.3%, for leptin 5.5-7.8%, 5.9-10.1%, for resistin 5.9-9.9%, 5.2-11.2%, respectively. Fasting glucose was measured by the glucose oxidase method, fasting insulin was measured using a radioimmunoassay (Tianjin Depp Co., PR CHN), with an intra-assay coefficient of variation of 5.7-9.6% and an inter-assay coefficient of variation of 7.2-11.8%. TCH was measured using the cholesterol oxidase method, and TG was measured using the triglyceride oxidase method.
Statistical analysis
The SPSS statistical software package for Window version 17.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses. The measurement data is expressed as (± s), and a paired t-test was used if the two groups met the normal distribution, which does not meet the paired non-parametric test (Wilcoxon). Count data using x2 test. Test level α = 0.05. The differences in age, gender, fasting plasma glucose (FPG), fasting insulin (FINS), TCH, TG, HOMA-IR, BMI, WHR, systolic blood pressure (SBP), diastolic blood pressure (DBP), adipokines including omentin-1, visfatin, adiponectin, leptin and resistin concentrations, and several variables concerning lifestyle and personal and family medical history between patients and controls were assessed using an x2 test or a Wilcoxon rank sum test. Spearman correlation coefficients were used to evaluate the correlations between omentin-1 and other variables separately for the controls and cases. The associations of the localizations and sizes of the tumors, the histological grades, as well as the clinical and pathological stages and plasma omentin-1 levels were assessed using a Wilcoxon rank sum test. To measure the associations between omentin-1 and the other variables with the risk of CRC, we calculated the adjusted odd ratios (OR) and their 95% CI using a conditional logistic regression model. Potential confounding factors were adjusted in the logistic regression analysis, including age, SBP, DBP, BMI, WHR, FPG, FINS, HOMA-IR, TCH, TG, and adiponectin, leptin, visfatin, and resistin levels, lifestyle characteristics, medications, and family history of CRC and diabetes. In the logistic regression analysis, omentin-1 and other variables were all analyzed as categorical variables and were classified into three categories, low, medium and high, based on the tertile distributions in the control group. Only adiponectin was inversely categorized (high, medium, and low). The values of fasting insulin, HOMA-IR, omentin-1, visfatin and resistin were natural log transformed when analyzed. Receiver operating characteristic (ROC) curves were established to see if omentin-1 could be a potential biomarker for CRC. The optimal sensitivity and specificity from ROC curves were determined by a commonly used method [16]. All P-values are two-sided and values less than 0.05 were considered statistically significant.
Results
Selected characteristics of the controls and patients
There were no significant differences in mean age, sex, the distribution of the numbers of current smokers, ex-smokers, habitual alcohol drinkers, habitual NSAID users, habitual exercisers, and habitual vegetable consumers between the two groups. No significant differences with respect to SBP, DBP, BMI, WHR, HOMA-IR, and insulin, glucose, total cholesterol, triglyceride or leptin concentrations were observed between the two groups. However, plasma omentin-1, adiponectin, visfatin, and resistin concentrations were all significantly different between the two groups. The omentin-1, visfatin, and resistin concentrations in the CRC patients were all higher than those in the control subjects (P = 0.005 for omentin-1, P = 0.026 for visfatin, P = 0.018 for resistin, respectively). By contrast, the adiponectin concentrations in the patients with CRC were lower than they were in the controls (P = 0.012) (Table 1).
Table 1.
