Abstract
Lactose is a disaccharide found in milk and thus a part of our daily food intake. Upon ingestion, it is hydrolyzed to glucose and galactose by the enzyme lactase and absorbed in the small intestine. People who suffer from lactose intolerance are unable to completely digest it due to deficiency of lactase, leading to intestinal problems such as diarrhoea, and bloating. Various studies have focused on treating these symptoms. However, the effects of lactose that diffuses passively into cells, on cellular senescence have largely remained unknown. Thus, the present study investigated the effects and mechanisms of lactose on senescence both in vitro and in vivo. The study was conducted in MRC-5 cells. The cellular senescence was estimated by determining the expression of SA-β-gal and p16ink4a. The cell viability of MRC-5 cells was determined by the CCK-8 Assay. Activity of intracellular reactive oxygen species was estimated by measuring the levels of superoxide dismutase (SOD), glutathione (GHS), and reactive oxygen species (ROS). The mechanism of lactose on cellular senescence was explored by western blotting. We also studied the effect of lactose on the lifespan of Caenorhabditis elegans. Increased activities of SA-β-gal and p16ink4a revealed the ability of lactose to induce senescence in MRC-5 cells. The elevated intracellular ROS level and decreased GSH and SOD levels in these cells were indicative of cellular oxidative stress induced by lactose. Furthermore, western blotting analysis of Nrf2 and mRNA expression of its downstream genes suggested the Nrf2/ARE pathway was involved in the oxidative stress induced by lactose. These results were further validated by the shortened lifespan of C. elegans after lactose supplement. Moreover, the lactose-induced senescence could be alleviated by an antioxidant, N-Acetyl-L-cysteine (NAC), both in vitro and in vivo. The present study observed a positive correlation between lactose and cellular oxidative stress, suggesting the latter to be an underlying mechanism of lactose-induced senescence.
Keywords: Lactose, ROS, oxidative stress, cellular senescence, Nrf2
Introduction
Aging is defined as an inescapable and irreversible phenomenon in mammals characterized by slow deterioration and degeneration of various physiologic functions [1]. Cellular senescence, a special state of irreversible cell cycle arrest, plays an important role in aging and is responsible for causing age-related diseases [2]. Many experimental studies have revealed that cellular senescence can induce functional degeneration of tissues and organs. For example, Baker and colleagues found that removal of senescent cells could prevent or delay dysfunction of tissues and thus extend the lifespan [3]. The ability of senescent cells to induce permanent proliferative arrest is now being exploited as a therapeutic target for major diseases [2]. This phenomenon of cellular senescence was first reported by Hayflick in serially cultured human fibroblasts in 1961 [4]. The cellular senescence is identified as a limited proliferative phase of lifespan caused by various types of stress in which cells stop proliferating and undergo various alterations. These include, but are not limited to, changes in morphology, failure of DNA replication, senescence-associated secretory phenotype (SASP), accumulation of senescence-associated heterochromatin foci (SAHF), high level of p16ink4a and increase of senescence-associated β-galactosidase (SA-β-gal) activity [5].
Advancements in medical sciences have made it evident to extend mammals’ lifespan by genetic, dietary, and drug interventions. However, genetic intervention or dietary restriction is difficult to realize in humans, and drug treatment is associated with some unacceptable side effects [6]. Therefore, recently the effects of nutrients taken as a part of our daily food intake on aging have drawn much attention of the scientific community [7,8]. Moreover, various nutrients have been reported to possess anti-aging properties. For example, Zwighaft and colleagues demonstrated the administration of spermidine to the diet, mice could extend their lifespan by regulating their circadian period [9]. On the other hand, nutrients such as galactose and so on can induce cellular senescence thereby shortening the lifespan in many animals [10-12].
Lactose is a disaccharide that is composed of galactose and glucose and is originally found in milk and milk products. It is therefore one of the most common nutrients taken by human beings as daily dietary intake. Upon absorption, it is hydrolyzed by lactase to glucose and galactose on the surface of the small intestine [13]. However, a deficiency of lactase would prevent its metabolism, leading to lactose intolerance. Approximately 15% of people in Northern European are reported as lactose intolerant, this percentage is even higher in other races, up to 80% in Latinos and nearly 100% in Chinese [14]. People with lactose intolerance are unable to digest it completely, leading to accumulation of lactose in the small intestine that is reflected by an array of intestinal distress [13]. However, effects of accumulated lactose on cellular senescence and aging remain largely unknown.
In the present study, we have studied the effects of lactose on the senescence of human fetal lung fibroblast cell lines (MRC-5 cell lines), a model for research on aging. We found lactose can increase the expression of SA-β-gal [15] and cyclin-dependent kinase (CDK) inhibitor p16ink4a, which are the most widely used biomarkers of cellular senescence [5,16]. Treatment of MRC-5 cells with lactose also increased the intracellular level of oxidative stress accompanied by activation of an antioxidant signaling pathway, nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. In addition, lactose treatment could shorten the lifespan of C. elegans, which could be rescued by treatment with antioxidants.