Selected characteristics of the patients with colorectal cancer and the controls n (%)
| Characteristic | CRC (n = 358) | Control (n = 286) | P-value |
|---|---|---|---|
| Age (yr) | 60.9 ± 11.8 | 58.8 ± 12.9 | 0.715 |
| Gender | |||
| Male | 202 | 169 | 0.591 |
| Female | 156 | 117 | |
| Smokers | |||
| Current- | 123 (34.36) | 82 (28.67) | 0.497 |
| Ex- | 31 (8.66) | 17 (5.94) | 0.268 |
| Alcohol | 86 (24.02) | 65 (22.72) | 0.736 |
| NSAIDs | 9 (2.51) | 4 (1.40) | 0.387 |
| Exercise | 95 (26.54) | 77 (26.92) | 0.703 |
| Vegetable | 120 (33.52) | 82 (28.67) | 0.631 |
| SBP, mmHg | 134 ± 12 | 128 ± 10 | 0.287 |
| DBP, mmHg | 83 ± 10 | 80 ± 7 | 0.301 |
| BMI (kg/m2) | 23.34 ± 1.44 | 23.11 ± 1.28 | 0.726 |
| WHR | 0.87 ± 0.08 | 0.86 ± 0.06 | 0.612 |
| FPG, mmol/L | 5.55 ± 0.31 | 4.77 ± 0.21 | 0.138 |
| FINS, mIU/mL | 11.21 ± 7.01 | 8.21 ± 4.57 | 0.193 |
| HOMA-IR | 2.77 ± 1.49 | 1.72 ± 1.02 | 0.108 |
| TCH, mmol/L | 4.59 ± 0.69 | 4.83 ± 0.81 | 0.421 |
| TG, mmol/L | 1.45 ± 0.65 | 1.46 ± 0.72 | 0.732 |
| Omentin-1 (ng/mL) | 67.28 ± 32.25 | 33.16 ± 19.93 | 0.005 |
| Adiponectin (mg/L) | 10.59 ± 3.27 | 13.26 ± 2.82 | 0.022 |
| Leptin (ng/mL) | 27.73 ± 9.19 | 22.84 ± 7.81 | 0.084 |
| Visfatin (mg/L) | 5.68 ± 1.18 | 3.94 ± 1.13 | 0.026 |
| Resistin (ng/mL) | 8.03 ± 4.99 | 5.69 ± 3.18 | 0.018 |
Age is expressed as means ± SD and the P-value was evaluated by ANOVA. The P-values were evaluated by χ2 test for current smokers, ex-smokers, habitual alcohol drinkers, users of non-steroid anti-inflammatory drugs (NSAID), habitual exercisers, and habitual consumers of vegetables and are expressed as the number of patients: controls. NSAID: non-steroid anti-inflammatory drug; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WHR, weight:hip ratio; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostatic model assessment of insulin resistance; TCH, total cholesterol; TG, triglyceride.
Correlations between omentin-1 levels and metabolic and anthropometric variables and other parameters
Among the patients with CRC, the omentin-1 levels were marginally negatively associated with WHR (r = -0.302, P = 0.059). When restricted to the control participants, the omentin-1 levels were negatively associated with FINS and WHR (r = -0.369, P = 0.041; r = -0.473, P = 0.018, respectively). We did not observe any significant correlations of the omentin-1 levels with age, SBP, DBP, fasting glucose, total cholesterol, triglyceride, fasting insulin, or adiponectin, leptin, visfatin, and resistin levels (P > 0.05) in the case and control groups (Table 2).
Table 2.
Correlations between omentin-1 levels and metabolic and anthropometric variables and other parameters
| Variables | CRC (n = 358) | Control (n = 286) | ||
|---|---|---|---|---|
|
|
|
|||
| ra | P value | ra | P value | |
| Age (yr) | -0.134 | 0.521 | -0.081 | 0.712 |
| SBP (mmHg) | 0.102 | 0.503 | 0.039 | 0.758 |
| DBP (mmHg) | 0.217 | 0.387 | 0.108 | 0.512 |
| FPG (mmol/L) | -0.085 | 0.614 | -0.052 | 0.785 |
| TCH (mmol/L) | 0.145 | 0.472 | 0.017 | 0.821 |
| TG (mmol/L) | 0.032 | 0.771 | -0.198 | 0.237 |
| FINS (mIU/L) | -0.147 | 0.346 | -0.369 | 0.041 |
| HOMA-IR | -0.164 | 0.291 | -0.186 | 0.195 |
| BMI (kg/m2) | -0.176 | 0.182 | -0.271 | 0.078 |
| WHR | -0.302 | 0.059 | -0.473 | 0.018 |
| Adiponectin (mg/L) | -0.171 | 0.284 | 0.287 | 0.086 |
| Leptin (ng/mL) | 0.238 | 0.096 | 0.095 | 0.643 |
| Visfatin (mg/L) | 0.283 | 0.065 | -0.038 | 0.761 |
| Resistin (ng/mL) | 0.164 | 0.337 | -0.122 | 0.426 |
Correlation coefficients and P values were determined using Spearman’s correlation analysis.
SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TCH, total cholesterol; TG, triglyceride; FINS, fasting insulin; HOMA-IR, homeostatic model assessment of insulin resistance; BMI, body mass index; WHR: waist:hip ratio.
The relationship between plasma omentin-1 levels and the clinicopathological features
The plasma omentin-1 levels were categorized into low (< 3.9 ng/mL) or high (≥ 3.9 ng/mL) according to a cut-off value calculated from the mean + 2SD of the values observed in the control subjects (The values of omentin-1 were natural log transformed when analyzed). Significantly positive associations were found between the plasma omentin-1 levels and a high stage of colorectal adenocarcinomas and depth of invasion (P = 0.005, 0.026, respectively). No significant correlation was observed between the plasma omentin-1 level and the other clinicopathological parameters (Table 3).
Table 3.
The relationship between plasma omentin-1 levels and clinicopathologic features
| Variables/categories | Plasma omentin-1 levelsa | |||
|---|---|---|---|---|
|
| ||||
| n | < 3.9 ng/mL | ≥ 3.9 ng/mL | P-value | |
| Gender | ||||
| Male | 202 | 90 | 112 | 0.240 |
| Female | 156 | 80 | 76 | |
| Size, cm | ||||
| ≤ 4 | 200 | 92 | 108 | 0.111 |
| > 4 | 158 | 87 | 71 | |
| Site | ||||
| Right | 187 | 96 | 91 | 0.672 |
| Left | 171 | 83 | 88 | |
| Histological grade | ||||
| 1-2 | 197 | 80 | 117 | 0.453 |
| 3 | 161 | 72 | 89 | |
| T | ||||
| T1-T2 | 196 | 80 | 116 | 0.026 |
| T3-T4 | 162 | 47 | 115 | |
| N | ||||
| N0 | 229 | 107 | 122 | 0.223 |
| N1-N2 | 129 | 51 | 78 | |
| M | ||||
| M0 | 267 | 121 | 146 | 0.221 |
| M1 | 91 | 34 | 57 | |
| Stage | ||||
| 1-2 | 192 | 90 | 102 | 0.005 |
| 3-4 | 166 | 53 | 113 | |
Categorized according to a cut-off value calculated from the mean value + 2SD of the values observed in the control subjects.
Numbers in parentheses represent percentages.
Evaluation of the risk for colorectal cancer
The ORs for CRC risk by control-defined tertiles of variables was 3.36 (95% CI, 1.67-9.06) for WHR, 1.97 (95% CI, 1.08-7.75) for fasting insulin, 3.47 (95% CI, 1.83-8.35) for HOMA-IR, 6.12 (95% CI, 2.86-10.47) for omentin-1, 2.11 (95% CI, 1.04-10.23) for leptin, 4.92 (95% CI, 2.26-11.68) for visfatin, and 3.74 (95% CI, 1.98-9.76) for resistin when comparing participants in the highest tertile with those in the lowest. Meanwhile, the OR was 3.61 (95% CI, 1.92-10.41) for adiponectin when comparing participants in the lowest tertile with those in the highest. A multivariate analysis (adjusted for age, BMI, WHR, SBP, DBP, TCH, TG, HOMA-IR, omentin-1, adiponectin, leptin, visfatin, resistin, lifestyle characteristics, medications, family history of CRC and diabetes) showed that WHR, HOMA-IR, omentin-1, adiponectin, visfatin, and resistin were all significant risk factors for CRC. The other factors, including BMI, fasting glucose, fasting insulin, SBP, DBP, TCH, TG, and leptin were not significant risk factors for CRC (Table 4).
Table 4.
Evaluation of risks for CRC
| Variable | Number | Unadjusted | Number | Adjusted | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Case/control | OR | 95% CI | Case/control | OR | 95% CI | |
| BMI by tertile (kg/m2) | ||||||
| ≤ 22.7 | 67/96 | 1.00 | 67/96 | 1.00 | ||
| 22.7-23.8 | 120/95 | 1.67 | 0.51-2.94 | 120/95 | 1.37 | 0.42-2.05 |
| > 23.8 | 171/95 | 2.89 | 0.94-7.02 | 171/95 | 2.11 | 0.87-6.28 |
| Trend test | P = 0.078 | Pa = 0.162 | ||||
| WHR by tertile | ||||||
| ≤ 0.84 | 62/95 | 1.00 | 62/95 | 1.00 | ||
| 0.84-0.87 | 111/96 | 1.97 | 1.17-6.28 | 111/96 | 1.73 | 1.04-5.92 |
| > 0.87 | 185/95 | 3.36 | 1.67-9.06 | 185/95 | 2.99 | 1.47-8.21 |
| Trend test | P = 0.025 | Pa = 0.045 | ||||
| SBP by tertile (mmHg) | ||||||
| ≤ 126 | 87/95 | 1.00 | 87/95 | 1.00 | ||
| 126-130 | 127/96 | 1.12 | 0.55-4.02 | 127/96 | 1.08 | 0.52-3.91 |
| > 130 | 144/96 | 1.68 | 0.43-4.99 | 144/96 | 1.42 | 0.42-4.56 |
| Trend test | P = 0.321 | Pa = 0.614 | ||||
| DBP by tertile (mmHg) | ||||||
| ≤ 82 | 89/95 | 1.00 | 89/95 | 1.00 | ||