Material and methods
Cell culture and drug treatment
The normal human fetal lung fibroblast line, MRC-5, was obtained from the Cell Bank of the Chinese Academy of Sciences at passage 18 (Shanghai, China). The cell line was used for experiments at passage 23 to 28. All fibroblasts were cultured in MEM (Life Technologies, Grand Island, United States) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, United States), sodium pyruvate, non-essential amino acids and antibiotics (penicillin and streptomycin; Biological Industries, Connecticut, United States) at 37°C with 5% CO2. Lactose (Macklin, Shanghai, China) was dissolved in sterile water and added to the growth medium at final concentration of 10 mmol/L, 25 mmol/L and 50 mmol/L, respectively. Rapamycin (Selleckchem, Houston, United States), doxorubicin (Selleckchem), N-Acetyl-L-cysteine (Selleckchem) were dissolved in sterile water and added to the growth medium at final concentration of 1 nmol/L, 50 μmol/L and 1 mmol/L, respectively. The drugs were replaced one day in between for 7 days, except where noted. The cultures were passaged 1:2 at 90% confluence and the medium was renewed thrice a week.
Senescence associated β-galactosidase activity assay
The SA-β-gal activity assay was performed according to the manufacturer’s protocol (Beyotime, Shanghai, China). In brief, fibroblast cells grown on a six-well plate were fixed in a fixative solution for 15 minutes, then rinsed with phosphate buffered saline (PBS) thrice, following a mixture containing 1% staining fluid A, 1% staining fluid B, 93% staining fluid C (fluids A, B and C were provided with the kit), and 5% X-gal was added. The cells were allowed to get stained overnight (12 hours) at 37°C in the dark. Images were captured using the Nikon Ni microscope.
Detection of intracellular lactose concentration by ELISA
After incubation with lactose, the cells were extracted using lysis buffer. The ELISA detection of lactose was performed as described. Briefly, 96-well flat-bottomed microtiter ELISA plates were coated with the streptavidin-HRP and the specific antibody, followed by incubation for 60 min at 37°C. After repeated washings, agents A and B were added. The plate was then incubated for 10 min at 37°C in darkness. Finally, 50 μL stop buffer was added to stop the reaction. The absorbance was detected using a microplate reader (Biotek, Vermont, United States) at 450 nm.
Determination of cell viability by the CCK-8 assay
The cell counting kit-8 (CCK-8) assay was performed according to the manufacturer’s protocol (Yeasen, Shanghai, China). A 100-μL cell suspension containing various concentrations of lactose was dispensed into a 96-well plate and incubated for 24 hours at 37°C with 5% CO2. After incubation, 10 μL of CCK-8 solution was added to each well of the plate and further incubated for 2 hours. The absorbance was then measured at 450 nm using a microplate reader (Biotek).
Measurement of anti-oxidative activities
After treatment with lactose for 7 days, activities of superoxide dismutase (SOD) and glutathione (GSH) were measured using the appropriate kits (Jiancheng, Nanjing, China). The absorbance was detected using a microplate reader (Biotek) at corresponding wavelengths.
Determination of cellular glutathione level
The formation of GSH was determined by a thiol probe, monochloromobimanem (mBCI), following the protocol described (Thermo, New York, United States). The thiol-reactive reagents of mBCI react with the thiol groups of living cells to form fluorescent products. For this, a 100 mmol/L stock solution of the thiol probe in dimethylsulfoxide was prepared before use. After exposure to lactose for 7 days, MRC-5 cells were rinsed with Hank’s balanced salt solution (HBSS) twice, and then mBCI was added to HBSS at a final concentration of 100 μmol/L. Pluronic F-127, a cell-permeable agent, was added at a final concentration of 0.04% to disperse the thiol dye. The cells were then incubated for 20 minutes at 37°C in the dark before fluorometric detection (excitation at 394 nm, emission at 490 nm) with a Nikon fluorescence microscope.
Determination of intracellular reactive oxygen species
After treatment with lactose for 7 days, levels of reactive oxygen species (ROS) were determined by an indicator, DCFH-DA dye, (Yeasen, Shanghai, China). The medium was replaced with 10 μmol/L of DCFH-DA dye (diluted in FBS-free medium) and incubated for 20 minutes at 37°C in the dark before fluorometric detection (excitation at 488 nm, emission at 525 nm) with a Nikon fluorescence microscope.