| 82-86 | 131/96 | 1.15 | 0.62-6.27 | 131/96 | 1.11 | 0.47-5.98 |
| > 86 | 138/95 | 1.23 | 0.85-5.34 | 138/95 | 1.25 | 0.67-4.21 |
| Trend test | P = 0.457 | Pa = 0.702 | ||||
| FPG by tertile (mmol/L) | ||||||
| ≤ 5.4 | 93/95 | 1.00 | 93/95 | 1.00 | ||
| 5.4-5.7 | 122/95 | 1.22 | 0.52-7.04 | 122/95 | 1.18 | 0.48-6.87 |
| > 5.7 | 143/96 | 1.39 | 0.65-9.22 | 143/96 | 1.29 | 0.57-8.21 |
| Trend test | P = 0.301 | Pb = 0.598 | ||||
| TCH by tertile (mmol/L) | ||||||
| ≤ 5.2 | 89/96 | 1.00 | 89/96 | 1.00 | ||
| 5.2-5.6 | 118/95 | 1.26 | 0.38-7.04 | 118/95 | 1.17 | 0.42-6.11 |
| > 5.6 | 151/95 | 1.61 | 0.58-8.11 | 151/95 | 1.54 | 0.63-6.92 |
| Trend test | P = 0.428 | Pa = 0.779 | ||||
| TG by tertile (mmol/L) | ||||||
| ≤ 1.5 | 67/96 | 1.00 | 67/96 | 1.00 | ||
| 1.5-1.7 | 120/95 | 1.78 | 0.84-7.86 | 120/95 | 1.59 | 0.77-6.82 |
| > 1.7 | 171/95 | 2.54 | 0.97-8.25 | 171/95 | 1.96 | 0.92-7.63 |
| Trend test | P = 0.148 | Pa = 0.253 | ||||
| Ln (FINS) by tertile (mIU/L) | ||||||
| ≤ 1.9 | 71/94 | 1.00 | 71/94 | 1.00 | ||
| 1.9-2.3 | 120/96 | 1.62 | 0.92-5.62 | 120/96 | 1.33 | 0.88-5.14 |
| > 2.3 | 167/96 | 1.97 | 1.08-7.75 | 167/96 | 1.68 | 0.94-6.91 |
| Trend test | P = 0.045 | Pb = 0.161 | ||||
| Ln (HOMA-IR) by tertile | ||||||
| ≤ 1.1 | 62/94 | 1.00 | 62/94 | 1.00 | ||
| 1.1-1.4 | 125/96 | 2.24 | 1.47-7.62 | 125/96 | 1.98 | 1.28-6.08 |
| > 1.4 | 171/96 | 3.47 | 1.83-8.35 | 171/96 | 2.85 | 1.65-7.22 |
| Trend test | P = 0.014 | Pb = 0.026 | ||||
| Ln (omentin-1) by tertile | ||||||
| ≤ 3.14 | 48/96 | 1.00 | 48/96 | 1.00 | ||
| 3.14-3.52 | 91/95 | 1.98 | 1.58-11.83 | 91/95 | 1.83 | 1.44-9.76 |
| > 3.52 | 219/95 | 6.12 | 2.86-10.47 | 219/95 | 5.76 | 1.81-8.95 |
| Trend test | P ≤ 0.001 | Pa = 0.001 | ||||
| Adiponectin (mg/L) | ||||||
| > 14.12 | 59/95 | 1.00 | 59/96 | 1.00 | ||
| 11.35-14.12 | 116/95 | 2.23 | 1.68-9.53 | 116/95 | 2.25 | 1.62-8.04 |
| ≤ 11.35 | 183/96 | 3.61 | 1.92-10.41 | 183/95 | 3.04 | 1.93-8.37 |
| Trend test | P = 0.002 | Pa = 0.019 | ||||
| Leptin (ng/mL) | ||||||
| ≤ 18.56 | 75/94 | 1.00 | 75/94 | 1.00 | ||
| 18.56-23.29 | 129/96 | 1.72 | 0.98-7.55 | 129/96 | 1.17 | 0.74-6.94 |
| > 23.29 | 154/96 | 2.11 | 1.04-10.23 | 154/96 | 1.98 | 0.96-8.73 |
| Trend test | P = 0.042 | Pa = 0.125 | ||||
| Ln (visfatin) by tertile (mg/L) | ||||||
| ≤ 1.42 | 49/96 | 1.00 | 49/96 | 1.00 | ||
| 1.42-1.57 | 131/95 | 3.28 | 1.92-9.02 | 131/95 | 2.74 | 1.78-7.83 |
| > 1.57 | 178/95 | 4.92 | 2.26-11.68 | 178/95 | 3.97 | 1.93-9.97 |
| Trend test | P = 0.001 | Pa = 0.008 | ||||
| Ln (resistin) by tertile (mg/L) | ||||||
| ≤ 1.65 | 54/95 | 1.00 | 54/95 | 1.00 | ||
| 1.65-1.94 | 127/95 | 2.68 | 1.73-10.47 | 127/95 | 1.99 | 1.48-9.36 |
| > 1.94 | 177/96 | 3.74 | 1.98-9.76 | 177/96 | 3.02 | 1.62-7.81 |
| Trend test | P = 0.007 | Pa = 0.016 | ||||
Adjusted for age, body mass index (BMI), waist:hip ratio (WHR), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TCH), triglyceride (TG), homeostatic model assessment of insulin resistance (HOMA-IR), omentin-1, adiponectin, leptin, visfatin, resistin, lifestyle characteristics, medications, family history of CRC and diabetes.