RNA purification, reverse transcription, and real-time PCR
The cells were harvested after being exposed to lactose for 7 days. Total RNA was extracted by the Eastep Super Total RNA extraction kit following manufacturer’s instruction (Promega, Wisconsin, United States). The cDNA was synthesized using an oligo (dT) primer, random primer and reverse transcriptase. All primers were designed and synthesized using Primer Premier Software 5.0 (PREMIER Biosoft International) by Sangon Biotech (Shanghai) Co., Ltd. The sequences were: GCLC FW: 5’-CAAGAGAAGGGGGAAAGGAC-3’, RV: 5’-GACCTCGGGCAGTGTGAAC-3’; GCLM FW: 5’-TCAGGGAGTTTCCAGATGTC-3’, RV: 5’-CAATAGGAGGTGAAGCAATG-3’; GPx7 FW: 5’-ACTTCAAGGCGGTCAACATC-3’, RV: 5’-GGCAAAGCTCTCAATCTCC-3’; GSR FW: 5’-CCCAAGCCCACAATAGAGG-3’, RV: 5’-ACCTGCACCAACAATGACG-3’; GSTM1 FW: 5’-GCATGATCTGCTACAATCC-3’, RV: 5’-CTTGGGCTCAAATATACGG-3’; GSTM4 FW: 5’-CCTTGCTCCCTGAACACTC-3’, RV: 5’-GTCGTCACTTCCAACCAAC-3’; HO-1 FW: 5’-AAGACTGCGTTCCTGCTCAAC-3’, RV: 5’-AAAGCCCTACAGCAACTGTCG-3’; NQO-1 FW: 5’-GGCATTCTGCATTTCTGTG-3’, RV: 5’-GGCGTTTCTTCCATCCTTC-3’; TALDA FW: 5’-GGGCCGAGTATCCACAGAAG-3’, RV: 5’-GGCGAAGGAGAAGAGTAACG-3’; TBP FW: 5’-CGCCAGCTTCGGAGAGTTC-3’, RV: 5’-ACAACCAAGATTCACTGTGGATACA-3’; β-actin FW: 5’-CTGGAACGGTGAAGGTGACA-3’, RV: 5’-AAGGGACTTCCTGTAACAATGCA-3’. β-actin was used as the housekeeping gene. The real-time PCR was performed using 4 μL of cDNA, 0.4 μL of each primer, and 10 μL SYBR Green PCR master mix in a 20 μL reaction volume. PCR runned for a 2 min activation step at 95°C, followed by 40 cycles of 95°C for 15 s, 60°C for 60 s, and finally at 60°C to 95°C for dissociation. Every sample was run in triplicate. The -ΔΔCt method was applied for quantification of RNA relative expression.
Western blotting analysis of p16ink4a and Nrf2
After incubation with 0, 10, 25, and 100 mmol/L of lactose for 7 days, the proteins were extracted using the lysis buffer (Yeasen) and quantified by the bicinchoninic assay (Yeasen). Protein samples (15 μg) were electrophoresed on a 12% SDS-polyacrylamide gel and transferred to polyvinyldiflouride membranes. After blocking for 1 hour in 5% (wt/vol) skim milk in TBST, membranes were labeled with corresponding antibodies. β-actin was used as the loading control. The molecular sizes of the immunoreactive proteins were confirmed by molecular weight markers separated, and all the bands were visualized by enhanced chemiluminescence using hyperfilm and enhanced chemiluminescence reagent (Yeasen) according to the manufacturer’s instruction.
C. elegans lifespan assay
The N2 C. elegans were obtained from Caenorhabditis Genetics Center. These were cultivated at 20°C on standard NGM plates with OP50 Escherichia coli and developmentally synchronized by hypochlorite treatment. E. coli OP50 was used as the food for worms. 250 to 300 synchronized L4 hermaphrodites were transferred to a fresh Petri dish containing 12.5 mg/L 5-fluoro-2’-deoxyuridine to eliminate self-progeny. Then, 200 mg/mL streptomycin was added to the plates to prevent any bacterial contamination. The first day of adulthood was counted as day 1 in survival curves, following which the hermaphrodites were transferred to a new Petri dish every 3 days during the reproductive period (approximately the first 7 days). Each hermaphrodite was scored as alive, dead, or lost every day. Worms were judged to be dead when they no longer responded to gentle prodding. Plates containing experimental treatments were prepared from the same batch of NGM agar as the control plates except that the respective chemical was added to a final concentration of 10, 25, 50 and 100 mmol/L from a sterile 500 mmol/L stock solution of lactose and to a final concentration of 5 mmol/L from a 500 mmol/L aqueous stock of NAC (all from Selleckchem). 15 worms were placed on NGM plates with lactose for 10 days, and then removed on NGM plates with or without NAC for 10 days. Worms viability was counted every day. A total of 90 worms were used per condition in three independent experiments.
Statistical analysis
All results were reported as average of three biological replicates, each consisting at least three technical replicates, unless otherwise stated, and analyzed by ANOVA in Graphpad Prism 7.0.