Adjusted for age, BMI, WHR, SBP, DBP, TCH, TG, omentin-1, adiponectin, leptin, visfatin, resistin, lifestyle characteristics, medications, family history of CRC and diabetes.
Marker validation
To further verify the discriminating power of omentin-1 identified for CRC diagnosis, the plasma levels of omentin-1 were assessed on an independent group of 644 plasma samples including 358 CRC patients and 286 normal controls. ROC curve analyses showed that the ROC curve areas for omentin-1 in CRC were 0.884 (95% CI 0.839-0.928). The optimal sensitivity and specificity obtained by movement of the cutoff value of plasma omentin-1, which was 56.9 ng/mL, were 81.2% (95% CI 72.6%-87.6%) and 69.8% (95% CI 63.8%-76.5%) in discriminating CRC from the normal control (Figure 1).
Figure 1.
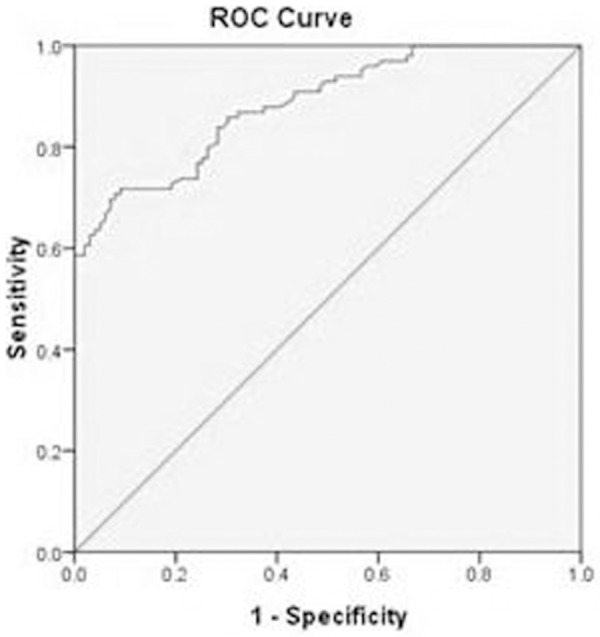
A receiver operating characteristics curve analysis using plasma omentin-1 in CRC for discriminating between CRC and the healthy subjects. Diagonal segments are produced by ties. ROC curve analyses showed the ROC curve areas for omentin-1 in CRC 0.884 (95% CI 0.839-0.928). The optimal sensitivity and specificity obtained by movement of the cutoff value of plasma omentin-1, which was 56.9 ng/mL, were 81.2% (95% CI 72.6%-87.6%) and 69.8% (95% CI 63.8%-76.5%) in discriminating CRC from the normal control.
Discussion
The results showed that the mean omentin-1 levels of the CRC group were significantly higher than they were in the control group. Patients with a higher plasma omentin-1 present an elevated risk of CRC independent of age, SBP, DBP, BMI, WHR, FPG, FINS, HOMA-IR, TCH, TG, lifestyle characteristics, medications, family history of CRC and diabetes, and adiponectin, leptin, visfatin, and resistin concentrations, suggesting that there is little confounding by the environmental factors and other adipokines. In addition, we observed the association between the high omentin-1 levels with the increasing stage of colorectal adenocarcinomas and depth of invasion. Moreover, we also found the plasma omentin-1 levels could provide a potential biomarker to predict CRC with an ROC curve area of 88.4% and discriminating CRC from the control subjects with the optimal sensitivity was 81.2% and the optimal specificity was 69.8%. Therefore, the results of this case-control study suggest that an increased level of omentin-1 not only is a strong risk factor for CRC but also could represent a potential biomarker for staging progression and diagnosing CRC in Chinese patients.