Results
Lactose could induce senescence signs in normal fibroblast cells
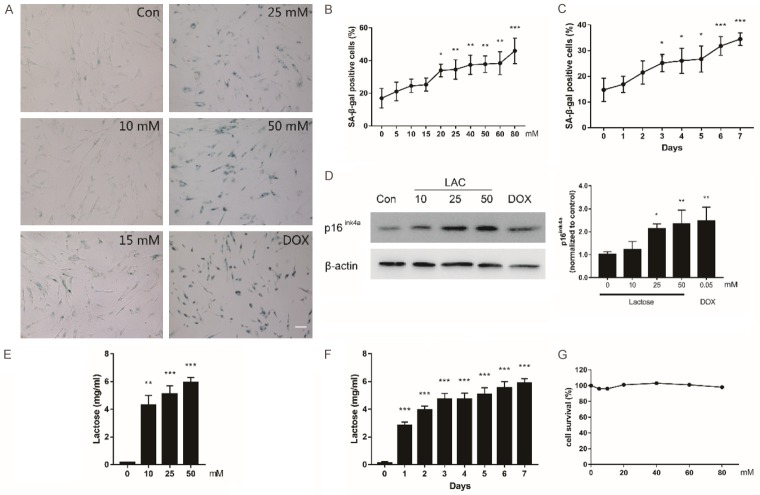
To examine whether lactose could induce the senescence in normal middle-age diploid human fibroblast cells, MRC-5 cell line was incubated with lactose at different concentrations for 7 consecutive days. Dairy products tend to contain dozens, or even hundreds of millimoles per liter of lactose [17]. Meanwhile, considering the concentration-dependent involvement of other monosaccharide [18,19] in the research of cellular senescence, 0 to 80 mmol/L lactose was administrated to MRC-5 cells. Then β-galactosidase staining was performed to evaluate the senescent characteristics in MRC-5 cells treated with lactose. The percent of SA-β-gal+ cells was observed to increase with the increasing concentration and incubation time of lactose (Figure 1A-C). At a lactose concentration of 20 mmol/L, the percentage of SA-β-gal+ cells was significantly elevated compared with control group (P<0.05; Figure 1B). Moreover, the percent of SA-β-gal+ cells was significantly elevated as of the third day compared with the control group (P<0.05; Figure 1C). Further, the expression of p16ink4a was assessed, which is indicative of irreversible cell cycle exit. It is therefore used as a biomarker of cellular senescence in combination with SA-β-gal expression [16]. The protein expression of p16ink4a increased in a dose-dependent manner (Figure 1D), which was in agreement with the results of SA-β-gal staining. Doxorubicin, as a positive control, caused senescence in diploid human fibroblasts, as reported previously [20]. In conclusion, lactose could induce senescence in MRC-5 cells, as evident by SA-β-gal staining and increased p16ink4a expression, two widely used senescent biomarkers. The intracellular concentration of lactose was examined using ELISA. It was found to increase with the exogenous lactose in time- and concentration-dependent manners (Figure 1E, 1F) indicating the ability of lactose to reach the cytoplasm. To exclude the effect of cell proliferative activity, lactose with concentrations ranging from 0 to 80 mmol/L was added to cells for 24 hours followed by measuring cell proliferation. The data showed that lactose had no effects on the survival of MRC-5 cells (Figure 1G).
Figure 1.
Lactose induced senescence signs in normal fibroblast cells. A. Senescence-associated β-galactosidase (SA-β-gal) staining in normal, middle-age diploid human cells (MRC-5 cell lines, Passage 23-25) treated with vehicle, lactose, and doxorubicin for 7 days (10×). Scale bar =100 μm. B. The cells incubated with lactose (7 days) and cellular senescence examined by SA-β-gal staining. C. The cells incubated (from 0-7 days) with 25 mM lactose and cellular senescence examined by SA-β-gal staining. D. MRC-5 cells treated with lactose and doxorubicin for 7 days. Whole cell lysates analyzed by western blotting with antibodies p16ink4a. β-actin was used as a loading control. Results (mean ± SD) obtained from three independent experiments. E. Concentration of lactose in cells (104 cell equivalent per ELISA well) with varying concentrations of lactose added to the cells for 7 days. F. Concentration of lactose in cells (104 cell equivalent per ELISA well) incubated with 25 mM lactose. G. Cell viability determined by CCK-8 assay kit. MRC-5 cells were treated with the indicated concentration of lactose for 24 hours. *P<0.05, **P<0.01, ***P<0.001 (ANOVA): *Lactose versus vehicle.
Lactose induced cellular oxidative load in MRC-5
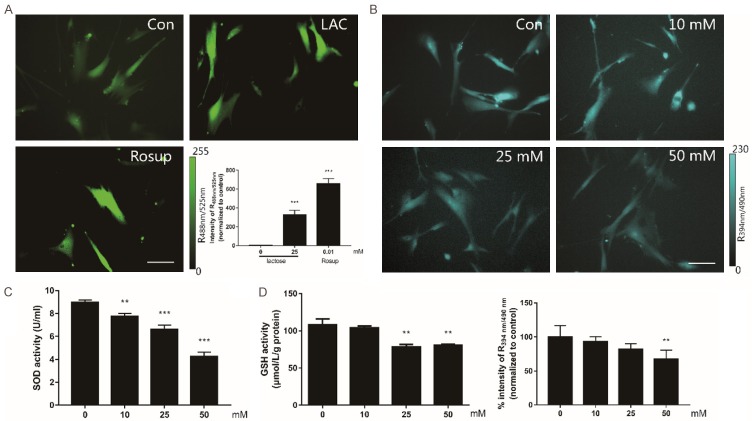
To examine whether the lactose-induced senescence was triggered by intracellular redox state, we investigated the effects of lactose on ROS (Figure 2A). The cells were treated with 25 mmol/L lactose for 7 days, and the intracellular ROS levels were indicated by DCFH-DA dye. The fold change of fluorescent brightness revealed that lactose could significantly increase the ROS levels in MRC-5 cells (P<0.01). Moreover, GSH (Figure 2B, 2D) and SOD (Figure 2C), the key anti-oxidative enzymes in redox metabolism, reported a dose-dependent decrease, as detected by microscope analysis and microplate reader. All assays suggested that lactose induces ROS generation in a dose-dependent manner.
Figure 2.
Lactose induced intracellular oxidative stress. A. The cells treated with 25 mM lactose for 7 days. 100 μM Rosup treated as positive control. ROS stained by DCFH-DA dye and imaged by inversion fluorescence microscope. Scale bar =100 μm. B. The GSH level of living cells treated with lactose and imaged by inversion fluorescence microscope. Fluorescent image of GSH stained with mBCI dye. Scale bar =100 μm. C. The levels of SOD tested by SOD assay kit. Results obtained from three independent experiments and represented as an average ± SD. D. The levels of GSH tested by GSH assay kit and results obtained from three independent experiments and represented as average ± SD. *P<0.05, **P<0.01, ***P<0.001 (ANOVA): *Lactose versus vehicle.
NAC can rescue the lactose-induced oxidative stress and cellular senescence
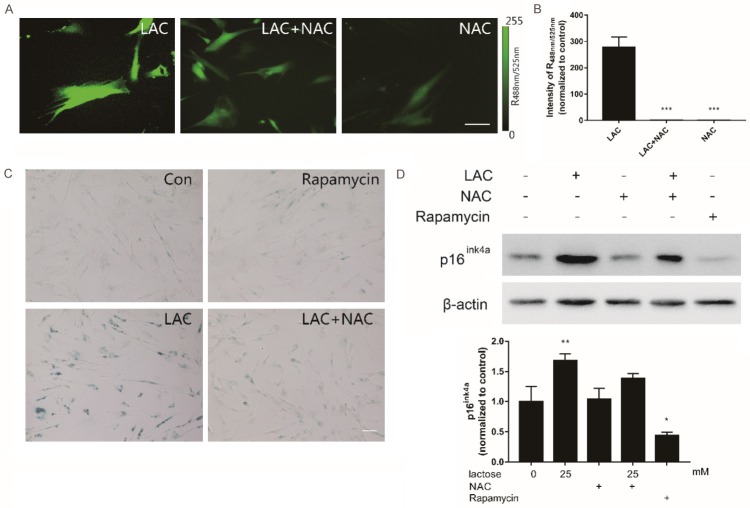
We next investigated whether the oxidative damage induced by lactose was the main reason of premature senescence. For this, NAC, a widely used ROS scavenger, was supplemented to the culture medium at a final concentration of 1 mmol/L to eliminate lactose-induced ROS. We initially detected the ROS levels using the DCFH-DA dye, and found that the addition of NAC resulted in a significant reduction in ROS levels (P<0.01 versus high lactose; Figure 3A, 3B). In parallel, NAC could also rescue cellular senescence induced by lactose. Also, accumulation of SA-β-gal decreased in the presence of NAC (Figure 3C). Similarly, western blotting analysis showed that p16ink4a expression significantly decreased to the basal level as compared with stable high lactose after incubation of NAC (P<0.01; Figure 3D).
Figure 3.
The oxidative stress induced by lactose could be abrogated by anti-oxidant. A. The cells incubated with lactose (7 days), and then treated with 1 mM NAC for 24 hours. Fluorescent image of DCFH-DA dye examined by inversion fluorescence microscope. Scale bar =100 μm. B. The optical density analysis of fluorescence imaging. C. The cells treated with 1 mM NAC, 1 nM Rapamycin, 1 mM NAC and 25 mM lactose. The rate of senescence stained by SA-β-gal staining and analyzed by SA-β-gal positive cells (%). D. The expression of p16ink4a analyzed by western blotting. Values represented average ± SD from at least 3 experiments. *P<0.05, **P<0.01, ***P<0.001 (ANOVA): *Lactose versus vehicle.