The results from different studies on the potential role of omentin-1 in gastrointestinal carcinogenesis were incompletely consistent among them, even paradoxical. Fazeli et al. demonstrated that serum circulating omentin-1 levels were significantly high in patients with cancer independent of measures of obesity and proposed that omentin-1 could play a role in the development of CRC [13]. Aleksandrova et al. reported that a higher omentin-1 concentration was associated with a higher risk of cancer in a prospective cohort study [14]. However, several studies revealed that omentin-1 may act as a tumor suppressor in gastrointestinal cancers. Li et al. found that high omentin-1 levels were significantly correlated with better outcomes in patients with gastric cancer [9]. Similarly, Zheng et al. revealed that the abnormal expression of omentin-1 in gastric cancer has a negative correlation with the depth of invasion, lymph node metastasis, and clinical stage, and omentin-1 may be a bio-marker for predicting the outcomes of gastric cancer patients [10]. Kim et al. reported that the downregulation of omentin-1 was related to a poor prognosis among patients with colorectal carcinoma at an advanced stage [11]. Maeda et al. and Kawashima et al. demonstrated that a lack of TMEM207, which participates in the processing of omentin-1, leads to insufficient omentin-1 production, thus promoting colorectal carcinogenesis, and is significantly associated with poor prognosis, so the omentin-1/TMEM207 axis might be a prognostic biomarker of colorectal carcinomas, especially in the case of the mucinous type [12,17]. In addition, Uyeturk et al. found that plasma omentin-1 levels are significantly increased in stage III colon carcinoma patients without diabetes who were treated with surgery and chemotherapy, independent of the basic risk factors associated with elevated omentin levels [18]. These controversial findings may reflect the “Janus-faced” pathobiological properties of omentin-1 in various types of carcinogenesis. Thus, further studies are needed to determine whether omentin-1 acts as a tumor suppressor or promoter in colorectal carcinogenesis.
Our study firstly assessed the association of CRC risk with circulating levels of omentin-1, simultaneously taking into account known risk factors, including visfatin, adiponectin, leptin, resistin, and other risk factors such as physical activity and lifestyle characteristics. In addition, we also found that the plasma omentin-1 levels could serve as a potential biomarker for predicting CRC. Furthermore, one important finding of our study is the correlation of high omentin-1 levels with the increasing TNM stages of CRC. Thus, our data suggest that omentin-1 may be a promising biomarker for CRC diagnosis and prognosis. However, in this context, we can’t conclude that there is a causality relationship between omentin-1 and CRC, but, on the other hand, we can’t be sure whether the increase in omentin-1 levels observed was a consequence of a reactive elevation in response to CRC, or whether an increase in omentin-1 can trigger CRC development, so further verification is worth conducting in the future to elucidate the underlying mechanisms of high plasma omentin-1 levels in CRC.
A correlation analysis showed that there is no significant correlation between plasma omentin-1 levels and anthropometric (including measures of obesity, such as BMI, WHR) or metabolic parameters among the case participants, which is consistent with previous studies [13,18]. In contrast, we revealed that the mean omentin-1 levels are negatively correlated with the FINS and WHR in the control participants, which is consistent with previous studies, indicating that omentin-1 is inversely related to obesity [8] and is down-regulated by insulin [19].
Moreover, our data showed an association of increased OR for CRC risk with lower adiponectin and higher visfatin and resistin levels, and higher WHR and HOMA-IR, according to a multivariate analysis, which is in agreement with previous studies [15,20-22]. Adiponectin is a pleiotropic adipocytokine with insulin-sensitizing, anti-inflammatory, antiatherogenic, and antiproliferative properties. Several studies have indicated that there is an association between low levels of adiponectin and an increased risk of CRC [15,20]. Visfatin was originally identified as a growth factor for immature B cells; however, a recent study demonstrated that visfatin could directly interact with insulin receptors and subsequently promote the proliferation of cancer cells. A few studies found that plasma visfatin levels are significantly increased in patients with CRC compared to the controls and gradually and significantly increase with tumor stage progression [21]. Resistin is secreted from adipose tissue and monocytes/macrophages, and is reportedly involved in the process of inflammation which can increase the risk of cancer. A recent meta-analysis revealed a positive association with elevated resistin levels with CRC [22].