Nrf2/ARE pathway is involved in oxidative stress induced by lactose in MRC-5
The Nrf2 pathway is known to regulate the total antioxidant system through modulating the expression of antioxidant-associated genes involved in repairing oxidative damage. Once released, it migrates to the cell nucleus and binds the DNA to activate antioxidant response elements (AREs), which works as antioxidant and detoxicant. Western blotting analysis showed the levels of total and cytoplasmic Nrf2 significantly increase in the cells exposed to 10 mmol/L, 25 mmol/L and 50 mmol/L of lactose (Figure 4A, 4B). The addition of NAC did not affect the total Nrf2 level but decreased its cytoplasmic level, whereas increased its nuclear level (Figure 4A-C). These results indicated that NAC might function by activating the Nrf2 translocation to the nucleus.
Figure 4.
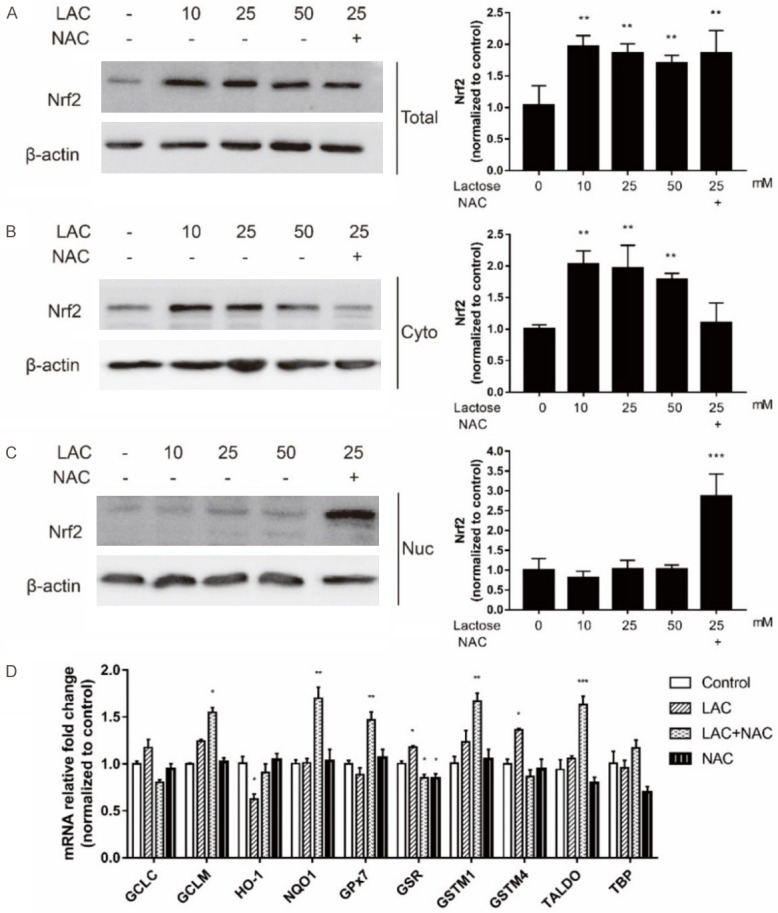
Nrf2 pathway was activated to resist oxidation. A. Western blotting showed the effect of drugs on total Nrf2 protein. B. Western blotting showed the effect of the drugs on cytoplasmic Nrf2 protein. C. Western blotting showed the effect of the drugs on nuclear Nrf2 protein. D. Real time PCR analysis of Nrf2 downstream ARE-containing mRNA was affected by lactose and/or NAC. *P<0.05, **P<0.01, ***P<0.001 (ANOVA): *Lactose versus vehicle.
We also examined the mRNA expression of Nrf2 downstream ARE-containing genes in fibroblasts. Phase II enzymes and endogenous antioxidants had been well reported to be Nrf2-dependent [21]. The regulation of phase II enzymes, such as glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit (GCLM), heme oxygenase 1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), would deactivate and detoxify foreign compounds and were referred to as detoxification enzymes [22]. Moreover, endogenous antioxidants, such as glutathione peroxidase 7 (GPx7), glutathione-disulfide reductase (GSR), glutathione S-transferase Mu (GSTM), and transaldolase (TALDO) play a significant role in detoxification by catalyzing radicals into non-toxic substances and scavenging ROS. RT-PCR analysis revealed lactose to have no effects on the mRNA expression of many Nrf2 targeting genes. However, addition of NAC induced a significant increase in the mRNA expression of these genes, including NQO1, TALDO, GCLM, GPx7 and GSTM1, which are members of antioxidative proteins and protect the cells from oxidative stress (Figure 4D). In brief, lactose could affect the intracellular redox state by activating Nrf2 signaling, which was rescued by NAC.
Lactose supplement shortens the lifespan of C. elegans
Lactose treatment significantly induced ROS (Figure 3). However, treatment with NAC attenuated the phenomenon. We observed similar results in C. elegans. All worms were administered with various concentrations of lactose from middle age onward to 10 days. Lactose at 10, 25, 50 and 100 mmol/L of concentrations decreased the mean lifespan of worms by 10.4%, 17.4% (P<0.05), 22.8% (P<0.01), 16.5% (P<0.05) respectively (Figure 5A). However, after co-administration of NAC and lactose, the mean lifespan of worms increased by 28.2% (P<0.01) as compared with 50 mmol/L of lactose treatment. It was consistent with our in vitro data which showed that only NAC treatment had no effect on the lifespan of worms (Figure 5B). Taken together, the presence of NAC could completely rescue the decreased longevity induced by the high concentration of lactose, indicating redox stress to be, at least in part, responsible for the lactose-induced senescence.
Figure 5.
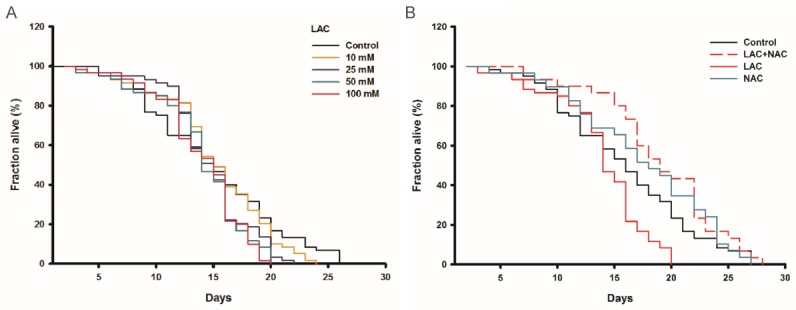
Lactose shortened lifespan of C. elegans through inducing oxidative stress in vivo. A. The supplement of lactose shortened the lifespan of C. elegans, but not in a dose-dependent manner. B. The lifespan was extended by NAC, an antioxidant, treated in NGM and all the worms were treated for 10 days with 50 mM lactose in the presence or absence of 5 mM NAC. The results were analyzed by 15 worms per group, 2 group each experiment, of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001 (ANOVA): *Lactose versus vehicle.
Discussion
The present study reported that treatment of lung fibroblasts (MRC-5 cells) with lactose induced cellular senescence through an increase in the generation of intracellular ROS. Lactose has been known to reduce the lifespan of C. elegans. Indeed, lactose seemed to increase dose-related ROS accumulation both in vitro and in vivo, which promoted imbalance of redox state and affected the Nrf2 pathway. This also suggested that activation of Nrf2 could resist aging induced by oxidants, and could thus act as a potential therapeutic target.
Lactose is a distinctive carbohydrate in milk and dairy products. It is an essential marker for the evaluation of dairy quality. Studies have focused on its physiological function in facilitating assimiliation of nitrogen, calcium and phosphorus markedly, as well as promoting the development of brain tissue [23,24]. Dairy products contain a varying amount of lactose, such as 4 to 5% lactose in human milk, 2 to 7% lactose in yogurt and 3 to 8% lactose in ice cream. The concentration of lactose in skimmed milk has been reported to be considerably high, up to 47 g/L [17,25] (equivalent to 137 mmol/L lactose, greater than what we reported). Thus, lactose can realistically exert a significant effect on the cells.
Upon ingestion, lactose is metabolized and distributed through passive diffusion. Normally lactose is hydrolyzed into glucose and galactose by lactase and gets absorbed in the small intestine. Unfortunately, lactase deficiency is associated with race and age worldwide. Pochart and colleagues studied the extent of lactose absorption in people suffering from lactose intolerant to exclude the distribution of lactase. They analyzed the absorption of lactose after administration of 18 g of lactose meals (without lactase in food), and collected the lactose from terminal ileum. The results showed that only 1.74 to 2.83 g of lactose was left [26], which suggested that passive diffusion was another important way to transport lactose, besides catabolism.
We also studied the long-term effect of lactose on cells and C. elegans. It was noted that lactose exposure would significantly induce, in a dose-dependent manner, cellular ROS and decrease the levels of GSH and SOD. As proven by traditional oxidative stress theory (OST), increases in mitochondrial ROS and oxidative damage would disrupt normal functions and accelerate cellular senescence, thereby damaging cells, proteins, and DNA [27]. This might serve as one of the mechanisms by which lactose induces senescence by increasing ROS generation. The results obtained in the present study indicated cellular dysfunction, which, in turn, speculated the involvement of redox-related pathways. We then tried to study the influence of lactose on Nrf2-ARE pathway, a key pathway for oxidation resistance, which can induce several cytoprotective proteins against toxicities and chronic diseases linked with oxidative stress [28]. According to the results of our research, lactose, as a chemical inducer, activated the expression of Nrf2 protein and ARE-containing genes, such as HO-1, GSR, and GSTM4. We speculated it could disrupt the combination of Nrf2 and Keap1, thereby activating the Nrf2-ARE signaling. We found that the effects of lactose on the Nrf2 pathway could be rescued by the antioxidant, NAC. So, it was reasonable to hypothesize that intracellular redox state regulated by NAC might stimulate uncoupling of Nrf2 from Keap1. Nrf2 then translocated to the nucleus, where it activated an array of antioxidant response element-regulated genes expression, including endogenous antioxidative system (TALDO, GPx7, GSR and GSTM1) and phase II detoxifying enzymes (GCLM, NQO-1). These results further supported the fact that lactose accelerated the onset of senescence and the protective effect of NAC in vitro and in vivo.