So far, several interesting reports on the molecular mechanism of connecting omentin-1 and carcinoma including colorectal tumors have been published. It was reported that omentin-1 could enhance Akt phosphorylation/activation in the absence of insulin [7]. It has been shown that Akt signaling pathway is not only vital for the regulation of essential cellular functions, including proliferation, apoptosis, metabolism, but also for the regulation of vascular permeability, angiogenic response and subsequent vascular maturation [23,24]. Neoangiogenesis and vascular leakage are important for angiogenesis-dependent pathologies, Akt signaling is believed to play a crucial role in carcinogenicity [25]. Itoh et al. reported that Akt activation plays an important role during the progression of CRC by enhancement of cell proliferation activity and the blocking of apoptosis [26]. In addition, endothelial nitric oxide synthase (eNOS), a downstream target of Akt, has been shown to be important in tumorigenesis, including colorectal carcinogenesis [27]. Mogal et al. have demonstrated that omentin-1 can suppress prostate tumorigenicity via inhibiting the effect of Nkx3.1 on prostate tumors and found that knockdown of endogenous omentin-1 suppressed the proliferation of prostate cancer cells [28]. The involvement of omentin-1 in cancer is further supported by a recent study showing that omentin can promote apoptosis by regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells [29]. It is necessary for future research to elucidate the precise molecular mechanisms connecting omentin-1 and colorectal tumors because of the major clinical implications.
Conclusions and limitations
In conclusion, this study has analyzed the risk factors for CRC. Our findings suggest an increased concentration of plasma omentin-1 is associated with the processing of CRC in Chinese patients. And we also demonstrated that plasma omentin-1 may be a potential biomarker for predicting CRC stage progression and diagnosis. However, as this study was done with a small number of patients, a large-scale study is needed to clarify the relationship between high expressions of omentin-1 and colorectal tumors, particularly including controlled prospective studies. Similar studies are also needed with other ethnic groups, including Caucasians and other Asians to confirm and expand the findings. Moreover, when using a case-control design with plasma measurement, causality cannot be inferred. Further investigations as to whether the change in omentin-1 level was the result and/or effects of colorectal cancer development are needed, and the elucidation of this causative association will undoubtedly clarify the correlation between obesity and cancer. Also, in the context of a case-control design, there is a potential selection bias, particularly in control groups. It is difficult to match for all covariates, and the uncontrolled differences between the groups may mask an association of plasma omentin-1 with CRC. Histological studies on the expression of omentin-1 in cancer tissues should be conducted to determine whether omentin-1 derived from cancer tissues or adipose tissues can affect the carcinogenesis and tumor progression. In addition, it is important to examine the association between the omentin-1 expression in CRC tissue with scientific and rational clinical outcome. Therefore, the implications of our findings should be carefully evaluated, considering these limitations. Nevertheless, our study can provide some methodological strengths, such as the strict criteria for the inclusion of the case and control participants, the adjustment for major known risk factors, including other adipocytokines, which can reinforces the observed associations, and the assessment of adipokines in future studies, which we made using well-validated assays in the same laboratory and in a blinded fashion, which also eliminated uncontrolled confusion from these sources.
Acknowledgements
Funding for this project was provided by the Natural Science Foundation of Anhui Province in China (1508085MH150) and the National Natural Science Foundation Youth Science Fund Cultivation Project of The First Affiliated Hospital of Anhui Medical University in China (2018kj19). We thank the participants of this study including the doctors, nurses, administrative staff, and researchers from the Department of Endocrinology, Surgery, and the Division of Endoscopy in the First Affiliated Hospital of Anhui Medical University.
Disclosure of conflict of interest
None.
References
- 1.Kant P, Hull MA. Excess body weight and obesity-the link with gastrointestinal and hepatobiliary cancer. Nat Rev Gastroenterol Hepatol. 2011;8:224–238. doi: 10.1038/nrgastro.2011.23. [DOI] [PubMed] [Google Scholar]
- 2.Goh LY, Goh KL. Obesity: an epidemiological perspective from Asia and its relationship to gastrointestinal and liver cancers. J Gastroenerol Hepatol. 2013;28(Suppl 4):54–58. doi: 10.1111/jgh.12293. [DOI] [PubMed] [Google Scholar]
- 3.Kelsldis T, Kelesldis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152:93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Invest. 2015;21:57–74. doi: 10.1515/hmbci-2014-0037. [DOI] [PubMed] [Google Scholar]
- 5.Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20:5177–5190. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Housa D, Housová J, Vernerová Z, Haluzík M. Adipocytokines and cancer. Physiol Res. 2006;55:233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 7.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 8.de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Zhao X, Xiao Y, Mei H, Pu J, Xiang X, Jiao W, Song H, Qu H, Huang K, Zheng L, Tong Q. Intelectin 1 suppresses tumor progression and is associated with improved survival in gastric cancer. Oncotarget. 2015;6:16168–16182. doi: 10.18632/oncotarget.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L, Weng M, Qi M, Qi T, Tong L, Hou X, Tong Q. Aberrant expression of intelectin-1 in gastric cancer: its relationship with clinicopathological features and prognosis. J Cancer Res Clin Oncol. 2012;138:163–172. doi: 10.1007/s00432-011-1088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Kang UB, Lee H, Jung JH, Lee ST, Yu MH, Kim H, Lee C. Profiling of differentially expressed proteins in stage IV colorectal cancers with good and poor outcomes. J Proteomics. 2012;75:2983–2997. doi: 10.1016/j.jprot.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Maeda K, Saigo C, Kito Y, Sakuratani T, Yoshida K, Takeuchi T. Expression of TMEM207 in colorectal cancer: relation between TMEM207 and intelectin-1. J Cancer. 2016;7:207–213. doi: 10.7150/jca.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazeli MS, Dashti H, Akbarzadeh S, Assadi M, Aminian A, Keramati MR, Nabipour I. Circulating levels of novel adipocytokines in patients with colorectal cancer. Cytokine. 2013;62:81–85. doi: 10.1016/j.cyto.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Aleksandrova K, di Giuseppe R, Isermann B, Biemann R, Schulze M, Wittenbecher C, Fritsche A, Lehmann R, Menzel J, Weikert C, Pischon T, Boeing H. Circulating omentin as a novel biomarker for colorectal cancer risk: data from the EPIC-potsdam cohort study. Cancer Res. 2016;76:3862–3871. doi: 10.1158/0008-5472.CAN-15-3464. [DOI] [PubMed] [Google Scholar]
- 15.Chen MW, Ye S, Zhao LL, Wang SY, Li YX, Yu CJ, Xie HJ, Wang YM. Association of plasma total and high-molecular-weight adiponectin with risk of colorectal cancer: an observational study in Chinese male. Med Oncol. 2012;29:3129–3135. doi: 10.1007/s12032-012-0280-2. [DOI] [PubMed] [Google Scholar]
- 16.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cut-points obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;6163:670–75. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima K, Maeda K, Saigo C, Kito Y, Yoshida K, Takeuchi T. Adiponectin and Intelectin-1: important adipokine players in obesity-related colorectal carcinogenesis. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uyeturk U, Alcelik A, Aktas G, Tekce BK. Post-treatment plasma omentin levels in patients with stage III colon carcinoma. J BUON. 2014;19:681–685. [PubMed] [Google Scholar]
- 19.Tan BK, Adya R, Farhatullah S, Lewandowski KC, O’Hare P, Lehnert H, Randeva HS. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–808. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 20.Aleksandrova K, Boeing H, Jenab M, Bueno-de-Mesquita HB, Jansen E, van Duijnhoven FJ, Fedirko V, Rinaldi S, Romieu I, Riboli E, Romaguera D, Westphal S, Overvad K, Tjønneland A, Boutron-Ruault MC, Clavel-Chapelon F, Kaaks R, Lukanova A, Trichopoulou A, Lagiou P, Trichopoulos D, Agnoli C, Mattiello A, Saieva C, Vineis P, Tumino R, Peeters PH, Argüelles M, Bonet C, Sánchez MJ, Dorronsoro M, Huerta JM, Barricarte A, Palmqvist R, Hallmans G, Khaw KT, Wareham N, Allen NE, Crowe FL, Pischon T. Total and high-molecular weight adiponectin and risk of colorectal cancer: the european prospective investigation into cancer and nutrition study. Carcinogenesis. 2012;33:1211–1218. doi: 10.1093/carcin/bgs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M, Wang Y, Li Y, Zhao L, Ye S, Wang S, Yu C, Xie H. Association of plasma visfatin with risk of colorectal cancer: an observational study of Chinese patients. Asia Pac J Clin Oncol. 2016;12:e65–74. doi: 10.1111/ajco.12090. [DOI] [PubMed] [Google Scholar]
- 22.Joshi RK, Lee SA. Obesity related adipokines and colorectal cancer: a review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:397–405. doi: 10.7314/apjcp.2014.15.1.397. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 2009;10:610–616. doi: 10.1111/j.1467-789X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 25.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127–3134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- 27.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogal AP, van der Meer R, Crooke PS, Abdulkadir SA. Haploinsufficient prostate tumor suppression by Nkx3.1: a role for chromatin accessibility in dosage-sensitive gene regulation. J Biol Chem. 2007;282:25790–25800. doi: 10.1074/jbc.M702438200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang YY, Zhou LM. Omentin-1, a new adipokine, promotes apoptosis through regulating sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur J Pharmacol. 2013;698:137–144. doi: 10.1016/j.ejphar.2012.11.016. [DOI] [PubMed] [Google Scholar]