In conclusion, we confirmed that high lactose for an extended period would result in oxidative stress in fibroblasts and model animals. The present study also emphasized that Nrf2 could serve as an essential therapeutic target to resist aging induced by oxidants.
Acknowledgements
This work was supported by the Exploratory Research Foundation of ECUST (Grant number: 222201714058) and National Natural Science Foundation of China (Grant number: 81772689).
Disclosure of conflict of interest
None.
References
- 1.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 5.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 6.Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson SJ, Le Couteur DG, Raubenheimer D. Putting the balance back in diet. Cell. 2015;161:18–23. doi: 10.1016/j.cell.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwighaft Z, Aviram R, Shalev M, Rousso-Noori L, Kraut-Cohen J, Golik M, Brandis A, Reinke H, Aharoni A, Kahana C, Asher G. Circadian clock control by polyamine levels through a mechanism that declines with age. Cell Metab. 2015;22:874–885. doi: 10.1016/j.cmet.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Elzi DJ, Song M, Shiio Y. Role of galactose in cellular senescence. Exp Gerontol. 2016;73:1–4. doi: 10.1016/j.exger.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W, Liu J. D-Galactose-caused life shortening in Drosophila melanogaster and Musca domestica is associated with oxidative stress. Biogerontology. 2004;5:317–325. doi: 10.1007/s10522-004-2570-3. [DOI] [PubMed] [Google Scholar]
- 12.Parameshwaran K, Irwin MH, Steliou K, Pinkert CA. D-galactose effectiveness in modeling aging and therapeutic antioxidant treatment in mice. Rejuvenation Res. 2010;13:729–735. doi: 10.1089/rej.2010.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y, Misselwitz B, Dai N, Fox M. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients. 2015;7:8020–8035. doi: 10.3390/nu7095380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swagerty DL Jr, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65:1845–50. [PubMed] [Google Scholar]
- 15.Maier AB, Westendorp RG, VAN Heemst D. Beta-galactosidase activity as a biomarker of replicative senescence during the course of human fibroblast cultures. Ann N Y Acad Sci. 2007;1100:323–332. doi: 10.1196/annals.1395.035. [DOI] [PubMed] [Google Scholar]
- 16.Xia X, Chen W, McDermott J, Han JJ. Molecular and phenotypic biomarkers of aging. F1000Res. 2017;6:860. doi: 10.12688/f1000research.10692.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conzuelo F, Gamella M, Campuzano S, Ruiz MA, Reviejo AJ, Pingarron JM. An integrated amperometric biosensor for the determination of lactose in milk and dairy products. J Agric Food Chem. 2010;58:7141–8. doi: 10.1021/jf101173e. [DOI] [PubMed] [Google Scholar]
- 18.Cui X, Li W, Zhang B. Studies on cell senescence induced by D-galactose in cultured neurons and fibroblasts. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 1997;13:131–133. [PubMed] [Google Scholar]
- 19.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Baar MP, Brandt RM, Putavet DA, Klein JD, Derks KW, Bourgeois BR, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IWF, Houtsmuller AB, Pothof J, de Bruin RW, Madl T, Hoeijmakers JH, Campisi J, de Keizer PL. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147. e116. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 22.Shen G, Kong AN. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos. 2009;30:345–355. doi: 10.1002/bdd.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talbot FB, Hill LW. The influence of lactose on the metabolism of an infant: With special reference to fat, nitrogen and ash. American Journal of Diseases of Children. 1914;VIII:218–227. [Google Scholar]
- 24.Jarvis B. Milk sugar in infant feeding: a study of the effects of the routine use of milk sugar in infant feeding. American Journal of Diseases of Children. 1930;40:993–999. [Google Scholar]
- 25.Fox PF, McSweeney P. Advanced Dairy Chemistry. 2009 [Google Scholar]
- 26.Marteau P, Flourie B, Pochart P, Chastang C, Desjeux JF, Rambaud JC. Effect of the microbial lactase (EC 3.2.1.23) activity in yoghurt on the intestinal absorption of lactose: an in vivo study in lactase-deficient humans. Br J Nutr. 1990;64:71–9. doi: 10.1079/bjn19900010. [DOI] [PubMed] [Google Scholar]
- 27.Ameziane-El-Hassani R, Dupuy C. Detection of reactive oxygen species in cells undergoing oncogene-induced senescence. Methods Mol Biol. 2017;1534:139–145. doi: 10.1007/978-1-4939-6670-7_13. [DOI] [PubMed] [Google Scholar]
- 28.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]